Abstract
Purpose
Inhibition of VEGF may effect transient “normalization” of tumor vasculature by pruning immature vessels, resulting in improved tumor perfusion and oxygenation. This may improve the efficacy of adjuvant ionizing radiation (IR). We tested this hypothesis using bevacizumab, an anti-VEGF antibody, in rhabdomyosarcoma (RMS) xenografts.
Methods
Mice bearing orthotopic alveolar RMS xenografts were treated with a single dose of bevacizumab, IR, or a combination of the two on different schedules. Tumors were then evaluated for changes in microvessel density, vessel maturity, vessel permeability, intratumoral oxygenation, as well as altered growth.
Results
After bevacizumab treatment, a significant decrease in tumor microvessel density and a significant increase in tumor vessel maturity, defined as the ratio of pericytes to endothelial cells, was observed, suggesting pruning of immature vessels lacking pericytes. Tumor vessel permeability was also significantly decreased and intratumoral oxygen tension increased two and five days after bevacizumab due to a transient improvement in tumor perfusion. Treatment with IR 2 or 5 days after bevacizumab resulted in the greatest antitumor activity.
Conclusion
Our findings support the hypothesis that VEGF inhibition with bevacizumab transiently normalizes the dysfunctional vasculature of RMS xenografts, improving tumor oxygenation and increasing tumor sensitivity to adjuvant IR.
Keywords: Rhabdomyosarcoma, Bevacizumab, VEGF inhibition, Ionizing Radiation
Introduction
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children, accounting for nearly 50% of soft tissue sarcomas in this population [1]. RMS has two major histologic subtypes, embryonal (ERMS) and alveolar (ARMS). The alveolar subtype accounts for 20-30% of newly diagnosed cases of RMS and has a poorer prognosis [2]. An analysis of patients with nonmetastatic RMS from Intergroup Rhabdomyosarcoma Studies III and IV demonstrated a 5-year failure free survival (FFS) of 82% for ERMS compared to 65% for ARMS, even with intensified therapy [3].
Treatment for ARMS currently consists of three modalities: surgical resection, radiation therapy, and systemic chemotherapy. Radiation therapy and chemotherapy are often used to reduce tumor size prior to surgical resection and/or to eliminate residual gross or microscopic disease [2]. Ionizing radiation is a critical component of multimodal therapy for ARMS. However, the efficacy of IR depends on the presence of oxygen in the target tumor tissue to create the free radicals that cause DNA injury leading to apoptosis. Findings of the Intergroup Rhabdomyosarcoma Studies I-IV support the use of ionizing radiation for all RMS except localized, completely resected group I ERMS [4]. Considering the failure rate of 35% over 5 years for ARMS, improvement in the activity of ionizing radiation and/or chemotherapy is clearly needed to improve survival of children with ARMS. An agent that enhances the efficacy of ionizing radiation could significantly improve patient outcomes and survival by decreasing the likelihood of local tumor recurrence without increasing the radiation dose and the associated side effects.
Bevacizumab is a humanized monoclonal antibody that targets and inhibits vascular endothelial growth factor (VEGF), a pro-angiogenic cytokine. VEGF plays a central role in mediating endothelial cell proliferation, migration, and survival necessary for tumor blood vessel formation and therefore tumor growth. VEGF is also a potent stimulator of vascular permeability [5]. Studies have shown VEGF to be important for autocrine and paracrine stimulation of vessel growth required for progression of most solid tumors, including RMS [6-7]. However, the vasculature that is created is generally dysfunctional, resulting in heterogeneous tumor perfusion. The areas of hypoxia within the tumor contribute to the development of radioresistance. The proposed mechanisms of action of bevacizumab, via VEGF inhibition, include regression of existing microvessels to help arrest tumor growth and reduce tumor size, relative “normalization” of the surviving mature tumor vasculature, and inhibition of new vessel growth [5].
Several preclinical studies [6-7] have shown that RMS is responsive to VEGF inhibition; however, no guidelines exist for optimal scheduling of VEGF inhibition as an adjuvant in the treatment of RMS. In our previous studies, we have shown that bevacizumab transiently improves tumor perfusion and oxygenation in neuroblastoma during a brief period of relative normalization of the tumor vasculature leading to improved chemotherapy delivery [8]. We hypothesized that bevacizumab would have a similar effect on ARMS xenografts and that improved tumor oxygenation would enhance the efficacy of adjuvant ionizing radiation.
