Abstract
Ipilimumab is a humanized antibody to CTLA4, used to treat cancers refractory to conventional treatment. We treated 21 patients with refractory melanoma or prostate cancer with anti-CTLA4 antibody (ipilimumab), with subsequent development of significant colitis in 9. Two of these nine did not respond rapidly to high-dose (2 mg/kg/day) glucocorticoids or infliximab. They required additional immunosuppression, and one ultimately died of opportunistic infection, representing a more refractory course than has previously been described complicating ipilimumab therapy. Both patients had received radiation to the pelvis for prostate cancer less than one year prior to receiving ipilimumab. We performed immunohistochemical analysis of colon biopsies from ipilimumab recipients to determine if colitis correlates with depletion of intramucosal FOXP3+ regulatory T cells (Tregs), which normally express CTLA4. However, we found no evidence of FOXP3+ T cell depletion in any of the 9 patients who developed colitis.
Keywords: Ipilimumab, CTLA4, FOXP3, Colitis
Introduction
CTLA4, a transmembrane protein expressed by T cells(1), plays a critical role in maintaining immune self-tolerance(2). A fully humanized version of a monoclonal IgG1 antibody to CTLA4 (ipilimumab, MDX010, Medarex, Inc., Princeton, NJ) has been developed for the purpose of breaking immune tolerance to potentially immunogenic malignancies, and thus, facilitating antitumor immunotherapy(3). This medication has shown some efficacy against a variety of advanced cancers, including melanoma(4-6), renal cell cancer(7;8), colon cancer, lymphoma and prostate cancer(9;10). However, in the largest of these series, 21% of recipients developed enterocolitis, presenting as diarrhea(7). All of these cases were reportedly responsive to glucocorticoids, infliximab, or colectomy(7).
CTLA4 is expressed constitutively and most highly by FOXP3+ regulatory T cells (Tregs)(11;12), which represent a small subset of CD4+ T cells. Tregs differ from other T cells in that they do not promote an immune response upon activation, but instead suppress the activation of other T cells, and thus limit inflammation(13). Although the mechanism through which Tregs inhibit other T cells is currently unknown, CTLA4 has been suggested to play an important role in their ability to inhibit colitis(11;14).
We have encountered colitis as a frequent complication of ipilimumab therapy for prostate cancer and melanoma. We have found this colitis to be unresponsive to glucocorticoids and infliximab in some cases. We present data on the frequency of gastrointestinal symptoms, the endoscopic and histologic features of colitis, and an analysis of intramucosal FOXP3+ T cell frequency in ipilimumab recipients. These cases illustrate that anti-CTLA4 therapy may be associated with gut inflammation that is protracted far beyond the half-life of the drug, but does not appear to be mediated by local depletion of Tregs at the site of inflammation.
Materials and Methods
Patient Selection
All individuals with metastatic melanoma or prostate cancer receiving ipilimumab as part of research protocols at the Seattle Cancer Care Alliance between May 2003 and September 2007 were included in this study. As controls for immunohistochemical (IHC) analyses, archived colon biopsies from non-cancer patients with lymphocytic colitis or histologically normal mucosa were selected at random from pathology records of Virginia Mason Medical Center (VMMC). The accession of clinical records and histologic specimens for analysis in this study was approved by the Institutional Review Boards of the Fred Hutchinson Cancer Research Center and VMMC.
Treatment Protocols
Subjects with prostate cancer were treated on one of two protocols. In the first study, patients were randomized to receive ipilimumab (10 mg/kg IV) (Medarex, Inc., Princeton, NJ) with or without a single dose of taxotere administered after the first dose of ipilimumab. Ipilimumab was then administered every 28 days for a total of four doses. In the second study, escalating doses of ipilimumab were administered starting at a dose of 3 mg/kg administered every three weeks for a total of four doses. Subjects who did not receive taxotere and failed to respond to the second or third dose of ipilimumab alone were allowed to receive taxotere with the first of up to four subsequent doses of ipilimumab.
