Abstract
Myoepithelial cells form a semi-continuous protective sheet separating the human breast epithelium and the surrounding stroma. They suppress stromal invasion of tumor cells by the secretion of various anti-angiogenic and anti-invasive factors. The disruption of this cell layer results in the release of the growth factors, angiogenic factors, and reactive oxygen species causing an alteration in the microenvironment. This helps in the proliferation of surrounding cells and increases the invasiveness of tumor cells. Two theories are proposed for the mechanism of tumor epithelial cells progression from in situ to invasive stage. According to the first theory, tumor cell invasion is triggered by the overproduction of proteolytic enzymes by myoepithelial cells and surrounding tumor cells. The second theory states that tumor invasion is a multistep process, the interactions between damaged myoepithelial cells and the immunoreactive cells trigger the release of basement membrane degrading enzymes causing tumor progression. Further studies in understanding of molecular mechanism of myoepithelial cell functions in tumor suppression may lead to the identification of novel therapeutic targets for breast cancer.
Keywords: Myoepithelial cells, DCIS, tumor progression, tumor microenvironment, basement membrane, matrix metalloproteinases, natural tumor suppressors, Review
2. INTRODUCTION
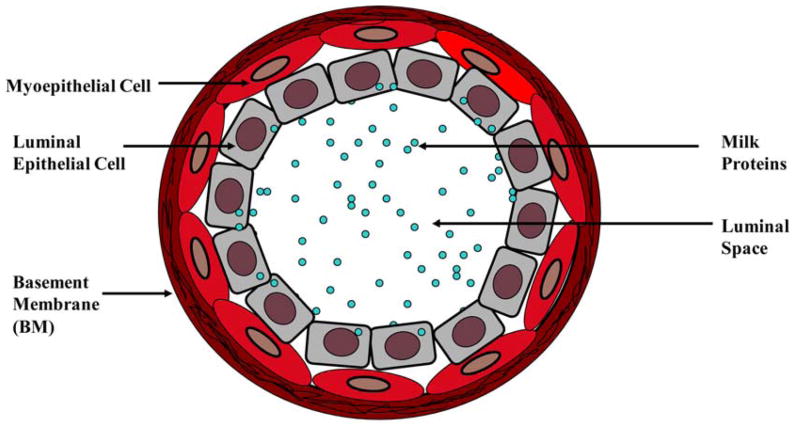
The mammary glands contain a branching ductal network which is composed of two epithelial cell types present in roughly equal numbers and are embedded in connective tissue. They are an incomplete outer layer of elongated myoepithelial cells and an inner layer of polarized luminal epithelial cells, and both of these cell layers are surrounded by a continuous lining called basement membrane (BM) (Figure 1) (1). Myoepithelial cells contribute to the synthesis of a surrounding BM, and their myogenic differentiation is responsible for the contractile phenotype mediated by oxytocin (2). BM is composed of the collagenous stroma and is rich in laminin, heparan sulfate proteoglycans, glycosaminoglycans and entactin. Myoepithelial cells are attached to luminal cells by desmosomes and to the BM by hemidesmosomes (1). They are contractile cells which contain alpha-smooth muscle actin (alpha-SMA) and adhere to the basement membrane. These cells are elongated in shape and joined to each other by intermediate or gap junctions and a number of intercellular adhesion molecules, forming a semi-continuous sheet or belt that encircles the epithelial cells. Thus, myoepithelial cells form a natural border to separate proliferating epithelial cells from the BM and underlying stroma (3).
Figure 1.
Cross section of a normal mammary gland duct.
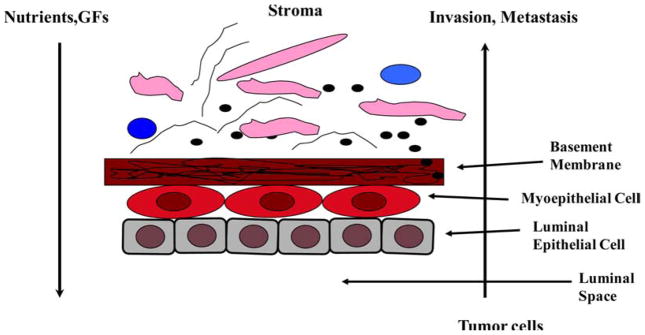
Both the myoepithelial cell layer and the BM act as a natural barrier and are selectively permeable to the passage of small molecules. Due to this structural feature, nutrients, growth factors and other chemotropic molecules must first pass through the BM then the myoepithelial cells to reach the inside of the duct, and most tumor epithelial cells have to first pass through the myoepithelial cell layer and then the BM in order to physically contact the stroma (Figure 2) (1,3). In other words, the disruption of both the BM and the myoepithelial cell layer is an absolute prerequisite for breast tumor invasion (4). Human breast myoepithelial cells have the property of self-renewal and they consistently undergo both proliferation and differentiation to replace injured, aged or dead myoepithelial cells. The division of myoepithelial cells in the adult breast in vivo is relatively slow whereas luminal epithelial cells multiply rather quickly with each menstrual cycle and the number is increased greatly during the lactating period. On the contrary, when the myoepithelial cells are cultured in in vitro, they divide more rapidly than the luminal cells which make it problematic to study the luminal cells in in vitro culture as the residual myoepithelial cells in sorted luminal cultures will divide rapidly and outgrow luminal cells (5, 6).
Figure 2.
Structural relationship between mammary gland duct cells, BM and stroma.
The importance of myoepithelial cells in breast tumor progression has always been under- estimated; however, it is now being recognized that besides milk ejection during lactation they have an important role in the suppression of tumor invasion. On the other hand, degradation of this layer promotes breast tumor progression and metastasis, although the exact mechanisms of this phenomenon are still unclear (7). In this review, we overview recent information about the general characteristics of myoepithelial cells, their roles in tumor suppression, tumor microenvironment alteration and progression in breast cancer. Understanding the underlying molecular mechanisms of the myoepithelial cell functions during tumor progression may lead to the identification of novel therapeutic targets.
