Abstract
Zinc is the second-most abundant transition metal within cells and an essential micronutrient. Although adequate zinc is essential for cellular function, intracellular free zinc (Zn2+) is tightly controlled, as sustained increases in free Zn2+ levels can directly contribute to apoptotic endothelial cell death. Moreover, exposure of endothelial cells to acute nitrosative and/or oxidative stress induces a rapid rise of Zn2+ with mitochondrial dysfunction and the initiation of apoptosis. This apoptotic induction can be mimicked through addition of exogenous ZnCl2 and mitigated by zinc-chelation strategies, indicating Zn2+-dependent mechanisms in this process. However, the molecular mechanisms of Zn2+-mediated mitochondrial dysfunction are unknown. Here we report that free Zn2+ disrupts cellular redox status through inhibition of glutathione reductase, and induces apoptosis by redox-mediated inhibition of the mitochondrial adenine nucleotide transporter (ANT). Inhibition of ANT causes increased mitochondrial oxidation, loss of ADP uptake, mitochondrial translocation of bax, and apoptosis. Interestingly, pre-incubation with glutathione ethyl ester protects endothelial cells from these observed effects. We conclude that key mechanisms of Zn2+-mediated apoptotic induction include disruption of cellular glutathione homeostasis leading to ANT inhibition and decreases in mitochondrial ATP synthesis. These pathways could represent novel therapeutic targets during acute oxidative or nitrosative stress in cells and tissues.
Keywords: Mitochondrial dysfunction, Apoptosis, Redox status
Introduction
Understanding the nature of how cells transduce, interpret, and respond to stress signals, both in terms of extracellular perception as well as signals from within, is fundamental for discovering and developing new therapies and interventions for situations where cells, tissues, and organs lose the ability to maintain homeostatic balance. It is now apparent reactive oxygen and nitrogen species (ROS, RNS, respectively) serve as signal transduction molecules in biological systems. ROS such as hydrogen peroxide (H2O2) and superoxide (O2•−) are also increasingly understood to have complex and highly nuanced signal transduction roles in cells and tissues (Forman and Torres 2001). However, these same chemical species can also initiate signaling pathways that result in apoptosis (Bauer 2000). We, and others, have previously reported that exposure of pulmonary artery endothelial cells to elevated levels of ROS and/or RNS leads to significant elevation in intracellular free zinc (Zn2+) (Tang et al. 2001; Wiseman et al. 2006, 2007; Bernal et al. 2008). Zinc is the second-most common transition metal in cells (Atwood and Steed 2004), and zinc ions function in multiple different intracellular processes. It is estimated that at least 3% of the known human genes encode proteins containing zinc-finger domains (Maret 2003). However, the concentration of unbound “free” intracellular zinc is typically found at picomolar levels (Simons 1991). Thus, although Zn2+ is classified as “redox inert,” if not adequately sequestered it can also function as a potent disruptive molecule within cells. Here, we present data that demonstrate that the effect of Zn2+ on mitochondrial dysfunction is indirect. Further, we show that a key mechanism for this indirect effect is disruption of glutathione homeostasis. Interestingly, we find that a direct consequence of Zn2+-mediated depletion of reduced cellular glutathione is the inhibition of mitochondrial adenine nucleotide transport. Together, these data illustrate a cell-death mechanism whereby mitochondrial dysfunction is initiated through a Zn +-induced loss of cellular antioxidant capacity, resulting in mitochondria deprived of adequate metabolites for normal function.
Materials and methods
Cell culture
Primary cultures of ovine fetal pulmonary artery endothelial cells (PAECs) were isolated and identified as described previously (Wedgwood et al. 2003). Cells between passages 3–10 were maintained in DMEM supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), antibiotics and antimycotics (MediaTech, Herndon, VA) at 37°C with 5% CO2–95% air. Cells were seeded at ~50% confluence, and at ~90% confluence. Before experimental treatment, except as noted, cells were trypsinized, counted, re-plated in 6-, 24-, or 96-well plates (Costar, Corning, NY) at a density of 5 × 105 cells/cm2, and incubated 18 h. Cells were serum starved in serum-free, phenol red-free DMEM (SF/PRF-DMEM, GIB-CO-BRL, Gaithersburg, MD) and incubated overnight. During serum deprivation, no exogenous sources of Zn2+ are available to the cells. Cells were pretreated for 2 h with SF/PRF-DMEM ± glutathione ethyl ester (GSH-EE, 2 mM, Sigma, St. Louis, MO), and then exposed 0–4 h with 0–1 mM ZnCl2 (200 mM in PBS, diluted in SF/PRF-DMEM), 0–1 mM N-[2-aminoethyl]-N-[2-hydroxy-2-nitroso-hydrazino]-1,2-ethylenediam-ine (spermine NONO-ate; Calbiochem, San Diego, CA) in SF/PRF-DMEM. Doses were selected to examine the entire range of potential response in PAECs as we (Wiseman et al. 2006, 2007), and others (Koh and Choi 1994) previously described. At designated time points, cells were subjected to immediate analysis, unless noted.
Mitochondrial isolation
PAECs were scraped, washed PBS, and pelleted by centrifugation 850×g at 4°C for 2 min. Mitochondria were isolated via the manufacturer’s protocol (Pierce Biotechnology, Rockford, IL). Mitochondrial fractions were resuspended in 0.25 M sucrose, 10 mM Tris-C1, pH 7.4, and 0.5 mM EDTA. In order to avoid potential issues with chelation of Zn2+ ions by EDTA, immediately prior to the beginning of each experiment, mitochondrial fractions were centrifuged and resuspended in EDTA-free buffer, and assayed as described below.
Fluorescence microscopy
A PC-based imaging system consisting of: an Olympus IX51 microscope equipped with a charge-coupled camera (Hamamatsu Photonics, Hamamatsu City, Japan) was used for acquisition of fluorescent images. Fluorescent-stained cells were observed using appropriate excitation and emission, measuring at least 300 cells per sample, and the average fluorescent intensities (to correct for differences in cell number) were quantified using ImagePro Plus v5.0 software (Media Cybernetics, Silver Spring, MD). High-resolution cellular images were obtained using an Applied Precision, Inc. (Issaquah, WA) DeltaVision™ imaging system, fitted with an environmental control chamber. Data obtained were quantified using DeltaVision™ software and compared by statistical analysis.
Detection of bax translocation
Ten hours following treatment, mitochondria were isolated and resuspended in lysis buffer (20 mM Tris base, pH 7, 2.5 mM EDTA, 1% Triton-X, 1:100 protease inhibitor cocktail [Sigma]). Lysates were assayed for protein concentration by BCA protein assay (Pierce Biotechnology, Rockford, IL) and normalized for concentration. Sixty microgram of mitochondrial protein per sample was subjected to SDS–PAGE and analyzed by standard Western blot analysis as described previously (Brennan et al. 2003) using anti-bax antibody (Cell Signaling Technology, Boston, MA).
