Abstract
There are a variety of factors and conditions that predispose cattle to pneumonia. Cattle have anatomic and cellular differences from humans and other species and are managed in groups/herds, all of which can increase susceptibility to microbial pathogens. This article highlights the basic innate immune response of the respiratory tract and newer developments in the understanding of adaptive immune responses of the bovine respiratory tract, placing special emphasis on features unique to cattle.
Keywords: Bovine, Innate immunity, Lung, Pneumonia
Pneumonia is a leading cause of loss to the cattle industry in the United States and Europe. Of cattle diseases, it has the greatest economic impact. Respiratory pathogens can cause serious outbreaks of acute pneumonia in neonatal, weaned, and growing calves. Chronic infection leads to debilitation, decreased performance, and culling in older animals. The means to enhance effective and noninjurious immune responses are needed because of the high incidence of pneumonia in cattle, ubiquity of respiratory pathogens, the increasing frequency of antibiotic resistance, and the general expectation by consumers for producers to use antibiotics less frequently. The lung has a wide array of both innate and adaptive immune responses to airborne particulates, vapors, and microbial pathogens. Vaccines can effectively enhance resistance to some pathogens, but not all. More recently, additional attention has been given to innate immune responses and methods/regimens that increase innate immune activity. Despite advances in managerial practices, vaccines, and clinical therapies, pneumonia remains a widespread problem and methods to enhance host resistance to pathogen colonization and pneumonia are needed.
There are a variety of factors and conditions that predispose cattle to pneumonia. Cattle have anatomic and cellular differences from humans and other species and are managed in groups/herds, all of which can increase susceptibility to microbial pathogens. This article highlights the basic innate immune response of the respiratory tract and newer developments in the understanding of adaptive immune responses of the bovine respiratory tract, placing special emphasis on features unique to cattle.
Innate immune responses
Commensal Microflora
The upper respiratory tract is colonized by a variety of bacterial pathogens that are inhaled and/or replicate in the tonsillar crypts and nasal/sinus mucin. Colonization of these organisms within regions of the upper respiratory tract mucosa may occupy micronutrient and receptor sites resulting in reduced colonization by pathogens. In a study from 1964, bacterial isolates from trachea and lung were colonized by Bacillus sp, Streptococcus sp, Streptomyces sp, Micrococcus sp, and Pseudomonas sp.1 A 1991 study demonstrated Pasteurella (Mannheimia) haemolytica, Pasteurella multocida, Mycoplasma bovine, and M bovirhinis, Histophilus somni, Streptomycetes sp, Neisseria spp, and Bacillus spp in nasopharyngeal swabs of healthy calves.2 The deeper lung remains relatively sterile in healthy cattle; however, in a recent study of bronchoalveolar lavage fluid from cattle in Denmark,3 63% of healthy cattle harbored bovine bacterial pathogens (Pasteurella multocida, Histophilus somni, Mannheimia haemolytica, Arcanobacterium pyogenes, and Mycoplasma sp).
Pathogenic Microflora
Bovine respiratory disease complex (BRDC) is a complex infectious disease caused by the interaction of several microbial agents. These include viruses that have a tendency to infect immunocompromised lung: bovine herpesvirus-1 (BHV-1), bovine respiratory syncytial virus, bovine parainfluenza virus 3, bovine coronavirus, bovine adenovirus A-D, and bovine viral diarrhea virus 1 and 2. Many herds of cattle are colonized by Mycoplasma spp (bovis, dispar, bovirhinis), which inhibit function and activity of ciliated respiratory epithelial cells. Initial viral infections, toxins such as 3-methyl indole, or other immunosuppressive conditions allow increased replication of other bacterial pathogens: Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, Arcanobacterium pyogenes, and Chlamydiaceae. Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni can colonize the tonsil and mucous of the nasal meatus and sinuses. With stress, their replication increases and their area of colonization spreads.
Respiratory Airways
Cattle have a relatively long tracheobronchial tree, which increases the amount of dead space volume in comparison with dogs, pigs, and horses.4 Increased dead space affects the amount of fresh oxygen that can be delivered to lung and increases the risk of alveolar hypoventilation with partial obstruction. The increased dead space in cattle may not affect respiratory tract immunity per se; however, it may allow increased surface area for particulate deposition and increased transit time of inhaled vapors, gases, and particulate matter.
Hairs
Hairs along the external nares provide a physical barrier to inhalation of large particulate matter. Squamous cells that line the anterior nares form a layer of stratified squamous epithelium that is more resistant to microbial adhesion compared with pseudostratified epithelium.
Mucociliary Apparatus
The air-surface liquid (ASL) lining the upper respiratory tract and pulmonary airways is generated largely from submucosal glands and goblet cells and provides a layer of protection against inhaled particulate matter, aerosols, vapors, and microbial pathogens (Fig. 1 ). The antimicrobial activity of the ASL is becoming increasingly understood. The ASL has 2 layers, a periciliary sol layer close to the apical cell surface and a gel/mucus layer that is toward the airway lumen. Ciliary beat occurs principally within the sol layer, which is less viscous than the gel layer. Dehydration can enhance the viscosity of the ASL as can aggregates of DNA and filamentous actin that accumulate from degraded neutrophils, leukocytes, and necrotic epithelial cells along with bacterial biofilms. Sneezing and coughing help to expel particulates and ASL/mucin aggregates and also induce some dilation of the airway lumens.
Fig. 1.