3. CHARACTERISTICS OF MYOEPITHELIAL CELLS
3.1. Structural features of myoepithelial cells
Myoepithelial cells contain a large amount of microfilaments and smooth muscle-specific cytoskeletal proteins such as alpha-actin and myosin that are responsible for the contractile phenotype mediated by oxytocin during lactation (2). Myoepithelial cells are slightly spindle or elongated in shape with pale cytoplasm and nuclei and are keratin-positive. They are distinct from myofibroblasts which are mesenchymal cells and positive for vimentin and are also generally positive for alpha-SMA, cytokeratin 5/6 and other high molecular weight cytokeratins, p63 and caldesmon (8). Each myoepithelial cell has long cytoplasmic processes that wrap around a secretory unit and hence, contraction of the myoepithelial processes can eject secretory product from the secretory unit into its duct. The myoepithelial cells play an active role in branching morphogenesis of the mammary gland, and their correct recognition and detection is crucial in the diagnosis of a number of pathological breast lesions (9). Several markers have been reported for the immunohistochemical detection of breast myoepithelial cells such as alpha-SMA, smooth muscle myosin heavy chain, h-caldesmon, S100 protein, p63, maspin and specific cytokeratins like CTK14 and CTK17. Other myoepithelial cell-specific proteins include CD10/CALLA, calponin, and epidermal growth factor receptor (EGFR); however, the specificity and sensitivity of these markers vary widely. Of these, maspin and p63 are generally considered the most promising markers for myoepithelial cells. (10). In addition to these genes, Barsky et al. used microarrays to analyze established myoepithelial cell lines and compared them to normal and cancerous cell lines as well as primary tumors. Using this approach, they identified many genes that encode extracellular matrix proteins (collagens, laminin, fibronectin and osteonectin), proteins involved in angiogenesis (thrombospondin-1 and plasminogen) and protease inhibitors (maspin and PAI-1). The suppression profile of these specific genes in myoepithelial cells strongly suggest that they act as natural tumor suppressors (11).
3.2. Normal functions of myoepithelial cells
Myoepithelial cells normally surround ducts and acini of glandular organs and contribute to the synthesis of a surrounding basement membrane (12). The most obvious and important function of the myoepithelial cell in the breast is contraction of mammary gland duct. Myoepithelial cells are attached to the luminal cells and control many aspects of luminal functions. They regulate the flow of fluid and control the entry and exit of nutrients, electrolytes and other growth factors. Myoepithelial cells also process signals of endocrine or paracrine nature, and perhaps as an intermediary in such signaling processes by passing information both inwards and outwards in a paracrine fashion or via intra-epithelial gap-junctions (1). Another important function of myoepithelial cells is to form a structural barrier between stroma and lumen as they generate a continuous layer like a sheet. Because of this location, the tumor cells can invade the stroma only after the disruption of myoepithelial cells and the basement membrane which is an absolute requirement for tumor invasion. Importantly, myoepithelial cells also modulate the expression of matrix metalloproteinases (MMPs) in the tumor as well as in the surrounding cells and help in the prevention of tumor invasion which may be mediated by these proteolytic enzymes (13). Several angiogenesis-related MMPs such as MMP19 are secreted by the normal myoepithelial cells and this enzyme is also reported to participate in the turnover of extracellular matrix (ECM) (14).
Ductal elongation requires the production and organization of new BM, and myoepithelial cells play a key role in this process. Myoepithelial cells synthesize BM components such as collagen IV, laminin-1, laminin-5 and fibronectin that regulate ductal growth, and facilitate the sculpting of new BM through the production of MMP2 and MMP3 (13, 14). Myoepithelial cells also express morphogens and growth factors that are activated in a coordinated manner during morphogenesis (1). Normal myoepithelial cells are critical for correct polarity of luminal epithelial cells, most likely via production of laminin-1. On the other hand, myoepithelial cells present in invasive breast carcinoma have many traits in common with normal myoepithelial cells but they show either complete absence or reduced expression of laminin-1. Laminin-1 is strongly expressed around normal breast epithelial structures and thus tumor myoepithelial cells are unable to induce the polarization of luminal epithelial cells (15). Interestingly, recent work from the Polyak laboratory has shown that myoepithelial cells isolated from DCIS are drastically altered and secrete many cytokines and other potential tumor promoting molecules (10).
3.3. Myoepithelial cells are natural tumor suppressors
Myoepithelial cells, which surround ducts and acini of glandular organs, form a natural border separating proliferating epithelial cells from basement membrane and underlying stroma, thus physically preventing tumor cell invasion. Several lines of evidence suggest that differentiated myoepithelial cells block proliferation of breast carcinoma cells by inducing growth arrest and apoptosis. In addition, myoepithelial cells also secrete various effector and inhibitor molecules that interfere with the invasive behavior of tumor cells, block angiogenesis and the BM degradation (1, 3). Myoepithelial cells have also been shown to modulate the gene expression of both tumor cell and fibroblast by antagonizing the tumor-stroma interactions that have been demonstrated as a critical factor in tumor progression (13). The modulation of MMP expression is perhaps the most important effect of myoepithelial cells. The MMPs are inhibited by specific endogenous tissue inhibitor of metalloproteinases (TIMPs), which comprise a family of four protease inhibitors: TIMP1, TIMP2, TIMP3 and TIMP4. TIMP-1 is mainly secreted by the myoepithelial cells (11, 21). However, the exact mechanism by which they block MMP expression is not clearly understood. In addition, myoepithelial cells constitutively express high amounts of proteinase inhibitors that include maspin, activin, connexin, thrombospondin, TIMP-1, protease nexin-II, alpha-1 antitrypsin and neogenin (Table 1). These proteinase inhibitors act by blocking the activity of the released enzyme rather than by inhibiting the proteolytic enzyme synthesis (12, 13).
Table 1.
Major tumor suppressor genes expressed in myoepithelial cells
| Gene | Tumor suppressor function(s) | Reference |
|---|---|---|
| Maspin | Protease inhibitor, anti-angiogenic, anti-locomotory | 15–17 |
| T1MP-1 | MMP inhibitor, decreases invasiveness of tumor cells | 11,21 |
| Caveolin-1 | Inhibition of growth factor signaling pathways in tumor cells, Inhibits primary tumor growth and metastasis | 22–25 |
| Cytokeratin-5/6 | Regulates cytoskeletal structure, regulates cell growth and decreases survival in tumor cells | 26–27 |
| alpha-SMA | Regulates cytoskeletal structure, suppresses cell growth and motility | 28–29 |
| Relaxin | Anti-tumor activity by production of nitric oxide (NO) | 30 |
| Activin | Growth inhibition in tumor cells, regulation of cell proliferation and apoptosis | 31–33 |
| Connexin-43 | Inhibits tumor cell proliferation, formation of gap junctions and induce E-cadherin expression | 34–35 |
| TSP-1 | Anti-angiogenic, regulated by p53 | 36 |
| MEPI | Inhibits tumor growth and metastasis, decreases invasive potential of tumor cells preventing tumor dissemination | 37 |
| Neogenin | Closely related to DCC tumor suppressor, decreases mammary tumorigenesis | 38–39 |
Maspin is one of the most important tumor suppressors that are secreted by myoepithelial cells. It is a member of the serpin family of serine proteases which inhibits tumorigenesis, tumor cell migration and metastasic spread thus it functions as a tumor suppressor. It is also detectable in breast ductal lavage fluid and breast nipple aspirates (15). Maspin is secreted in large quantities by the normal cells whereas tumor cells do not secrete it. This suggests that maspin has tumor suppressor function in normal cells but this function is lost in tumor cells. In addition, maspin acts as an angiogenesis inhibitor and locomotion inhibitor (16, 17). These properties may explain the anti-angiogenic and anti-invasive effects of myoepithelial cells on tumor and precancerous cells. Therefore, myoepithelial cells are considered to be natural suppressors of invasion and metastasis and may specifically inhibit the progression of precancerous disease states to invasive cancer (15–20).