Detection of apoptotic events
PAECs in 96-well plates were treated as described. Caspase activation was visualized by co-treating cells with 1 µM CaspACE FITC-vad-FMK (Promega, Madison, WI). This fluorescent analog of the pancaspase inhibitor N-benzyloxycarbonyl-Val-Ala-Aspfluor-omethylketone (Z-vad-FMK) readily enters cells and binds irreversibly to activated caspases. After treatment, cells were washed with ZnCl2-free DMEM and incubated overnight in PRF-DMEM media with 1% FBS. Eighteen hours post-exposure, cells were incubated in media with 10 µM FITC-conjugated caspase inhibitor (FITC-vad-FMK). After 20 min incubation at 37°C in dark conditions, cells were washed with fresh media and visualized using fluorescence microscopy. A second method involved TUNEL analysis. PAECs were incubated for ~18 h in PRF-DMEM supplemented with 1% FBS. Following incubation, analysis was performed as we have previously described (Wedgwood and Black 2003).
EPR detection of mitochondrial O2•− levels
Mitochondrial-specific O2•− production was measured using electron paramagnetic resonance (EPR) spectroscopy with spin probe, 1-hydroxy-3-methox-ycarbonyl-2,2,5,5-tetramethyl-pyrrolidine•HCl (CMH, Alexis Biochemicals, San Diego, CA) as described (Wiseman et al. 2007). Following exposure, mitochondrial isolation was performed as described above. Mitochondrial fractions were incubated for 1 h in the presence of CMH (PBS, pH 7.4 + 25 µM deferrioxamine mesylate [Calbiochem], CMH 5 mg/ml). Thirty five microliter of sample was loaded into a 50 µl capillary tube and analyzed with a MiniScope MS200 EPR (Magnettech, Berlin, Germany) at a microwave power of 40 mW, modulation amplitude of 3,000 mG, and modulation frequency of 100 kHz. EPR spectra were analyzed measured for amplitude using ANALYSIS software (v2.02; Magnettech).
Detection of cellular and mitochondrial redox status
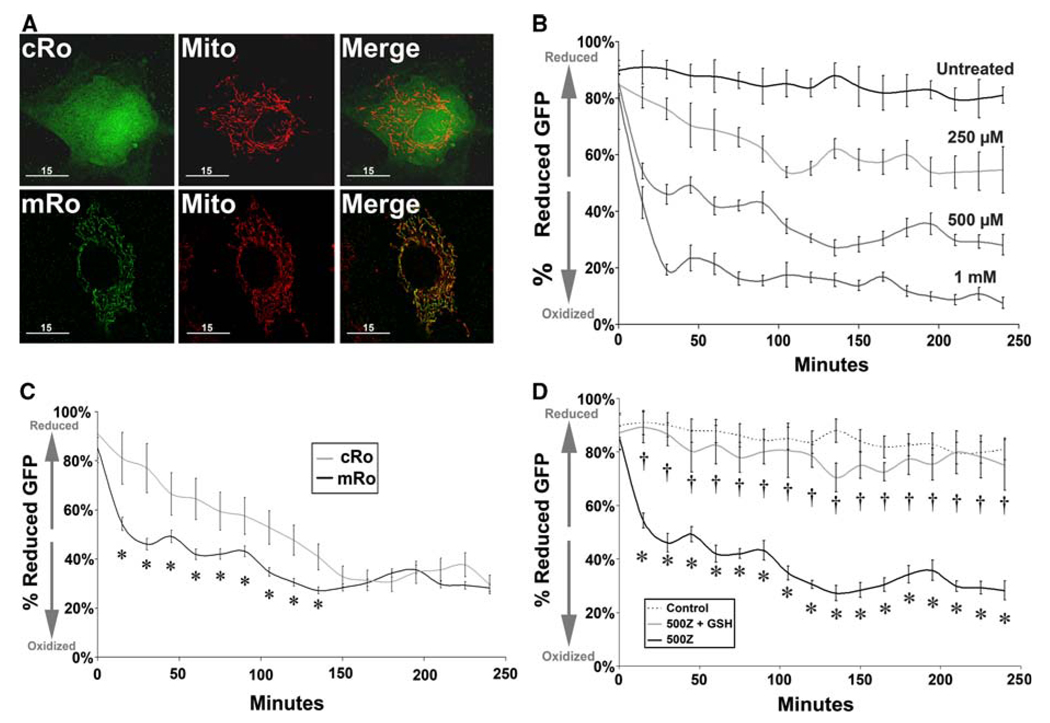
PAECs were plated into 10 cm dishes at a density of ~15,000 cells/cm2 (~1.25 × 106 cells per dish) and transfected with expression constructs of roGFP, redox-sensitive GFP analogs where surface-exposed residues are substituted with serine, allowing the ability to monitor the cellular thiol redox status of cells. These GFP analogs demonstrate a redox-specific shift in fluorescence spectra directly correlated to cellular oxidation status (Hanson et al. 2004). Redox-sensitive constructs (generously provided by S. James Remington, University of Oregon) delivered either general cytosolic roGFP expression (“cRo,” Fig. 3a), or expression of roGFP specifically within mitochondria (“mRo,” Fig. 3a). Transfections were performed via Effectene® lipid-based delivery protocols (Qiagen). Following transfection with either cRo or mRo constructs, cells were subdivided into three groups and re-plated onto cover glass. One group was treated as described above to assess cellular redox status for up to 4 h post-onset of exposure, while the other two groups were used to calibrate maximal reduction (using 1 mM dithiothreitol, Sigma) and maximal oxidation (using 1 mM t-butyl hydroperoxide, Sigma) (Farrow et al. 2008), and cellular fluorescence at 470 and 360 nm excitation wavelengths in order to determine the ratio of reduced versus oxidized GFP for up to 4 h. In addition, an untransfected sample of cells was quantified to determine nonspecific background. Each experimental group was evaluated as a percentage of oxidation, relative to fluorescence values obtained for fully reduced and fully oxidized conditions.
Fig. 3.
