Abstract
It is becoming increasingly clear that cholesterol-independent effects of statins also contribute to the cardioprotective effects, but these mechanisms remain poorly understood. We investigated the effects of lovastatin on vascular smooth muscle phenotype. We have previously shown that mammalian target of rapamycin complex 1 (mTORC1) inhibition with rapamycin induces vascular smooth muscle cell (VSMC) differentiation. We found that lovastatin also inhibits mTORC1 signaling and that this inhibition is required for VSMC differentiation. Lovastatin inhibition of mTORC1 was farnesylation dependent, suggesting the farnesylated G protein Rheb (Ras homologue enriched in brain), a known upstream activator of mTORC1. Rheb overexpression induced mTORC1 activity and repressed contractile protein expression, but a farnesylation-deficient mutant (C18S) elicited the opposite effect. Rheb knockdown with small interfering RNA was also sufficient to inhibit mTORC1 and induce contractile protein expression, and it prevented statin-induced VSMC differentiation. Notably, mTORC1 activity was elevated in VSMC isolated from an intimal hyperplastic patient lesion compared with normal media, and lovastatin treatment inhibited mTORC1 activity in these cultures. Furthermore, lovastatin inhibited mTORC1 activity and prevented the downregulation of contractile protein expression in an ex vivo angioplasty model. In conclusion, these findings illustrate a mechanism for the cardioprotective effects of lovastatin through inhibition of Rheb and mTORC1 and promotion of a differentiated VSMC phenotype.
Keywords: vascular smooth muscle cell differentiation, statin, mammalian target of rapamycin
statin use has been linked to improved patency of coronary and lower extremity vein bypass grafts (1, 6, 17). The beneficial effects of statins may not be entirely through reduction of cholesterol as recently it has been shown that patients with normal cholesterol and elevated C-reactive protein levels had reductions in risk of stroke and cardiovascular intervention by 50%, as well as a 20% reduction in risk of cardiovascular death (25).
The beneficial effects of statins are assumed to result from their ability to reduce cholesterol biosynthesis. Mevalonic acid is the precursor not only of cholesterol, but also of many nonsteroidal isoprenoid compounds. The mevalonate pathway yields a series of isoprenoids that are vital for diverse cellular function, including cell signaling, myelination, cytoskeleton dynamics and endocytotic/exocytotic transport (32). These proteins must be prenylated as a prerequisite for membrane association, which is required for their function.
Intimal hyperplasia remains the most common reason for early graft failure following bypass surgery and angioplasty. Vascular smooth muscle cells (VSMC) in normal vessels exhibit a differentiated, quiescent, contractile phenotype. Markers of differentiation are therefore contractile proteins such as smooth muscle (SM) α-actin, calponin, h-caldesmon, and smooth muscle myosin heavy chain (SM-MHC). SM-MHC is the most stringent of the differentiation markers (18). VSMC retain plasticity, enabling them to dedifferentiate into a proliferative, migratory, synthetic phenotype in response to injury, thus being an important contributor to the intimal hyperplastic process (24).
We have previously reported that inhibition of the mTORC1 pathway induced VSMC differentiation (22). The mammalian target of rapamycin complex 1 (mTORC1) signaling pathway regulates translation initiation (15, 20). This pathway is regulated by amino acids, growth factors, and energy levels and coordinately regulates protein synthesis and cell growth with cell cycle progression and proliferation (20, 28). Recently, Rheb (Ras homologue enriched in brain) was identified as an essential regulator of mTORC1, and farnesylation of Rheb is required for its ability to promote mTORC1 signaling (30). Rheb is unique, compared with many small GTPases, in that it exists in a highly activated state in mammalian cells (31). Farnesyltransferase catalyzes the farnesylation of Ras and Ras-like small G proteins, which is required for their membrane localization (13). Others have shown that farnesylation inhibitors prevent Rheb prenylation and S6 kinase 1 (S6K1) activation (5).
In this study, we tested the hypothesis that lovastatin, through its inhibition of Rheb farnesylation and consequent inhibition of mTORC1, may exert cardioprotective effects by modulating VSMC phenotype. We report that lovastatin induces VSMC differentiation through inhibition of Rheb and mTORC1, and that lovastatin can inhibit elevated mTORC1 activity in intimal hyperplastic VSMC, as well as inhibit mTORC1 activity and promote VSMC differentiation in an ex vivo angioplasty model.
MATERIALS AND METHODS
Cell Culture
Human VSMC were isolated from arterial or intimal hyperplastic segments of bypass grafts from vascular surgery patients or organ donors and cultured in M199 medium with 10% fetal bovine serum, l-glutamine, penicillin-streptomycin, human (h) EGF, and hFGF (Clonetics) (11). VSMC at passage 2-6 were used for experiments. For experiments, cells were cultured in 2.5% calf serum for the duration of drug treatment. Untreated VSMC proliferate and do not spontaneously differentiate under these conditions.
