Abstract
Little is known of transcriptional mechanisms underlying development of the trigeminal (V) principal sensory nucleus (PrV), the brainstem nucleus responsible for development of the whisker to barrel cortex pathway. Lmx1b, a LIM homeodomain transcription factor, is expressed in embryonic PrV. In Lmx1b knockout (−/−) mice, V primary afferent projections to PrV are normal, albeit reduced in number, whereas the PrV-thalamic lemniscal pathway is sparse and develops late. Excess cell death occurs in the embryonic Lmx1b−/− PrV, but not in Lmx1b/Bax double null mutants. Expression of Drg11, a downstream transcription factor essential for PrV development and pattern formation, is abolished in PrV, but not in the V ganglion. Consequently, whisker patterns fail to develop in PrV by birth. Rescued PrV cells in Lmx1b/Bax double −/−s failed to rescue whisker-related PrV pattern formation. Thus, Lmx1b and Drg11 may act in the same genetic signaling pathway that is essential for PrV pattern formation.
Keywords: Lmx1b, Drg11, transcription factor, pattern formation, barrels, whiskers
Introduction
The pattern of the whiskers on the face in rodents is faithfully recapitulated by neuronal aggregates in the trigeminal (V) brainstem complex, ventrobasal thalamus and cerebral cortex (reviews in Jones and Diamond, 1995). These somatotopic aggregations of cells and fibers are called barrelettes, barreloids and barrels, respectively (Woolsey and Van der Loos, 1970; Ma 1991; see Erzurumlu et al., 2006, for a recent review). In the brainstem, barrelettes occur in the V brainstem subnuclei principalis (PrV), interpolaris (SpVi) and caudalis (SpVc). The PrV is noteworthy because, during development, it is responsible for conveying the whisker-related pattern to the thalamus which, in turn, conveys and establishes the barrel pattern in layer IV of the S1 somatosensory cortex (Killackey and Fleming, 1985). The similarly patterned spinal V subnuclei subserve a “paralemniscal” function (Williams et al., 1994) and, together with the PrV, provide parallel pathways for processing and relaying whisker-related information to higher-order structures in the brain.
Within the past decade, a number of patterning mechanisms involving specific molecules and genes have been revealed in the developing whisker-barrel pathway (see Jacquin et al., 2008, for a review). However, most of these molecules and their genetic bases have been studied within the context of the developing cerebral cortex. Further study of the mechanisms controlling subcortical barrelette formation is desirable insofar as the PrV is the indispensable bridge between the periphery and the thalamocortical pathway and whisker-related patterns appear first in the PrV in development. Thus, a clear understanding of barrel formation requires an elucidation of PrV mechanisms. Recent studies of transgenic mice have begun to uncover several key molecules that are required for PrV pattern formation. Most are related to the requirement that glutamatergic neurotransmission be intact, possibly to convey requisite electrical activity-dependent competitive interactions known to serve as a patterning mechanism in other systems (Purves and Lichtman, 1985; Erzurumlu and Kind, 2001; but see Henderson et al., 1994). For example, the NMDA NR1 receptor (Li et al., 1994; Iwasato et al., 1997) has been shown to be necessary for development of barrel-like patterns in the PrV. The axon guidance molecule, Hoxa2, has also been implicated in PrV development (Oury et al., 2006).
Transcriptional mechanisms that regulate whisker-related pattern formation are also now being revealed. Drg11, a paired homeodomain-containing protein, is the first transcription factor that has been shown to be necessary for the development of the PrV-based lemniscal pathway (Ding et al., 2003). In Drg11−/− mice that survive into adulthood, whisker-related patterns fail to develop in the PrV, thalamus and cortex, but they do develop in the SpVi and SpVc. Such specificity is unprecedented in the study of the developing V system in the sense that it reveals differing genetic substrates for patterning different portions of the V brainstem complex. Other transcription factors, including Tlx3 and Ebf1, 2 and 3 have also been shown to be expressed in the developing PrV (Qian et al., 2002; Ding et al., 2003), but their function has yet to be ascertained.