Exogenous zinc induces a rapid oxidation of the mitochondrial environment. PAECs were transfected with DNA expression constructs of redox sensitive GFP targeted either to the cytosolic (“cRho”) or mitochondrial (“mRho”) compartments. a High-resolution fluorescent microscopic images of cells counterstained with MitoTracker™ Red mitochondrial-specific fluorescent marker demonstrate the non-specific and mitochondrial-specific expression of GFP in cRho or mRho-transfected cells, respectively (bar = 15 µm). b Real-time fluorescent analysis of mRho within ECs incubated at 37°C, 5% CO2 in DMEM ± 0–1 m ZnCl2. c Although both compartments exhibit a shift to a more oxidized environment on exposure to ZnCl2, the mitochondria appears to be more susceptible, as mRho oxidation occurs more rapidly than cRho. d mRho oxidation is protected from Zn 2+-induced oxidation through pre-incubation with glutathione ethyl ester (1 mM, 2 h). Graphs represent mean intracellular ratio of reduced versus oxidized Rho-GFP (three independent replicates) relative to intensity of fully reduced samples (100%, 1 mM DTT, 1 h) and fully oxidized cells (0%, 1 mM t-BH, 1 h) at the onset of the experiment. Error bars represent ±SD. * P < 0.05 versus control; †P < 0.05 versus RhoGFP + ZnCl2
Measurement of glutathione reductase activity
The assay for glutathione reductase activity is based on oxidation of NADPH to NADP+ catalyzed by a limiting concentration of glutathione reductase. One GR activity unit is defined as the amount of enzyme catalyzing reduction of one micromole of GSSG per minute at pH 7.6 and 25°C. Given that one molecule of NADPH is consumed for each molecule of GSSG reduced, reduction of GSSG is determined indirectly by measurement of NADPH consumption, observed as a decrease in absorbance at 340 nm (A340) over time. Briefly, PAECs were treated and harvested by scraping and centrifugation at 1,000×g for 10 min at 4°C. Cell pellets were homogenized in 200 µl of ice-cold KPO4 buffer (100 mM, 0.1% BSA, 5 mM EDTA, pH 7.5) and centrifuged at 10,000×g for 15 min at 4°C, and supernatants kept on ice for immediate assay. To each sample, a known amount of NADPH was added, and A340 was continuously monitored 60 s. Extinction coefficients were calculated and activity determined via comparison to known standards of purified glutathione reductase. To determine if the effect of Zn2+ is acting directly upon the glutathione reductase enzyme, samples of purified glutathione reductase was exposed or not to 50 µM ZnCl2 and analyzed as described.
Determination of cellular GSH:GSSG ratio
This assay used is based on reaction of GSH with DTNB (Ellman’s reagent, [5,5′-dithiobis-2-nitrobenzoic acid]), which produces a detectable product with a maximal absorbance at 412 nm. The rate of product formation is proportional to the concentration of GSH. As GSH typically far exceeds the amount of oxidized GSSG within cells, this assay uses a thiol-scavenging reagent, 1-methyl-2-vinylpyridinium trifluoromethanesulf-onate1 (M2VP) to scavenge GSH. Samples are then incubated with a known amount of purified glutathione reductase in the presence of DTNB, and ratios calculated according to the manufacturer’s protocol (Oxis International). For both GSSG and GSH, absorbance curves were generated and concentrations were extrapolated via comparison to known GSH standards.
Determination of mitochondrial ATP production
Following exposure to Zn2+ (500 µM, 4 h), isolated mitochondria were incubated in physiological conditions with exogenous ADP (100 µM, Sigma, St. Louis, MO) in the presence of ATP-dependent luciferase, according to the manufacturer’s protocol (Sigma, St. Louis, MO). Mitochondrial isolates were volume adjusted to 800 µl, pH 7.8. Hundred microliter of ATP Assay Mix solution was added to isolates, and allowed to incubate at room temperature for ~3 min, during which any endogenous ATP is hydrolyzed, decreasing background signal. The assay was performed by rapidly adding 100 µl of either ATP standard or ADP, with mixing, and measured with a luminometer. Negative controls were performed using mitochondrial-free reactions and mitochondrial fractions absent of exogenous ADP.
In addition, we directly measured mitochondrial adenine uptake. [3H]ADP uptake was measured by carboxyatractyloside inhibitor stop-technique described previously (Chan and Barhour 1979). Approximately 50 µg of mitochondria were suspended in 200 µl buffer containing 116 mM KC1, 21 mM Tris/HCI, pH 7.4, 1.05 mM EDTA (KCl buffer), 5.26 mM 2-oxoglutarate, and 5.26 µM p-ruthenium red. Following 5-min incubation at RT, 10 pg oligomycin was added to the suspension and chilled to 2°C. Various concentrations (5 µl) of [3H]ADP were added to 45 µ aliquots of mitochondrial suspension under constant mixing. After 12 s, reactions were stopped by 10 µl injection of 200 µM p-carboxy-atractyloside, and mitochondria were pelleted at 12,000×g for 4 min. Supernatant was removed and mitochondria were washed with 200 µl KC1 buffer containing 10 nM carboxyatractyloside and re-pelleted. The mitochondrial pellet was dissolved in 100 µl of 2% SDS, transferred to a scintillation vial containing 3.0 ml of counting fluid, and counted by liquid scintillation.
Statistical analyses
Statistical calculations were performed using Graph-Pad Prism V. 4.01 software. The mean ± SD was calculated for all samples and significance determined by either by the unpaired t test or ANOVA. For ANOVA, Neuman-Kuehls post-hoc testing was utilized. A value of P < 0.05 was considered significant.
Results
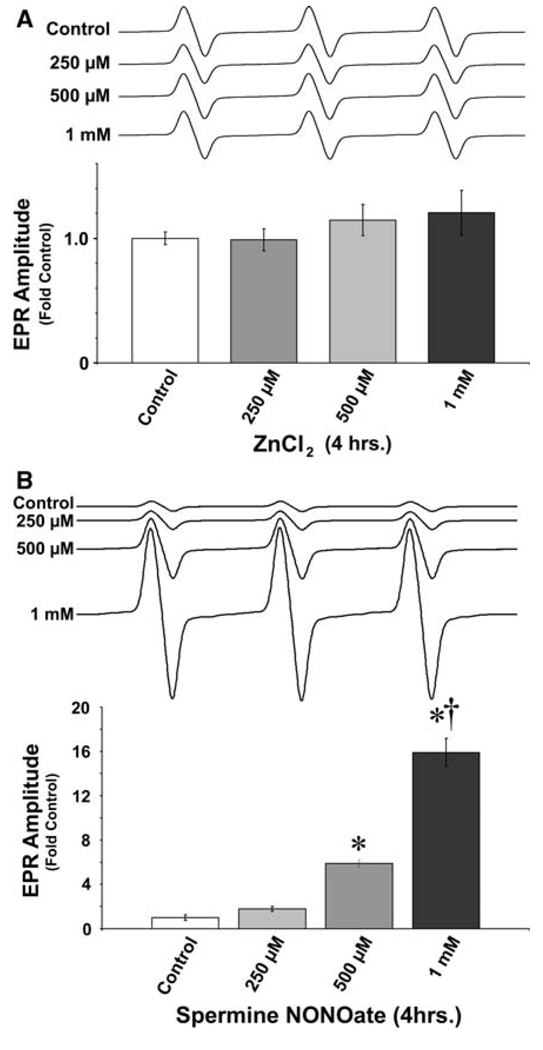
Our previous findings indicate that pulmonary artery endothelial cells (PAECs) undergo mitochondrial dysfunction and apoptosis when exposed to either oxidative or nitrosative stress, and this effect can be mimicked if cells are exposed in the same fashion to exogenous Zn2+. Initially, we determined if elevated intracellular Zn2+ induces mitochondrial dysfunction via a direct or indirect mechanism. Isolated mitochondrial fractions (from ~2 × 107 cells) were suspended in metabolic buffer (free from metal chelators, such as EDTA) and acutely exposed to either ZnCl2 (Fig. 1a) or spermine NONOate (Fig. 1b, as a positive control) or for 1 h and superoxide levels determined using the spin-trap, CMH, a cyclic hydroxylamine with relatively specific affinity for O2•− (Fink and Dikalov 2002). CMH-O2•− products were detected by EPR analysis as we have described (Wiseman et al. 2006). Our data indicate that although NO increases mitochondrial-derived O2•− (Fig. 1b), Zn 2+ alone does not (Fig. 1a), indicating that Zn 2+-mediated mitochondrial dysfunction occurs indirectly.