Materials and Methods
Animal Model
All murine experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee at St. Jude Children's Research Hospital (protocol 273). A model of orthotopic rhabdomyosarcoma was established by injection of 2 × 106 Rh-30 human alveolar rhabdomyosarcoma cells (P. Houghton, Memphis, TN) in 200 uL PBS into the right calf muscle of 4- to 6-week-old male CB-17 SCID mice (Charles River Laboratories, Wilmington, MA). Intramuscular tumor size was estimated by measuring the size of the normal left calf and subtracting that volume (width2 × length × 0.5) from the volume of the tumor-injected right calf. After approximately one month of tumor growth, mice were divided into 8 groups of equivalent tumor burden (~400mm3) and treatment was initiated. Mice were treated with either 200ug bevacizumab (Genentech, South San Francisco, CA) in 200uL PBS or 200uL PBS alone via tail vein. Control and bevacizumab treated groups were evaluated on days 0, 2, 5 and 10. Bevacizumab vs. control experiments were done in triplicate.
An additional cohort of tumor bearing mice was divided into six treatment groups in order to evaluate the effect of bevacizumab combined with ionizing radiation (IR) at different time points. Treatment groups were as follows: control, bevacizumab only, IR only, bevacizumab given 2 days before IR, bevacizumab given 5 days before IR, and bevacizumab given 10 days before IR. A single dose of 4 Gy of ionizing radiation was administered using the Orthovoltage D3000 X-ray tube (Gulmay Medical Ltd., UK) on the same day for all groups receiving that therapy (23 days after tumor cell injection) based on tumor growth patterns from the first set of experiments. Bevacizumab was also given via tail vein on the day of radiation to the bevacizumab only group.
Immunohistochemistry
Formalin-fixed, paraffin-embedded 4um thick tumor sections were stained for CD34 (Santa Cruz, sc-18917) and α-smooth muscle actin (αSMA) (DAKO, M0851) as previously described [9]. Three fields per tumor sample were digitally photographed at 200X, focusing on endothelial cell “hotspots” without tumor necrosis. All images were analyzed using Adobe Photoshop (Adobe Systems, Inc. San Jose, CA) to remove background staining and to convert the images to grayscale, and then monochrome images. Positive staining was then quantified using ImageJ (NIH analysis software) and reported as the number of pixels per high power field (HPF). Vessel maturity index (VMI) was calculated as the ratio of αSMA to CD34 staining.
Evans Blue Dye Assay
Tumor vessel permeability was assessed using an Evans blue dye assay [10]. One hundred microliters of 2% Evan's blue due (MP Biomedicals) was injected via tail vein and allowed to circulate for 20 minutes. The chest cavity was opened surgically and 10 mL of PBS was infused into the left ventricle and vented through the right atrium to remove the remaining intravascular dye. Tumors were surgically removed and divided into pieces weighing 0.15 g. Each tumor piece was then placed in 1 mL of formamide (Fisher Scientific) for 72 hours to allow dye extraction. Tumor pieces were removed and the remaining extract was centrifuged at 1200 rpm for 5 minutes. Two 200 uL aliquots per tumor piece were then quantified using a spectrophotometer at a wavelength of 620 nm.
In Vivo Oxygen Tension Measurement
Intratumoral oxygen tension was measured using the OxyLab fiber optic probe (Oxford Optronics, UK). Mice were anesthetized with ketamine/xylazine. After the mice were sedated, they were placed on 2 liters of continuous oxygen via nose cone. A small incision (<2 mm) was made in the skin at the base of the tumor and the oxygen probe was placed into the base of the tumor and positioned 3/4 of the way into the tumor. The probe was allowed to equilibrate for up to 20-30 minutes, and then a timer was set for 5 minutes and the measurement was recorded.
Statistical Analysis
SigmaPlot (SPSS, Inc., Chicago, IL) was used to perform unpaired Student's t-tests and to graphically present data. A p value of less than 0.5 was considered significant.
