Abstract
Purpose
Tear film composition depends on water and ion transport across ocular surface epithelia and on fluid secretion by lacrimal glands. The purpose of this study was to establish in situ fluorescence methods to measure tear film ionic concentrations and pH in mice and to determine whether tear film composition is sensitive to deficiency of the major ocular surface aquaporin water channels.
Methods
Tear film ionic concentrations and pH were measured in anesthetized mice by ratio imaging fluorescence microscopy after topical application of ion/pH-sensing, dual-wavelength fluorescent indicators. [Na+], [K+], and [Cl−] were measured with membrane-impermeant indicators developed by our laboratory, and pH was measured with bis(carboxyethyl)-carboxyfluorescein fluorescence-conjugated dextran. Measurements were performed on wild-type mice and on knockout mice lacking aquaporins AQP1, AQP3, and AQP5.
Results
In wild-type mice, tear film [Na+] was 139 ± 8 mM, [K+] was 48 ± 1 mM, [Cl−] was 127 ± 4 mM, and pH was 7.59 ± 0.2 (SE; n = 5–8). pH did not differ significantly in the AQP knockout mice. [Na+] was increased by approximately twofold in AQP5 null mice (230 ± 20 mM) and was greatly reduced after exposure of the ocular surface to a humidified atmosphere. [K+] was mildly reduced in AQP1 null mice.
Conclusions
These results establish an in situ optical methodology to measure tear film [Na+], [K+], [Cl−], and pH in living mice, without the need for fluid sampling. Tear film hypertonicity in AQP5 deficiency is likely caused by reduced transcorneal water secretion in response to evaporative water loss.
The composition of the tear film depends on the water and ion transport properties of the cornea and conjunctiva, rates of tear fluid production and drainage, and ocular surface evaporation. Tear film osmolality is consistently elevated in evaporative dry eye (caused by excessive tear fluid evaporation rate) and tear-deficient dry eye (caused by inadequate tear fluid secretion) and is considered an objective, quantitative index of the severity of keratoconjunctivitis sicca (KCS).1,2 We previously reported a mathematical model relating tear film osmolality to tear fluid secretion, drainage, evaporation, and ocular surface transport.3 As expected, the model predicted increased tear fluid osmolality in response to increased evaporation or reduced lacrimal gland secretion and to reduced osmotic water permeability of the ocular surface.
Water transport at the ocular surface largely involves aquaporin (AQP) water channels expressed in cornea and conjunctiva.4 AQP1 is expressed in corneal endothelium, AQP3 in corneal and conjunctival epithelia, and AQP5 in corneal epithelium.5–8 Studies in transgenic mice lacking these AQPs individually have indicated their importance in osmotically driven water transport across the cornea and conjunctiva into the tear film.3 Analysis of AQP knockout mice has also shown the involvement of AQP1 in the maintenance of corneal hydration and transparency after stress9 and of AQP3 in corneal epithelial cell migration and proliferation during wound healing.10
Previous studies of tear film ionic content have relied on microsampling of tear fluid,11,12 an approach that introduces potential artifacts. Tear fluid samples are collected from the tear meniscus, which likely has a lower osmolarity than the average tear film, producing an underestimate in hyperosmolality in KCS.13 Physical manipulation of the ocular surface, which stimulates tear fluid secretion, also confounds the interpretation of sampled tear fluid. In mice, the total tear fluid volume has been estimated as approximately 0.5 μL per eye,14 making meaningful collection difficult. The purpose of this study was to establish a methodology to measure the concentrations of the major ions, Na+, K+, and Cl− and the pH in tear fluid film in situ in living mice. We used membrane-impermeant fluorescent dyes developed previously by our laboratory for measurements of [Na+] and [Cl−] in the airway surface liquid in mice15 and a new fluorescent [K+] indicator developed recently by our laboratory.16,17 Tear film pH was measured with the commercial fluorescent dye bis(carboxyethyl)-carboxyfluorescein (BCECF) fluorescence-dextran, as has been measured previously in humans and rabbits.18,19 Ion concentrations and pH were measured by ratio imaging fluorescence microscopy on indicator-stained eyes in anesthetized control mice and mice lacking the major ocular surface aquaporins AQP1, AQP3, and AQP5. A remarkable finding was significant tear film hypertonicity in AQP5 knockout mice.
Methods
Mice
Measurements were made on adult wild-type mice (age range, 6–10 weeks; weight range, 22–30 g) in a CD1 genetic background and on knockout mice individually lacking AQP1, AQP3, and AQP5, generated by targeted gene disruption as described previously.20–22 Mice were matched by age and sex and were maintained on standard mouse chow in air-filtered cages. Protocols were approved by the University of California at San Francisco Committee on Animal Research and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Fluorescent Indicators for Ratiometric Measurements of pH and Ion Concentrations
Tear film pH measurements were performed using the commercially available dual-excitation wavelength pH indicator BCECF (2′,7′-bis-[carboxyethyl]-5-carboxyfluorescein) conjugated to dextran (10 kDa; Molecular Probes, Eugene, OR). pH was determined from green images (535 nm emission) collected at two excitation wavelengths, 440 and 490 nm.