Lmx1b is a LIM homeodomain transcription factor that has been implicated in the development of several neuronal systems, such as the serotonin-containing raphe nuclei (e.g. Zhao et al., 2006). Here, we show that in mouse embryonic development, Lmx1b is also abundantly expressed in the PrV. But, unlike the case for Drg11, Lmx1b is not expressed in the V ganglion. Therefore, genetic manipulations targeting Lmx1b have implications for PrV development exclusive of any confounding effects upon its presynaptic V primary afferent inputs. Thus, we report on the development of the PrV-based lemniscal pathway in Lmx1b−/− embryos. Similarities between the latter and Drg11−/− mice, along with known actions of Lmx1b upon Drg11 expression, suggest that these two genes function within the same genetic signaling cascade to form barrelettes within the developing PrV.
Results
Lmx1b is expressed in postmitotic PrV neurons, but not in the V ganglion
The spatiotemporal expression patterns of Lmx1b were studied at varied ages in the embryonic PrV by the use of in situ hybridization. At E12.5, many neurons that have completed their ventrolateral migration from the ventricular zone and are beginning to form the nascent PrV display intense Lmx1b expression (Fig. 1A). This continues through E15.5 when Lmx1b staining becomes more widespread throughout the PrV and appears to also extend dorsomedially into the supratrigeminal region abutting the PrV (Fig. 1B). In the SpVi, Lmx1b is only weakly expressed and this occurs in SpVi’s dorsomedial extent (Fig. 1C). Lmx1b staining was never observed in the V ganglion or motor nucleus (Figs. 1A–C). To assess the mitotic status of Lmx1b expressing cells, BrdU pulse-labeling was followed by Lmx1b immunohistochemistry. BrdU/Lmx1b double labeled cells were never observed in the PrV at E11.5 (Fig. 1D), indicating that Lmx1b is only expressed in postmitotic PrV neurons.
Figure 1.
Expression of Lmx1b in wild-type (WT) developing trigeminal nuclei detected by in situ hybridization (A–C) and immunocytochemical staining (D). A, At E12.5, Lmx1b is detected in the presumptive PrV. B, At E15.5, Lmx1b expression has expanded concurrent with the increase in the size of PrV. It is also present in the supratrigeminal nucleus (SuV), but not in the trigeminal motor nucleus (Mo). C, Lmx1b is also expressed in SpVi, but much weaker compared to PrV. Lmx1b is not expressed in TG (small frame). D, Lmx1b (red, arrowheads) is not colocalized with BrdU staining (arrows, green). Scale bar: 100 μm (A–C); 50 μm (D).
PrV cell survival in Lmx1b mutant mice
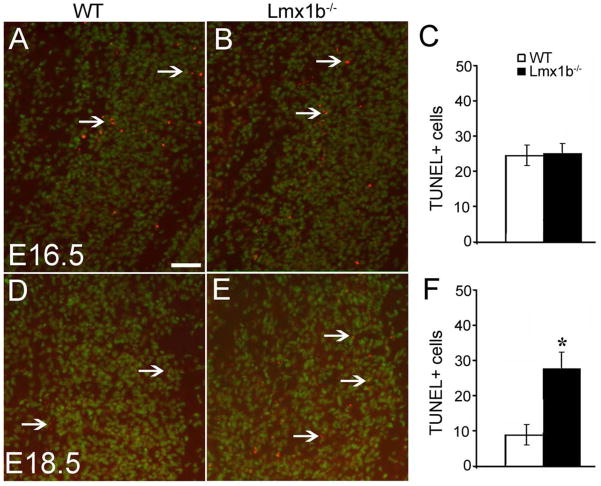
The preceding section revealed Lmx1b expression coinciding with PrV morphogenesis. To assess whether Lmx1b impacts PrV cell morphogenesis, PrV integrity was evaluated in Lmx1b−/− embryos. Until E17.5 there were no obvious cytoarchitectural differences between the mutant and wildtype PrV (Figs. 2A,B). However, at E18.5, the Lmx1b−/− PrV as a whole was narrower and less densely populated than the PrV of littermate controls (Figs. 2C,D). This could reflect a reduced genesis of postmitotic neurons. However, BrdU pulse-labeling produced equal numbers and distributions of labeled PrV cells in mutant and wild-type embryos (Figs. 2E–G). The reduced size of, and decreased cell density within, the Lmx1b−/− PrV might then reflect increased cell death there. At E16.5, the spatial distributions and numbers of TUNEL+ PrV cells was indistinguishable between mutant experimental and control cases (Figs. 3A–C). However, by E18.5, significantly increased PrV cell death was detected in the mutant group (Figs. 3D–F). This was reflected in a dramatically reduced total number of PrV cells on the day of birth. The mutant PrV contained 21,734 +/− 2,303 cells (mean +/− standard deviation; N = 6), which is significantly lower (one-tailed t-test, p < .05) than the 48,439 +/− 6,515 PrV cells estimated in wildtype littermate controls (N = 8). Consequently, the total number of V ganglion cells (most of which project to PrV) was significantly reduced (p < .05) in these same Lmx1b −/−s at birth (23,350 +/− 2,563) vs. wildtype controls (40,829 +/− 3,755).