Fig. 1.
Zinc does not directly increase mitochondrial superoxide generation. Mitochondria were isolated from PAECs as described in “Methods and materials” and resuspended in EDTA-free physiological buffer containing either a 0–1 mM ZnCl2 or b 0–1 mM Spermine NONOate (as a positive control) and incubated at 37°C for 2 h. Following incubation, superoxide levels were determined using EPR. The direct addition of ZnCl2 does not increase mitochondrial superoxide levels while the NO donor does. Graphs represent mean amplitude of superoxide-CMH product EPR waveforms (n = 5), with representative individual waveforms illustrated above. Error bars represent ±SEM. * P < 0.05 versus control samples; † P < 0.05 versus previous dose
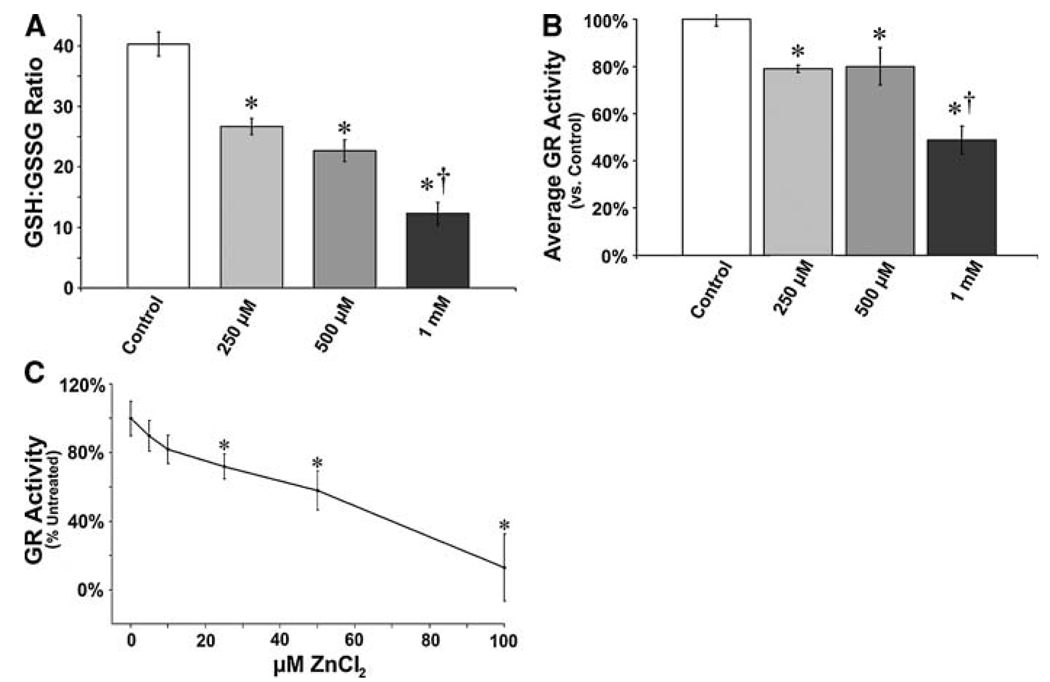
Previous studies show an important role for reduced GSH in maintaining mitochondrial function (Garcia-Ruiz et al. 1995). Thus, we next determined if the indirect effect of free Zn2+ on mitochondrial function could be due to alterations in cellular GSH homeostasis. We found that Zn2+ exposure caused a decrease in the GSH-to-GSSG ratio (Fig. 2a). As the rate-limiting enzyme in the homeostatic maintenance of cellular GSH-to-GSSG ratio is glutathione reductase (GR), we next ascertained if the mechanism by which increased free Zn2+ produces elevated GGSG is mediated through inhibition of glutathione reductase (GR) enzymatic activity. Our data indicate that elevated free Zn 2+ leads to the inhibition of GR activity in PAECs (Fig. 2b). Furthermore, to verify that Zn2+ is a direct biochemical inhibitor of GR, we determined if Zn 2+ inhibits GR in vitro. We found the presence of Zn2+ directly inhibits the ability of GR to reduce GSSG to GSH (Fig. 2c).
Fig. 2.
Zinc-mediated disruption of glutathione homeostasis is mediated by the inhibition of glutathione reductase activity. PAECs exposed to ZnCl2 (0–1 mM) were analyzed to determine GSH-to-GSSG ratio (a) and GR enzymatic activity (b). ZnCl2 causes a dose dependent decrease in the GSH-to-GSSG ratio that is correlated with a decrease in GR activity. Confirming the ZnCl2 mediated inhibition of GR, purified bovine GR was inhibited in vitro when exposed to ZnCl2 (0–100 µM, c). Graph represents average enzymatic activity versus control reaction over 5 min. Error bars ±SD (n = 4). * P < 0.05 versus control; † P < 0.05 versus previous dose
In order to examine the effect of the decreased GSH-to-GSSG ratio induced by elevated free Zn2+ on cellular redox status, we utilized redox-sensitive GFP constructs targeted to either cytosol (“cRo”) or to mitochondria (“mRo”) (Fig. 3a). Following transfection with either cRo or mRo redox-sensitive GFP, we exposed PAECs to Zn2+ (0–1 mM ZnCl2) and monitored average cellular fluorescence for 4 h. Firstly, we observed a dose-dependent, significant shift in mitochondrial roGFP to a more oxidized state (Fig. 3b). Secondly, we observed a shift in both cytosolic and mitochondrial redox status to a more oxidized state, but mitochondrial oxidation occurred earlier (Fig. 3c). Lastly, in cells treated with 1 mM glutathione ethyl ester prior and during onset of exposure to Zn2+, we found significant protection of mitochondrial roGFP from Zn2+-induced oxidation (Fig. 3d).