[Cl−] was measured using the ratioable, Cl−-sensitive fluorescent indicator BAC-TMR-dextran, synthesized as described previously,23 in which the Cl−-sensitive, green fluorescent chromophore BAC (10,10′-bis[3-carboxypropyl]-9,9′-biacridinium) was conjugated covalently to dextran (40 kDa), together with the Cl−-insensitive, red fluorescent chromophore tetramethylrhodamine (TMR). BAC fluorescence was quenched by Cl− by a collisional mechanism. [Cl−] was determined from red (TMR) and green (BAC) images of the BAC-TMR-dextran–stained tear film.
[Na+] was measured with a ratioable, Na+-sensitive fluorescent indicator prepared by incubation of carboxyl-modified latex beads (200-nm diameter; Polymer Laboratories Inc., Amherst, MA) with an Na+-insensitive, green fluorescent chromophore (BODIPY-fl-EDA; Molecular Probes) and an Na+-sensitive, red fluorescent chromophore (CoroNa Red; Molecular Probes), as described previously,15 with minor modifications. Na+-insensitive, green fluorescent chromophore was dissolved in dimethylsulfoxide (DMSO) and added at a final concentration of 20 μM to a 5% (vol/vol) suspension of beads in 3 mL water. After shaking the Na+-insensitive, green fluorescent chromophore–bead mixture for 3 days at room temperature, Na+-sensitive, red fluorescent chromophore was added at 100 μM from a DMSO stock solution. Beads were shaken for an additional 1 to 2 hours, centrifuged at 14,000g for 30 minutes, washed three times with water, dispersed by brief sonication, and resuspended in 30 μL water. The chromophores remained quantitatively immobilized on the beads for more than 2 weeks during storage in water at 4°C in the dark. [Na+] was determined from red and green images taken of the indicator-stained tear film.
[K+] was measured with the ratioable, K+-sensitive fluorescent indicator TAC-Lime-dextran-TMR, synthesized as described recently,17 in which the green fluorescence of the K+-sensitive, modified boron-dipyrromethene chromophore increases on K+ binding to its nearby triazacryptand chromophore. TMR red fluorescence is K+ insensitive, such that tear film [K+] is determined from green and red images of the TAC-Lime-dextran-TMR–stained tear film.
Tear Film Ion Concentration and pH Measurements
Mice were anesthetized by gas anesthesia (2% isoflurane). To induce sedation, mice were placed in a box gassed with 0.8 L/min oxygen containing 4% isoflurane (Minrad, Bethlehem, PA) and were transferred to a respirator (Surgivet, Waukesha, WI) delivering 2% isoflurane in oxygen at the same rate. By fixing the respirator to the bench, mice remained immobilized throughout the experiment, with the central corneal surface positioned perpendicularly to the optical axis. Most measurements took less than 5 minutes per mouse (including anesthesia induction). In some studies mice were kept in a humidified chamber (100% humidity) for approximately 20 minutes before measurements. Eyelids were blinked periodically with the use of forceps, and core body temperature was monitored with a rectal probe and maintained at 37°C with a heating pad. Three methods were used to stain the tear film with fluorescent indicators. In the first method, as performed for staining of the airway surface liquid,15 approximately 2 mg BCECF-dextran was dispersed in 1 mL low-boiling perfluorocarbon (compound FC-72, boiling point 56°C; 3M Company, St. Paul, MN) with brief probe sonication. Just before measurements, 50 to 100 μL perfluorocarbon was pipetted onto the ocular surface, and the lids were briefly closed to disperse and dissolve the dye in the tear film. In the second method, very small amounts of finely dispersed dextran-conjugate powders (K+ and Cl− indicators) were applied directly into the outer canthus with a needle tip and dissolved by repeated mechanical blinking. Last, the Na+ indicator was added to tear fluid by direct pipetting of 0.25 μL concentrated bead suspension into the outer canthus and was followed by blinking. Dyes were redistributed between measurements by blinking.
Fluorescence Microscopy
Ratio imaging was performed with a stereo-zoom epifluorescence microscope (SMZ1500; Nikon, Tokyo, Japan) equipped with a tungsten halogen light source (X-Cite 120; EXFO, Mississauga, ON, Canada), 1.6× objective lens (numerical aperture, 0.21; working distance, 24 mm), cooled charge-coupled device (CCD) camera (EM-CCD; Hamamatsu, Bridgewater, NJ), and custom filter sets (Chroma, Rockingham, VT) for BAC, tetramethylrhodamine (TMR), Na+-sensitive, red fluorescent chromophore, Na+-insensitive, green fluorescent chromophore, and BCECF. For ratio image analysis, images (1000-ms acquisitions for pH; 1500-ms acquisitions for Cl−, Na+, and K+) were obtained from pairs of filter sets that were manually switched. Tear fluid fluorescence ratios were computed over at least three randomly selected regions after subtraction of background (determined from the same regions of the ocular surface imaged before the fluorescent indicator was added). Ion concentrations and pH were determined from fluorescence ratios using the calibrations described. Photobleaching, which was insignificant for the Na+, K+ and pH indicators (<1% in 2-minute continuous illumination), was minimized for the Cl− indicator by limiting BAC excitation to periods of image acquisition.