Figure 2.
Nissl staining and BrdU immunostaining of developing PrV in wild-type and Lmx1b−/− mice. A,B. At E17.5, little difference is found in the morphology of the PrV of wild-type (A) and Lmx1b−/− (B) mice; From E18.5, Lmx1b−/− PrV (D) appears to have fewer cells than wild-type mice (C); E,F, BrdU injected at E11 and stained at E14.5. G, There is no difference in the number of BrdU+ PrV cells in wild-type (WT) (209.5± 10.7) and Lmx1b−/− (203.2±7.9) mice at E14.5 (P>0.05). Scale bar: 100 μm.
Figure 3.
Examination of cell death in the PrV by TUNEL. A,B, At E16.5 no major difference is found in the number of TUNEL+ cells (red, arrows) in Lmx1b−/− (B) and wild-type (WT) mice (A). D,E, At E18.5 the number of TUNEL+ cells (red, arrows) is significantly larger in Lmx1b−/− mice (E) relative to wild-type mice (D). Green (A, B, D, E) indicates Hoechst counterstaining. C,F, Quantitative analysis reveals no significant difference in the number of TUNEL+ cells of E16.5 Lmx1b−/− (25.3±3.5) and wild-type PrVs (24.3±4.3) (C, P>0.05), whereas cell death is significantly increased in the E18.5 Lmx1b−/− PrV (28.3±5.2 labeled cells per randomly selected transverse section) relative to the wild-type PrV (8.7±4.1). (F, P<0.01). Scale bar: 100 μm.
PrV cell death mechanism in Lmx1b mutant mice
The preceding section revealed extensive PrV cell death that might be attributed to Lmx1b deletion-induced activation of an apoptotic pathway. To test this hypothesis, mice lacking the proapoptotic gene, Bax, were crossed with Lmx1b heterozygotes. On the day of birth (P0), the Lmx1b/Bax−/− PrV had a normal shape, size and cell density, unlike the Lmx1b single null PrV (Figs. 4A–D). Cell counts in random transverse sections taken through the single null PrV on the day of birth (Fig. 4E) revealed significant cell loss that was not observed in the Lmx1b/Bax−/− cases. Such PrV cell counts did not differ between Bax single −/− and wild-type mice in this non-stereological assessment. To provide independent validation of Bax deletion-induced rescue of V neurons based upon stereological estimates, total numbers of V ganglion cells were estimated. In 3 Lmx1b/Bax double null mutants, total ganglion cell numbers (31,451 +/− 1,460) fell at a midpoint between above-listed totals obtained from Lmx1b single nulls and wildtype controls (23,350 and 40,829, respectively).
Figure 4.

Nissl staining of PrV of wild-type (A), Lmx1b−/− (B), Lmx1b−/−/Bax−/− (C) and Bax−/− (D) mice, indicating that there are more PrV cells in wild-type (A), Lmx1b−/−/Bax−/− (C) and Bax−/− (D) mice than in Lmx1b−/− mice. E, Statistical analysis reveals decreased PrV cell numbers in P0 Lmx1b−/− mice (775.13±67.02) compared with wild-type (1738.75±100.87) (P<0.001), Lmx1b−/−/Bax−/− mice (1598.25±89.81) (P<0.001) and Bax−/− mice, (1999.13±74.19, P<0.001). WT: wild-type. Scale bar: 100 μm.
Normal primary afferent projections in Lmx1b mutant mice
Bulk labeling of the peripheral projections of the Lmx1b−/− V ganglion revealed a qualitatively normal innervation pattern of the whiskers (Fig. 5A–D). Di-I labeled axons displayed the characteristic and dense circumferential projections to individual whisker follicles when viewed either in tangential or transverse sections. No differences were observed between mutant and wild-type cases at this rather gross level of analysis.