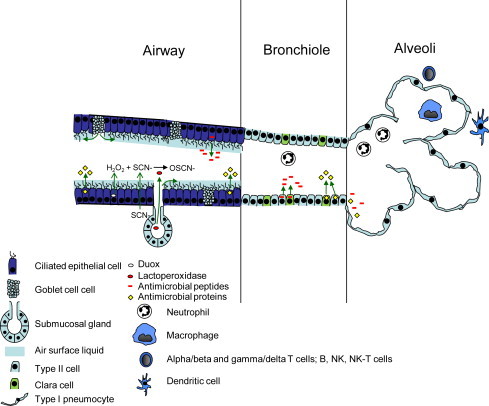
Schematic illustration of the bovine respiratory immune system emphasizing the innate components. Depicted are immune systems within 3 main regions of the respiratory tract: the airways (upper respiratory tract to the bronchi), bronchiole, and alveoli. Airway: Conducting airways are lined by pseudostratified ciliated epithelium that transports the air-surface liquid (ASL) anteriorly toward the pharynx or nares. ASL is produced by secretion products from goblet cells and submucosal glands. Within the ASL are mucins (see text), as well as antimicrobial proteins (eg, lysozyme, lactoferrin, surfactant proteins A and D, SLPI, PLUNC, and others) and antimicrobial peptides (defensins and RNAase 7), which are produced by ciliated epithelial cells. The oxidative defense system (ODS) is composed of lactoperoxidase (LPO) produced by submucosal glands, dual function oxidases 1 and 2 (Duox1 and Duox2) produced by epithelia, and thiocyanate (SCN-) transport proteins produced by epithelia. Duox1 and 2 produce hydrogen peroxide (H2O2), which, in the presence of SCN-, is converted to OSCN-, which has potent antimicrobial activity. Bronchioles: Bronchioles are lined by Clara and Type II cells, both of which also produce antimicrobial proteins and peptides. Clara cells also produce CC10, an immunomodulatory protein, and express cytochrome P450 enzymes that biometabolize toxins. With injury, Type II and Clara cells proliferate and replace damaged cells. Alveoli: Type I pneumocytes line the alveolar lumen and are covered by surfactant phospholipids that are admixed with surfactant proteins, antimicrobial proteins, and antimicrobial peptides. With injury, Type I pneumocytes are replaced by Type II cells, which then differentiate into Type I cells. Effector cells: Neutrophils, alveolar and intravascular macrophages, alpha/beta T (CD4 and CD8) cells, and gamma/delta T cells along with B, NK, and dendritic cells are effector cells present along the respiratory tree for induction of adaptive immune responses. With inflammation, neutrophils and macrophages are present in the alveolar lumen, bronchioles, and airways. Other immune responses such as pattern recognition receptors as well as cytokine, interferon, and chemokine responses are not included in this figure.
Air Surface Liquid
Once considered simply a lubricant for airway function, the ASL has a very active role in innate defense. Water accumulates within the periciliary sol layer after secretion of chloride ions by epithelial cells, submucosal glands, and serous cells and water is resorbed as sodium is removed from the layer by epithelial Na+ channels (ENaC). In addition to providing a microenvironment for ciliary activity, the periciliary sol layer maintains a proper pH (slightly more acidic than blood). The gel layer of the ASL is composed of mucin glycoproteins and proteoglycans secreted by goblet cells and submucosal glands. The amount of this layer can increase with chronic inflammatory conditions, allergic conditions, and cholinergic stimulation. The mucin glycoproteins are either tethered to the apical membrane of the subjacent epithelial cell or secreted. The protein backbone of the mucin glycoproteins encoded by MUC genes contain repeating serine and threonine amino acids that form O-linkages with oligosaccharides. In humans, MUC1 and MUC4 (and also MUC 11, 13, 15, and 20) proteins are associated with the apical surface, whereas MUC5AC is secreted from goblet cells and MUC5B is secreted from mucous cells of submucosal glands.5 Variations of MUC gene expression change with inflammation and lung injury. MUC proteins serve as receptors and signaling molecules and can bind bacteria. For example, MUC1 is a receptor for Pseudomonas aeruginosa flagellin protein, activates Src tyrosine kinases, and activates phospholipase C and protein kinase C. MUC7 is a non–gel-forming secreted mucin.
The ASL contains numerous molecules that mediate antimicrobial as well as pro- and anti-inflammatory activity, immunomodulation, and wound healing.6 The osmotic gradient is maintained by sodium and chloride levels regulated by sodium and chloride transport pumps and channels, which allows proper protein, enzyme, and peptide activity. The presence and activity of some of these, such as lactoferrin and lysozyme have been known for many years. Lactoferrin binds and sequesters iron from microbial agents, whereas lysozyme can disrupt membranes of bacteria. Additional components of the ASL have been indentified in cattle and other species and increasingly appreciated for the immune and immunomodulatory role. These include antimicrobial peptides such as defensins, cathelicidins, and larger proteins.7 There are 3 major subclassifications of defensins and 2, the alpha and beta defensins, are present in cattle.