Subjects with melanoma received up to four doses (10 mg/kg IV) of ipilimumab, 2-4 weeks apart, with one subject (subject #2, defined below and in Table 1) receiving a second course of four doses of ipilimumab eight months after completion of an initial course of four doses (eight doses total). Subjects with melanoma were randomized to receive prophylactic budesonide (9 mg/day) or placebo daily after initiating ipilimumab therapy. Subjects who developed grade 2 diarrhea (defined below) stopped ipilimumab and were switched to open label budesonide, while those with grade 3 or 4 diarrhea were given systemic glucocorticoids (1-2 mg/kg/day, per Table 1). Additionally, all patients with melanoma underwent flexible video sigmoidoscopy, without enema or other colon preparation, approximately one week after initiating ipilimumab, regardless of symptoms, and 1-4 biopsies were obtained approximately every 10 cm for the extent of the sigmoidoscopy (20-60cm).
Table 1.
Characteristics, symptoms, and treatment of ipilimumab recipients who developed diarrhea.
| Patient | Cancer1 | Age/ Sex |
Therapy Prior to Ipilimumab | #doses ipilimumab |
Signs and symptoms | Treatment of colitis2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormone/ cytokine3 |
cytotoxic chemo |
Irradiation | Diarrhea (max) |
Abd. pain |
Rectal blood |
Initial therapy |
Secondary therapies |
Outcome | ||||
| #1 | Mel | 38/F | Yes | Yes | Axilla | 2 | Grade 2 | No | No | B | None | Resolution |
| #2 | Mel | 58/M | Yes | Yes | None | 8 | Grade 2 | No | No | B | None | Resolution |
| #3 | Mel | 48/F | Yes | No | None | 3 | Grade 3 | No | No | B | 2mg/kg/d G, T | Resolution |
| #4 | Mel | 64/M | No | Yes | Brain | 4 | Grade 3 | No | Yes | 2mg/kg/d G | None | Resolution |
| #5 | Prost | 81/M | Yes | No | None | 2 | Grade 3 | No | No | 1mg/kg/d G | None | Resolution |
| #6 | Mel | 64/F | No | Yes | None | 1 | Grade 3 | Yes | No | 2mg/kg/d G | None | Improvement4 |
| #7 | Mel | 68/M | No | Yes | None | 2 | Grade 3 | Yes | No | 2mg/kg/d G | I | Resolution |
| #8 | Prost | 62/M | Yes | Yes | Pelvic | 2 | Grade 3 | No | No | 1mg/kg/d G | I, T | Improvement but relapse off therapy |
| #9 | Prost | 52/M | Yes | Yes | Pelvic | 1 | Grade 3 | Yes | Yes | 2mg/kg/d G | I, T, R | Persistent symptoms5 |
Mel=melanoma. Prost=Prostate cancer.
B=budesonide, G=glucocorticoids (prednisone or methylprednisolone), I=infliximab, T=tacrolimus, R=rapamycin
Hormonal therapy was used exclusively in prostate cancer patients. Cytokine therapy was used exclusively in patients with melanoma.
Patient died of cancer before completing a course of prednisone
Patient died from opportunistic Aspergillus infection
Clinical and endoscopic evaluation
The timing and severity of diarrhea in ipilimumab recipients was noted, including the number of diarrheal bowel movements per day and whether diarrhea was accompanied by abdominal pain or hematochezia. Diarrhea was graded according to Common Toxicity Criteria: Adverse Experiences, version 3 (NIH/NCI). Stool was sent for Clostridium difficile toxin assay and pathogenic bacterial culture to exclude infectious etiologies of diarrhea. All patients complaining of diarrhea subsequent to initiating ipilimumab therapy underwent sigmoidoscopy or colonoscopy, with mucosal biopsies. Patients complaining of nausea, vomiting, anorexia or epigastric pain underwent esophagogastroduodenoscopy with gastroduodenal biopsies. Histology and, in certain patients, IHC and/or viral culture of mucosal biopsy tissue was used to further exclude infection or neoplasia as a cause of diarrhea.
Histology
Formalin-fixed, paraffin-embedded mucosal biopsies from both ipilimumab recipients and control subjects were sectioned and stained with hematoxylin and eosin for microscopic evaluation. Additional serial sections of colorectal biopsies were stained by IHC with a monoclonal antibody to FOXP3 (clone 236A/E7, eBioscience, San Diego, CA) or polyclonal antiserum to CD3 (Cell Marque, Rocklin, CA), using the Envision+ system (Dako, Glostrup, Denmark) in a Dako autostainer. Total tissue area of IHC-stained slides was digitally photographed at low power and analyzed with ImageJ software (NIH), employing a color deconvolution plugin to isolate and count DAB-stained cells. CD3+ cells were too confluent in many areas to count individual positive cells, so to estimate the total CD3+ cell count, a total count of DAB+ pixels was recorded instead, and divided by the average pixel area per cell determined in fields where isolated CD3+ cells could be found.