Calibration Protocols
In vitro calibrations were performed to relate ion concentrations and pH to fluorescence ratios. Ratio imaging of [Na+], [K+], [Cl−], and pH was performed on 10-μL droplets of buffer standards of specified composition containing the respective indicator dyes. The base buffer, designed to approximate native tear ion composition, consisted of 120 mM NaCl, 30 mM KCl, 20 mM HEPES, 0.7 mM MgCl2, and 0.7 mM CaCl2 (pH 7.4). For Na+ calibrations, a Na+-free buffer (choline chloride replacing NaCl in base buffer) and a high Na+ buffer (NaCl replacing KCl) were mixed in appropriate proportions. Additional NaCl was added to approximate the tear film in a hypertonic state. For K+ calibrations, a K+-free buffer (NaCl replacing KCl) and a high K+ buffer (KCl replacing NaCl) were used. For Cl− calibrations, a Cl−-free buffer (sodium nitrate replacing NaCl) was mixed with the base buffer. For pH calibration, the base buffer was titrated with nitric acid or potassium hydroxide (sodium hydroxide for K+ buffer).
Results
Figure 1 shows chemical structures and fluorescence spectra for each of the indicator dyes; chromophores are shown in the colors of their fluorescence emission. The pH, [K+], and [Cl−] indicators are covalent dextran conjugates. The pH-sensitive chromophore BCECF is intrinsically ratioable,24 with the pH determined from BCECF green fluorescence (535 nm) measured at excitation wavelengths of 440 and 490 nm. The K+-sensing dextran consists of the green-fluorescing K+-sensitive TAC-lime chromophore and the red-fluorescing K+-insensitive TMR chromophore. Increased [K+] produces increased green fluorescence by abrogation of charge transfer quenching between the triazacryptand ionophore and the Na+-insensitive, green fluorescent chromophore.17 The Cl−-sensing dextran consists of a green-fluorescing, Cl−-sensitive BAC chromophore and a red-fluorescing, Cl−-insensitive TMR chromophore. Increased [Cl−] reduces BAC green fluorescence by a collisional quenching mechanism.23 The [Na+] indicator consists of the red-fluorescing Na+-sensitive chromophore and the green-fluorescing Na+-insensitive chromophore immobilized on 200-nm diameter latex carboxyl beads.15
Figure 1.

Chemical structures and fluorescence spectra of the ion and pH indicators used for tear film fluorescence measurements. (A) [Na+], (B) [K+], (C) [Cl−], and (D) pH.
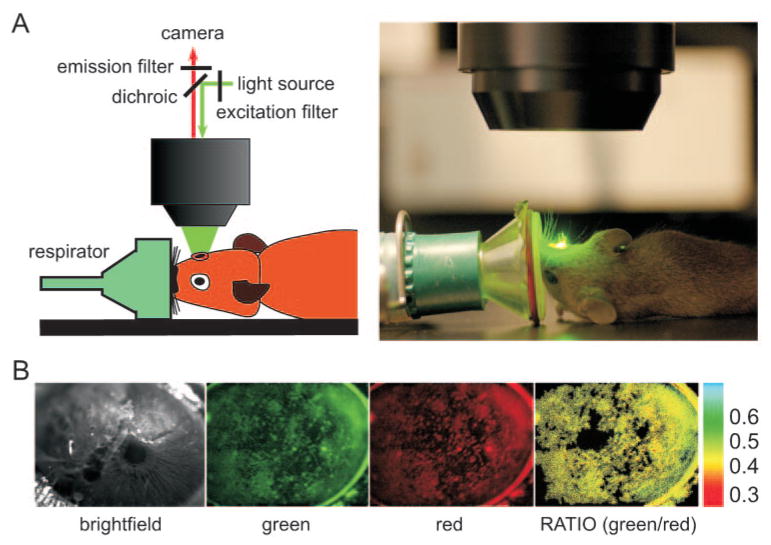
Fluorescence measurements of tear film ion concentrations and pH in mice involved gas anesthesia, staining of the tear film with indicator dyes (without significant addition of aqueous volume), and fluorescence image acquisitions at two sets of wavelengths. Figure 2A shows a schematic and a photograph of the instrumentation, which included a stereo-zoom epifluorescence microscope with a cooled CCD camera detector and a respirator for gas administration and immobilization of the upward-facing mouse ocular surface. Figure 2B shows examples of light and fluorescence images of the ocular surface after tear film staining with the K+ indicator TAC-lime/TMR. Generally, background fluorescence before dye staining, which was subtracted for computation of fluorescence ratios, was less than 5% of fluorescence after staining. The pseudocolored fluorescence ratio image (Fig. 2B, rightmost panel) shows fairly uniform ratio values throughout the tear film.