Three beta-defensins of cattle include tracheal antimicrobial peptide (TAP), lingual antimicrobial peptide (LAP), and enteric antimicrobial peptide. TAP and LAP are produced by respiratory epithelia and TAP has the highest level of expression. In fact, TAP was the first mammalian defensin identified, being isolated and cloned by Gill Diamond and colleagues in 1991.8 Both TAP, LAP, and other defensins can form pores in the membranes of bacteria, enveloped viruses, and other microbial agents resulting in rapid lysis. Beta defensins have numerous other activities that range from exacerbating the acute inflammatory reaction by triggering histamine release from mast cells, to triggering the adaptive immune response through their chemotactic properties to dendritic cells, to inducing wound healing via their mitogenic function in epithelial cells. Cercropin is an antimicrobial peptide from insects that, when delivered by vector to bovine mucosa, reduces colonization by Mannheimia haemoyltica.9 Bovine neutrophils have numerous alpha defensins as well; however, these are produced in neutrophils and are present in the ASL only after neutrophil recruitment and degranulation. Cathelicidin antimicrobial peptides are also produced in neutrophils and can be released by lung epithelia; however, epithelial expression is apparently low and limited. Airway epithelial cells also produce RNAase 7, an RNA helicase with antimicrobial properties; however, the expression levels of RNAase in cattle are not known. Bovine neutrophils express 13 defensins that are released upon degranulation and contribute to microbial killing and the acute inflammatory reaction. Binding of defensins and other antimicrobial peptides to host membranes is limited, presumably because of the higher cholesterol content of mammalian cells and, thus, autotoxicity by these peptides is not a feature of the their activity.
In alveoli of deep lung, alveolar macrophages engulf particulate matter and pathogens that manage to be inhaled into the deep lung. Once activated, the macrophages release cytokines, chemokines, and other soluble mediators that stimulate an inflammatory and/or immune response. Cells lining alveoli (type I pneumocytes) are covered by a layer of surfactant, composed of phosphatidylcholine and other phospholipids, which prevents alveolar collapse by its effect on apical membrane surface tension. Surfactant is secreted by airway type II cells and Clara cells along with surfactant proteins. Intracellularly, surfactant proteins B and C are associated with surfactant and maintain surfactant folding/structure until release. Surfactant proteins A and D are also associated with surfactant and released by pseudostratified ciliated, type II, and Clara cells into the airway and/or alveolar lumen and have very potent antimicrobial and immunomodulatory roles. Surfactant protein A (SP-A) and D (SP-D) can bind and inactivate microbial agents. Both of these proteins have a carbohydrate recognition domain (CRD) that binds to mannose residues of microbial pathogens. Once bound, the pathogen and surfactant protein complex can aggregate and be taken up by alveolar macrophages. This is well documented for respiratory syncytial virus (RSV) in humans,6 and individual altered surfactant proteins A and/or D attributable to nucleotide polymorphisms have increased numbers of RSV infection and increased severity. Once released, SP-A can activate macrophages and enhance macrophage uptake and killing of microbial pathogens. In addition, SP-A present within the alveolar lumen liquid can be taken up by the pulmonary lymphatic drainage system and enter the blood. Increased levels of SP-A in blood is associated with pneumonia and could be a useful biomarker of pneumonia severity.
Airway and Lung Alveolar Epithelia
As indicated previously, the ASL is produced by subjacent airway and lung epithelial layer that is specialized along the respiratory tract. The inner nares is lined by stratified squamous epithelium that transitions into pseudostratified ciliated epithelium that lines the nasal meatus and conchae (turbinates). Intervening within the pseudostratified ciliated epithelium are goblet cells that release mucin products discussed earlier and also submucosal glands. In the ethmoid region, additional nerve sensory fibers enter the respiratory epithelia and mediate olfactory sensations. Pseudostratified ciliated epithelia, along with submucosal glands, line the frontal and ethmoid sinuses, trachea, and bronchi. Lung bronchiole mucosa has a thinner epithelial layer (1 cell-layer thick) lined by both type II and Clara cells (see Fig. 1). Both cell types produce surfactant and surfactant proteins and can serve as progenitor cells; type II cells are progenitors for other type II cells and type I cells that line lung alveoli. Clara cells produce daughter cells that can be Clara cells or type II cells. Clara cells also express mixed function oxidases (cytochrome p450 isoenzymes such as CYP1A1 and CYP1A2) that detoxify inhaled toxins and/or toxins that are spread hematogenously. This is a beneficial feature for some toxins; however, for others, such as 3-methyl indole (3 MI), a toxic metabolite, 3-methylindololamine is formed that causes Clara and airway epithelial cell injury and necrosis. With loss of these cells, there is concomitant loss of the epithelial barrier and the overlying air-surface liquid containing antimicrobial factors. The 3 MI is present in certain feedstuffs such as foggage, rape, and kale. Clara cells also produce Clara cell secretory protein 10 (CC10). CC10 is an important, yet subtle, immunomodulator of lung physiology, influencing synthesis, secretion, and function of molecules such as phospholipase A2, interferon gamma, and locally produced IgA. Generally, it is an immunosuppressive and anti-inflammatory protein that is important in limiting the collateral damage caused by inflammatory cells such as neutrophils.
Microbial Pattern Recognition Molecules
Epithelial cells are central to respiratory systems sensing of the external environment. Most particulate matter and microbial agents are removed from the inhaled air in the nares, nasal conchae, and trachea leaving the deeper lung sterile and relatively free from particulate material. These substances, along with mists, vapors, and gases once through the ASL can bind the lung epithelia and trigger activation, cell injury, metaplasia, or cell death. Microbial agents produce a number of conserved molecular patterns, termed pathogen-associated molecular patterns (PAMPs) that include substances such as teichoic acid from gram-positive bacteria, lipopolysaccharide (LPS) from gram-negative bacteria, cytokine-phosphate-guanine (CpG) DNA, single- and double-stranded RNA (dsRNA), flaggelin, fungal zymosan, and lipopeptides from mycobacteria. Major pathogens of cattle, Histophilus somni, Mannheimia haemolytica, Pasteurella multocida, Mycoplasma bovis, bovine herpesvirus-1 (BHV-1; infectious bovine rhinotracheitis), parainfluenza virus, bovine respiratory syncytial virus, and bovine viral diarrhea virus (BVD), all produce some type of PAMP that are recognized by epithelia, alveolar macrophages, and intravascular macrophages. Intravascular macrophages are macrophages within the small capillaries of alveolar walls, attached to the underlying endothelial cells that are very active in metabolic generation of inflammatory mediators such as prostaglandins and leukotrienes. With acute inflammation, neutrophils also recognize PAMPs, and with adaptive immune responses, the various lung dendritic cells (DCs), including plasmcytoid DCs, natural killer (NK) cells, NK T cells, alpha/beta and gamma/delta T cells, and B cells, all interact with microbial PAMPs.