Results
Effect of Bevacizumab on Tumor Growth in Vivo
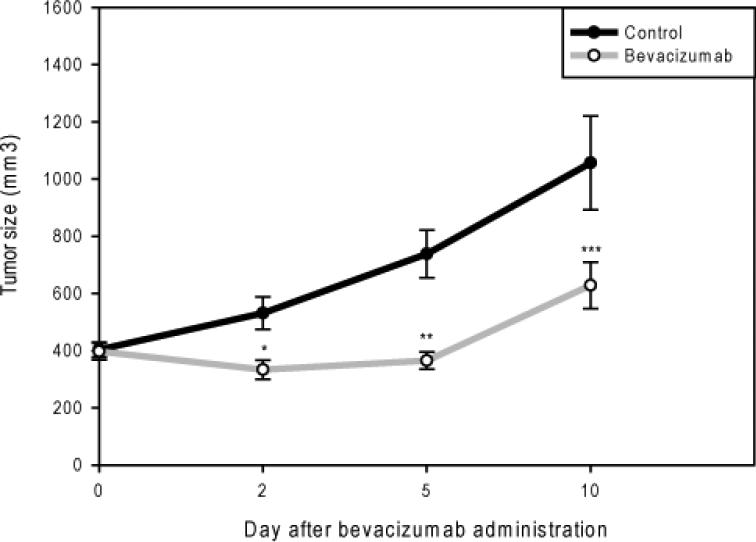
Established, sized matched intramuscular Rh-30 tumors were divided into a bevacizumab treatment group (n=20) and a vehicle control group (n=20) approximately 1 month after tumor cell injection when tumors were about 400 mm3. Tumors were harvested (n=5) from each group on days 2, 5, and 10 after bevacizumab administration and subjected to the analysis discussed below. Bevacizumab treated tumors were significantly smaller compared to control tumors on day 2 (333±33 vs. 531±57 mm3, p=0.003), 5 (365±30 vs. 738±84 mm3, p=0.00003), and 10 (628±81 vs. 1,056±164 mm3, p=0.02), demonstrating that a single dose of bevacizumab alone could significantly restrict further growth of established, orthotopic ARMS xenografts (Figure 1).
Figure 1. A single dose of bevacizumab restricts orthotopic alveolar rhabdomyosarcoma growth.
Growth curves showing no significant difference in tumor size between the bevacizumab treated and control groups at the start of therapy (p=0.88). Bevacizumab treated tumors were significantly smaller compared to control tumors on day 2 (*p=0.003), 5 (**p=0.00003), and 10 (***p=0.02).
Effect of Bevacizumab on Tumor Vessel Number and Function
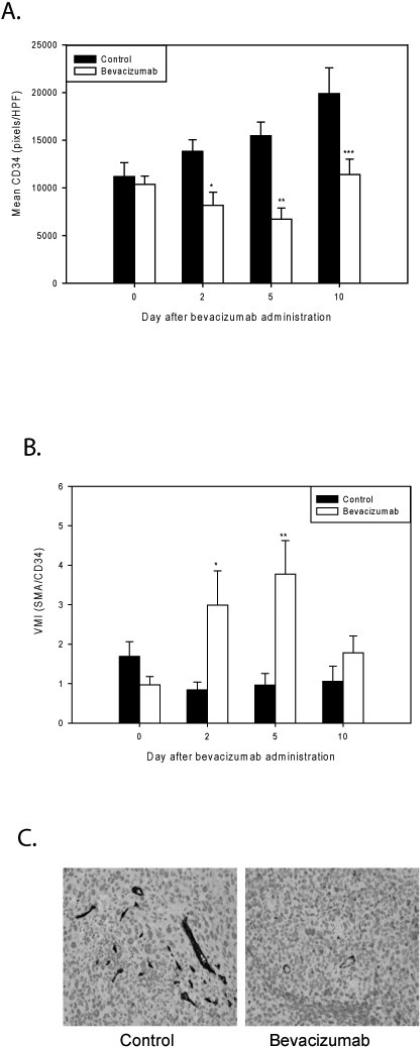
On days 0, 2, 5, and 10, one group of 5 mice from each of the bevacizumab treated and control cohorts were sacrificed and tumor tissue was harvested for immunohistochemical analysis. Experiments were performed in triplicate. CD34 staining for endothelial cells was used to evaluate microvessel density and αSMA staining was used to identify perivascular cells. On day 0, there was no significant difference in microvessel density between the bevacizumab treated and control tumors (10,371±883 vs. 11,185±1454 pixels/HPF, p=0.62). A subsequent significant decrease in microvessel density was observed in the bevacizumab treated tumors compared to controls on day 2 (8,183±1,355 vs. 13,834±1,207 pixels/HPF, p=0.005), which persisted on day 5 (6,710±1,167 vs. 15,485±1,420 pixels/HPF, p=0.00008), and day 10 (11,400±1,619 vs. 19,899±2,692 pixels/HPF, p=0.01) (Figure 2A). No significant difference in αSMA staining was seen during this