Cell Area Measurement
VSMC were cultured on coverslips in six-well plates and fixed with paraformaldehyde, stained with toluidine blue, and mounted on slides. Ten separate high-power fields from each slide were photographed and digitized, and the cell area was determined in a blinded manner by planimetry. National Institutes of Health (Bethesda, MD) image software was used to outline cell dimensions and compute the two-dimensional area.
Cell Immunohistochemisty
VSMC were cultured on glass coverslips. Cells were washed with phosphate-buffered saline and fixed in 50:50 cold methanol:acetone 10 min at −20°C. Cells were blocked with 3.0% BSA in Tris-buffered saline (TBS) and then incubated with primary antibodies (as described in Western blot analysis) in TBS overnight at 4°C. After cells were washed, anti-mouse ALEXA 568-conjugated secondary antibody (Molecular Probes) was incubated for 1 h then washed with TBS. Coverslips were then processed for immunofluorescence confocal microscopy.
Western Blot Analysis
Lysates were harvested for Western blot analysis as previously described (21). The Bradford assay was used to determine total protein concentration. Equal amounts of protein per lane were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane, and immunoblotted using primary antibodies to SM-MHC SM2 (NeoMarker, Fremont, CA), SM α-actin, calponin, h-caldesmon (Sigma), P-S6, P-S6K1 S240/244, pS6K1 T389, 4E-binding protein-1 (4E-BP1), pmTOR S2448, Rheb (Cell Signaling Technology), horseradish peroxidase-conjugated secondary antibody, and enhanced chemiluminescence detection reagents (Pico or Femto, Pierce).
Transient Transfection of Small Interfering RNA for Gene Knockdown
Transient transfection of siRNA in human VSMC via Nucleofector (Amaxa, Gaithersburg, MD) was as published (10) Human VSMC were transfected with 0.5–0.65 μg Rheb or 1.0–1.3 μg small interfering (si)CONTROL for 48 h. Cells were treated with vehicle or lovastatin for 8 h and harvested for Western blot analysis. The sequence for Rheb was 5′-GGGAAAUCCUCAUUGACGAUU-3′ (Dharmacon). Microarray-validated nontargeting duplex siRNA (siCONTROL; Dharmacon) was used as negative control.
Adenoviral Transfection for Gene Overexpression
We have generated recombinant adenoviruses encoding hemagglutinin (HA)-rapamycin-resistant mutant (ED3E) p70 S6K1 (21). These viruses coexpress green fluorescent protein (GFP) to easily assess infection efficiency using the AdEasy system (16). Virus encoding GFP alone serves as a control. VSMC were infected with adenoviral supernatant (at 1:20–1:100 dilutions) in DMEM with 10% calf serum overnight, washed, and treated with 20 nmol/l rapamycin, 5 μM lovastatin, or vehicle for 48 h in 2.5% calf serum medium. Optimal titers have been predetermined for each virus to yield infection of 75% of VSMC.
Plasmid Transient Transfection for Gene Overexpression
Plasmids.
Wild-type (WT) Rheb (with both HA and glutathione S-transferase epitope tags) was purchased from Addgene (27). Rheb C181S (with myc and His tags) harbors a substitution of the CAAX box cysteine residue to a nonfarnesylatable serine (generous gift from Vuk Stambolic). Human VSMC (1–1.5 × 106 cells) were transfected with WT or mutant Rheb plasmids using the basic SMC Nucleofector Solution and the Nucleofector Device (Amaxa). VSMC were transfected with 1 μg of WT Rheb or Rheb C18S for 48 h.
In Vitro Angioplasty Model
Institutional Animal Care and Use Committee approval was obtained to harvest porcine femoral arteries after nonsurvival surgery. The artery was cut into two segments. One segment was immediately injured by angioplasty with a 3-mm balloon at 8 atm for 1 min. The other uninjured segment served as a control. Each segment was then cultured in M199 medium with 10% fetal calf serum for 1 wk. Protein lysates were made by homogenization of tissue in cell lysate buffer [10 mM Tris·HCl, pH 7.5, 5 mM MgCl2, 2 mM EDTA, 1 mM DTT, 1 mM 4-(2-aminoethylbenzenesulfonyl fluoride), 20 μg/ml leupeptin, and 20 μg/ml aprotinin].
Data and Statistical Analysis
All experiments were performed in triplicate, using three different patient samples, and representative blots are shown. Data were averaged, unless otherwise specified, and are presented as means ± SE. Significant differences between groups were tested by Student's unpaired t-test or analysis of variance and post hoc t-test where appropriate. P < 0.05 was considered significant.