Figure 5.

Normal peripheral and central primary afferent projections. Coronal (A,B) and tangential (C,D) views of DiI labeled TG innervation of the whiskers of E15.5 wild-type (A,C), and Lmx1b−/− (B,D) mice. The typical circumferential projection is similar for both wild-type and Lmx1b−/− mice. Topographic organization of TG afferents revealed by DiI and DiA double labeling (E–H). DiI and DiA were applied to the dorsal (B row) and ventral (D row) vibrissae follicles of wild-type (E,G) and Lmx1b−/− mice (F,H) at E12.5 (E,F) and E16.5 (G,H). DiI-labeled (red) and DiA-labeled (green) axons are segregated in the trigeminal root entry zone (E,F) and PrV (G,H) in both mutant (F,H) and wild-type mice (E, G), although the labeling in the former is somewhat less abundant. Scale bar: 100 μm.
To assess gross topography in the central projections of V primary afferents at the time when they are growing into the brainstem, DiI was applied to the dorsal B-row whisker follicles and DiA was applied to the more ventral D-row follicles. Normal differential foci of these two labeled fiber populations were observed in the Lmx1b−/− V ganglion and root entry zone (Fig. 5E, F), as well as in the PrV (Fig. 5G, H) where dorsal whisker afferents terminated in the ventral PrV and more ventral whisker afferents terminated in more dorsal PrV.
In more mature embryos, fine-grain topography to the PrV and SpVi was revealed by applying DiI to only the B3 and C3 whisker follicles. Circumscribed terminal foci were revealed in the topographically appropriate portions of the PrV and SpVi in the mutant and wild-type cases (Fig. 6).
Figure 6.
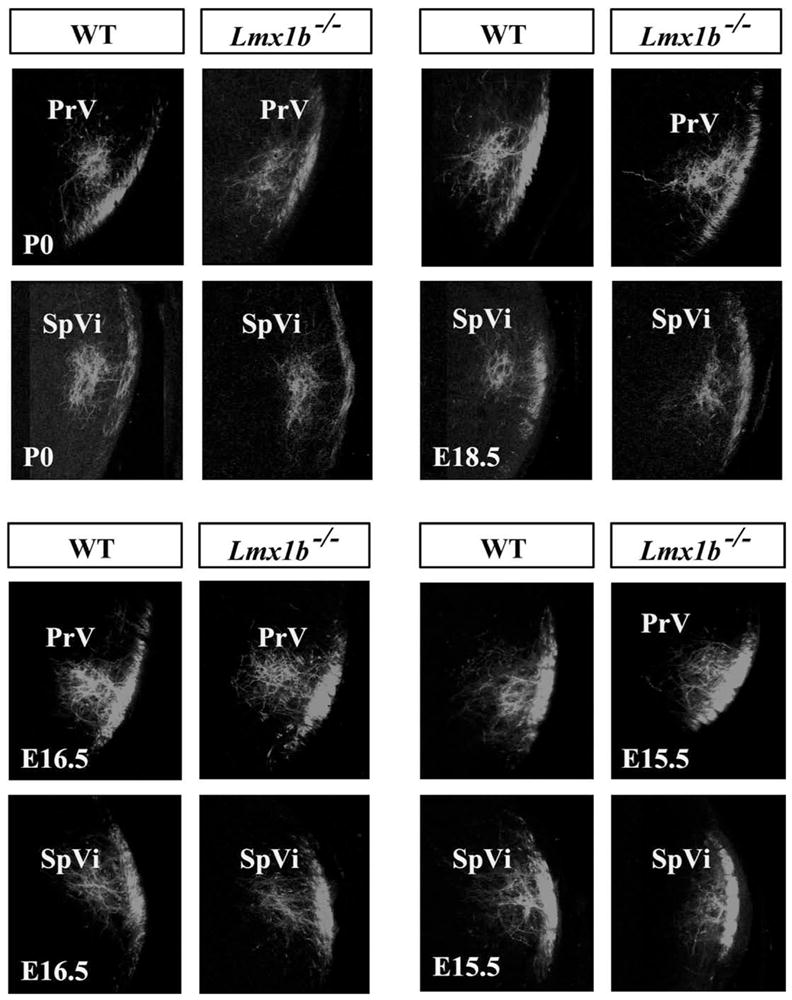
Fine-grain topography of primary afferents in PrV and SpVi of wild-type and Lmx1b−/− mice shown after applying DiI to the B3 and C3 whisker follicles only. Both nuclei are depicted at developmental ages P0, E18.5 (top), E16.5 and E15.5 (bottom).