time period, although there was a slight decrease in αSMA positive cells in the bevacizumab treated group compared to control on day 10 (not shown). Therefore, the vessel maturity index, defined as the ratio of pericytes to endothelial cells, also significantly increased due to the decline in the endothelial cell count. A significant increase in VMI was seen in the bevacizumab treated group, as measured on days 2 (2.99±0.87 vs. 0.84±0.20, p=0.03) and 5 (3.78±0.85 vs. 0.96±0.30, p=0.005). However, this effect had abated by day 10 (1.78±0.43 vs. 1.06±0.38, p=0.25), suggesting that by day 10 even some of the more mature vessels were dropping out (Figure 2B).
Figure 2. Bevacizumab decreases intratumoral endothelial cell counts and increases the vascular maturity index in RMS.
A) CD34 staining for vascular endothelial cells reflects microvessel density. No significant difference in microvessel density between the bevacizumab treated and control tumors was seen on day 0 (p=0.62). A significantly lower microvessel density was observed among the bevacizumab treated tumors on days 2 (*p=0.005), 5 (**p=0.00008), and 10 (***p=0.01). B) The vessel maturity index is the ratio of perivascular cells to endothelial cells. A significant increase in VMI between the treated and control groups was measured on days 2 (*p=0.03) and 5 (**p=0.005), but abated by day 10 (p=0.25). C) Representative slides stained with an anti CD34 antibody of a control and bevacizumab treated tumor 5 days after treatment, 200X.
Effect of Bevacizumab on Tumor Vessel Permeability and Intratumoral Oxygenation
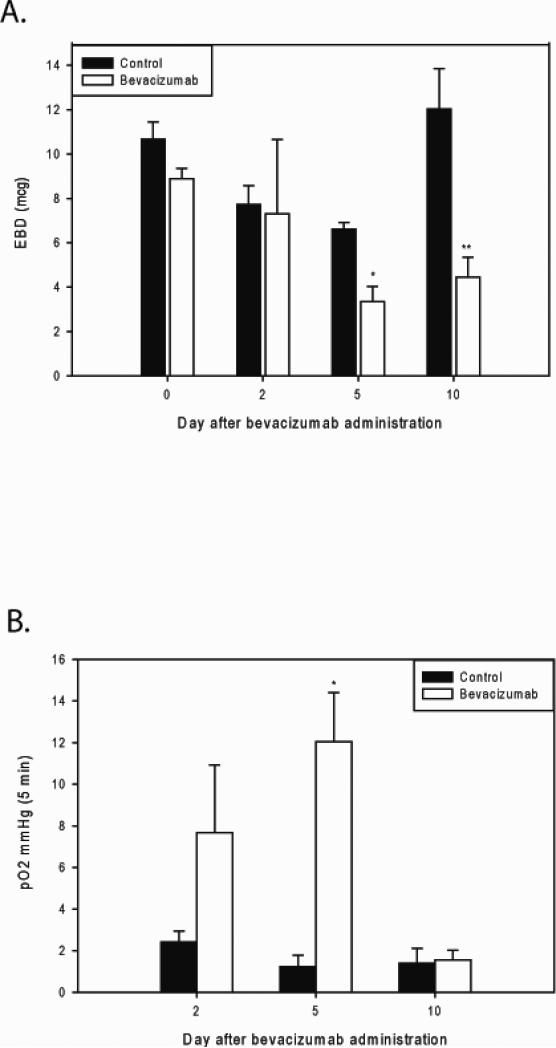
VEGF is a potent stimulator of vascular permeability [11]. We assessed the effect of VEGF inhibition and the normalization of the treated RMS tumor vasculature noted above on vessel leakiness. When using an Evans blue dye assay, the amount of extravasation of blue dye into the parenchyma of the tumors reflects tumor vessel permeability. We found that bevacizumab treated tumors demonstrated a significant decrease in tumor vessel permeability 5 and 10 days after treatment compared to control tumors, 3.34±0.69 vs. 6.61±0.31 A.U., p=0.01, and 4.45±0.89 vs. 12.02±1.82 A.U., p=0.02 respectively (Figure 3A).