RESULTS
Lovastatin Induces VSMC Differentiation
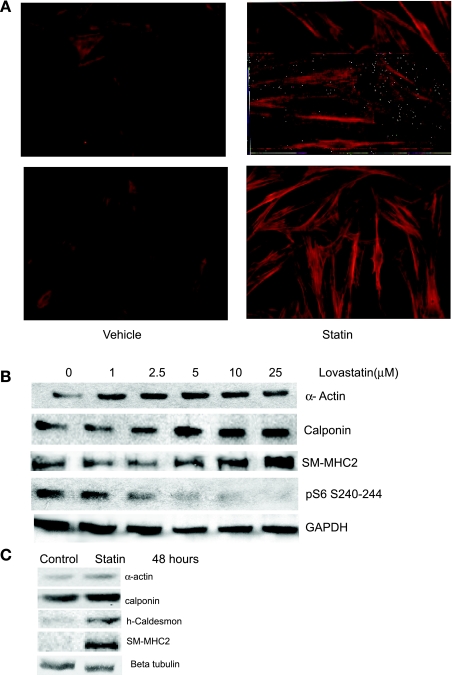
The exact mechanism of the effect of statins on VSMC phenotype has not been completely studied. We have a well-characterized model of phenotypic modulation in which VSMC isolated from human vessels and cultured with serum exhibit the synthetic phenotype of VSMC in intimal hyperplastic lesions, including an enlarged, nonmyocytic morphology, loss of contractile protein expression, and elevated rates of proliferation, migration, and protein synthesis (10, 21). We employed this model to test the hypothesis that statins may beneficially influence VSMC phenotype. Treatment of human primary VSMC in the synthetic phenotype with 5 μM lovastatin increased expression of calponin, a calcium-binding protein and marker of differentiation, as measured by immunohistochemistry (Fig. 1A). Dose-dependent contractile protein induction, including SM α-actin, calponin, and SM2-MHC, the most stringent marker of VSMC differentiation (9, 18), was also evident in Western blot analysis (Fig. 1B). On the basis of this result, 5 μM lovastatin was chosen for subsequent experiments. Furthermore, long-term upregulation of contractile protein expression was noted with lovastatin at 48 h (Fig. 1C).
Fig. 1.
Lovastatin induces vascular smooth muscle cell (VSMC) differentiation. A: human VSMC were cultured with ethanol vehicle (left) or 5 μM lovastatin (Statin; right) for 48 h and subjected to immunohistochemical staining with an anti-calponin primary antibody and FITC-conjugated secondary antibody. Duplicate slides representative of 3 experiments are shown. Magnification, ×40. B: lovastatin induces VSMC differentiation in a dose-dependent manner. Human iliac artery VSMC were cultured in the presence of vehicle (0) or the indicated dose of lovastatin for 8 h. Equal amounts of protein (as determined by Bradford analysis) were loaded and analyzed by immunoblotting using antibodies against smooth muscle (SM) α-actin, calponin, SM2-myosin heavy chain (SM-MHC2), or ribosomal S6 phosphorylated at Ser240/244 (pS6) as indicated. C: lovastatin induces VSMC contractile proteins long term. Human coronary VSMC were cultured in the presence of vehicle (control) or lovastatin (5 μM) for 48 h. Equal amounts of protein were loaded and analyzed by immunoblotting using antibodies against SM α-actin, calponin, h-caldesmon, and SM2-MHC as indicated. β-Tubulin was used for loading control.
Lovastatin Inhibits mTORC1
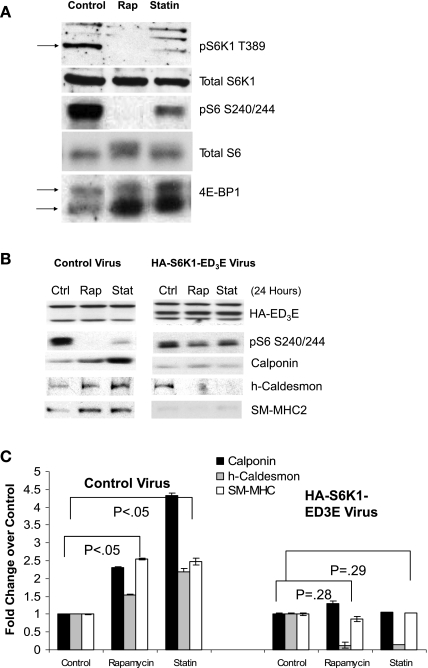
We assessed mTORC1 activity in response to lovastatin treatment through Western analysis of rapamycin-sensitive phosphorylation sites in known mTORC1 direct substrates (S6K1, 4E-BP1) and downstream effectors (ribosomal protein S6). Interestingly, treatment with lovastatin reduced phosphorylation of 4E-BP1 (as indicated by the classic change in electrophoretic mobility) (12), S6K1 (Thr389), and its substrate ribosomal protein S6 (Ser240/244), indicating inhibition of mTORC1 (Fig. 2A). However, statins inhibited these mTORC1 targets to a lesser extent than the direct mTORC1 inhibitor rapamycin (Fig. 2A). The dose-dependent inhibition of S6 phosphorylation correlated with the induction of contractile proteins in Fig. 1B. Using adenoviral overexpression of a rapamycin-resistant and constitutively active S6K1 mutant (S6K1 ED3E) which retains activity in the presence of mTORC1 inhibition (21), we determined that S6K1 inhibition is necessary for lovastatin-induced differentiation. Inhibition of mTORC1 with rapamycin or lovastatin statistically significantly induced contractile protein expression in the presence of control virus, but not when S6K1 activity was maintained by overexpression of the mutant (Fig. 2, B and C). The S6 phosphorylation despite the presence of rapamycin or lovastatin confirmed the activity of the S6K1 ED3E construct.