Caveolins are a family of proteins that are involved in receptor independent endocytosis and high expression of caveolins leads to inhibition of growth factor signaling pathways (22). Caveolin-1 was found to be downregulated in breast cancer specimens as well as in human breast cancer cell lines compared with matched normal tissue and normal epithelial cell lines (23–24). It has also been demonstrated that caveolin-1 reduces both primary tumor growth and spontaneous metastasis to lungand bone (25). myoepithelial cells secrete another class of tumor suppressors, cytokeratins (CK) which are intermediate filament keratins found in the intracytoplasmic cytoskeleton of epithelial tissue. Steffansson et al. found that loss of CK5/6 was associated with features of aggressive tumors and lack of CK5/6 was significantly associated with reduced survival in large population-based series of 276 endometrial carcinomas with long and complete follow-up (26). It was reported in another study that there was a highly significant relationship between loss of CK5/6 expression and reduced membranous beta-catenin staining and that the reduction of membranous beta-catenin expression was associated with aggressive tumors and decreased survival in this tumor series (27). Relaxin is a peptide hormone secreted by Myoepithelial cells which is capable of stimulating the production of nitric oxide (NO) in several cell types. NO has been reported to have anti-tumor activity by inhibiting proliferation, promoting differentiation, and reducing the metastatic spread of some tumor cell types such as in MCF-7 cells (30).
Myoepithelial cells express activin, which belongs to the TGF-beta superfamily. Mutations in activin receptors have been indeed associated with pancreatic and pituitary tumors (31–32). Activin has been reported to inhibit growth of breast cancer cells by activating Smad proteins and by blocking the p38 mitogen-activated protein kinase (MAPK) pathway (33). Connexins (Cx), or gap junction proteins, belong to a family of structurally-related transmembrane proteins that assemble to form gap junctions. Connexins are tumor suppressors, and Cx-26 and Cx-43 gap junctions are often down-regulated in breast cancer. The downregulation of connexin expression is often observed in tumors and transformed cell lines and is believed to contribute to the loss of cell growth control (34). Xu et al. reported that Cx-43 may induce E-cadherin expression and inhibit cell proliferation and progression of lung cancer (35). Thrombospondin-1 (TSP-1) is another natural tumor suppressor secreted by myoepithelial cells which is a 430-kd glycoprotein that is an important component of the ECM and is known to be a potent inhibitor of angiogenesis both in vitro and in vivo. TSP-1 possesses tumor suppressor function, possibly through its ability to inhibit tumor neovascularization and this gene appears to be regulated by p53, a gene which is mutated in as high as 50% of advanced breast cancers (36).
A new protein known as myoepithelium-derived serine proteinase inhibitor (MEPI) was recently identified exclusively in the myoepithelial cells on the normal and noninvasive mammary epithelial side of the basement membrane, while MEPI expression was not detected in the malignant breast carcinomas (37). Expression of MEPI in human breast cancer cells, blocks their growth, decreases their invasive potential and prevents tumor dissemination in vivo (37). Thus, the expression of MEPI in myoepithelial cells may prevent breast cancer progression and metastasis. Myoepithelial cells also express neogenin which is a receptor initially identified to act in short and long range neuronal guidance and cap cells in terminal end buds (TEBs), a specialized structure at the end of growing ducts. Neogenin is a member of the N-CAM family of cell adhesion molecules and is closely related to the DCC tumor suppressor gene product (38). In fact, Lee et al. reported that the expression of neogenin was inversely related to the tumorigenicity of human breast cancer (39). They used tissue array and found that all of the normal breast tissues showed a strong neogenin expression, while most cancer tissues showed weaker neogenin expression compared to normal tissues. These results suggest that neogenin expression in myoepithelial cells has an important function in suppression of mammary tumorigenesis. In addition to producing these anti-invasive and anti-angiogenic molecules, myoepithelial cells have been shown to possess CD44 shedding activity by producing soluble CD44, which blocks the adhesion and migration of human carcinoma cells on hyaluronic acid-coated surfaces (40). All these observations strongly support the notion that myoepithelial cells function as tumor suppressors.
4. ROLES OF MYOEPITHELIAL CELLS IN TUMOR PROGRESSION
Tissue microenvironment has profound effects on the progression of cancer cells by its paracrine signaling. Molecular characterization of various cell types from the normal breast tissue, ductal carcinoma in situ (DCIS) and invasive breast tumor revealed significant changes in gene profile in all cell types during breast tumor progression. Microenvironment changes influence tumor progression as well as the efficacy of various cancer therapies. Alteration in tissue organization and homeostasis can precede and increase the chance of tumor initiation as exemplified by the increased cancer risk associated with chronic inflammation and wound healing (41). Paracrine factors such as extracellular proteins, protease inhibitors, various growth factors, angiogenesis regulators and other unidentified factors secreted by myoepithelial cells exert their effects on the tumor epithelial cells. Such regulation may be important in determining tumor cell behavior in vivo and may be mediated by specific ECM molecules, matrix associated growth factors or host cell themselves (42). Myoepithelial cells contribute to the synthesis and remodeling of the basal lamina and the basement membrane. They exert important paracrine effects on normal glandular epithelium, and there are several lines of evidence to indicate that myoepithelial cells regulate the progression of DCIS to invasive breast cancer (1). On the contrary, myoepithelial cells also exhibit a tumor suppressor phenotype and they rarely transform themselves; however, when they transform, they generally give rise to benign neoplasms that grow without degrading ECM (11).
The comparison of myoepithelial cells derived from normal breast tissue with myoepithelial cells that surround spaces involved by DCIS have showed a significant difference in the gene expression profiles in several respects (43). Most of the over-expressed genes in DCIS- associated myoepithelial cells encode secreted cell surface proteins, several of which are chemokines, such as, SDF1/CXCL12 and CXCL14 which bind to receptors on epithelial cells and enhance their proliferation, migration, invasion and stromal angiogenesis whereas a variety of genes are downregulated in the DCIS- associated myoepithelial cells which are involved in normal functions including those for oxytocin receptors, laminin and TSP-1. DCIS- associated myoepithelial cells also upregulate the synthesis of enzymes involved in the degradation of ECM and BM such as MMPs (43). Myoepithelial cell lines such as HMS-16 which are derived from benign myoepithelial tumors, express high levels of active anti-angiogenic factors that include maspin, TIMP-1, TSP-1 and soluble bFGF receptors but very low levels of angiogenic factors thus, secretory factors from myoepithelial cells can change the tumor microenvironment and the signaling molecules such as chemokines may play a role in breast tumorigenesis by acting as paracrine factors (44). These results provide strong evidence that DCIS- associated myoepithelial cells show abnormal behavior and because of this, they lost their normal tumor suppressor functions and lead to the progression of DCIS to invasive breast cancer (10–11, 43–44).