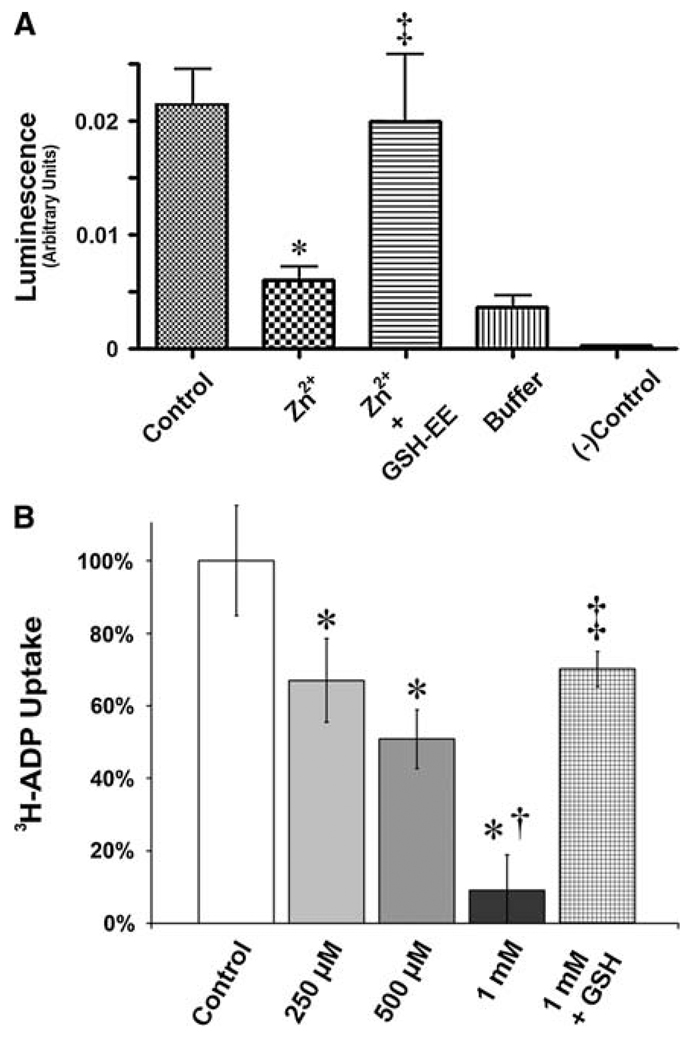
GSH is known to be important in preventing oxidation and subsequent inhibition of the adenine nucleotide translocator (ANT) protein in neuronal cells (Vesce et al. 2005). Thus, we next we determined if the shift of the mitochondria to a more oxidized environment had an effect on ANT function. We utilized two assays to measure of ANT function: Initially we examined the ability of mitochondrial isolates to oxidatively phosphorylate ADP into ATP. We found that mitochondria isolated from Zn2+-treated PAECs have a significantly diminished capacity to generate ATP relative to controls (Fig. 4a). However, preloading with exogenous GSH (in the form of cell-permeant GSH-ethyl ester [GSH-EE]) restored ANT activity (Fig. 4a). To corroborate this finding, we incubated mitochondrial isolates in the presence of [3H]-ADP, and determined the rate of adenine translocation into mitochondria. We found that Zn2+-exposed mitochondria showed significant, dose-dependent reductions in ADP translocation rate. Furthermore, GSH-EE pre-incubation significantly protected ANT function from Zn2+-mediated effects (Fig. 4b).
Fig. 4.
Zinc-mediated oxidation of the mitochondrial environment leads to the inhibition of adenine nucleotide translocator activity and this is prevented by reduced GSH. PAECs were exposed to increasing concentrations of ZnCl2 (0–1 mM, 2 h) in the presence and absence of a cell permeable GSH analogue (glutathione ethyl ester [GSH-EE], 1 mM), then ANT activity determined using either an ATP synthetic activity assay (a) or [3H]-ADP mitochondrial uptake (b). Both ATP generation and [3H]-ADP uptake is inhibited by ZnCl2 and this is prevented by preincubation with GSH-EE. The “buffer” sample represents mitochondrial fraction in reaction buffer in the absence of ADP. Graphs represent mean ± SD (n = 4). * P < 0.05 versus control; † P < 0.05 versus previous dose; ‡ P < 0.05 versus Zn2+ in the absence of GSH-EE
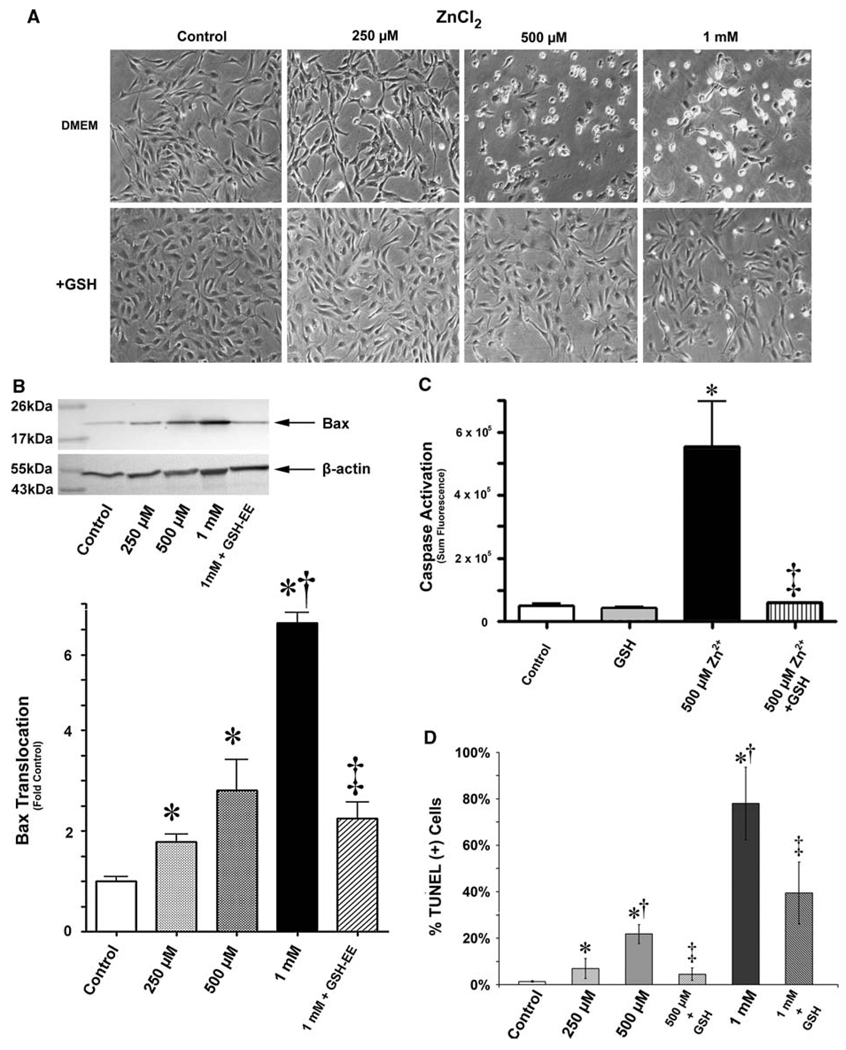
To determine if glutathione could ultimately protect PAECs from apoptotic events induced by increased free Zn2+, we examined translocation of pro-apoptotic bax proteins to mitochondria. We found pre-incubation of PAECs with GSH-EE maintained the morphology of PAECs exposed to Zn2+ (Fig. 5a). We also observed significant reductions in Zn2+-mediated bax translocation to mitochondria (Fig. 5b). Furthermore, when we examined caspase activation, which we previously found to be elevated in PAECs following acute Zn2+ exposure, GSH-EE-preloaded cells showed significant resistance to Zn2+ (Fig. 5c). Similarly, GSH-EE preloaded cells had significantly fewer TUNEL positive nuclei versus Zn2+-exposed cells alone (Fig. 5d).