Fig. 2.
Lovastatin inhibits mammalian target of rapamycin complex 1 (mTORC1) activity A: human iliac VSMC were cultured with vehicle (control), 20 nM rapamycin (Rap), or 5 μM lovastatin (Statin) for 24 h and subjected to Western blotting with antibodies to p70 S6 kinase 1 (S6K1) phosphorylated at Thr389 (T389) (arrow indicates specific band), total S6K1, pS6 S240/244, total S6, or total 4E-binding protein-1 (4E-BP1) (mobility shift reflects phosphorylation; top arrow indicates phosphorylated form and bottom arrow indicates dephosphorylated form). B: lovastatin-induced VSMC differentiation requires inhibition of mTORC1 signaling to S6K1. Human VSMC were infected overnight with adenovirus encoding green fluorescent protein (control) or the hemagglutinin (HA)-S6K1-ED3E mutant (constitutively active). VSMC were then treated with vehicle (Ctrl), 20 nM rapamycin (Rap), or 5 μM lovastatin (Stat) for 24 h before Western analysis with antibodies to the HA epitope tag (S6K1), phospho-S6, calponin, h-caldesmon, and SM-MHC. The nonspecific bands on the HA blot serve as confirmation of even gel loading and transfer. Data are representative of 5 independent experiments. C: lovastatin-induced VSMC differentiation requires inhibition of mTORC1 signaling to S6K1: quantification of results. Rapamycin and lovastatin statistically significantly (P < 0.05) induced contractile protein expression in VSMC infected with control virus. Human VSMC with overexpression of HA-S6K1-ED3E mutant had no significant induction of contractile protein expression in the presence of rapamycin or lovastatin (n = 3).
Lovastatin Inhibits mTORC1 and Induces VSMC Differentiation Through Inhibition of Rheb
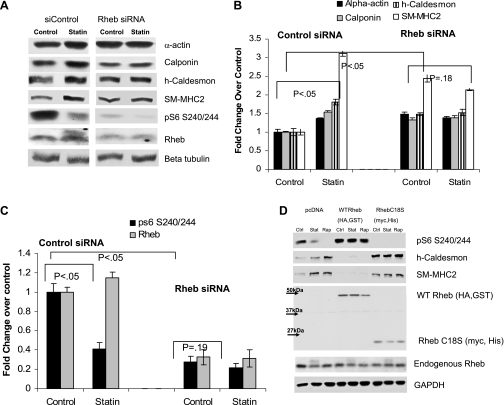
To determine whether lovastatin-induced differentiation is dependent on Rheb, we used siRNA to knock down Rheb expression. Using transient transfection, we were able to achieve a 60% reduction in Rheb expression (Fig. 3, A and B). In the presence of control siRNA, lovastatin statistically significantly induced expression of the differentiation marker contractile proteins SM α-actin, h-caldesmon, calponin, and SM-MHC, and it inhibited mTORC1 activity (S6 phosphorylation) and Rheb farnesylation (mobility shift). Rheb knockdown inhibited pS6 phosphorylation at baseline, which led to a statistically significant increase in baseline contractile protein expression. Lovastatin induction of contractile proteins was markedly inhibited after Rheb knockdown (Fig. 3, A and B), and the effect on S6 phosphorylation was attenuated (Fig. 3C). Complementary overexpression studies yielded dramatic results. Overexpression of WT Rheb markedly induced mTORC1 activity and inhibited contractile protein expression, and these effects could not be overcome by lovastatin or rapamycin (Fig. 3D). However, overexpression of a Rheb mutant that cannot be farnesylated (C18S)(4) showed the opposite phenotype. mTORC1 activity was dramatically suppressed and contractile protein expression was induced. Rapamycin or lovastatin had no additional effect in the context of this mutant (Fig. 3D). Collectively, the results in Fig. 3 suggest a model in which lovastatin promotes VSMC differentiation through suppression of Rheb farnesylation and subsequent inhibition of mTORC1.
Fig. 3.
Rheb is required for lovastatin-induced VSMC differentiation. A: human VSMC were transfected with small interfering RNA (siRNA) to Rheb, or negative control siRNA (siCONTROL), for 48 h in M199 medium with 2.5% serum. Cells were then treated with 5 μM lovastatin for 12 h and harvested for Western blot analysis for SM α-actin, calponin, h-caldesmon, SM2-MHC, pS6 S235/236, Rheb, and loading control β-tubulin. B: graph shows quantitation of Western blots from 3 experiments, with a representative blot shown in A. C: graph shows quantitation of Western blots from 3 experiments, with a representative blot shown in A. D: VSMC were transfected with pcDNA (empty vector control) or plasmids encoding wild-type (WT) Rheb (with HA and glutathione S-transferase epitope tags) or the Rheb mutant C18S (with myc and His tags) for 48 h, before treatment with vehicle (Ctrl), 20 nM rapamycin (Rap), or 5 μM lovastatin (Stat) for 12 h and Western blot analysis with antibodies to pS6 S240/244, h-caldesmon, SM2-MHC, Rheb, and GAPDH (loading control). Note that overexpressed Rheb migrates with different mobilities depending on the different epitope tags compared with endogenous Rheb, which migrates at 20 kDa.