4.1 Roles of myoepithelial cells in the alteration of microenvironment and breast tumor progression
As already mentioned, myoepithelial cells act as natural tumor suppressors by secreting the various molecules that have inhibitory effects on tumor cell growth, invasion and angiogenesis. They also act as a physical barrier to prevent the invasion of tumor cells from the duct to the stroma. It is generally accepted that primary breast carcinomas show a dramatic increase in the ratio of luminal epithelial cells to myoepithelial cells. In the later stages of breast cancer, the ducts completely lack myoepithelial cells (45). Thus, it is mandatory for this layer to be disrupted in order for the tumor to spread and metastasize. It has been speculated that most of these secretory molecules act in a paracrine manner so that a disruption in the myoepithelial cell layer can result in the release of these factors and possibly alter the tumor microenvironment which ultimately results in metastasis to other organs (3, 7). Numerous studies in cell culture and in xenograft models have demonstrated that paracrine interaction between stromal and tumor epithelial cells promote the proliferation, invasiveness, tumorigenicity and metastatic potential of immortalized epithelial cells or cancer cells (46–48).
It is generally believed that development of breast cancer is a multi-step process which progresses from normal to hyperplastic to in situ and finally to invasive stages (49). One distinct feature of the invasive tumor is disappearance of the myoepithelial cell layer although the exact mechanism for this phenomenon is unknown. It may be possible that the myoepithelial cells are degraded by the overproduction of the degradative enzymes or they are selectively eliminated by apoptotic mechanism (50–51). Alternatively, the stem cells in that area stop differentiating into myoepithelial cells. In any of these cases, once this protective layer is lost, the tumor cells can easily invade the stroma and metastasize to other organs. Tumor progression occurs when there is a change in microenvironment due to the secretions from myoepithelial cells (51).
There are two major hypotheses that explain the mechanism of tumor progression from in situ to stromal tumor invasion. One is proteolytic enzymes theory which is based on the overproduction of MMPs by the myoepithelial cells and surrounding tumor cells and the other theory is known as focal myoepithelial cell layer disruption (FMCLD theory) (4, 7, 50). FMCLD theory states that the breast tumor invasion is a multistep mechanism which occurs in a series of events when myoepithelial cells, damaged by genetic abnormalities or any physical injuries, secrete various effector molecules which alter the microenvironment. Myoepithelial cells could be the target of external and internal insults and are also affected by a variety of normal and pathologic changes. For instance, exposure of myoepithelial cells to oxytocin results in the enhancement of myoepithelial cell differentiation and proliferation whereas exposure to lambda carrageenans, naturally occurring sulfated polysaccharides which are used in the commercial food preparation, could result in the disassembly of the filaments and loss of myoepithelial cells; however, the exact mechanism by which these chemicals cause the destruction of myoepithelial cells is still unknown (51–52).
4.1.1. Proteolytic enzymes theory
In order to invade the stroma and metastasize, tumor cells have to cross several barriers like BM, myoepithelial cell layer, interstitial tissues and extracellular matrices, which are composed primarily of collagen, proteoglycans, laminin, elastin, and other glycoproteins. Tumor cells over-express and secrete proteases which are capable of degrading the components of these barriers and thus facilitate their migration. According to the proteolytic enzyme theory, the progression from the in situ to invasive stage is believed to be triggered by the overproduction of various proteolytic enzymes by the tumor cells, such as MMPs, serine proteases and cathepsins resulting in the degradation of the BM (53). The most important among these proteolytic enzymes are MMPs, which are able to degrade the BM and make it easier for the tumor cells to cross the previously intact barrier and to invade the stroma. Ultimately, the invaded cells metastasize to other distant organs and colonize there resulting in micro or macrometastases (54–55). It was found that the level of proteolytic enzymes increases linearly with tumor progression and reaches the highest level at the in situ stage in which invasion occurs (54). MMPs are not solely responsible for BM degradation because the inhibitors of these proteolytic enzymes do not inhibit the tumor invasion completely. It is possible that there are some other secretory factors or mechanisms by which the normal microenvironment is changed, which results in degradation of the BM. In the case of DCIS, it may be possible that the tumor cells induce myoepithelial cells and luminal cells to secrete additional factors which can degrade the BM.
4.1.2. Focal myoepithelial cell layer disruption (FMCLD) theory
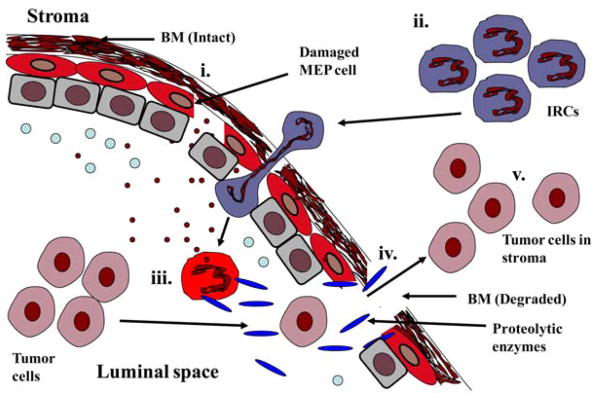
Recently, a new model of tumor invasion of stroma by the epithelial cells was proposed by Man et al. (Figure 3) (4). According to this model, tumor invasion is triggered by a series of events which begin when the myoepithelial cells are damaged by any genetic abnormalities, inflammation, mutations, localized trauma or other physical/chemical injuries which result in the disruption of the myoepithelial cell layer or impairs the normal replacement process. In fact, it is now known that disruption in the myoepithelial cell layer is the most distinct sign of tumor invasion in breast cancer. The death of myoepithelial cells results in a localized loss of tumor suppressors like maspin, TIMPs and other proteases and paracrine inhibitory factors. The diffusion of these molecules in the microenvironment increases permeability for oxygen, nutrients, and growth factors, and a localized increase in leukocyte infiltration, leading to substantial alterations of the microenvironment that facilitate cell proliferation, locomotion and stromal invasion (4,7). The infiltration of immunoreactive (IRC) cells such as leucocytes and macrophages into the damaged site results in the release of digestive enzymes that destroy the myoepithelial cells. The damaged myoepithelial cells also release MMPs and other proteases which in turn cause the focal disruption in the myoepithelial cell layer and ultimately degrade the BM producing a gap. Once the BM integrity is compromised, tumor cells can cross the damaged BM and come in contact with the stroma and colonize there. This altered microenvironment leads to variable consequences in overlying tumor and adjacent myoepithelial cells, depending on the nature of these cells. Indeed this stromal-epithelial interaction is another important factor in the alteration of microenvironment that plays a significant role in the transition from DCIS to invasive breast cancer. The direct physical contact between the tumor cells and stromal cells stimulates the production of tenascin and other invasion-associated molecules that influence tumor cell proliferation, differentiation, angiogenesis, facilitate tissue remodeling and epithelial-mesenchymal transition by providing a favorable microenvironment in the stromal region (4, 7).