Data Analysis
We hypothesized that ipilimumab induced long-lasting colitis in some subjects by eradicating a slowly-renewing population of FOXP3+ Treg cells that express CTLA-4 and are essential for preventing spontaneous colitis. We compared the number of FOXP3+ Tregs and other T cells in the colonic mucosa of ipilimumab recipients who developed colitis to the number in those who did not, or to the number in the mucosa of non-cancer patients who did not receive ipilimumab. Because tissue sections were of variable size, the total number of CD3+ or FOXP3+ cells identified in the section was divided by the total tissue area (in pixels) to determine the absolute mucosal density of T cells and Tregs, respectively. To determine the percent of T cells expressing FOXP3, the number of FOXP3+ cells was divided by the number of CD3+ T cells in a serial section. These values were compared between ipilimumab recipients who did versus did not develop diarrhea from therapy, using a Student's t-test.
Results
Patient Characteristics
Twenty-one patients with cancer refractory to conventional treatment received ipilimumab, on investigational protocols. Thirteen of these patients had melanoma (age 36-72, mean 55, 23% female), for which ten had previously been treated with cytotoxic chemotherapy, six cytokine therapy (interferon-alpha or interleukin-2 or -21), and five local radiation therapy (usually to brain or spine metastases). Eight patients had prostate cancer (age 49-85, mean 69, all male), for which all had previously received hormonal therapy, and two had received cytotoxic chemotherapy prior to ipilimumab. Five of these prostate cancer patients received their first cytotoxic therapy (a single dose of taxotere) with a dose of ipilimumab. Six of the prostate cancer patients had also received pelvic irradiation at some point prior to ipilimumab, with external beam irradiation in five and brachytherapy in two.
Frequency of Intestinal Symptoms
New gastrointestinal symptoms developed after ipilimumab administration in 9 patients (43%), with diarrhea occurring in all patients, abdominal pain in five, and hematochezia in three. Table 1 describes the characteristics, presenting symptoms, treatments, and clinical course of these 9 patients. The onset of diarrhea occurred after one dose of ipilimumab in two recipients, after two doses in four recipients, and after three or more doses in three patients. The interval between the last ipilimumab dose and diarrhea onset ranged from 5 to 36 days, with a median of 14 days. Subjects with diarrhea reported anywhere from 2-30 bowel movements per day. Maximal diarrheal symptoms were deemed grade 3 in seven subjects, while two never exceeded grade 2.
Response of Colitis to Treatment
Three subjects (#1, #2, and #3), who initially presented with grade 2 diarrhea, were started on open label oral budesonide (9 mg/day). Subjects #1 and #2 had symptomatic improvement, while subject #3 progressed to grade 3 diarrhea, received systemic prednisone (2 mg/kg/day), and then improved only after the addition of tacrolimus.
Six other subjects, who presented with grade 3 diarrhea, were initially placed on methylprednisolone or prednisone (1-2 mg/kg/day). For three of these subjects (#4, #5, and #6), this therapy resulted in improvement, with subsequent reduction and eventual discontinuation of glucocorticoids in subjects #4 and #5; subject #6 died from metastatic melanoma prior to completing a course of prednisone. However, persisting or worsening symptoms in the other three patients with grade 3 diarrhea (#7, #8, and #9) led to treatment with one dose of infliximab (5mg/kg), with a prompt resolution of diarrhea and gradual discontinuation of glucocorticoids only in subject #7.
In subject #8, who had received pelvic irradiation as salvage treatment for prostate cancer as recently as 10 months prior to ipilimumab infusion, persisting diarrhea after a single dose of infliximab prompted the addition of tacrolimus to ongoing prednisone. Symptomatic and endoscopic evidence of colitis resolved on this regimen, and allowed subject #8 to gradually taper his prednisone dose over the next 9 months. However, complete cessation of both glucocorticoids and tacrolimus resulted in recurrent diarrhea and colitis, up to 294 days after his second and last dose of ipilimumab. Consequently, subject #8 has since been maintained on chronic tacrolimus, and oral budesonide.