Figure 2.
Experimental setup for ratio imaging measurements of the fluorescently stained tear film. Mice were anesthetized and immobilized using a custom device with the eye under study facing upward. Tear film was stained with fluorescent indicators. Fluorescence images were recorded at two excitation or emission wavelengths. (A) Schematic (left) and photograph (right) of experimental setup. (B) Representative white light (left) and fluorescence images of mouse eyes after the addition of K+ indicator (middle), with corresponding fluorescence ratio image (right). Ratios were computed only for those pixels whose intensity in the red image was greater than an arbitrary cutoff. Pixels in the ratio image are black to indicate ratio not computed.
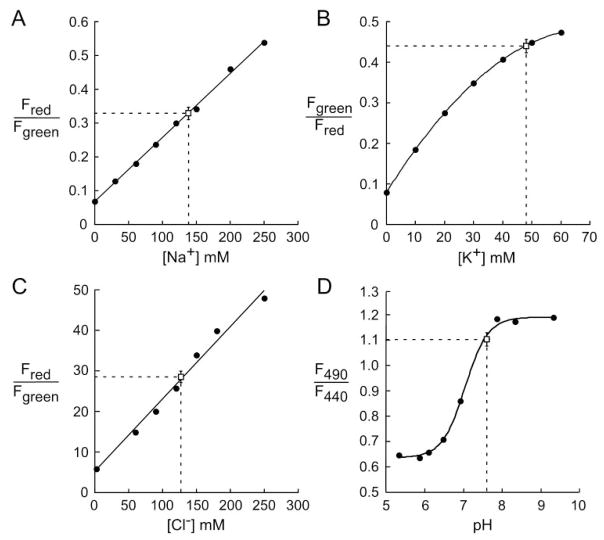
Figure 3 shows fluorescence ratios measured on “calibration” solutions for each of the ions and for pH. For Na+ measurements, bead red-to-green fluorescence ratio (R/G) increased linearly with [Na+] from 0 to more than 200 mM (Fig. 3A). In agreement with previous data,15 R/G was not affected by pH or by [K+] (not shown). For K+ measurements, the TAC-lime-dextran-TMR green-to-red fluorescence ratio (G/R) increased in a curvilinear manner with [K+] from 0 to 200 mM (Fig. 3B). In agreement with previous data,16 G/R was not affected by pH in the range 6 to 8 or by [Na+] (not shown). For Cl− measurements, indicator R/G increased linearly with [Cl−] from 0 to more than 200 mM (Fig. 3C). In agreement with previous data,23 R/G was not affected by pH, [K+], or organic solutes (not shown). The excitation fluorescence ratio (F490/F440) for BCECF-dextran fluorescence was pH sensitive and had a pKa of approximately 7.0 (Fig. 3D), as reported previously for BCECF.24
Figure 3.
Calibrations of indicator fluorescence ratios with (A) [Na+], (B) [K+], (C) [Cl−], and (D) pH. Solution calibrations were performed as described. Superimposed on each calibration are background-corrected fluorescence ratios measured in the tear film of wild-type mice (mean ± SE; n = 4 mouse eyes), along with deduced ion concentrations and pH.
Overlaid onto the calibration data in Figure 3 are mean ± SE for background-subtracted fluorescence ratios measured in wild-type mice. SE values were computed using the number of different mouse eyes used for each condition (four to eight eyes), with ratio measurements in each eye averaged over at least three different regions determined from three sets of ratio images. Fluorescence ratios were mapped, using the calibration data, onto the abscissas to give tear film [Na+] of 139 ± 8 mM, [K+] of 48 ± 1.3 mM, [Cl−] of 127 ± 4 mM, and pH of 7.59 ± 0.2.