Figure 3.
Degradation of the BM resulting in the stromal invasion by tumor cells. (i). Myoepithelial cells are damaged by various factors releasing the inner contents like the diffusible molecules and chemoattractants. (ii). Immunoreactive cells (IRC) attracted to the luminal space by these chemoattractants. (iii). IRCs activated by coming in contact with chemoattractants and secrete different proteolytic enzymes (iv). These proteolytic enzymes then degrade the basement membrane resulting in gaps. (v). Tumor cells enter the stromal region through these gaps.
The proteolytic enzyme theory was widely accepted in the past; however, it is becoming clear that some other factors and interactions also take place during BM degradation and stromal invasion. The interactions between the myoepithelial cells in the ducts and the fibroblasts present in the stromal compartment are known to influence tumor progression; tumor-associated fibroblasts (TAFs) have been shown to promote tumor cell invasion by releasing extracellular matrix (ECM) degrading proteases and these enzymes consequently modify the composition of the ECM and facilitate tumor cell motility (56–57). Besides MMPS, some other proteolytic enzymes like serine proteases and cathepsins are also indicated for BM degradation. But the exact mechanism of the interactions between these cell types is not fully understood (53). There were several clinical trials for various MMP inhibitors; however, they failed to block the tumor invasion in patients which suggest that this proteolytic enzyme theory is inadequate to completely reflect the molecular mechanisms of the tumor invasion. Therefore, the FMCLD theory has some advantages over proteolytic theory because it focuses on the interaction of the different types of cells present in the tumor microenvironment. The localized death of myoepithelial cells causes the release of its inner contents like the proteolytic enzymes and growth factors. The resulting immunoreactions that accompany an external environmental insult or internal genetic alterations are triggering factors for further disruptions of the myoepithelial cell layer, BM degradation, and subsequent tumor progression and invasion (4, 7). However, the FMCLD theory failed to determine the significance of acidic microenvironment and how it affects the tumor invasiveness and progression.
4. CLINICAL IMPLICATIONS OF MYOEPITHELIAL CELL STAINING
The presence of myoepithelial cells has long been recognized as a prominent feature of benign breast diseases using different types of staining procedures. Presence or absence of an intact myoepithelial cell layer around the luminal cells is by far the major diagnostic criteria that pathologists use to differentiate in situ from invasive carcinomas (58). Confirmation of the myoepithelial cell layer on routine cytology or histology can be done with the help of alpha-SMA immunostaining; however, these cells can also be identified by S-100 (59), calponin (60), h-caldesmon (60–61), smooth muscle heavy chain (SMMHC) antibodies (60–61) and CD10 (62). Foschini et al. (60) showed that SMMHC is more specific for myoepithelial cells in the breast, while Masood et al. demonstrated significant difference in the number of myoepithelial cells in benign versus malignant tumors (63). Bofin et al. also noted that myoepithelial cells are virtually absent or markedly reduced in invasive carcinoma compared to DCIS lesions, therefore, the myoepithelial cell is an important marker to distinguish benign, proliferative breast diseases (PBD) and frank malignant breast lesions (64). Furthermore, Yu et al. quantified the number of myoepithelial cells and showed that solitary myoepithelial cell could be seen in both benign and malignant lesions. They also noted that paired myoepithelial cells were a feature of only benign lesions which were absent in malignant lesions (58). The number of myoepithelial cells may be helpful in distinguishing between PBD versus DCIS and invasive carcinoma on fine needle aspiration cytology (FNAC) smears which is a useful tool for rapid and accurate diagnosis of various benign and malignant breast lesions with high sensitivity and specificity (65). Finally, the analysis of myoepithelial marker expression remains a commonly used approach to distinguish between benign and malignant tumors, or to detect stromal invasion. Therefore, early detection of the myoepithelial cell layer disruption may help in the identification of the status of breast carcinoma to select the patients for optimal treatment.
6. DISCUSSION AND CONCLUSION
Myoepithelial cells are present in almost all glandular organs; however, the exact role of these cells in tumor progression is yet to be clarified. It is believed that myoepithelial cells present in mammary glands have a role in the secretion of milk and in the synthesis of the basement membrane (1). The contractile function of the myoepithelial cells are always taken into account; however, it is now clear that myoepithelial cells are also important in some other physiological functions like regulation of growth, differentiation and morphogenesis of neighboring cells as they also secrete growth factors and cytokines such as basic fibroblast growth factor (bFGF), transforming growth factor-alpha (TGF-alpha) and various cytokines (2, 5, 11). The growth and development of tumors are determined not only by the specific oncogene activation or by the loss of tumor suppressor but also by some microenvironment factors that play important roles. The interaction of the secreted factors of the myoepithelial cells with the surrounding epithelial cells results in the promotion of proliferation of the endothelial cells and following angiogenesis which also increases the invasiveness of the tumor cells and helps in the metastatic spread (3, 5). It is now evident that myoepithelial cells have a role in stromal tumor invasion by secretion of various molecules resulting in degradation of BM, hypoxia and inflammation; however, many questions still remain unanswered like what are the exact mechanisms of the degradation of the myoepithelial cell layer? Why is there the overproduction of the degrading enzymes and other molecules? It is likely that the tumor cells can induce the myoepithelial cells to produce the degradative enzymes or the tumor cells themselves produce such molecules and enzymes.