Subject #9 had also received salvage pelvic irradiation for recurrent prostate cancer, and had experienced grade 2+ diarrhea acutely during radiation therapy. Diarrhea resolved shortly after completion of radiation therapy, 8 months before ipilimimab was infused. After receiving his first and only dose of ipilimumab, he developed severe diarrhea, which improved only in response to high-dose (2 mg/kg/day) steroids, and worsened with any attempt to reduce prednisone dosage below 1.5 mg/kg/day, even after a first dose of infliximab. He was placed on total parenteral nutrition, and six weeks after the first infliximab, two more doses of infliximab were administered, two weeks apart. Ultimately tacrolimus and then rapamycin were added to the regimen, allowing a reduction in prednisone dose to 0.5 mg/kg/day. Despite extensive immunosuppression, stool studies and mucosal biopsies were repeatedly negative for infectious or neoplastic causes of diarrhea. However, this patient continued to have grade 3 diarrhea and distal colonic ulcers up until he died of disseminated Aspergillus infection, 138 days after a single dose of ipilimumab.
Endoscopic Findings
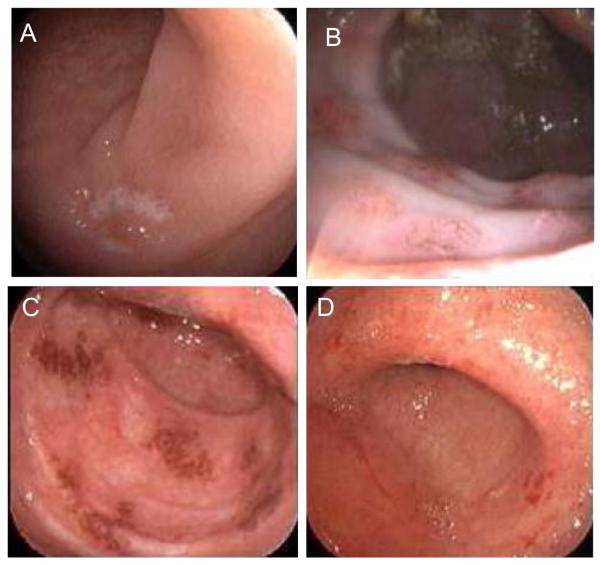
Most subjects with diarrhea had endoscopic evidence of colitis (Figure 1), although subject #1, with grade 2 diarrhea, had only histologic evidence of colitis, with grossly normal appearing rectosigmoid mucosa. Endoscopic and histologic findings in ipilimumab recipients with diarrhea are summarized in Table 2. Colitis, when evident, was consistently identified in the distal colon, evaluable by flexible sigmoidoscopy, although in some cases mucosal involvement was non-contiguous or patchy, and subject #4 demonstrated greater abnormalities in the right than left colon. Abnormalities included erythema (in 95% of abnormal sigmoidoscopies), edema (in 73%), friability (in 53%), ulcers (in 39%), exudate (in 33%), erosions (in 24%), or visible bleeding (in 6%). In 12 patients with melanoma, protocol sigmoidoscopies were performed 2-10 days after a first dose of ipilimumab in the absence of diarrhea, and these were consistently normal, even in five subjects who later developed diarrhea. Therefore, in the absence of symptoms, gross endoscopic appearance shortly after ipilimumab administration did not predict eventual colitis onset.
Figure 1.
Variable endoscopic appearance of ipilimumab-mediated colitis: A. Ulceration (Subject #8). B. Nodular erythema (Subject #2). C. Patchy erythema (Subject #7). D. Diffuse erythema, granularity, and friability (Subject #9).
Table 2.