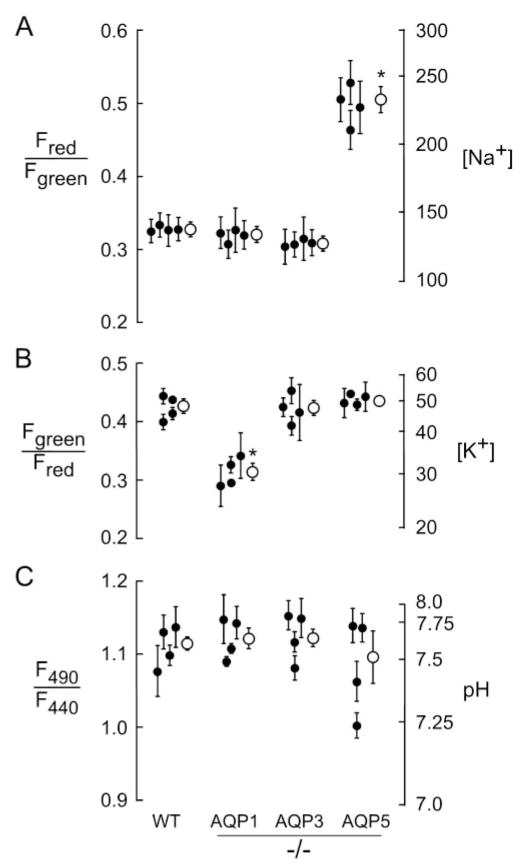
Similar measurements were made in transgenic knockout mice lacking each of the ocular surface aquaporins (AQP1, AQP3, and AQP5). Figure 4 summarizes tear film ion [Na+], [K+], and pH on a series of mice of each genotype compared with values in wild-type mice (Fig. 3). Deletion of each individual AQP did not significantly alter tear film pH. Most remarkably, there was a significant, approximately twofold, elevation in tear film [Na+] in AQP5 null mice (223 ± 20 vs. 139 ± 8 mM), which probably resulted from reduced corneal osmotic water permeability in AQP5 null mice. Tear film [Na+] in AQP1 and AQP3 null mice did not differ significantly from that in wild-type mice. We also found a small but significant reduction in tear film [K+] in AQP1 null mice (33 ± 0.9 vs. 45 ± 1.3 mM in wild-type mice). Tear film [K+] was similar in wild-type, AQP3, and AQP5 null mice. The reduced tear film [K+] in AQP1 deficiency is an unexpected finding because AQP1 is not expressed in ocular surface epithelia and because fluid secretion by lacrimal glands in mice is AQP1 independent.25
Figure 4.
Tear film ion concentrations and pH in AQP knockout mice. (A) [Na+], (B) [K+], and (C) pH were determined by ratio imaging fluorescence microscopy, as in Figure 3. Data shown are mean ± SE for five to eight ratio image measurements performed on four eyes (each from a different mouse) of each genotype (filled circles). Averaged (± SE) values are shown as open circles. *P < 0.005 compared with wild-type mice.
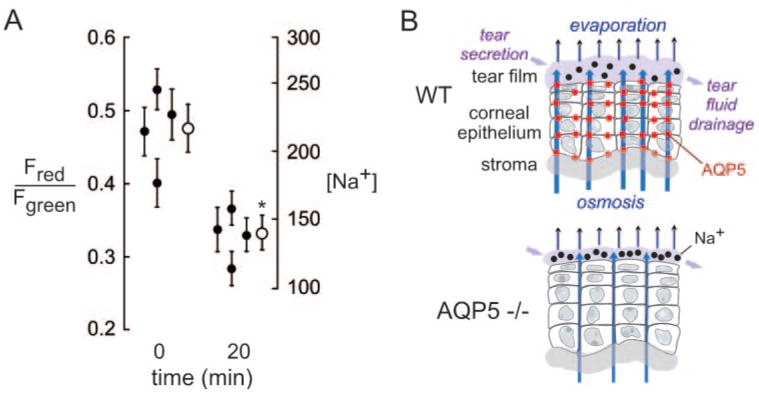
To investigate whether the elevated tear film [Na+] in AQP5 deficiency is related to evaporative water loss, we prevented evaporation by placing mice in a 100% humidified atmosphere for 20 minutes before measurements. As shown in Figure 5A, prevention of tear fluid evaporation produced a reduction in tear film [Na+] in AQP5 null mice that was marked compared with reductions found in wild-type mice. Figure 5B shows a schematic of the determinants of tear film osmolality, including evaporative water loss, osmotically driven water secretion across the AQP5-expressing corneal epithelium, lacrimal gland fluid secretion, and tear fluid drainage. As explained in the Discussion, our data support the involvement of AQP5 in the replacement of solute-free water into the tear film by transcorneal osmosis. AQP5 deficiency thus produces a relatively low-volume, hypertonic tear film layer.
Figure 5.
Reduced tear film hypertonicity in AQP5 knockout mice by prevention of evaporation. (A) Tear film [Na+] measured before and after 20-minute exposure of mice to a 100% humidified atmosphere (SE, four eyes from two mice; filled circles). Averaged (± SE) values are shown as open circles. *P < 0.005). (B) Schematic of the determinants of tear film osmolality show the replacement of evaporative water loss by AQP5-facilitated osmotic water transport across the ocular surface epithelium.
Discussion
This study establishes the methodology to measure the concentrations of the major ions (and pH) in the tear film in situ in living, anesthetized mice. Notwithstanding the need for staining of the tear film with very small amounts of fluorescent dyes, our method can be considered minimally invasive or noninvasive because sampling of the tear fluid is not required, with repeated measurements possible in situ and without direct contact with the eye. Each of the indicator dyes selected for tear film measurements is ratioable such that the measurements are insensitive to the amount or distribution of dyes within the tear film. Fluorescence ratio imaging of the dye-stained tear film made here provided the first in situ measurements of tear film [Na+], [K+], [Cl−] and pH in mice and allowed examination of the role of ocular surface AQPs in tear film composition. An important finding in these measurements was marked tear film hypertonicity in AQP5-deficient mice.