Lovastatin Inhibits mTORC1 Through Inhibition of Rheb Farnesylation
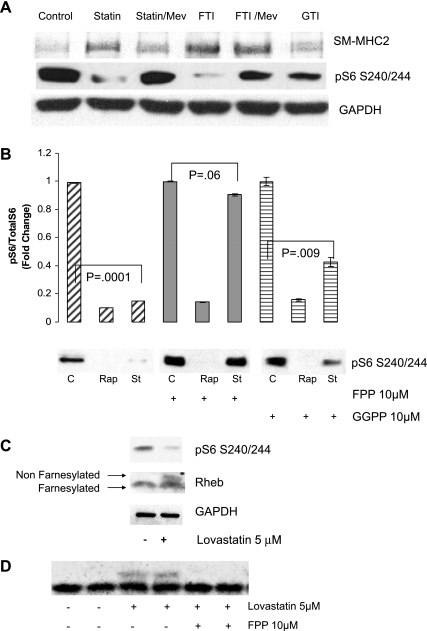
To determine whether farnesylation or geranylation is involved in VSMC differentiation, VSMC were treated with chemical inhibitors of farnesylation (FTI277) or geranylation (GTI 298). Notably, the farnesylation inhibitor FTI277 alone was sufficient to dramatically induce expression of SM2-MHC and to inhibit S6 phosphorylation. This effect could be rescued by addition of mevalonate (Fig. 4A). In contrast, inhibition of geranylation had only a modest effect on induction of expression of SM2-MHC (Fig. 4A). Similar to mevalonate, the addition of farnesylpyrophosphate (FPP) completely rescued the lovastatin inhibition of mTORC1 (S6 phosphorylation), whereas geranylgeranyl pyrophosphate (GGPP) did not (Fig. 4B). Notably, these isoprenoids could not rescue the effects of rapamycin.
Fig. 4.
Role for farnesylation in lovastatin-induced VSMC differentiation and mTORC1 inhibition. A: human iliac VSMC were cultured in the presence of vehicle (ethanol), 5 μM lovastatin, 10 μM FTI277 (FTI), or 10 μM GTI 298 (GTI) for 8 h. Where indicated, VSMC were pretreated for 1 h with 10 μM mevalonate (Mev) before the other drug treatment. Equal amounts of protein (as determined by Bradford analysis) were loaded for each lane and analyzed by immunoblotting using antibodies against SM2-MHC and pS6 (S240/244). GAPDH served as a loading control. B: human VSMC were pretreated with 10 μM farnesylpyrophosphate (FPP) or 10 μM geranylgeranyl pyrophosphate (GGPP) for 1 h. Vehicle (ethanol; C) or 5 μM lovastatin (St) was then added for an additional 8 h and analyzed by immunoblotting as above using pS6 (S240/244) antibody. GAPDH served as a loading control. A representative blot is shown. The graph shows quantitation of Western blots from 3 experiments. C: VSMC were treated with 5 μM lovastatin or vehicle for 24 h as indicated. Lysates were analyzed by immunoblotting as above using antibodies to Rheb, pS6, and GAPDH. Top arrow indicates nonfarnesylated Rheb and bottom arrow indicates farnesylated Rheb (Rheb cleavage precedes farnesylation). D: human VSMC were pretreated with 10 μM FPP. Vehicle (ethanol) or 5 μM lovastatin was then added for an additional 8 h. Lysates were analyzed by immunoblotting as above using anti-Rheb antibody.
The farnesylation status of Rheb can be assessed by mobility shift. Because Rheb must be cleaved before farnesylation, farnesylated Rheb migrates in SDS-PAGE with a more rapid mobility (30). Western blotting revealed that lovastatin inhibited Rheb mobility in a manner consistent with inhibition of Rheb farnesylation (Fig. 4C). This inhibition could be rescued by addition of excess FPP (Fig. 4D). The results of these experiments in Fig. 4 suggest that statin inhibition of Rheb farnesylation may mediate the mTORC1 inhibition and differentiation response.