The lung, like other organs, expresses a wide variety of extracellular, cell surface, endosomal, and cytoplasmic receptors that recognize microbial PAMPs, termed pattern recognition receptors (PRRs). Extracellular PRRs include LPS binding protein, mannan-binding lectins such as ficolin and collectins, and also C-reactive protein and serum amyloid protein. In swine, single nucleotide polymorphisms (SNP) in these mannan-binding lectin C are associated with increased respiratory disease.10
Cell surface PRR on respiratory epithelia include Toll-like receptors (TLRs), which are transmembrane receptors that have outer leucine-rich repeats (LRR) and cytoplasmic Tll/interleukin-1 (IL-1) receptor homology domains that transmit signals to the nucleus once the outer LRR are activated by PAMPs. The TLR activate nuclear factor (NF)-kappa B or the interferon regulatory factors (IRF)-3 and -7. Cattle have 10 TLRs that have specific and sometimes overlapping PAMP affinities. In short, TLR 1 has affinity for triacyl lipopeptide of mycobacteria; TLR 2 has affinity for peptidoglycans of gram-positive organisms and lipoarbinomannan of mycobacteria and zymosan of fungi; TLR 3 has affinity for dsRNA; TLR 4 has affinity for LPS; TLR 5 has affinity for flagellin; TLR 6 has affinity for diacyl lipopeptides of mycoplasma; TLR 7 and 8 have affinity for single-stranded RNA (ssRNA); TLR 9 has affinity for CpG; and TLR 10 is not yet fully assessed. As indicated, several TLRs1, 2, 5, 6, 7, 8, 9 signal through MyD88, which leads to NF-kappa B activity and inflammatory reactions, whereas other TLRs3 are MyD88 independent (signaling through TRIF/TRAF) and induce IRF-3 and -7 and type I interferon genes. TLR 4 can signal through MyD88 or TRIF/TRAF, depending on the stimulus. LPS, a bacterial component of Histophilus somni, Mannheimia haemolytica, and Pasteurella multocida, first binds LPS binding protein, soluble CD14, and the TLR 4 cofactor MD2 before fully activated TLR 4. TLR 4 is also bound by the F protein of RSV.
Bovine respiratory viruses, BHV-1, PI-3, RSV, and BVD, can infect lung epithelial cells. Viruses can induce formation of noncapped, 5' triphosphated RNA, long dsRNA, ssRNA, and viral DNA, along with their capsids, matrix proteins, and nonstructural proteins. These signal through TLR 3, 7, 8, and 9 as indicated previously but recent work has also characterized cytosolic viral pathogen recognition receptors, retinoic acid–induced gene-I (RIG-I), and melanoma differentiation associated gene-5 (MDA-5). These proteins have an RNA helicase and caspase recruitment (CARD) structural domains and recognize noncapped 5' triphosphated RNAs (RIG-I) and dsRNA (MDA-5). RIG-I and MDA-5 activate NF-kappa B and IRF-3 and -7 via mitochondrial antiviral signaling adaptor (MAVS), interferon-beta promoter stimulator (IPS-1), virus-inducing signaling adaptor, and Cardif.
Other cytosol receptors include the nucleotide-binding domain, leucine-rich repeat (NOD)-like receptors that can detect viral, bacterial, and other pathogens that enter the cytoplasm. Over 20 NOD-like receptors have been identified. NOD 1 and 2 bind peptidoglycan, Iinterleukin 1β-converting enzyme and protease activating factor (IPAF) recognizes flagellin, and NACHT, LRR, and PYD contain protein3 (NALP3) recognizes a very broad range of exogenous and endogenous molecules including viruses, low intracellular potassium, toxins, ultraviolet light, asbestos, silica aluminum, and urate crystals. NOD-like receptors have leucine-rich repeat domains that recognize pathogens. The NOD-like receptors stimulate cell activation. In macrophages, NALP3, IPAF, and NALP1 are components of the inflammasome. The inflammasomes induce activity by triggering caspase 1 activation that results in cleavage of pro-interleukin 1 beta (IL-1 beta) to active IL-1 beta and active IL-18. IL-1 beta and IL-18 bind their respective receptors on leukocytes and other cells, which triggers proinflammatory responses.
Although NF-kappa β and IRF-3 or -7 can be activated by TLR, RIG-I, MDA-5, and NOD-like proteins, endogenous molecules such as heat shock proteins 60 and 70, urates, adenosine, and other molecules can also activate epithelial cells, macrophages, and other inflammatory cell types in the lung. These are termed danger-associated molecular patterns (DAMPs) or alarmins. Although DAMPs activate these inflammation transcription factors, their activation signal is modulated by simultaneous binding to CD24, which interacts with Siglec, which reduces the intensity of NF-kappa B activation.