Endoscopic and histologic findings in 9 patients
| Patient | Colon mucosal findings1 | Colon Histology2 | T cells % FOXP3+ |
|||
|---|---|---|---|---|---|---|
| Surface | Crypts | Lamina Propria | Other | |||
| #1 | Normal | PMN | 4.80 | |||
| #2 | Erythema, Edema | Increased PMN, eosinophils | 5.63 | |||
| #3 | Erythema, Edema, Friability, Erosions |
Apoptotic bodies | Increased lymphocytes, plasma cells, eosinophils |
3.06 | ||
| #4 | Erythema, Edema, Friability | Lymphocytes | Microabscesses, PMN, eosinophils |
Increased plasma cells | 8.81 | |
| #5 | Erythema, Friability | Microabscesses, PMN, lymphocytes, eosinophils |
Increased PMN, eosinophils, lymphocytes, plasma cells, |
1.51 | ||
| #6 | Erythema, Ulcers | PMN | PMN | Increased PMN, lymphocytes, plasma cells |
4.02 | |
| #7 | Erythema, Edemea, Exudate, Friability, Ulcers, Bleeding |
PMN | Increased PMN, eosinophils, lymphocytes, plasma cells, |
3.90 | ||
| #8 | Erythema, Edema, Exudate, Ulcers | Lymphocytes | Apoptotic bodies, architectural distortion, microabscesses |
Increased lymphocytes, plasma cells, eosinophils |
1.21 | |
| #9 | Erythema, Edema, Friability, Exudate, Ulcers, Denuded mucosa |
Thin epithelium |
Apoptotic bodies, architectural distortion, microabscesses |
Increased PMN, lymphocytes, plasma cells |
Crypt loss |
3.56 |
Based on the findings at all endoscopic examinations following ipilimumab.
Based on the histologic findings from all colon mucosal biopsies. PMN= neutrophils.
Subjects #2, #3, and #8 also underwent esophagogastroduodenoscopy, to evaluate epigastric discomfort, nausea and/or vomiting occurring at some point after ipilimumab administration. In each case, only mild antral erythema and edema were seen.
Histological Findings
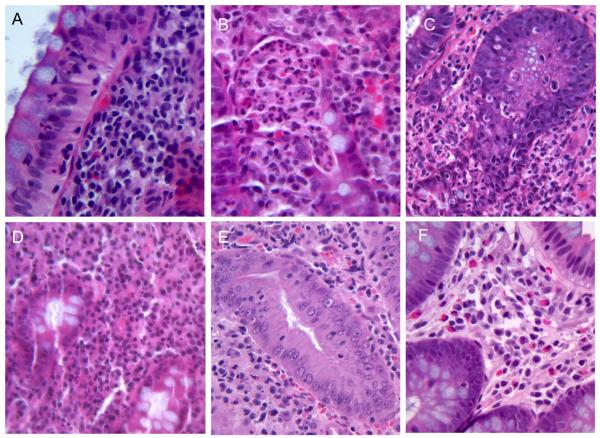
Rectosigmoid mucosal biopsies taken prior to diarrhea onset in five patients with melanoma did not reveal findings predictive of subsequent colitis. An increased lamina propria cellularity and large lymphoid follicles were sometimes seen in endoscopically normal mucosa, but these did not correlate with whether subjects later developed diarrhea. Intraepithelial neutrophils were seen in surface epithelium over highly cellular areas of lamina propria (Figure 2A) in subject #1, prior to onset of mild diarrhea, although neutrophils were actually less prominent by the time she was symptomatic. Subject #4, who eventually developed prednisone-responsive colitis, demonstrated increased lamina propria eosinophils (Figure 2F) prior to diarrhea onset.
Figure 2.
Variable histology preceding or concurrent with ipilimumab-mediated colitis: A. Intra-epithelial neutrophils (Subject #1). B. Crypt microabscesses (Subject #7). C. Neutrophilic crypt invasion and destruction (Subject #9). D-F. Lymphocytic (D, subject #8), neutrophilic (E, subject #5), or eosinophilic (F, subject #4) lamina propria infiltrates.
Microscopic findings in subjects with diarrhea ranged from normal histology to severe inflammation, with neutrophilic invasion and destruction of surface epithelium and crypts (Figure 2C), in the most severely affected ipilimumab recipient (Subject #9). In the latter case, the lamina propria was heavily infiltrated by lymphocytes and neutrophils, while residual epithelium was atrophic and thin, with absence of goblet cells throughout the mucosa except near the crypt bases, which were comparatively spared. However, in most cases who underwent biopsies at the time of diarrhea, epithelial damage was less prominent, and abnormalities consisted primarily of a lamina propria infiltrate (Figure 2D, E), which variably consisted of lymphocytes, plasma cells, eosinophils, or neutrophils. Crypt microabscesses were also occasionally seen (Figure 2B). Epithelial cell apoptotic bodies were noted only in those patients who ultimately required tacrolimus (Subjects C, H, and I), two of whom had refractory or relapsing diarrhea (Subjects #8 and #9) associated with crypt architectural distortion as well.