Lovastatin Inhibits mTORC1 and Promotes VSMC Contractile Protein Expression Following Arterial Injury
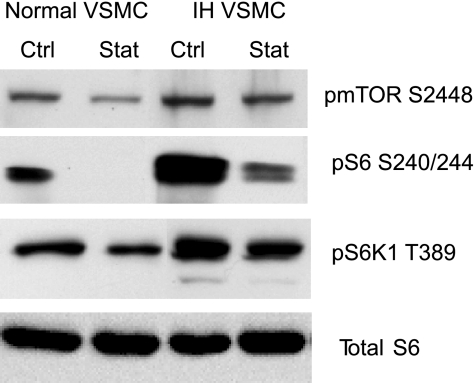
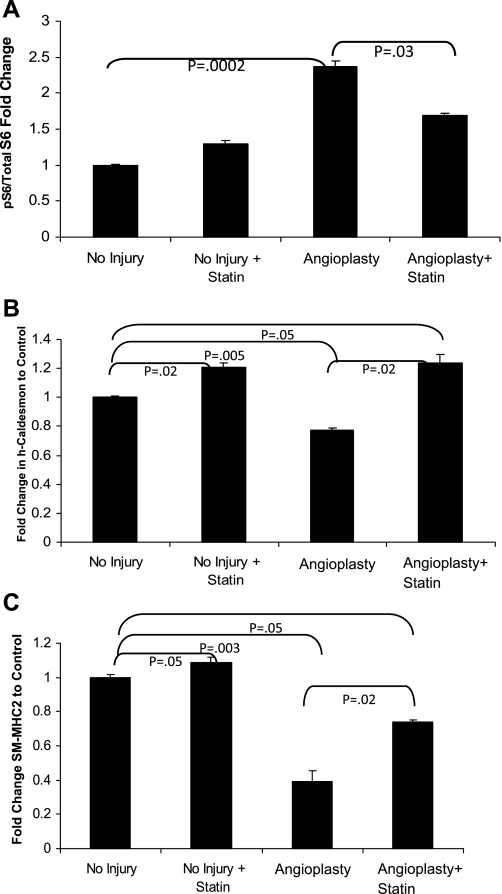
We next evaluated mTORC1 activity and lovastatin response in VSMC derived from a human intimal hyperplastic lesion versus normal vascular media from the same patient. The VSMC isolated from an intimal hyperplastic (IH) lesion exhibited elevated mTORC1 activity as compared with the normal VSMC. Lovastatin inhibited mTORC1 activity in the normal and IH VSMC, but the inhibition in IH was incomplete (Fig. 5). In Fig. 6, we employed an ex vivo angioplasty method to study lovastatin response. Segments of isolated porcine femoral artery were subjected to balloon angioplasty followed by organ culture in serum for 7 days in the presence or absence of lovastatin. Similar to an in vivo injury model, the ex vivo balloon angioplasty induced neointimal formation (not shown), mTORC1 activity (S6 phosphorylation 240/244, Fig. 6A), and repressed contractile protein expression after 1 wk (Fig. 6, B and C). Notably, lovastatin inhibited the injury-induced mTORC1 activity and rescued the contractile protein levels. Lovastatin treatment rescued h-caldesmon expression to greater than baseline levels, while SM2-MHC returned to near baseline. Lovastatin treatment even augmented contractile protein expression in the absence of injury in the cultured vessel segments. Just as rapamycin, through inhibition of mTORC1, is highly effective in inhibiting in-stent restenosis, these data reveal that lovastatin inhibition of mTORC1 similarly attenuates the phenotypic modulation characteristic of the intimal hyperplastic response. This new role for lovastatin inhibition of Rheb and mTORC1, resulting in VSMC differentiation, suggests an important new mechanism by which statins may protect patients from cardiovascular disease.
Fig. 5.
Intimal hyperplasic (IH) VSMC exhibit enhanced, lovastatin-sensitive mTORC1 activity. VSMC derived from an intimal hyperplastic lesion or from a normal vessel in the same patient were grown in primary culture in 10% fetal calf serum following explant or enzymatic digestion. VSMC were treated with 5 μM lovastatin (Stat) or vehicle (Ctrl) for 12 h and harvested for Western blot analysis using phospho-mTOR S2448 (a surrogate for mTORC1 activity), pS6 S240/244, or pS6K1 T389 and total S6 antibodies.
Fig. 6.
Statins inhibit mTORC1 activity and prevent loss of contractile proteins in an ex vivo injury model. Porcine femoral arteries were removed and angioplastied with a 3-mm balloon to 8 atm for 1 min. Artery pieces were then cultured in M199 medium with 10% fetal calf serum for 1 wk with or without 5 μM lovastatin. Arteries were then homogenized in lysis buffer. Western blot analysis of vessels in A was performed using pS6 S240/244 (B) and h-caldesmon (C) and SM2-MHC antibodies and normalized to GAPDH. Error bars = SE; n = 4.
DISCUSSION
We report that lovastatin induces VSMC differentiation through inhibition of Rheb and mTORC1, and that lovastatin can inhibit mTORC1 activity and prevent VSMC dedifferentiation in an ex vivo angioplasty model. Several studies have documented that statins inhibit intimal hyperplasia, but the mechanism underlying this effect is unknown. Our work reveals a novel mechanism by which lovastatin can dramatically modulate VSMC phenotype, a key component of intimal hyperplasia, and atherosclerosis (7, 8, 23). This study also identifies Rheb inhibition of mTORC1 as the critical pathway by which lovastatin induces VSMC differentiation in culture and prevents dedifferentiation in an injury model. Elucidation of this new mechanism of lovastatin cardioprotection could have implications for development of new therapies. This suggests a novel, cholesterol-independent cardioprotective mechanism for statin therapy and has implications for understanding the mTORC1 signal transduction pathway.