Fig. 5.
Elevation of reduced intracellular glutathione protects ECs from Zn2+-induced induction of apoptosis. The analysis of cell morphology using brightfield microscopy shows extensive cell rounding and reduced plate adherence 18 h after an acute exposure to exogenous Zn2+ (ZnCl2, 0–1 mM), whereas cells pre-incubated in 1 mM glutathione ethyl ester (GSH) generally retain a morphology similar to that of untreated cells (a). PAECs were exposed to increasing concentrations of ZnCl2 (0–1 mM, 4 h) in the presence and absence of a GSH-EE (1 mM). After an additional 12 h the cells were analyzed for mitochondrial bax translocation (b), caspase activation (c), and the presence of apoptotic nuclei (d). The increases in mitochondrial bax translocation, caspase activation, and apoptotic nuclei induced by Zn 2+ are reduced in the presence of GSH-EE. Graphs represent mean ± SD (n > 3). * P < 0.05 versus control; † P < 0.05 versus next lesser dose; ‡ P < 0.05 versus control DMEM pre-incubation
Discussion
There is an increasing understanding regarding the role of intracellular free zinc (Zn2+) in mediating cell protection versus cell death. Zn2+ is the second-most abundant transition metal in biological systems, and although it is thought to be redox inert, it is kept under extraordinarily tight control. With multiple functional roles within cells (Nyborg and Peersen 2004), free Zn2+, like other transition metals such as calcium, demonstrates different effects depending on its concentration. Thus, free Zn2+ could represent a form of biological “rheostat” whereby relatively low release events serve a signaling and/or protective role, while a more profound release within the cell triggers cell death responses. We have previously demonstrated that Zn2+, when elevated to high enough levels, induces apoptosis in endothelial cells (Wiseman et al. 2006, 2007). In this study, we identify new key events by which elevations in free Zn2+ contribute to induction of mitochondrial dysfunction and the apoptotic process. Specifically, we show that elevated Zn2+ concentrations disrupt glutathione homeostasis though inhibition of glutathione reductase enzymatic activity. Interestingly, with decreased endogenous reduced glutathione, we observe an associated loss of mitochondrial import of ADP, measured not only by the ability of mitochondria to synthesize ATP in physiological conditions, but also by direct measurement of [3H]-ADP uptake. Importantly, this entire process was reversed by supplementation with glutathione ethyl ester, and allowed endothelial cells to withstand extremely high Zn2+ concentrations. Here our data not only corroborate the findings of others, which show Zn2+-mediated disruption of glutathione homeostasis, but also extend understanding of this process to include a novel specific outcome of glutathione disruption: inhibition of ADP transport into mitochondria. This new finding explains how Zn2+, or any other stimulus which deprives mitochondria the ability to import metabolites like ADP properly, could trigger a negative cycle of mitochondrial dysfunction with subsequent generation of superoxide, increased oxidative stress, and ultimately apoptosis. This mechanism also provides an explanation for why mitochondrial uncoupling proteins such as UCP-2 can provide protective effects during periods of cell stress, and oxidative stress in particular (Echtay et al. 2002). Furthermore, this role of Zn2+ levels contributing to glutathione deprivation and oxidative stress appears to be evolutionarily conserved in both animals and plants (Lange et al. 2002; Bittsanszky et al. 2005), indicating that although we utilized a pulmonary cell model system, this mechanism has broad applicability.
Adenine nucleotide translocase (ANT) is the major mitochondrial carrier facilitating ADP and ATP transport across the inner mitochondrial membrane (Shertzer and Racker 1976). In mammals, there are multiple isoforms of ANT, including four known in humans, and these isoforms appear to be differentially expressed across tissues. In addition to functioning as a nucleotide transporter, ANT complexes are involved in regulation of apoptosis (Lunardi and Attardi 1991). Specific interaction of ANT protein with both pro- and anti-apoptotic proteins have been reported and demonstrate a highly complex, nuanced mechanism of cell death regulation (Belzacq et al. 2003). Notably, sulfhydryl moieties within the ANT peptide appear to be required for proper ANT activity (Li et al. 2006), and thus represent a significant vulnerability in the face of loss of oxidative protection from glutathione. Previous studies report that ANT proteins can be oxidatively modified even under relatively mild conditions (Giron-Calle et al. 1994). Therefore, given our data demonstrating that the mitochondrial are rapidly altered to a more oxidized environment during exposure to free Zn2+, we speculate that Zn2+-mediated inhibition of glutathione homeostasis allows inappropriate oxidation of ANT, with subsequent reduction of adenine nucleotide transport. Although our data indicate that elevated Zn2+ can have a pathologic effect on PAEC it must be emphasized that these findings should not be misconstrued as suggesting that dietary supplementation of zinc is necessarily harmful. Oral zinc intoxication events are rare in humans, and are normally only observed following ingestion of zinc-containing items such as coins (Bennett et al. 1997). This is likely due to the fact that the diet is the single biological source of zinc and metals, and a highly regulated uptake and dietary excretion mechanisms in place which mitigates all but extreme levels of uptake.