Gastric biopsies obtained from three subjects, were histologically normal or only mildly atrophic in two of these (Subjects #2 and #8); lymphocytic infiltrate, eosinophils, and apoptotic epithelial cells were seen in the third (Subject #3).
Colonic FOXP3+ T Cell Content
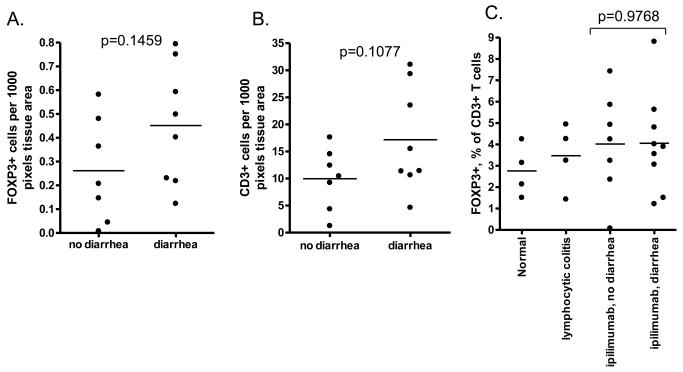
Rectosigmoid mucosal biopsies, taken 2-35 days after a dose of MDX010, consistently revealed mononuclear cells in the lamina propria with nuclear staining for FOXP3, which were not depleted in the setting of colitis (Figure 3). Some patients with ipilimumab-induced diarrhea actually had an increase in total intramucosal FOXP3+ cell numbers (Figure 4A), although this correlated with an increase in CD3+ T cells in the mucosa (Figure 4B), such that the percent of T cells expressing FOXP3 was no different between ipilimumab recipients who did versus did not develop diarrhea (Figure 4C). Furthermore, the percent of colon T cells expressing FOXP3 was no lower in ipilimumab recipients than in non-cancer patients with either an unrelated cause of diarrhea (lymphocytic colitis) or no intestinal pathology (Figure 4C). The frequency of FOXP3+ Tregs, as a percentage of CD3+ T cells, also did not correlate with patient age, time after MDX010 administration, diarrhea grade, or histologic inflammation severity (data not shown).
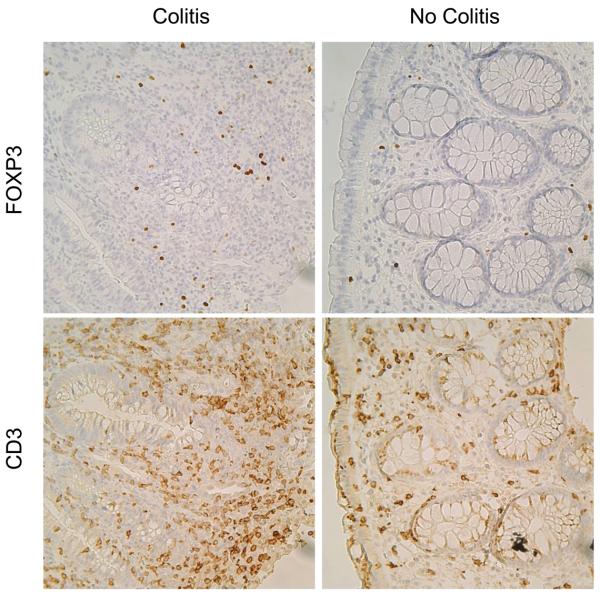
Figure 3.
FOXP3+ cells (upper panels) were present in the colonic mucosa in subjects with colitis (left panels) or without (right panels). The number of FOXP3+ cells correlated with the density of CD3+ T cells present in the lamina propria (lower panels), which were increased in the setting of colitis.
Figure 4.
Immunohistochemistry (IHC) for FOXP3 and CD3 revealed a trend towards more Tregs (A) and T cells (B), respectively, infiltrating the colonic mucosa of ipilimumab recipients who developed diarrhea than in those who did not. However, IHC did not show a lower fraction of these T cells to be FOXP3+ Tregs in ipilimumab recipients relative to controls, nor in ipilimumab recipients who developed diarrhea relative to those who did not (C). P-values were calculated by two-tailed unpaired Student's t-test.