We have previously reported that inhibition of the mTOR pathway, using rapamycin, induced VSMC differentiation as determined by induction of contractile protein expression and morphology. Rapamycin binds FKBP12, and this complex inhibits mTORC1, the mammalian target of rapamycin (20). mTORC1 is a ubiquitously expressed protein kinase that regulates translation initiation of specific growth-related mRNA subsets through the effectors p70 S6K1 and 4E-BP1/eIF4E. Other mTOR-regulated effectors included S6K2, SKAR, and eIF2 kinase. It remains to be determined whether these might also be affected by lovastatin. However, since Rheb is upstream of mTORC1, it is likely that they would be. The growth factor and nutrient and energy sufficiency inputs to mTORC1 converge at the level of TSC2, a Rheb GTPase-activating protein. Recently, Rheb was identified as an essential regulator of mTORC1, and farnesylation of Rheb is required for its ability to promote mTORC1 signaling (5, 30, 31). Others have shown that farnesylation inhibitors prevented Rheb prenylation and S6K1 activation (5, 30, 31). The mechanism by which Rheb controls mTORC1 activity is not yet clear.
We show that lovastatin can also inhibit mTORC1 through a mechanism distinct from rapamycin. This may provide a useful tool to help to elucidate the mechanism by which Rheb controls mTORC1 activity. We show that lovastatin inhibits mTORC1 through farnesylation inhibition, because FPP rescues this inhibition. Notably, FPP does not rescue rapamycin inhibition because Rheb is upstream of mTORC1. We also show that rapamycin cannot rescue the inhibition of contractile protein and increased mTORC1 activity due to overexpression of WT Rheb, even though rapamycin acts downstream from Rheb. Similarly, overexpression of the Rheb C18S mutant acts as a dominant negative, inhibiting mTORC1 activity even in the presence of endogenous Rheb. These data suggest that Rheb regulates mTORC1 via a physical interaction, which can be disrupted by lovastatin. It is likely that inhibition of Rheb farnesylation prevents Rheb association with a membrane that is a requisite site of functional mTORC1 assembly.
Although studies strongly suggest that GTP-bound Rheb potently activates mTORC1, the precise molecular mechanism has been somewhat elusive. Rheb has been found to bind to mTOR in overexpression studies (19, 31). However, associations between endogenous Rheb and components of mTORC1 have not been detected. In general, Ras-related small G proteins bind to their downstream effectors only in the GTP-bound state. Paradoxically, Rheb has been found to bind more tightly to mTOR in its GDP-bound state (19). Recently, Rheb was found to directly associate with the FKBP12 homologue FKBP38 (also known as FKBP8), and this interaction appears to be stronger with GTP-loaded Rheb (2). This suggests an intriguing model in which Rheb-GTP binds to FKBP38, which relieves the FKBP38-mediated repression of mTORC1, thereby stimulating mTORC1 activation. Our study would suggest that if binding of FKBP38 is critical for Rheb function, lovastatin inhibition of Rheb farnesylation inhibition could inhibit the ability of FKBP38 to interact with Rheb, which could stabilize the inhibitory association of FKBP38 with mTORC1. Alternately, a new in vitro study reveals that mTORC1 kinase activity toward substrates is enhanced by the presence of recombinant Rheb, but not other small G proteins, and that this effect occurs even in the absence of FKBP38 (29). This study also supports our hypothesis that proximity of mTORC1 to Rheb, which is likely determined by Rheb farnesylation status, could determine the ability of mTORC1 to phosphorylate its substrates, and it is this function that is disrupted by lovastatin.
Our study confirms that the mTORC1 pathway is a key pathway to target in the treatment of intimal hyperplasia, as inhibition of mTORC1 through lovastatin, a mechanism distinct from rapamycin or its analogs, prevented the phenotypic modulation characteristic of intimal hyperplasia. We further show that overexpression of S6K1 and Rheb were mutually exclusive with lovastatin-induced VSMC differentiation. These findings suggest that mTORC1 inhibition through methods distinct from rapalogues is possible. Determination of the exact mechanism of action of statins′ beneficial effect on VSMC phenotypic modulation may allow for more localized drug delivery via drug-eluting stents as has been demonstrated with rapamycin. Because of the ability to achieve high local concentration, delivery of rapamycin via drug-eluting stents has been far more successful than oral delivery in treatment of restenosis (3, 26). This concept is of particular importance given the current controversy over the safety of rapamycin family drugs or paclitaxel, which have the potential to inhibit reendothelialization, thought to be a key factor in the pathogenesis of late-stent thrombosis. Hence, augmentation of reendothelialization and amelioration of endothelial dysfunction represent effective strategy to overcome potential unfavorable effects associated with local sirolimus treatment. Recently, it has been shown that fluvastatin significantly promoted proliferation and migration of mature endothelial cells treated with sirolimus. Furthermore, fluvastatin increased endothelial nitric oxide synthase expression and decreased plasminogen activator inhibitor type 1 expression from human umbilical vein endothelial cells in the presence of sirolimus (14). Our finding that lovastatin inhibits mTORC1 activity and promotes VSMC differentiation furthers our understanding of the basic signaling mechanisms regulating VSMC phenotype and suggests new avenues for discovery of cardiovascular therapeutics.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant 5K08HL076658-03 and by Pfizer Atorvastatin Research Award 2005.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Vuk Stambolic for the gift of the Rheb plasmids.