As intracellular Zn2+ has both catalytic roles and structural roles in proteins, despite being considered “redox inert,” Zn2+ can bind to cysteine residues to create protein folds important in protecting molecules from oxidation. From both our previous data showing Zn2+-mediated increases in mitochondrial-derived superoxide, as well as data presented here with redox-sensitive GFP, Zn2+ appears to be both directly and specifically involved in regulation of cellular redox homeostasis. Several reports have concluded that zinc serves as a cytoprotective agent, defending cells against both oxidative insult and against stimuli that induce apoptosis (Zalewski et al. 1991; Bao and Knoell 2006). Indeed, a recent study found that low-level zinc release enhances glutathione synthesis through an Nrf2-mediated transcriptional mechanism (Cortese et al. 2008), seemingly in contradiction to our current findings. However, this same report shows that above a given threshold, similar to our own findings, cell viability drops precipitously. Thus, our data here actually corroborate and extend what was reported. Together these findings suggest that cellular Zn2+ release is important cell fate determinant in the face of stress. Furthermore, these data reinforce that Zn2+ level must remain within a specific physiological window, and both sub- and super-optimal intracellular Zn2+ can result in similar apoptotic consequences. The complexity and difficulty of elucidating Zn2+-specific roles in this mechanism are further illustrated in reports where Zn2+ is concluded to serve as a caspase and/or pro-caspase enzymatic inhibitor (Truong-Tran et al. 2001; Peterson et al. 2009). There are multiple possibilities for this apparent “zinc paradox,” where Zn2+ serves simultaneously as both a pro- and/or anti-antiapoptotic regulator. We speculate that this paradox is the result of differences in cellular availability of zinc homeostatic mechanisms (i.e., zinc transporters, metallothionein, etc.), antioxidant systems, and ultimately different proteomic milieus across cells, even similar cells in different metabolic circumstances. The presence—even transient presence—of a particularly zinc-sensitive protein within a cell could potentially have significant impact on how zinc manifests pathological or beneficial effect. Clearly, further examination and clarification of the fundamental biochemical interaction of Zn2+ and proteins in the context of cellular physiology is merited.
Also notable is the significance of glutathione homeostasis during Zn2+-mediated oxidative stress, despite the fact that several intracellular antioxidant systems are known. These include the thioredoxin (Watson et al. 2004) and peroxyredoxin (Novoselov et al. 2000) antioxidant systems, along with canonical systems of superoxide dismutase and catalase (Machlin and Bendich 1987). As we find that addition of exogenous GSH protects cells from an apoptotic induction, this suggests the glutathione system is more important than these other systems. We further speculate that if glutathione levels could be enhanced, this might provide a comprehensive mechanism to protect cells from the induction of apoptosis. It would also be interesting to determine if enhancing GSH levels can protect cells from necrotic cell death. However, further studies will be required to evaluate GSH as a therapeutic agent. It should be noted that the doses of exogenous Zn2+ used here and in our previously published studies appear to be high, and the highest doses administered are likely unattainable in normal physiological situations. This is due to the difficulty of raising intracellular Zn2+ without need of chemical agents, notably zinc pyrithione (bis(2-pyridylthio)zinc 1,1′-dioxide), thus allowing Zn2+ doses that are more “physiological” (Tang et al. 2001). However, while use of these compounds is possible, they are extremely toxic to cells and introduce the possibility of confounding pharmacological effects which may be mistakenly attributed to Zn2+. Furthermore, using ZnCl2 in our “direct” fashion is both direct and effective, given that chloride ions are already present in multiple millimolar concentrations, so addition of up to one additional millimole of chloride is relatively innocuous, especially when compared to potential effects of anions of other zinc compounds, such as sulfate, although relatively abundant, are orders of magnitude more rare than chloride (Markovich 2001). So as long as pH and osmotic pressures are accounted for, this system provides a simple, repeatable method of modulating intracellular Zn2+. It should also be noted that our ability to protect cells even in the face of super-physiological levels of exposure further reinforces the validity of this protective mechanism. The ramifications of this robust protection may have particular significance in instances of acute respiratory distress syndrome (ARDS) where patients are exposed to toxic levels of metal fumes (Frutos-Vivar et al. 2004) or exposure to aerosolized zinc nanoparticles in smoke (Wilson et al. 2007). Indeed, we speculate that virtually any non-enteric delivery of bioavailable zinc particles/ions may pose a potential hazard, as illustrated by recent events involving nasal neuroreceptor toxicity arising from popular over-the-counter nasally-delivered cold remedies containing significant concentrations of bioavailable zinc, and that caution is merited.
In conclusion, this study illustrates potentially new avenues for understanding the specific role of intra-cellular Zn2+ as a key mediator of the cell-fate decision, and the specific mechanism by which Zn2+ can function in a pro-apoptotic role. We speculate that developing novel methods of intervention based on reducing free Zn2+ levels could be considered in cases of acute cellular oxidative or nitrosative stress.
Acknowledgments
This research was supported by a Ruth L. Kirschstein National Research Service Award Individual Fellowship, F32HL090198 (to D. A. Wiseman); National Institute of Health Grants HL-60190, HL-67841, HL-72123, HL084739, R21HD057406, and HL-70061, a Transatlantic Network Development Grant from Fondation LeDucq (all to S. M. Black), and an AHA Southeast affiliates Beginning Grant In Aid Award (09BGIA2310050, to S. Sharma).