Oxidative Killing by Respiratory Epithelia
More recently, an oxidative defense system (ODS) has been identified in the human and ovine respiratory tracts. Because it is active in sheep, it is likely also present in cattle. The ODS requires activity of epithelial enzymes dual oxidases1 and 2 (Duox1 and Duox2), which are members of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family and generate hydrogen peroxide production onto the ASL. In the presence of thiocyante (SCN-), the hydrogen peroxide that is weakly microbicidal is then converted to a more toxic compound, hypothiocyanite (OSCN-), which is short-lived but highly microbicidal.
Cytokines, Chemokines, Interferons
Acute inflammatory cytokines such as IL-1, tumor necrosis factor (TNF), and IL-6 invoke activation of nearby cells including endothelial cells, epithelial cells, alveolar macrophages, and lung dendritic cells. With activation there is release of chemokines that attract migration of neutrophils and monocytes into the affected area and with time also attract DCs and NK, T, and B cells. Inflammatory chemokines include IL-8 (CXCL8), GCP-2 (CXCL6), ENA-78 (CXCL5), Gro (CXCL1-3), IP-10 (CXCL10), I-Tac (CXCL11), RANTES (CCL5), MIP-alpha (CCL3) and -beta (CCL4), MCP 1-5 (CCL7, 8, 12, 13), and eotaxins 1-3 (CCL24, 26) depending on the stimulus with receptors that include CXCR1, 2, and 3 and CCR1, 2, 3, and 5. Lymphocytes that home to the lung use a different set of chemokines (eg, naïve cells express CCR1-10, CSCR1-3 and memory cells express CCR8-10, CSCR1, 2, 4, and 5). Chemerin is a chemotactic factor for DCs and macrophages through binding of chemokine-like receptor 1 (CMKLR1).
Some viral infections activate viral receptors that invoke IRF-3 and -7, which stimulate production of type I interferons (IFN alpha and beta) that are released by numerous cells types. These interferons bind Jak/Stat receptors of adjacent cells to induce expression of antiviral substances that include the ISGlyation pathway, which complexes with IRF-3 and prevents degradation; MxA protein, which binds and traps viruses; 2′,5′-oligoadenylate synthetase 1 (OAS1) activates RNaseL, which cleaves viral RNA; and protein kinase R (PKR), which dimerizes in the presence of viral RNA and inhibits eukaryotic translation initiation factor 2 alpha (EIF2 alpha) to reduce viral replication.
Effector Cells
In addition to respiratory epithelia, major innate immune effector cells include the vascular endothelium, neutrophils, alveolar and intravascular macrophages, dendritic cells, NK cells, NK T cells, eosinophils, and mast cells. With acute inflammatory responses, endothelial cells become activated by inflammatory mediators, open gaps, and allow passage of serum factors into the lung, especially alveolar lumens. This fluid contains dilute microbial agents and toxins physically, and contains numerous molecules with protective immune function such as complement, hydrolytic enzymes, IgG, IgM, IgA, IgE, collectins, acute phase proteins, and others. Endothelial cells also release inflammatory mediators and express adhesion molecules that mediate passage of leukocytes into sites of infection/injury. Neutrophils internalize microbial agents, foreign substances, and other particulates, release their granule contents, and upon death release neutrophil extracellular traps (NETs) composed of DNA, histones, and antimicrobial peptides (alpha defensins) that entrap microbes. NK cells do not require previous antigenic exposure to kill virus-infected cells. They express CD161 and CD56 and are activated by interferon and macrophage cytokines to produce interferon gamma and release perforin to kill virus-infected cells. In many species, activation of macrophages occurs in a classical, proinflammatory cell–mediated Th1-type response induced by interferon gamma and TNF; an alternative Th2-type response induced by IL-4, and an anti-inflammatory, regulatory response induced by IL-10. In cattle, however, the Th1/Th2 paradiagm is not as clearly distinct, functionally, as in other species. Despite this, alveolar and intravascular macrophages of the bovine do function in generation of cell-mediated, humoral, and regulatory (inhibitory) responses. In allergic (Th2-type) conditions, eosinophilic and mast cell infiltration occurs with release of granule content. Once established, inhaled antigen can cross-link IgE receptors on mast cells and basophils resulting in cellular activation.
Factors That Can Alter Respiratory Immunity
The bovine lung has anatomic features that predispose cattle to respiratory infections. This is reviewed elsewhere.4 Briefly, cattle have a relatively increased area of dead space, as indicated, and they also have a right tracheal bronchus. The bovine lung has interlobular septae with limited interdependence, increased resistance, and decreased compliance, and collateral ventilation is reduced because of a lack of (1) bronchoalveolar communication (channels of Lambert), (2) alveolar pores (pores of Kohn), and (3) interbronchiolar connections (channels of Martin). Because of the lack of collateral ventilation, atelectasis occurs readily and these areas of lung remain consolidated and lack functional gaseous exchange. Regions of atelectasis are also under hypoxic vasoconstriction whereby arterioles shunt blood flow away from these areas to better perfused regions with adequate gaseous exchange. Thus, these anatomic and physiologic conditions increase the demands for protective immune responses.
There are several environmental factors and managerial issues that also can lower respiratory tract immunity. These include transportation, weaning, overcrowding, changes in social structure, precipitation, fluctuations in temperature, humidity, air exchange, lighting, sounds, changes in feedstuffs, feedlot floor conditions, and other microbial agents. These can affect even basic aspects of the immune system. For example, although corticosteroids (dexamethasone), catecholamines, acetylcholine, and substance P do not affect basal level of TAP and LAP expression by respiratory epithelial cells, dexamethasone exposure reduces TAP and LAP expression on exposure to LPS.11 In addition, infection with type 2 bovine viral diarrhea virus (BVDV) also reduces LPS-induced expression of TAP (and lactoferrin) but a type 1 noncytopathic BVD strain had no effect.12
Other conditions that impair immune responses may include single nucleotide polymorphisms within genes encoding innate and adaptive immune responses of the lung as studied in swine; however, no large-scale studies have been completed in cattle, to date.