REFERENCES
- 1.Abbruzzese TA, Havens J, Belkin M, Donaldson MC, Whittemore AD, Liao JK, Contes MS. Statin therapy is associated with improved patency of autogenous infrainguianl bypass grafts. J Vasc Surg 39: 1178–1185, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science 318: 977–980, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Brara PS, Moussavian M, Grise MA, Reilly JP, Fernandez M, Schatz RA, Teirstein PS. Pilot trial of oral rapamycin for recalcitrant restenosis. Circulation 107: 1722–1724, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Buerger C, DeVries B, Stambolic V. Localization of Rheb to the endomembrane is critical for its signaling function. Biochem Biophys Res Commun 344: 869–880, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Castro A, Rebhun J, Clark G, Quilliam L. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem 278: 32492–32496, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Christenson J. Preoperative lipid control with simvastatin reduces the risk of graft failure already 1 year after myocardial revascularization. Cardiovasc Surg 9: 33–43, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Corpataux JM, Naik J, Porter KE, London NJ. A comparison of six statins on the development of intimal hyperplasia in a human vein culture model. Eur J Vasc Endovasc Surg 29: 177–181, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Corpataux JM, Naik J, Porter KE, London NJ. The effect of six different statins on the proliferation, migration, and invasion of human smooth muscle cells. J Surg Res 129: 52–56, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther 84: 413–428, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Fetalvero KM, Shyu M, Nomikos AP, Chiu YF, Wagner RJ, Powell RJ, Hwa J, Martin KA. The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am J Physiol Heart Circ Physiol 290: H1337–H1346, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Fillinger MF, Sampson LN, Cronenwett JL, Powell RJ, Wagner RJ. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J Surg Res 67: 169–178, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev 16: 1472–1487, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu H, Casey P. Enzymology and biology of CaaX protein prenylation. Recent Prog Horm Res 54: 315–343, 1999 [PubMed] [Google Scholar]
- 14.Fukuda D, Enomoto S, Shirakawa I, Nagai R, Sata M. Fluvastatin accelerates re-endothelialization impaired by local sirolimus treatment. Eur J Pharmacol 612: 87–92, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev 15: 807–826, 2001 [DOI] [PubMed] [Google Scholar]
- 16.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509–2514, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henke PK, Blackburn S, Proctor MC, Stevens J, Mukherjee D, Rajagopalin S, Upchurch GR, Jr, Stanley JC, Eagle KA. Patients undergoing infrainguinal bypass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: effect on graft patency, limb salvage and mortality. J Vasc Surg 39: 357–365, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kuro-o M, Nagai R, Tsuchimochi H, Katoh H, Yazaki Y, Ohkubo A, Takaku F. Developmentally regulated expression of vascular smooth muscle myosin heavy chain isoforms. J Biol Chem 264: 18272–18275, 1989 [PubMed] [Google Scholar]
- 19.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol 15: 702–713, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Adv Cancer Res 86: 1–39, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol 286: C507–C517, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Martin KA, Schalm SS, Richardson C, Romanelli A, Keon KL, Blenis J. Regulation of ribosomal S6 kinase 2 by effectors of the phosphoinositide 3-kinase pathway. J Biol Chem 276: 7884–7891, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Miyauchi K, Kasai T, Yokayama T, Aihara K, Kurata T, Kajimoto K, Okazaki S, Ishiyama H, Daida H. Effectiveness of statin-eluting stent on early inflammatory response and neointimal thickness in a porcine coronary model. Circ J 72: 832–838, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487–517, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359: 2195–2207, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez AE, Alemparte MR, Vigo CF, Pereira CF, Llaurado C, Russo M, Virmani R, Ambrose JA. Pilot study of oral rapamycin to prevent restenosis in patients undergoing coronary stent therapy: Argentina Single-Center Study (ORAR Trial). J Invasive Cardiol 15: 581–584, 2003 [PubMed] [Google Scholar]
- 27.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol cell 25: 903–915, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol 17: 596–603, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Nakashima A, Guo L, Tamanoi F. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem 284: 12783–12791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tee A, Manning B, Roux P, Cantley L, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13: 1259–1268, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Tee AR, Blenis J, Proud CG. Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1. FEBS Lett 579: 4763–4768, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Zhang F, Casey P. Protein prenylation:molecular mechanisms and functional consequences. Annu Rev Biochem 65: 241–269, 1996. [DOI] [PubMed] [Google Scholar]