We used commercially available dextran-bound BCECF to measure tear film pH. Although other probes, such as SNARF-dextrans, if appropriately validated, could have provided improved sensitivity for measurement of pH changes when pH was above 7.5, we used BCECF-dextran because it had been successfully used for pH in the airway surface liquid15 and showed good sensitivity in the pH range reported in the tear film.
To measure tear film K+, we used a dextran-bound fluorescent K+ indicator consisting of a triazacryptand K+-binding ionophore conjugated covalently to a chromophore whose fluorescence is quenched by the ionophore by a charge-transfer mechanism.17 K+-chromophore binding reduces the charge transfer quenching, resulting in increased chromophore fluorescence. Fluorescence correlation spectroscopy indicated that K+ binding to the triazacryptand ionophore occurs within a few milliseconds.26 The TAC-lime dextran conjugate used here has good K+-sensitivity and selectivity for measurements of [K+] in the range found in the tear film.
To measure tear film Na+, we used the Na+-sensing chromophore, which was immobilized noncovalently on latex beads as originally reported by our laboratory for measurements of Na+ in the airway surface liquid15 and which was used subsequently for measurements of [Na+] in colonic crypts.27,28 Sodium red fluorescence has good sensitivity to Na+ over a wide range of concentrations, including that found in the tear film. The crown ether in the Na+-sensing chromophore confers Na+ selectivity. Although a Na+-sensing dextran would be preferable to the beads used here in terms of ease of tear film staining, such a dextran is unavailable and presents a major challenge in conjugation chemistry.
Measurement of tear film Cl− was most challenging technically because of the relatively dim fluorescence of the BAC chromophore and its relatively poor photostability compared with other chromophores used in this study that required subtraction of significant background fluorescence and minimization of light exposure to reduce photobleaching. The BAC-TMR-dextran conjugate used here was applied successfully to measure [Cl−] in the airway surface liquid of cultured bronchial cells, mouse trachea, and distal bronchioles15,29 and to measure endosomal [Cl−] after cellular internalization by fluid-phase endocytosis.23 We used similar conjugates to define the determinants of [Cl−] in early and late endosomes by conjugations with ligands (transferrin and macroglobulin) of receptor-mediated endocytosis30 and to test the proton-sponge hypothesis of nonviral gene delivery by conjugation with various polycation-DNA complexes.31 Given the technical challenge in accurately measuring tear film [Cl−] because of the low BAC fluorescence, particularly when [Cl−] is high, we measured tear film [Cl−] only in wild-type mice.
As mentioned in the Introduction, the determinants of tear film ionic content and pH include lacrimal gland fluid secretion, tear fluid drainage through the nasolacrimal duct, evaporation, and water/ion transport between the tear film and the cornea and conjunctiva. Fluid produced by the lacrimal gland is enriched in K+ and Cl− relative to serum as a consequence of acinar secretion through an apical maxi-K+ channel and likely apical Cl− channels.32–34 Ion activities of secreted lacrimal fluid collected from cannulated mouse lacrimal ducts and measured with microelectrodes were 38 mM for K+, 144 mM for Na+, and 140 mM for Cl−,35 in agreement with earlier measurements in the rat (46 mM K+, 135 mM Na+, 123 mM Cl−), and rabbit (42 mM K+, 107 mM Na+, 126 mM Cl−).32,33 The similar ion concentrations found in situ in the tear film in this study support the conclusion that lacrimal gland fluid secretion is an important determinant of tear film ionic composition.
The high tear film [Na+] in AQP5 null mice provides the first direct evidence for a physiologically important role of an AQP in ocular surface fluid balance. We previously showed greater corneal thickness in AQP5 null mice than in wild-type mice (144 vs. 123 μm),9 with increased thickness in epithelial and stromal compartments. Increased corneal thickness in AQP5 deficiency was proposed to be a consequence of chronically impaired exit of water out of the cornea because of reduced corneal epithelial water permeability. In support of this possibility was the finding that transcorneal water permeability was reduced approximately fivefold in AQP5 null mice, which was measured as the rate of corneal water secretion in response to corneal surface hyperosmolality.9 We propose, as shown schematically in Figure 5B, that the tear film hypertonicity in AQP5 knockout mice found here is the consequence of impairment in osmotically driven water secretion across the cornea to offset evaporative water losses. Defective lacrimal gland fluid secretion is unlikely to account for tear film hypertonicity in AQP5 deficiency; data from our laboratory25 and other laboratories36 show that lacrimal fluid secretion is unimpaired in AQP5 null mice. As predicted by our proposed mechanism, tear film osmolality was reduced when evaporation was prevented by exposure of mice to a humidified atmosphere. Our results thus establish AQP5 as an important determinant of tear film tonicity. Because our measurements were made exclusively in mice, the importance of corneal AQP5 in the human eye remains speculative, as does its involvement in ocular surface abnormalities such as KCS.