Diagnostic Biomarkers
Some innate immune molecules have use or potential use in assessing respiratory tract function/activity or stage/severity of clinical disease. Surfactant protein A (SP-A) production by lung epithelia increases markedly in fetal lung near term and thus roughly delineates the level of lung maturation at birth. SP-A production can also be increased with viral pneumonia and enters the pulmonary lymphatic vessels where it is carried by the thoracic duct to the systemic blood circulation. Thus, serum SP-A measurement has potential to be a marker of respiratory tract maturation and also pneumonia severity.
Recent work has shown that acute-phase proteins are clinical markers of pneumonia severity. Serum-associated amyloid (SAA), haptoglobin, alpha 1-acid glycoprotein produced by liver in response to IL-1, and TNF alpha are produced during pneumonia. SAA and haptoglobin can differentiate acute and chronic pneumonia and alterations in haptoglobin and apolipoprotein A1 are associated with viral infections.13 Metabolic and elemental compounds are biomarkers of viral infection (glucose, low density lipoprotein [LDL], valine, phosphorus, and iron) and disease outcome (lactate, glucose, iron).13
Recent developments in adaptive immune responses
Several excellent reviews of respiratory adaptive immune responses to bovine pathogens have detailed advanced concepts in cellular and molecular immunology in this text series14 and other journals.15, 16 These pathogens can evade respiratory immune responses by modulating (1) the host immune response, and/or (2) the microbial phenotype or location.15 In addition, it is commonplace for investigators to study neutrophils, alveolar macrophages, and other airway cells retrieved by bronchoalveolar lavage. In contrast, few investigative studies have examined the precise role of adaptive immune cells (dendritic, gamma/delta T, alpha/beta T, B, NK, and NK-T cells) in the respiratory mucosa, bronchus-associated lymphoid tissue (BALT), and tracheobronchial lymph nodes in response to bovine pulmonary pathogens. Such studies could be completed with laser capture microdissection coupled with flow cytometric analysis and microarray. Moreover, the dynamic interaction among these cells and their migration to and from the tracheobronchial lymph node have not been fully assessed. Clearly, there is antigen exposure in the lung airways that triggers differentiation, maturation, and responses by these cells that have a kinetic progression with time. The progression and cellular/molecular responses are likely very different in neonatal lung upon initial encounter to a newly inhaled antigen and repeated exposure of an antigen in an older animal. This dynamic is further complicated by vaccination.
Passive Transfer (Colostrum)
Adaptive responses of the bovine neonate including failure of passive transfer (FPT) have been reviewed.17, 18 With FPT there are inadequate levels of maternal IgG, particularly IgG1, which comprises 80% of IgG, and other immunoglobulins that increase risk of respiratory infection. More recently, additional components of colostrum appear to influence the development and activity of the neonatal calf immune system. Maternal colostral leukocytes reduce CD11a+ lymphocytes in neonatal calf blood and these calves have higher CD25-, CD26-, and MHC I–expressing lymphocytes. Thus, maternal colostral leukocytes accelerate fetal lymphocyte development.19
Proinflammatory cytokines are also present in colostrum. IL-beta, IL-6, TNF alpha, and IFN gamma are significantly increased in colostrum but reduced to zero or nearly zero by day 5 of lactation. The high colostral levels of these cytokines correspond to increased levels in the serum of neonatal calves.20 Serum cytokine levels of IL-1 beta, IL-1 alpha, TNF alpha, IL-6, and IFN gamma peak at 12–24 hours after birth and progressively decrease to zero or near zero by day 21. One study has shown high levels of IL-18 in colostrum and increased serum levels of IL-18 in neonates by 6 hours post colostral ingestion.21 The increased colostral cytokines appear to trigger neutrophil function, as colostral whey enhances neutrophil functional assays (cytochrome C reduction and iodination).22 This finding suggests that the colostral cytokines are important for neutrophil “readiness” in newborn animals. This likely has a systemic protective effect because neutrophil function is vital for the protection of respiratory airways, intestine, and other mucosal surfaces against microbial pathogens.
Gamma/Delta T Cells
The generation of humoral and cellular responses of the adaptive immune system by the bovine are described by Srikumara and colleagues15 and Kindt and collegues.23 These occur simultaneously with innate immune responses. The adaptive responses are contingent upon antigen recognition, antigen processing and presentation by dendritic cells, and involvement of gamma/delta T cells and alpha/beta (CD 4 and CD8) cells. Of these, gamma/delta T cells of cattle are of special interest for at least two reasons. First, newborn calves have an unusually high number of circulating gamma/delta T cells and second, gamma/delta T cells of ruminants express WC-1 antigen. Gamma/delta T cells account for 60% of the peripheral blood mononuclear cells in young calves. WC1+ gamma/delta T cells are considered an inflammatory population, whereas CD1- gamma/delta T cells are regulatory with myeloid cell features. Within the WC1+ phenotype are WC1.1+ and WC1.2+ subsets that differ in their IFN gamma production and proliferative responses to stimuli.24, 25 The role of WC protein is not fully understood but it may be a homolog of CD4 and CD8 antigens on alpha/beta T cells by regulating gamma/delta T-cell responses or transmission of signals from the outside into the cell.26 It is possible that WC protein binds microbial PAMPs similar to other proteins with similarities to WC.