Figure 3. Bevacizumab decreases vascular permeability and increases intratumoral oxygen tension.
A) Evans blue dye assay was used to quantify tumor vessel permeability. Bevacizumab treated tumors demonstrated a significant decrease in tumor vessel permeability 5 and 10 days after treatment compared to control tumors (*p=0.01, **p=0.02). B) Oxygen tension was significantly higher in the bevacizumab treated tumors compared to control tumors on day 5 after bevacizumab treatment (*p=0.001), suggesting a transient, but significant increase in tumor perfusion and oxygenation.
Finally, we assessed the effect of the changes in vascular phenotype and decreased vessel permeability on intratumoral oxygenation. Intratumoral oxygen tension was measured as described above using an OxyLab probe. In the first set of experiments comparing bevacizumab treatment to controls, intratumoral oxygen tension was measured on days 0, 2, 5 and 10. Oxygen tension was significantly higher in the bevacizumab treated tumors compared to control tumors on day 5 after bevacizumab treatment (12.05±2.36 vs. 1.24±0.54, p=0.001) but not on the other days, indicating a transient, but significant increase in tumor perfusion and oxygenation (Figure 3B).
Effect of Combined Treatment with Bevacizumab and Ionizing Radiation on ARMS Xenograft Growth
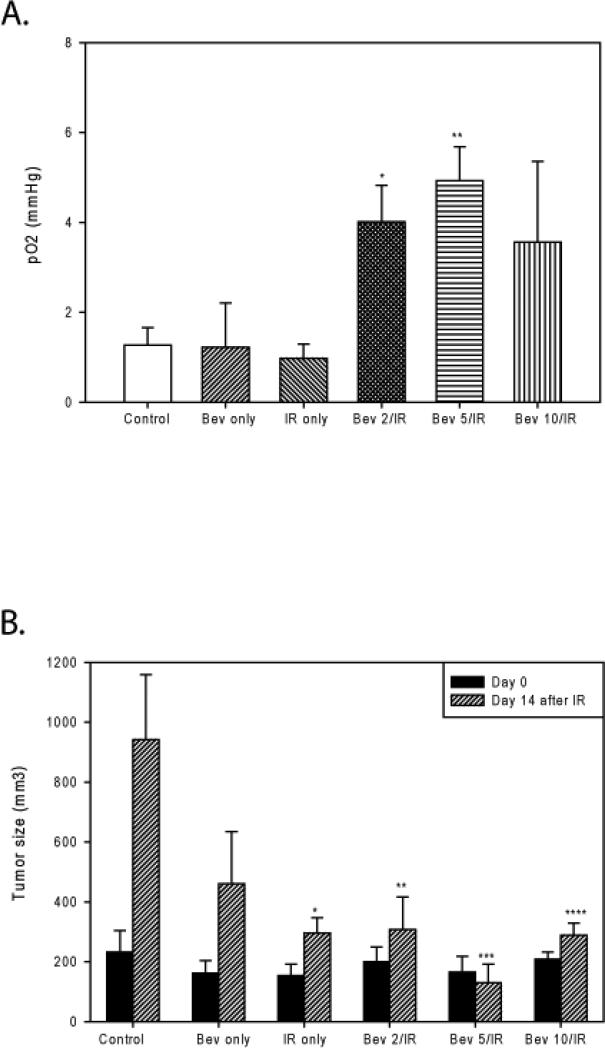
Six treatment groups were used to evaluate the differences the effect of the timing of bevacizumab administration with respect to IR would have on Rh-30 ARMS xenograft growth. These included: control, bevacizumab only, IR only, bevacizumab given 2 days prior to IR, bevacizumab given 5 days prior to IR, and bevacizumab given 10 days prior to IR. Intratumoral oxygen tension for all groups was measured just prior to the administration of IR. Again, as found in the earlier experiments, significantly greater intratumoral oxygenation was observed transiently in tumors that received bevacizumab prior to IR, compared to controls (4.01±0.82 [day 2] and 4.92±0.76 [day 5] vs. 1.27±0.39 mmHg [control], p=0.016 and p=0.0026, respectively). The IR only and bevacizumab only groups had not yet received adjuvant therapy at the time of measurement; therefore, the intratumoral oxygenation in these groups was similar, as expected (Figure 4A).