Discussion
Our experience with ipilimumab in 21 patients with melanoma or prostate cancer has revealed iatrogenic colitis to be a common complication of therapy that resulted in a prolonged, treatment-refractory course in two patients. In a prior report of anti-CTLA4 antibody-mediated colitis, 12 of 198 melanoma or renal cell cancer patients whose colitis was refractory to initial doses of glucocorticoids either responded to additional glucocorticoid therapy (n=5) or a single dose of infliximab (n=4) or developed spontaneous bowel perforation (n=3) (7). However, in our cohort, two of four glucocorticoid-refractory subjects were also refractory to infliximab. One of these patients also failed to respond to treatment with tacrolimus and rapamycin, and ultimately died of infection as a consequence of severe immune suppression, more than 4 months after a single infusion of CTLA4 antibody. While the prior larger series did not identify any cases of infliximab-refractory colitis, a high frequency of bowel perforations in steroid-refractory cases was reported(7), and it is possible that some of these perforating cases might have proven to be infliximab-refractory had they not gone to emergent colectomy. No patient in our series experienced bowel perforation.
Another novel aspect of our experience with ipilimumab has been the length of time that colitis persisted following receipt of drug, exceeding 130 days in two cases, which is more than ten times the 12.5 day average half-life of ipilimumab(10). Such a persistence of inflammation suggests that this drug may do more than simply antagonize CTLA-4, as the latter effect would presumably disappear with drug metabolism or clearance, allowing the immune system to return to normal. The observed prolonged time course formed the basis for our hypothesis that ipilimumab may instead eradicate mucosal FOXP3+ Tregs. However, we found no depletion of FOXP3+ Tregs in the colonic mucosa associated with either ipilimumab administration or the development of colitis in recipients. Furthermore, the frequency of FOX3+ Tregs was no lower in ipilimumab treated cancer patients that in untreated individuals without cancer. Others have reported that Tregs are not depleted from the spleens of mice(14) or the peripheral blood of humans(7) after administration of anti-CTLA4 antibodies. We cannot exclude the possibility that a small oligoclonal subset of Tregs, that may normally be responsible for preventing colitis, is not being selectively eliminated by anti-CTLA4. However, it is difficult to speculate as to how ipilimumab could achieve such selectivity.
CTLA4 is expressed predominantly in intracellular vesicles, with very little on the cell surface at any given time(1). Although T cells do mobilize CTLA4 to their surface upon activation by an antigen presenting cell (APC), CTLA4 remains sequestered in areas deep within the “immunological synapse” interface between T cell and APC(15). Thus, CTLA4, and the ipilimumab molecules that bind it, are perhaps not sufficiently exposed to the extracellular environment to fix complement or cause antibody-dependent cell-mediated cytotoxicity. Instead, perhaps ipilimumab alters the long-term phenotype of mucosal T cells to favor colitis, by either changing how naïve T cells differentiate upon activation, or selectively enriching existing T cells with an antigen-specificity or other phenotype favoring colitis.
Both of the infliximab-refractory cases of colitis in our series were in prostate cancer patients who had received salvage external beam pelvic irradiation within a year of ipilimumab administration (Subjects #8 and #9). Furthermore, the most severe and treatment-refractory case (Subject #9) had developed transient symptoms of colitis during radiation treatment, although symptoms had resolved after cessation of radiation, months before receiving ipilimumab. We speculate that recent colonic irradiation in the setting of androgen and therefore estrogen deletion may be a risk factor for later developing severe or prolonged colitis after ipilimumab administration, particularly if there was symptomatic evidence of colitis during radiation treatment.
Histologically, colitis from ipilimumab demonstrated a variable pattern of inflammatory infiltrate that did not clearly correlate with clinical course. Some microscopic features, such as crypt microabscesses and architectural distortion, resemble spontaneous inflammatory bowel disease (IBD), although these features did not reliably identify patients who would respond to infliximab—a drug commonly used for IBD therapy. Epithelial cell apoptotic bodies, a histological feature of gastrointestinal graft versus host disease (GVHD) in hematopoietic cell transplant recipients(16), were noted only in the two patients with more prolonged or severe colitis (Subjects #8 and #9), and in one additional steroid-refractory patient who also ultimately required tacrolimus to control colitis (Subject #3), suggesting that a similar pathophysiological process may occur in both GVHD and more serious cases of ipilimumab-mediated colitis. Tacrolimus, an immunosuppressant widely used in the prevention and treatment of GVHD, was effective in controlling colitis in two of our ipilimumab recipients with apoptotic bodies in their colon biopsies (Subjects #3 and #8).