Clinical implications
A 2009 study of bovine respiratory disease (BRD) in an Oklahoma feedlot underscores the complexity of this disease and its clinical impact.27 BRD morbidity was 14.7% in that herd with 0.7% mortality. The mean fatal disease onset was 32.6 days and mean treatment interval was 29 days, mean number of antibiotic treatments were 2.6 days, and mean day of death was 61.8 days. All of the agents listed at the beginning of this article were isolated from the cattle. These agents were correlated with mortality, treatment, lesion, and association with other agents. Also correlated were the type of lesions with mortality, treatment, and infectious agent. Interestingly, along with BVDV-1a and 2b, BVDV-1b was identified in fatal cases and this was considered noteworthy because most current BVDV vaccines contain BVDV-1a and 2b but not BVDV1b. The work also identified BVDV-2b for the first time. This study confirms the continued presence of and damage caused by these microbial agents in 2009. Thus, despite advancements in managerial practices, vaccine development, antimicrobial agents, medicine, and molecular diagnostics, each of these agents remains a significant concern.
The innate immune response can be viewed as the basal, most basic immune response to pathogen but it is a response that is difficult to enhance therapeutically. As with adaptive immune responses, there is redundancy and overlap in the function of innate immune products that protect the respiratory tract. However, loss of some innate factors is clearly associated with increased incidence and/or severity of respiratory disease. Factors that influence this include dehydration, genetic changes resulting in altered expression of innate immune factors, stress, and primary infection.
Dehydration
It is well known that dehydration causes increased viscosity of respiratory secretions. The sol layer of the respiratory air-surface liquid is reduced resulting in increased mucinous material that can allow increased colonization of bacterial pathogens and accumulation of inhaled particulate matter because of reduced mucociliary function.
Genetic Changes Resulting in Altered Expression of Innate Immune Factors
Single nucleotide polymorphisms (SNPs) are single nucleotide changes in a particular animal that can result in production of a different amino acid in a protein. Although this is a very slight change, a single amino acid change may greatly change the function of an innate immune factor. As indicated, studies in pigs have demonstrated that SNPs in mannan-binding lectin C are associated with increased susceptibility to respiratory disease. Humans with SNPs in surfactant protein A and D have increased incidence and severity of respiratory syncytial virus pneumonia. Identifying an association between SNPs and increased respiratory disease often requires large-scale genetic studies matched to clinical disease scores and thus are often expensive. However, SNPs within a cattle herd that has minimal genetic variation could have profound effects.
Stress
Causes of stress are varied but include overcrowding, lack of shade in the hot summer, rapid and extreme fluctuations in temperature, wind, shipping, persistent startling (eg, dogs, coyotes, traffic noise), and other situations that change the physical and/or mental homeostasis of cattle. Stress affects cortisol levels, among other endogenous stress responses, and corticosteroids (dexamethasone) can reduce innate immune expression of defensin genes in response to LPS, as indicated. Thus, there appears to be a direct effect of stress on certain innate immune factors.
Primary Infection
As indicated, all animals have commensal microflora in the upper respiratory tract and nasopharynx. Changes in these secondary to stress, dehydration, or SNPs can allow increased proliferation of pathogens. For example, stressed cattle can have increased proliferation of Mannheimia haemolytica in the nasopharynx and tonsil. In addition, with reduced innate immune responses, organisms such as Mycoplasma bovis and viruses (PI-3, RSV, BVDv) have enhanced opportunities to colonize, proliferate, and damage the respiratory epithelia setting the stage for secondary bacterial pathogens with increased virulence.
Reducing the effects and duration of these factors are a first step in enhancing innate immune activity. With time, therapies aimed toward enhancing activity of some innate immune responses may become a viable option and adjunct to management, vaccination, and antimicrobial agents.
Summary
The bovine respiratory tract immune response is sophisticated and shaped by anatomic and cellular features unique to cattle/ruminants, management practices, and interactions with specific microbial pathogens. Because extensive inflammatory reactions impair gaseous exchange and lung function, innate immune responses are vital for sensing and handling pulmonary exposures to particulates, vapors, microbial agents, and other inhaled substances without invoking a marked inflammatory reaction. Cattle have anatomic features such as reduced collateral ventilation that reduce the effectiveness of the innate immune response and current managerial practices sometimes weaken the innate immune response. With persistence or repeated exposure to antigens or insults, adaptive immune responses are generated. Neonatal calves require colostrum for fully functional adaptive responses and have high levels gamma/delta cells that contribute significantly to adaptive responses in concert with dendritic cells, NK cells, macrophages, alpha/beta T cells, and B cells.
Footnotes
Funding acknowledgment: MRA: NIH NIAID RO1 AI062787.