References
- Atwood JL, Steed JW. Encyclopedia of supramolecular chemistry. New York: M. Dekker; 2004. [Google Scholar]
- Bao S, Knoell DL. Zinc modulates airway epithelium susceptibility to death receptor-mediated apoptosis. Am J Physiol Lung Cell Mol Physiol. 2006;290:L433–L441. doi: 10.1152/ajplung.00341.2005. [DOI] [PubMed] [Google Scholar]
- Bauer G. Reactive oxygen and nitrogen species: efficient, selective, and interactive signals during intercellular induction of apoptosis. Anticancer Res. 2000;20:4115–4139. [PubMed] [Google Scholar]
- Belzacq AS, Vieira HL, Verrier F, Vandecasteele G, Cohen I, Prevost MC, Larquet E, Pariselli F, Petit PX, Kahn A, Rizzuto R, Brenner C, Kroemer G. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 2003;63:541–546. [PubMed] [Google Scholar]
- Bennett DR, Baird CJ, Chan KM, Crookes PF, Bremner CG, Gottlieb MM, Naritoku WY. Zinc toxicity following massive coin ingestion. Am J Forensic Med Pathol. 1997;18:148–153. doi: 10.1097/00000433-199706000-00008. [DOI] [PubMed] [Google Scholar]
- Bernal PJ, Leelavanichkul K, Bauer E, Cao R, Wilson A, Wasserloos KJ, Watkins SC, Pitt BR, St Croix CM. Nitric oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circ Res. 2008;102:1575–1583. doi: 10.1161/CIRCRESAHA.108.171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittsanszky A, Komives T, Gullner G, Gyulai G, Kiss J, Heszky L, Radimszky L, Rennenberg H. Ability of transgenic poplars with elevated glutathione content to tolerate zinc(2+) stress. Environ Int. 2005;31:251–254. doi: 10.1016/j.envint.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Brennan LA, Wedgwood S, Bekker JM, Black SM. Nitric oxide activates p21ras and leads to the inhibition of endothelial NO synthase by protein nitration. DNA Cell Biol. 2003;22:317–328. doi: 10.1089/104454903322216662. [DOI] [PubMed] [Google Scholar]
- Chan SHP, Barhour KL. Membrane bioenergetics: based on the international workshop held at Cranbrook Schools, Bloomfield Hills, Michigan, July 5–7, 1979 in honor of Efraim Racker. Reading: Addison-Wesley; 1979. [Google Scholar]
- Cortese MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med. 2008;44:2002–2012. doi: 10.1016/j.freeradbiomed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Murphy MP, Smith RA, Talbot DA, Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J Biol Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res. 2008;102:226–233. doi: 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink B, Dikalov S. Detection of superoxide with new cyclic hydroxylamine CMH in plasma, cells, isolated heart. Free Radic Biol Med. 2002;33:S366. [Google Scholar]
- Forman HJ, Torres M. Redox signaling in macrophages. Mol Asp Med. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- Frutos-Vivar F, Nin N, Esteban A. Epidemiology of acute lung injury and acute respiratory distress syndrome. Curr Opin Crit Care. 2004;10:1–6. doi: 10.1097/00075198-200402000-00001. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernandez-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48:825–834. [PubMed] [Google Scholar]
- Giron-Calle J, Zwizinski CW, Schmid HH. Peroxidative damage to cardiac mitochondria. II. Immunological analysis of modified adenine nucleotide translocase. Arch Biochem Biophys. 1994;315:1–7. doi: 10.1006/abbi.1994.1463. [DOI] [PubMed] [Google Scholar]
- Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Zinc toxicity on cultured cortical neurons: involvement of N-methyl-d-aspartate receptors. Neuroscience. 1994;60:1049–1057. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Lange A, Ausseil O, Segner H. Alterations of tissue glutathione levels and metallothionein mRNA in rainbow trout during single and combined exposure to cadmium and zinc. Comp Biochem Physiol C Toxicol Pharmacol. 2002;131:231–243. doi: 10.1016/s1532-0456(02)00010-8. [DOI] [PubMed] [Google Scholar]
- Li Q, Sato EF, Kira Y, Nishikawa M, Utsumi K, Inoue M. A possible cooperation of SOD1 and cytochrome c in mitochondria-dependent apoptosis. Free Radic Biol Med. 2006;40:173–181. doi: 10.1016/j.freeradbiomed.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Lunardi J, Attardi G. Differential regulation of expression of the multiple ADP/ATP translocase genes in human cells. J Biol Chem. 1991;266:16534–16540. [PubMed] [Google Scholar]
- Machlin LJ, Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. Faseb J. 1987;1:441–445. [PubMed] [Google Scholar]
- Maret W. Cellular zinc and redox states converge in the metallothionein/thionein pair. J Nutr. 2003;133:1460S–1462S. doi: 10.1093/jn/133.5.1460S. [DOI] [PubMed] [Google Scholar]
- Markovich D. Physiological roles and regulation of mammalian sulfate transporters. Physiol Rev. 2001;81:1499–1533. doi: 10.1152/physrev.2001.81.4.1499. [DOI] [PubMed] [Google Scholar]
- Novoselov VI, Amelina SE, Kravchenko IN, Novoselov SV, Yanin VA, Sadovnikov VB, Fesenko EE. The role of peroxyredoxin in the antioxidant system of respiratory organs. Dokl Biophys. 2000:373–375. 64–66. doi: 10.1023/a:1026604830091. [DOI] [PubMed] [Google Scholar]
- Nyborg JK, Peersen OB. That zincing feeling: the effects of EDTA on the behaviour of zinc-binding transcriptional regulators. Biochem J. 2004;381:e3–e4. doi: 10.1042/BJ20041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson QP, Goode DR, West DC, Ramsey KN, Lee JJ, Hergenrother PJ. PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J Mol Biol. 2009;388:144–158. doi: 10.1016/j.jmb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shertzer HG, Racker E. Reconstitution and characterization of the adenine nucleotide transporter derived from bovine heart mitochondria. J Biol Chem. 1976;251:2446–2452. [PubMed] [Google Scholar]
- Simons TJ. Intracellular free zinc and zinc buffering in human red blood cells. J Membr Biol. 1991;123:63–71. doi: 10.1007/BF01993964. [DOI] [PubMed] [Google Scholar]
- Tang ZL, Wasserloos K, St Croix CM, Pitt BR. Role of zinc in pulmonary endothelial cell response to oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2001;281:L243–L249. doi: 10.1152/ajplung.2001.281.1.L243. [DOI] [PubMed] [Google Scholar]
- Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14:315–330. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- Vesce S, Jekabsons MB, Johnson-Cadwell LI, Nicholls DG. Acute glutathione depletion restricts mitochondrial ATP export in cerebellar granule neurons. J Biol Chem. 2005;280:38720–38728. doi: 10.1074/jbc.M506575200. [DOI] [PubMed] [Google Scholar]
- Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP. Thioredoxin and its role in toxicology. Toxicol Sci. 2004;78:3–14. doi: 10.1093/toxsci/kfh050. [DOI] [PubMed] [Google Scholar]
- Wedgwood S, Black SM. Molecular mechanisms of nitric oxide-induced growth arrest and apoptosis in fetal pulmonary arterial smooth muscle cells. Nitric Oxid. 2003;9:201–210. doi: 10.1016/j.niox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Wedgwood S, Mitchell CJ, Fineman JR, Black SM. Developmental differences in the shear stress-induced expression of endothelial NO synthase: changing role of AP-1. Am J Physiol Lung Cell Mol Physiol. 2003;284:L650–L662. doi: 10.1152/ajplung.00252.2002. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Foucaud L, Barlow PG, Hutchison GR, Sales J, Simpson RJ, Stone V. Nanoparticle interactions with zinc and iron: implications for toxicology and inflammation. Toxicol Appl Pharmacol. 2007;225:80–89. doi: 10.1016/j.taap.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitro-sative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol. 2006;291:C555–C568. doi: 10.1152/ajpcell.00509.2005. [DOI] [PubMed] [Google Scholar]
- Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2007;292:L165–L177. doi: 10.1152/ajplung.00459.2005. [DOI] [PubMed] [Google Scholar]
- Zalewski PD, Forbes IJ, Giannakis C. Physiological role for zinc in prevention of apoptosis (gene-directed death) Biochem Int. 1991;24:1093–1101. [PubMed] [Google Scholar]