Figure 4. Intratumoral oxygenation is significantly increased after bevacizumab administration and results in augmentation of the tumor response to ionizing radiation.
A) Significant increases in intratumoral oxygenation were seen in the bevacizumab given 2 days prior to IR and bevacizumab given 5 days prior to IR groups compared to controls (*p=0.016 and **p=0.0026). B) Murine tumors in all six treatment groups were measured on the day of IR (day 0) and two weeks after IR. No significant difference was seen between any of the groups on the day 0. On day 14 after IR, a significant decrease in tumor size was observed between the IR only, bevacizumab given 2 days prior to IR, bevacizumab given 5 days prior to IR, and bevacizumab given 10 days prior to IR groups compared to the control group (*p=0.04, **p=0.03, ***p=0.007, and ****p=0.02). Bev 2/IR= bevacizumab given 2 days before IR, Bev 5/IR = bevacizumab given 5 days before IR, Bev 10/IR = bevacizumab given 10 days prior to IR.
We tested our hypothesis that improved oxygenation at the time of IR administration in the groups treated with bevacizumab 2-5 days prior would lead to improved antitumor efficacy of radiation therapy. There was no significant difference in tumor size seen between any of the groups on the day of IR administration (day 0, 23 days after tumor cell injection). Larger tumors were selected to treat with bevacizumab on days -10, -5, and -2, so that all tumors would be approximately the same size on day 0. Fourteen days later, tumor sizes were compared. Bevacizumab and IR monotherapies restricted further tumor growth compared to control (460±175, p=0.12 and 296±51 mm3, p=0.04). All three cohorts that received combination therapy had significant tumor growth restriction compared to controls (307±110, p=0.03 [bevacizumab given 2 days before IR]; 130±62, p=0.007 [bevacizumab given 5 days before IR]; and 289±40 mm3, p=0.02m [bevacizumab given 10 days before IR] vs. 942±217 [control]). IR given 5 days after bevacizumab was the only treatment that actually effected tumor regression (Figure 4B). This finding is consistent with our previous data showing the greatest increase in tumor oxygenation occurring 5 days after bevacizumab administration.
Discussion
This is the first study to use VEGF inhibition via bevacizumab as an adjuvant to ionizing radiation in the treatment of ARMS. In addition, this study demonstrates that VEGF inhibition via bevacizumab transiently improves the dysfunctional vasculature of ARMS xenografts, creating a period of time during which there is improved intratumoral perfusion and oxygenation. This, in turn, results in an improvement in antitumor efficacy of ionizing radiation delivered during this period. Because the effect of bevacizumab on the tumor vasculature is transient, the enhancement of IR activity depends, at least in part, on the timing of bevacizumab administration relative to IR.
We observed a significant decrease in the endothelial cell density 2 days after bevacizumab administration; this persisted throughout the study period of 10 days. However, because the pericyte count remained relatively unchanged between the two groups on the same day, this resulted in a significant increase in vessel maturity 2-5 days after treatment, although this effect was abrogated by day 10. Thus, it appears that bevacizumab eradicated the immature microvessels comprised only of endothelial cells, but lacking stabilizing perivascular cells. Although there were fewer vessels, those that remained were more mature, functional vessels. This was confirmed by demonstrating significantly decreased vascular permeability after a single dose of bevacizumab which blocked VEGF's potent effect of increased vascular permeability. This normalization of the intratumoral vasculature also increased intratumoral oxygen tension, which was significantly increased on day 5 after bevacizumab treatment. However, this effect was also transient, likely due to dropout of even those vessels invested with pericytes by day 10.