Another important factor which may play a role in the breast tumor progression is the extracellular acidic pH of the tumor microenvironment which both the hypotheses failed to mention. Cancer cells proliferate rapidly and develop an acidic extracellular environment, which is believed to occur as a result of lactic acid accumulation produced during aerobic and anaerobic glycolysis. Several studies have indicated that cancer cells exposed to acidic pH in vitro may show increased expression of several genes known to promote invasive growth and metastasis, including genes encoding MMPs (MMP-2 and MMP-9), cysteine proteases like cathespin B and cathespin L. Several proangiogenic factors such as vascular endothelial growth factor-A (VEGF-A) and interleukin-8 (IL-8) are also induced due to acidity of the microenvironment (66, 67). It is possible that the myoepithelial cells present in the DCIS can also secrete these BM degrading and proangiogenic factors because of the acidic pH of the microenvironment. However, it is not clear whether this acidity-induced increase in gene expression is sufficient to enhance the metastatic potential of tumor cells. Rofstad et al., measured the secretion of these acidity-induced proteins in A-07, D-12 and T-22 human melanoma cells, cultured in acidic and normal medium and investigated whether treatment with proteinese inhibitors or neutralizing antibodies could inhibit acidity-induced invasiveness, angiogenesis and experimental metastasis. Their results showed that acidity indeed induced up-regulation of VEGF-A, IL-8, MMP-2, MMP-9, cathespin B and cathespin L resulting in the enhanced metastatic potential of these human melanoma cell lines thus providing significant evidence that acidity-induced up-regulation of these proteins is a possible mechanism for tumor invasiveness in melanoma cancer (68). This acidity-induced tumor invasiveness may also hold true for the tumor progression from DCIS to stroma but much works need to be done before coming to any conclusion.
Man et al. reported that the immunoreactive cells such as leucocytes and macrophages can produce MMPs when they come in contact with the damaged myoepithelial cells (4, 7). Another important question is how genetic abnormalities in the myoepithelial cells affect the secretion of these molecules? It is also possible that the secretions of the myoepithelial cells result in the degradation of their own however; no conclusive evidence has shown that host’s own enzymes are capable of degrading its own myoepithelial cells. Are there any preventive mechanisms for this myoepithelial cell layer not to be degraded? Are there any factors and molecules that play significant roles in this mechanism? The role of other signaling pathways in over-production of proteolytic enzymes is also not clearly understood. Do the tumor cells have the ability to induce the myoepithelial cells to secrete these degrading enzymes and other angiogenesis factors so that the tumor cells can exploit it for their own benefits? The identification of disruption of myoepithelial cells beforehand may have a clinical significance (60–65). This will make the prognosis of breast cancer easier but the question is how we can properly identify the early disruption of myoepithelial cells. There is an urgent need for some rapid and sensitive method for the detection of degraded myoepithelial cells. Several markers have been reported for the immunohistochemical detection of breast myoepithelial cells and their correct recognition and detection may serve as tools for the diagnosis of breast cancers although the sensitivity and specificity of these markers vary widely (5, 6). Maspin and p63 are most promising at this time and the identification of more sensitive and specific markers is necessary for the prompt and proper diagnosis of breast lesions.
The fact that myoepithelial cells constitutively express a large amount of tumor suppressors, proteinase inhibitors and angiogenesis inhibitors has changed the way we look at the myoepithelial cells (9, 11). It is also clear now that cancer cells come under the influence of important paracrine regulation from the host microenvironment (4, 7). Therefore, it seems that myoepithelial cells have an important role in the paracrine regulation of the normal and tumor cells present in the microenvironment; however the precise mechanism of these molecules in the suppression of tumor progression and how these functions are compromised during cancer development are yet to be clarified.
In conclusion, the potential role of myoepithelial cells in tumor progression has always been under-recognized; however, it is now believed that myoepithelial cells have an important regulatory role in breast cancer by influencing the epithelial and luminal compartments and ultimately altering the tissue microenvironment. Myoepithelial cells appear to have dual functions as tumor suppressor and promoter. However much remains to be learned about the molecular mechanism and physiological role of myoepithelial cells in the tumor invasion and metastasis, which may eventually lead to the development of novel approaches for the prevention and treatment of breast cancer.
References
- 1.Adriance MC, Inman JL, Petersen OW, Bissell MJ. Myoepithelial cells: good fences make good neighbors. Breast Cancer Res. 2005;7(5):190–7. doi: 10.1186/bcr1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murrell TGC. The potential for oxytocin (OT) to prevent breast cancer: a hypothesis. Breast Cancer Res Trea. 1995;35:225–229. doi: 10.1007/BF00668213. [DOI] [PubMed] [Google Scholar]
- 3.Deugnier MA, Teulière J, Faraldo MM, Thiery JP, Glukhova MA. The importance of being a myoepithelial cell. Breast Cancer Res. 2002;4(6):224–30. doi: 10.1186/bcr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Man YG, Sang QX. Significance of focal myoepithelial cell layer disruptions in human breast tumor invasion: a paradigm shift from the “protease-centered” hypothesis. Exp Cell Res. 2004;301(2):103–18. doi: 10.1016/j.yexcr.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Clarke C, Sandle J, Lakhani SR. Myoepithelial cells: pathology, cell separation and markers of myoepithelial differentiation. J Mammary Gland Biol Neoplasia. 2005;(3):273–80. doi: 10.1007/s10911-005-9587-3. [DOI] [PubMed] [Google Scholar]