Elevated tear film [K+] compared with serum [K+] is likely attributed, in part, to the high K+ content of lacrimal gland fluid secretions.32–34 Relatively high tear film [K+] has been suggested to be important for the health of the ocular surface epithelium.37 The reasons(s) for reduced tear film [K+] in AQP1 null mice are not clear because AQP1 deletion in mice does not impair lacrimal gland fluid secretion25 nor is AQP1 expressed in ocular surface epithelia.9 Perhaps AQP1 deficiency in mice is associated with changes in the expression of K+ channels and transporters in ocular surface epithelia that impair K+ secretion or increase K+ absorption.
The methodology developed here should be applicable to minimally invasive determination of tear film ionic composition and pH in mouse and larger animal models of ocular surface disorders, such as KCS, and in the testing of drug therapies. Because the eye is not subject to trauma, serial measurements can be made over minutes to hours, and repeated measurements can be made in the same animal over weeks or months, as may be required for some drug treatments (e.g., immunosuppressive agents).38 Another consequence of our study is the possibility of increasing AQP5 expression, by pharmacologic or gene delivery approaches, as a novel treatment for dry eye conditions.
Acknowledgments
Supported by National Institutes of Health Grants EY13574, DK35124, HL59198, EB00415, DK72517, and HL73856.
The authors thank Aaron Mills and Prashant Padmawar for synthesis of the ratioable K+ indicator, Nitin Sonawane for synthesis of the Cl− indicator, Peter Haggie for help in image analysis, and Liman Qian for mouse breeding and genotype analysis.
Footnotes
Disclosure: J. Ruiz-Ederra, None; M.H. Levin, None; A.S. Verkman, None
References
- 1.Lemp MA. Evaluation and differential diagnosis of keratoconjunctivitis sicca. J Rheumatol. 2000;61(suppl):11–14. [PubMed] [Google Scholar]
- 2.Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47:4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- 3.Levin MH, Verkman AS. Aquaporin-dependent water permeation at the mouse ocular surface: in vivo microfluorimetric measurements in cornea and conjunctiva. Invest Ophthalmol Vis Sci. 2004;45:4423–4432. doi: 10.1167/iovs.04-0816. [DOI] [PubMed] [Google Scholar]
- 4.Verkman AS, Ruiz-Ederra J, Levin MH. Functions of aquaporins in the eye. Prog Retin Eye Res. 2008;27:420–433. doi: 10.1016/j.preteyeres.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci U S A. 1995;92:4328–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamann S, Zeuthen T, La Cour M, et al. Aquaporins in complex tissues: distribution of aquaporins 1–5 in human and rat eye. Am J Physiol. 1998;274:C1332–C1345. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa H, Lian SC, Finkbeiner WE, Verkman AS. Extrarenal tissue distribution of CHIP28 water channels by in situ hybridization and antibody staining. Am J Physiol. 1994;266:C893–C903. doi: 10.1152/ajpcell.1994.266.4.C893. [DOI] [PubMed] [Google Scholar]
- 8.Patil RV, Saito I, Yang X, Wax MB. Expression of aquaporins in the rat ocular tissue. Exp Eye Res. 1997;64:203–209. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- 9.Thiagarajah JR, Verkman AS. Aquaporin deletion in mice reduces corneal water permeability and delays restoration of transparency after swelling. J Biol Chem. 2002;277:19139–19144. doi: 10.1074/jbc.M202071200. [DOI] [PubMed] [Google Scholar]
- 10.Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- 11.Gilbard JP, Farris RL, Santamaria J., 2nd Osmolarity of tear micro-volumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96:677–681. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- 12.Gilbard JP, Rossi SR, Gray KL. A new rabbit model for keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 1987;28:225–228. [PubMed] [Google Scholar]
- 13.Yamada M, Mochizuki H, Kawai M, Yoshino M, Mashima Y. Fluorophotometric measurement of pH of human tears in vivo. Curr Eye Res. 1997;16:482–486. doi: 10.1076/ceyr.16.5.482.7050. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan DA, Krenzer KL, Sullivan BD, Tolls DB, Toda I, Dana MR. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest Ophthalmol Vis Sci. 1999;40:1261–1265. [PubMed] [Google Scholar]