These findings support the concept of vascular normalization proposed by Jain [12]. Tumor vessels are structurally and functionally abnormal compared to vessels that perfuse normal tissues. Increased production of pro-angiogenic factors such as VEGF by the tumor cause formation of poorly organized and hyperpermeable tumor vessels. Blocking VEGF leads to increased apoptosis of endothelial cells and therefore elimination of immature blood vessels not fortified with pericytes that provide paracrine survival factors. This effectively creates tumor vessels with closer resemblance to normal vessels in structure and function [12]. Improved perfusion, and therefore oxygen delivery is essential for improved effectiveness of IR. Increased oxygen delivery augments the ability of the target tumor tissue to create free radicals to cause DNA injury and apoptosis.
It is interesting to note that the highest intratumoral oxygen tension on the morning of IR delivery occurred in the group treated with bevacizumab 5 days prior. This was also the group in which the greatest antitumor activity was observed, actually effecting tumor regression.
Our cumulative results suggest combination therapy with bevacizumab as an adjuvant to IR may be effective in the treatment of alveolar RMS and improve patient outcomes. Careful consideration of the timing of bevacizumab as an adjuvant to IR will be needed to optimize the effectiveness of combination therapy.
Acknowledgements
This work was supported by the Assisi Foundation of Memphis, the US Public Health Service Childhood Solid Tumor Program Project Grant No. CA23099, the Cancer Center Support Grant No. 21766 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the AAP National Conference & Exhibition (NCE), Surgical Section, Washington, DC, October 17-20, 2009.
References
- 1.Loeb DM, Thornton K, Shokek O. Pediatric Soft Tissue Sarcomas. Surg Clin N Am. 2008;88:615–627. doi: 10.1016/j.suc.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wexler LH, Meyer WH, Helman LJ. Rhabdomyosarcoma and the Undifferentiated Sarcomas. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th edition Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 971–1001. [Google Scholar]
- 3.Meza JL, Anderson J, Pappo AS, et al. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children's Oncology Group. J Clin Oncol. 2006;24:3844–51. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 4.Raney RB, Maurer HM, Anderson JR, et al. The Intergroup Rhabdomyosarcoma Study Group (IRSG): major lessons from the IRS-I through IRS-IV studies as background for the current IRS-V treatment protocols. Sarcoma. 2001;5:9–15. doi: 10.1080/13577140120048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duda DG, Batchelor TT, Willett CG, et al. VEGF-targeted cancer therapy strategies: current progress, hurdles and future prospects. TRENDS in Molecular Medicine. 2007;13:223–230. doi: 10.1016/j.molmed.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gee MFW, Tsuchida R, Eichler-Jonsson, et al. Vascular endothelial growth factor acts in an autocrine manner in rhabdomyosarcoma cell lines and can be inhibited with all-trans-retinoic acid. Oncogene. 2005;24:8025–37. doi: 10.1038/sj.onc.1208939. [DOI] [PubMed] [Google Scholar]
- 7.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 8.Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-Induced Transient Remodeling of the Vasculature in Neuroblastoma Xenografts Results in Improved Delivery and Efficacy of Systemically Administered Chemotherapy. Clin Cancer Res. 2007;13:3942–50. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 9.Spurbeck WW, Ng CY, Strom TS, et al. Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood. 2002;100:3361–8. doi: 10.1182/blood.V100.9.3361. [DOI] [PubMed] [Google Scholar]
- 10.Farrell C, Stewart P, Del Maestro R. A new glioma model in rat: the C6 spheroid implantation technique permeability and vascular characterization. J Neuro-oncol. 1987;4:403–15. doi: 10.1007/BF00195612. [DOI] [PubMed] [Google Scholar]
- 11.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 12.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nature Medicine. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]