The frequency with which colitis complicates ipilimumab therapy clearly implicates CTLA4 in human intestinal immunoregulation. However, it is unclear what pro-inflammatory signals may normally be held in check by CTLA4 to prevent spontaneous colitis. It is possible that immune cells may recognize either intestinal autoantigens, or foreign antigens for either food or enteral microbes, unless controlled by mechanisms requiring CTLA4. Indeed, the fact that only a subset of patients developed colitis following ipilimumab administration makes it attractive to speculate that variations in disease penetration may be due to person-to-person differences in diet or intestinal microflora. However, subject #9 continued to have severe, steroid-dependent colitis even after initiating total parenteral nutrition (TPN), which would be expected to dramatically reduce intestinal exposure to food antigens and diminish the intralumenal microbial burden by depriving it of nutrition. Therefore, is appears that continuous exposure to food and/or microbes in the GI tract may not be essential for maintaining colitis, once inflammation is induced by ipilimimab. Whether prophylactic TPN use surrounding ipilimumab administration would prevent the onset of spontaneous colitis, and thus implicate components of the fecal stream in disease induction, remains to be seen.
Ipilimimab has demonstrated efficacy in the treatment of certain advanced cancers and more widespread use of the agent is anticipated. Consequently, it is important to understand the natural history and treatment options for the complication of immune mediated colitis. Based upon our experience, radiation therapy to the pelvis within one year of therapy in prostate cancer patients who are androgen deprived may result in colitis that is more refractory to steroids and infliximab, and may last longer than cases in other cancers. Although the majority of cases of ipilimumab-mediated colitis are responsive to glucocorticoids, a fraction of those who are not may also not respond to a single dose of infliximab, and may benefit from the early addition of calcineurin inhibitors such as tacrolimus. In any case, we believe that prompt recognition and treatment of this potentially fatal complication of ipilimumab therapy, while avoiding protracted immune suppression, is essential to a good clinical outcome. When prolonged immunsuppression cannot be avoided, prophylaxis against opportunistic infections is indicated.
Acknowledgments
Grant Support:
This work was supported by the National Institutes of Health AI48779 AI007411 (JDL), and CA18029 (RCH, GBM), as well as clinical funds from the University of Washington and the Seattle Cancer Care Alliance.
Abbreviations
- CTLA4
Cytotoxic T Lymphocyte Antigen 4
- GVHD
Graft Versus Host Disease
- IBD
Inflammatory Bowel Disease
- Tregs
regulatory T cells
- FOXP3
Forkhead bOX Protein 3 (human nomenclature)
Footnotes
Financial Disclosures: None of the authors have personal financial relationships to disclose. However, subjects with melanoma were participants in a trial funded by the Bristol-Myers Squibb company, for which John Thompson was an investigator. Subjects with prostate cancer were participants in a trial funded by the Medarex company, for which Celestia Higano was an investigator, and Deborah Chielens was a coordinator.
Reference
- 1.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol.Rev. 2006;212:131–48. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 2.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 3.O'Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614–27. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 4.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann.Surg.Oncol. 2005;12:1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc.Natl.Acad.Sci.U.S.A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J.Clin.Oncol. 2005;23:741–50. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 7.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J.Clin.Oncol. 2006;24:2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J.Immunother. 2007;30:825–30. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Mahony D, Morris JC, Quinn C, Gao W, Wilson WH, Gause B, et al. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin.Cancer Res. 2007;13:958–64. doi: 10.1158/1078-0432.CCR-06-1974. [DOI] [PubMed] [Google Scholar]
- 10.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin.Cancer Res. 2007;13:1810–5. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 11.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J.Exp.Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J.Exp.Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J.Exp.Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J.Immunol. 2006;177:4376–83. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–13. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Epstein RJ, McDonald GB, Sale GE, Shulman HM, Thomas ED. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology. 1980;78:764–71. [PubMed] [Google Scholar]