- 6.O’Hare MJ, Ormerod MG, Monaghan P, Lane EB, Gusterson BA. Characterization in vitro of luminal and myoepithelial cells isolated from the human mammary gland by cell sorting. Differentiation. 1991;6(3):209–21. doi: 10.1111/j.1432-0436.1991.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 7.Man YG. Focal degeneration of aged or injured myoepithelial cells and the resultant auto-immunoreactions are trigger factors for breast tumor invasion. Med Hypotheses. 2007;69(6):1340–57. doi: 10.1016/j.mehy.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Kolar Z, Ehrmann J, Jr, Turashvili G, Bouchal J, Mokry J. A novel myoepithelial/progenitor cell marker in the breast? Virchows Arch. 2007;450(5):607–9. doi: 10.1007/s00428-007-0403-x. [DOI] [PubMed] [Google Scholar]
- 9.Joshi K, Smith JA, Perusinghe N, Monoghan P. Cell proliferation in the human mammary epithelium. Differential contribution by epithelial and myoepithelial cells. Am J Pathol. 1986;124(2):199–206. [PMC free article] [PubMed] [Google Scholar]
- 10.Polyak K, Hu M. Do myoepithelial cells hold the key for breast tumor progression? J Mammary Gland Biol Neoplasia. 2005;10(3):231–47. doi: 10.1007/s10911-005-9584-6. [DOI] [PubMed] [Google Scholar]
- 11.Barsky SH, Karlin NJ. Myoepithelial cells: autocrine and paracrine suppressors of breast cancer progression. J Mammary Gland Biol Neoplasia. 2005;10(3):249–60. doi: 10.1007/s10911-005-9585-5. [DOI] [PubMed] [Google Scholar]
- 12.Sternlicht MD, Barsky SH. The myoepithelial defense: a host defense against cancer. Med Hypotheses. 1997;48(1):37–46. doi: 10.1016/s0306-9877(97)90022-0. [DOI] [PubMed] [Google Scholar]
- 13.Jones JL, Shaw JA, Pringle JH, Walker RA. Primary breast myoepithelial cells exert an invasion-suppressor effect on breast cancer cells via paracrine down-regulation of MMP expression in fibroblasts and tumour cells. J Pathol. 2003:562–572. doi: 10.1002/path.1483. [DOI] [PubMed] [Google Scholar]
- 14.Djonov V, Högger K, Sedlacek R, Laissue J, Draeger A. MMP-19: cellular localization of a novel metalloproteinase within normal breast tissue and mammary gland tumours. J pathol. 2001;195:147–155. doi: 10.1002/path.927. [DOI] [PubMed] [Google Scholar]
- 15.Zou Z, Anisowicz A, Hendrix MJC, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–9. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins PCR, Whisstock J. Function of maspin. Science. 1994;265:1893–4. [PubMed] [Google Scholar]
- 17.Pemberton PA, Wong DT, Gibson HL, Kiefer MC, Fitzpatrick PA, Sager R, Barr PJ. The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases: evidence that maspin is not a protease inhibitory serpin. J Biol Chem. 1995;270:15832–7. doi: 10.1074/jbc.270.26.15832. [DOI] [PubMed] [Google Scholar]
- 18.Andreasen PA, Georg B, Lund LR, Riccio A, Stacey SN. Plasminogen activator inhibitors: hormonally regulated serpins. Mol Cell Endocrinol. 1990;68:1–19. doi: 10.1016/0303-7207(90)90164-4. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka H, Seguchi K, Iwamura T, Moriyama T, Nabeshima K, Koono M. Reverse-zymographic analysis of protease nexin-II/amyloid b protein precursor of human carcinoma cell lines, with special reference to the grade of differentiation and metastatic phenotype. Int J Cancer. 1995;60:123–8. doi: 10.1002/ijc.2910600118. [DOI] [PubMed] [Google Scholar]
- 20.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477(1–2):267–83. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 22.Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279(49):51630–46. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 23.Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16(11):1391–7. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- 24.Hurlstone AF, Reid G, Reeves JR, Fraser J, Strathdee G, Rahilly M, Parkinson EK, Black DM. Analysis of the Caveolin −1 gene at human chromosome 7q31.1 in primary tumours and tumour-derived cell lines. Oncogene. 1999;11; 18(10):1881–90. doi: 10.1038/sj.onc.1202491. [DOI] [PubMed] [Google Scholar]
- 25.Sloan EK, Stanley KL, Anderson RL. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene. 2004;23(47):7893–7. doi: 10.1038/sj.onc.1208062. [DOI] [PubMed] [Google Scholar]
- 26.Stefansson IM, Salvesen HB, Akslen LA. Loss of p63 and cytokeratin 5/6 expression is associated with more aggressive tumors in endometrial carcinoma patients. Int J Cancer. 2006;118(5):1227–33. doi: 10.1002/ijc.21415. [DOI] [PubMed] [Google Scholar]
- 27.Stefansson IM, Salvesen HB, Akslen LA. Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol. 2004;22:1242–52. doi: 10.1200/JCO.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 28.Ronnov-Jessen L, Petersen OW. A function for filamentous alpha-smooth muscle actin: retardation of motility in fibroblasts. J Cell Biol. 1996;134:67–80. doi: 10.1083/jcb.134.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto-Inoue M, Kamada S, Kimura G, Taniguchi S. The induction of smooth muscle alpha actin in a transformed rat cell line suppresses malignant properties in vitro and in vivo. Cancer Lett. 1999;142:173–178. doi: 10.1016/s0304-3835(99)00150-0. [DOI] [PubMed] [Google Scholar]
- 30.Bani D, Masini E, Bello MG, Bigazzi M, Sacchi TB. Relaxin activates the L-arginine-nitric oxide pathway in human breast cancer cells. Cancer Res. 1995;55(22):5272–5. [PubMed] [Google Scholar]
- 31.Zhou Y, Sun H, Danila DC. Truncated activin type I receptor Alk4 isoforms are dominant negative receptors inhibiting activin signaling. Mol Endocrinol. 2000;14:2066–75. doi: 10.1210/mend.14.12.0570. [DOI] [PubMed] [Google Scholar]
- 32.Su GH, Bansal R, Murphy KM. ACVR1B (ALK4, activin receptor type 1B) gene mutations in pancreatic carcinoma. Proc Natl Acad Sci. 2001;98:3254–7. doi: 10.1073/pnas.051484398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cocolakis E, Lemay S, Ali S, Lebrun JJ. The p38 MAPK pathway is required for cell growth inhibition of human breast cancer cells in response to activin. J Biol Chem. 2001;276:18430–6. doi: 10.1074/jbc.M010768200. [DOI] [PubMed] [Google Scholar]
- 34.McLachlan E, Shao Q, Wang HL, Langlois S, Dale WL. Connexins act as Tumor Suppressors in Three-dimensional Mammary Cell Organoids by Regulating Differentiation and Angiogenesis. Cancer Research. 2006;66(20):9886–94. doi: 10.1158/0008-5472.CAN-05-4302. [DOI] [PubMed] [Google Scholar]
- 35.Xu HT, Li QC, Zhang YX, Zhao Y, Liu Y, Yang ZQ, Wang EH. Connexin 43 recruits E-cadherin expression and inhibits the malignant behaviour of lung cancer cells. Folia Histochem Cytobiol. 2008;46(3):315–21. doi: 10.2478/v10042-008-0057-9. [DOI] [PubMed] [Google Scholar]
- 36.Grossfeld GD, Ginsberg DA, Stein JP, Bochner BH, Esrig D, Groshen S, Dunn M, Nichols PW, Taylor CR, Skinner DG, Cote RJ. Thrombospondin-1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. J Natl Cancer Inst. 1997;89(3):219–27. doi: 10.1093/jnci/89.3.219. [DOI] [PubMed] [Google Scholar]