References
- 1.Collier J.R., Rossow C.F. Microflora of apparently healthy lung tissue of cattle. Am J Vet Res. 1964;25:391–393. [PubMed] [Google Scholar]
- 2.Allen J.W., Viel L., Bateman K.G. The microbial flora of the respiratory tract in feedlot calves: associations between nasopharyngeal and bronchoalveolar lavage cultures. Can J Vet Res. 1991;55:341. [PMC free article] [PubMed] [Google Scholar]
- 3.Angen O., Thomse J., Larsen L.E. Respiratory disease in calves: microbiological investigations on trans-tracheally aspirated bronchoalveolar lavage fluid and acute phase protein response. Vet Microbiol. 2009;137(1–2):165–171. doi: 10.1016/j.vetmic.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirschvink N. Respiratory function in cattle: impact of breed, heritability, and external factors. Dtsch Tierartzl Wochenschr. 2008;115:265–270. [PubMed] [Google Scholar]
- 5.Linden S.K., Sutton P., Karlsson N.G. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1(3):183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grubor B., Meyerholz D.K., Ackermann M.R. Collectins and cationic antimicrobial peptides of the respiratory epithelia. Vet Pathol. 2006;43:595–612. doi: 10.1354/vp.43-5-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartlett J.A., Fischer A.J., McCray P.B., Jr. Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- 8.Diamond G., Zasloff M., Eck H. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of cDNA. Proc Natl Acad Sci U S A. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudreux C.M., Corstvet R.E., Cooper R.K. Effects of cercropin B transgene expression on Mannheimia haemolytica serotype 1 colonization of the nasal mucosa of calves. Am J Vet Res. 2005;66(100):1922–1930. doi: 10.2460/ajvr.2005.66.1922. [DOI] [PubMed] [Google Scholar]
- 10.Lillie B.N., Keirstead N.D., Squires E.J. Gene polymorphisms associated with reduced hepatic expression of porcine mannan-binding lectin C. Dev Comp Immunol. 2007;31:830–846. doi: 10.1016/j.dci.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell G.B., Al-Haddawi M.H., Clark M.E. Effect of corticosteroids and neuropeptides on expression of defensins by tracheal epithelial cells. Infect Immun. 2007;75(3):1325–1334. doi: 10.1128/IAI.00686-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Haddawi M., Mitchell G.B., Clark M.E. Impairment of innate immune response of airway epithelium by infection with bovine viral diarrhea virus. Vet Immunol Immunopathol. 2007;116:153–162. doi: 10.1016/j.vetimm.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Aich P., Babiuk L.A., Potter A.A. Biomarkers for prediction of bovine respiratory disease outcome. OMICS. 2009;113:199–210. doi: 10.1089/omi.2009.0012. [DOI] [PubMed] [Google Scholar]
- 14.Ellis J.A. The immunology of the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract. 2001;17(3):535–550. doi: 10.1016/S0749-0720(15)30005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srikumaran S., Kelling C.L., Ambagala A. Immune evasion by pathogens of bovine respiratory disease complex. Anim Health Res Rev. 2008;8(2):215–229. doi: 10.1017/S1466252307001326. [DOI] [PubMed] [Google Scholar]
- 16.Makoschey B., Lekeux P., Lacroux C. Concepts in the prevention of bovine respiratory disease. Berl Munch Tierarztl Wochenschr. 2008;121(11/12):446–449. [PubMed] [Google Scholar]
- 17.Barrington G.M., Parish S.M. Bovine neonatal immunology. Vet Clin North Am Food Anim Pract. 2001;17(3):463–476. doi: 10.1016/S0749-0720(15)30001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortese V.S. Neonatal immunology. Vet Clin North Am Food Anim Pract. 2009;25:221–227. doi: 10.1016/j.cvfa.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reber A.J., Donovan D.C., Gabbard J. Transfer of maternal colostral leukocytes promotes development of the neonatal immune system. Part II. Effects on neonatal lymphocytes. Vet Immunol Immunopathol. 2008;123:305–313. doi: 10.1016/j.vetimm.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka J., Hagiwara K., Kirisawa R. Transient detection of proinflammatory cytokines in sera of colostrum-fed newborn calves. J Vet Med Sci. 2003;65(7):813–816. doi: 10.1292/jvms.65.813. [DOI] [PubMed] [Google Scholar]
- 21.Muneta J., Yoshihara K., Minagawa Y. Bovine IL-18 ELISA: detection of IL-18 in sera of pregnant cow and newborn calf, and in colostrums. J Immunoassay Immunochem. 2005;26:203–213. doi: 10.1081/IAS-200062487. [DOI] [PubMed] [Google Scholar]
- 22.Roth J.A., Frank D.E., Weighner P. Enhancement of neutrophil function by ultrafiltered bovine whey. J Dairy Sci. 2001;84:824–829. doi: 10.3168/jds.S0022-0302(01)74540-7. [DOI] [PubMed] [Google Scholar]
- 23.Kindt T.J., Goldsby R.A., Osborne B.A. 5th edition. W.H. Freeman and Company; New York: 2007. Kuby immunology. [Google Scholar]
- 24.Rogers A.N., Vanburen D.C., Hedblom E. Function of ruminant gammadelta T cells is defined by WC1.1 or WC1.2 isofomr expression. Vet Immunol Immunopathol. 2005;108:211–217. doi: 10.1016/j.vetimm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Rogers A.N., Vanburen D.B., Hedblom E.E. Gammadelta T cell function varies with the expressed WE1 coreceptor. J Immunol. 2005;174:3386–3393. doi: 10.4049/jimmunol.174.6.3386. [DOI] [PubMed] [Google Scholar]
- 26.Chen C., Herzig C.T.A., Telfer J.C. Antigenic basis of diversity in the gamma/delta cell co-receptor WC-1 family. Mol Immunol. 2009;46:2565–2575. doi: 10.1016/j.molimm.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Fulton R.W., Blood K.S., Panciera R.J. Lung pathology and infectious agents in the fatal feedlot pneumonias and relationships with mortality, disease onset, and treatments. J Vet Diagn Invest. 2009;21:464–477. doi: 10.1177/104063870902100407. [DOI] [PubMed] [Google Scholar]