- 15.Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J Clin Invest. 2001;107:317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padmawar P, Yao X, Bloch O, Manley GT, Verkman AS. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat Methods. 2005;2:825–827. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- 17.Namkung W, Padmawar P, Mills AD, Verkman AS. Cell-based fluorescence screen for K+ channels and transporters using an extracellular triazacryptand-based K+ sensor. J Am Chem Soc. 2008;130:7794–7795. doi: 10.1021/ja8014499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada M, Kawai M, Mochizuki H, Hata Y, Mashima Y. Fluorophotometric measurement of the buffering action of human tears in vivo. Curr Eye Res. 1998;17:1005–1009. doi: 10.1076/ceyr.17.10.1005.5239. [DOI] [PubMed] [Google Scholar]
- 19.Chen FS, Maurice DM. The pH in the precorneal tear film and under a contact lens measured with a fluorescent probe. Exp Eye Res. 1990;50:251–259. doi: 10.1016/0014-4835(90)90209-d. [DOI] [PubMed] [Google Scholar]
- 20.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 21.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aqua-porin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 22.Ma T, Song Y, Yang B, et al. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci U S A. 2000;97:4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonawane ND, Thiagarajah JR, Verkman AS. Chloride concentration in endosomes measured using a ratioable fluorescent Cl− indicator: evidence for chloride accumulation during acidification. J Biol Chem. 2002;277:5506–5513. doi: 10.1074/jbc.M110818200. [DOI] [PubMed] [Google Scholar]
- 24.Rink TJ, Tsien RY, Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982;95:189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore M, Ma T, Yang B, Verkman AS. Tear secretion by lacrimal glands in transgenic mice lacking water channels AQP1, AQP3, AQP4 and AQP5. Exp Eye Res. 2000;70:557–562. doi: 10.1006/exer.1999.0814. [DOI] [PubMed] [Google Scholar]
- 26.Magzoub M, Padmawar P, Dix JA, Verkman AS. Millisecond association kinetics of K+ with triazacryptand-based K+ indicators measured by fluorescence correlation spectroscopy. J Phys Chem B. 2006;110:21216–21221. doi: 10.1021/jp0633392. [DOI] [PubMed] [Google Scholar]
- 27.Thiagarajah JR, Jayaraman S, Naftalin RJ, Verkman AS. In vivo fluorescence measurement of Na+ concentration in the pericryptal space of mouse descending colon. Am J Physiol. 2001;281:C1898–C1903. doi: 10.1152/ajpcell.2001.281.6.C1898. [DOI] [PubMed] [Google Scholar]
- 28.Moreto M, Cristia E, Perez-Bosque A, Afzal-Ahmed I, Amat C, Naftalin RJ. Aldosterone reduces crypt colon permeability during low-sodium adaptation. J Membr Biol. 2005;206:43–51. doi: 10.1007/s00232-005-0772-5. [DOI] [PubMed] [Google Scholar]
- 29.Song Y, Thiagarajah J, Verkman AS. Sodium and chloride concentrations, pH, and depth of airway surface liquid in distal airways. J Gen Physiol. 2003;122:511–519. doi: 10.1085/jgp.200308866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonawane ND, Verkman AS. Determinants of [Cl−] in recycling and late endosomes and Golgi complex measured using fluorescent ligands. J Cell Biol. 2003;160:1129–1138. doi: 10.1083/jcb.200211098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 32.Alexander JH, van Lennep EW, Young JA. Water and electrolyte secretion by the exorbital lacrimal gland of the rat studied by micropuncture and catheterization techniques. Pflugers Arch. 1972;337:299–309. doi: 10.1007/BF00586647. [DOI] [PubMed] [Google Scholar]
- 33.Botelho SY, Martinez EV. Electrolytes in lacrimal gland fluid and in tears at various flow rates in the rabbit. Am J Physiol. 1973;225:606–609. doi: 10.1152/ajplegacy.1973.225.3.606. [DOI] [PubMed] [Google Scholar]
- 34.Rismondo V, Osgood TB, Leering P, Hattenhauer MG, Ubels JL, Edelhauser HF. Electrolyte composition of lacrimal gland fluid and tears of normal and vitamin A-deficient rabbits. CLAO J. 1989;15:222–228. [PubMed] [Google Scholar]
- 35.Walcott B, Birzgalis A, Moore LC, Brink PR. Fluid secretion and the Na+-K+-2Cl− cotransporter in mouse exorbital lacrimal gland. Am J Physiol. 2005;289:C860–C867. doi: 10.1152/ajpcell.00526.2004. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki Y, Tsubota K, Kawedia JD, Menon AG, Yasui M. The difference of aquaporin 5 distribution in acinar and ductal cells in lacrimal and parotid glands. Curr Eye Res. 2007;32:923–929. doi: 10.1080/02713680701733076. [DOI] [PubMed] [Google Scholar]
- 37.Bachman WG, Wilson G. Essential ions for maintenance of the corneal epithelial surface. Invest Ophthalmol Vis Sci. 1985;26:1484–1488. [PubMed] [Google Scholar]
- 38.Lekhanont K, Leyngold IM, Suwan-Apichon O, Rangsin R, Chuck RS. Comparison of topical dry eye medications for the treatment of keratoconjunctivitis sicca in a botulinum toxin B-induced mouse model. Cornea. 2007;26:84–89. doi: 10.1097/01.ico.0000240079.24583.a1. [DOI] [PubMed] [Google Scholar]