- 37.Xiao G, Liu YE, Gentz R, Sang QA, Ni J, Goldberg ID, Shi YE. Suppression of breast cancer growth and metastasis by a serpin myoepithelium-derived serine proteinase inhibitor expressed in the mammary myoepithelial cells. Proc Natl Acad Sci. 1999;96:3700–5. doi: 10.1073/pnas.96.7.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keeling SL, Gad JM, Cooper HM. Mouse Neogenin, a DCC-like molecule, has four splice variants and is expressed widely in the adult mouse and during embryogenesis. Oncogene. 1997;15(6):691–700. doi: 10.1038/sj.onc.1201225. [DOI] [PubMed] [Google Scholar]
- 39.Lee JE, Kim HJ, Bae JY, Kim SW, Park JS, Shin HJ, Han W, Kim SW, Kang KS, Noh DY. Neogenin expression may be inversely correlated to the tumorigenicity of human breast cancer. BMC Cancer. 2005;3(5):154. doi: 10.1186/1471-2407-5-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alpaugh ML, Lee MC, Nguyen M, Deato M, Dishakjian L, Barsky H. Myoepithelial-specific CD44 shedding contributes to the anti-invasive and antiangiogenic phenotype of myoepithelial cells. Exp Cell Res. 2000 Nov 25;261(1):150–8. doi: 10.1006/excr.2000.5056. [DOI] [PubMed] [Google Scholar]
- 41.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64(2):327–36. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 42.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, William OP. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. Journal of Cell Science. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen M, Lee MC, Wang JL, Tomlinson JS, Shao ZM, Alpaugh ML, Barsky SH. The human myoepithelial cell displays a multifaceted anti-angiogenic phenotype. Oncogene. 2000;19(31):3449–59. doi: 10.1038/sj.onc.1203677. [DOI] [PubMed] [Google Scholar]
- 45.Rudland PS. Stem cells and the development of mammary cancers in experimental rats and in humans. Cancer Metastasis Rev. 1987;6(1):55–83. doi: 10.1007/BF00047609. [DOI] [PubMed] [Google Scholar]
- 46.Cornil I, Theodorescu D, Man S, Herlyn M, Jambrosic J, Kerbel RS. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proc Natl Acad Sci. 1991;88(14):6028–32. doi: 10.1073/pnas.88.14.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cutler LS. The role of extracellular matrix in the morphogenesis and differentiation of salivary glands. Adv Dent Res. 1990;4:27–33. doi: 10.1177/08959374900040010401. [DOI] [PubMed] [Google Scholar]
- 48.Schnitt SJ. The transition from ductal carcinoma in situ to invasive breast cancer: the other side of the coin. Breast Cancer Res. 2009;27; 11(1):101. doi: 10.1186/bcr2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beckmann MW, Niederacher D, Schnürch HG, Gusterson BA, Bender HG. Multistep carcinogenesis of breast cancer and tumour heterogeneity. J Mol Med. 1997;75(6):429–39. doi: 10.1007/s001090050128. [DOI] [PubMed] [Google Scholar]
- 50.Li F, Strange R, Friis RR, Djonov V, Altermatt HJ, Saurer S, Niemann H, Andres AC. Expression of stromelysin-1 and TIMP-1 in the involuting mammary gland and in early invasive tumors of the mouse. Int J Cancer. 1994;59(4):560–8. doi: 10.1002/ijc.2910590421. [DOI] [PubMed] [Google Scholar]
- 51.Tobacman JK. Filament disassembly and loss of mammary myoepithelial cells after exposure to lambda-carrageenan. Cancer Re. 1997;15; 57(14):2823–6. [PubMed] [Google Scholar]
- 52.Sapino A, Macrì L, Tonda L, Bussolati G. Oxytocin enhances myoepithelial cell differentiation and proliferation in the mouse mammary gland. Endocrinology. 1993;133(2):838–42. doi: 10.1210/endo.133.2.8344220. [DOI] [PubMed] [Google Scholar]
- 53.Goel A, Chauhan SS. Role of proteases in tumor invasion and metastasis. Indian J Exp Biol. 1997;35(6):553–64. [PubMed] [Google Scholar]
- 54.Goldfarb RH, Liotta LA. Proteolytic enzymes in cancer invasion and metastasis. Semin Thromb Hemost. 1986;(4):294–307. doi: 10.1055/s-2007-1003570. [DOI] [PubMed] [Google Scholar]
- 55.Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2(4):252–7. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 57.Singer CF, Kronsteiner N, Marton E, Kubista M, Cullen KJ, Hirtenlehner K, Seifert M, Kubista EA. MMP-2 and MMP-9 expression in breast cancer-derived human fibroblasts is differentially regulated by stromal-epithelial interactions. Breast Cancer Res Treat. 2002;72(1):69–77. doi: 10.1023/a:1014918512569. [DOI] [PubMed] [Google Scholar]
- 58.Yu GH, Sethi S, Cajulis RS, Gokaslan ST, Frias-Hidvegi D. Benign pairs: A useful discriminating feature in fine needle aspiration of the breast. Acta Cytol. 1997;41:721–726. doi: 10.1159/000332692. [DOI] [PubMed] [Google Scholar]
- 59.Hijazi YM, Lessard JL, Weiss MA. Use of anti-actin and S100 protein antibodies in differentiating benign and malignant sclerosing breast lesions. Surg Pathol. 1989;2:125–35. [Google Scholar]
- 60.Foschini MP, Scarpellini F, Gown AM. Differential expression of myoepithelial markers in salivary, sweat and mammary glands. Int J Surg Pathol. 2000;8:29–37. doi: 10.1177/106689690000800108. [DOI] [PubMed] [Google Scholar]
- 61.Wang NP, Wan BC, Skelly M, Frid MG, Glukhova MA, Koteliansky VE, Gown AM. Antibodies to novel myoepithelium-associated proteins distinguish benign lesions and in situ carcinoma from invasive carcinoma of the breast. Appl Immunohistochem. 1997;5:141–151. [Google Scholar]
- 62.Kalof AN, Tam D, Beatty B, Cooper K. Immunostaining patterns of myoepithelial cells in breast lesions: a comparison of CD10 and smooth muscle myosin heavy chain. J Clin Pathol. 2004;57(6):625–629. doi: 10.1136/jcp.2003.013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masood S, Lu L, Assaf-Munasifi M, McCaulley K. Application of immunostaining for muscle specific actin in detection of myoepithelial cells in breast fine needle aspirates. Diagn Cytopathol. 1995;13:71–74. doi: 10.1002/dc.2840130115. [DOI] [PubMed] [Google Scholar]
- 64.Bofin AM, Lydersen S, Hagmar BM. Cytological criteria for the diagnosis of intraductal hyperplasia, ductal carcinoma in situ, and invasive carcinoma of the breast. Diagn Cytopathol. 2004;31(4):207–15. doi: 10.1002/dc.20098. [DOI] [PubMed] [Google Scholar]
- 65.Pattari SK, Dey P, Gupta SK, Joshi K. Myoepithelial Cells: Any role in aspiration cytology smears of breast tumors? Cytojournal. 2008;5:9. doi: 10.1186/1742-6413-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rofstad EK. Microenvironment induced cancer metasrasis. Int J Radiat Biol. 2000;76:589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- 67.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanges in metastasis. Nat Rev Cancer. 2005;5:786–77795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 68.Rofstad EK, Methiesen B, Kindem K, Galappathi G. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Can Res. 2006;66:6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]