Finally, DiI was applied to the entire infraorbital nerve on the day of birth to assess the integrity of the entire whisker-related primary afferent projection to the PrV and SpVi. As shown in Figure 7, save for occupying a smaller transverse areal extent in the PrV of the Lmx1b−/−, reflecting V ganglion and PrV cell death (see above), the DiI-labeled infraorbital nerve projection to PrV and SpVi was completely normal. Indeed, rudimentary whisker-related patterning of this projection is somewhat discernable in both V subnuclei in the wildtype and Lmx1b/Bax double null mutants illustrated in Figure 7.
Figure 7.

Labeling of primary afferents in PrV (top) and SpVi (bottom) after application of DiI to the entire infraorbital nerve on the day of birth showing a normal projection in wild-type (left), Lmx1b−/− (center) and Bax−/−/Lmx1b−/− (right).
Trigeminothalamic projections in Lmx1b mutant mice
Di-I labeling of the wild-type PrV projections to the contralateral thalamus revealed a dense input that spanned the entire ventroposteromedial thalamic nucleus on E17.5 (Fig. 8A). In six mutants, similar DiI injections into the PrV failed to label fibers in the contralateral thalamus (Fig. 8B). By E19.5, however, Di-I labeled fibers were observed in the mutant ventroposteromedial thalamus, but they displayed a reduced density and areal expanse relative to wild-type controls (Fig. 8C,D). Thus, the trigeminothalamic projection exhibited a delayed and sparse innervation of the thalamus in the Lmx1b−/− embryos.
Figure 8.
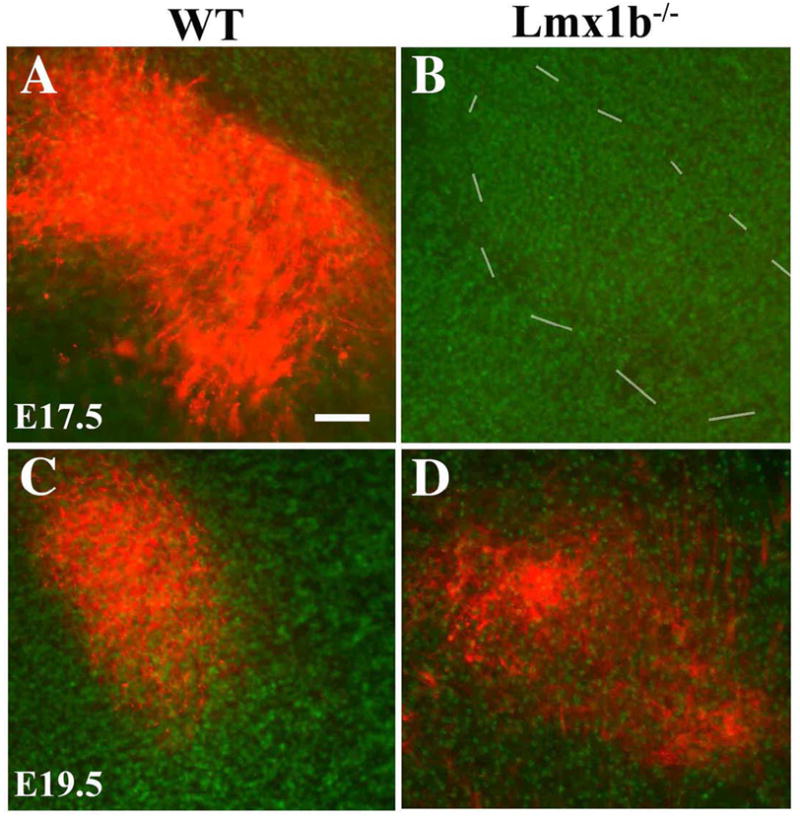
Axonal projections of PrV neurons to VPM (A–D) are stained by DiI labeling. At E17.5, projections of PrV efferents are detected in wild-type VPM (A), but not in Lmx1b−/− VPM (B). At E19.5, DiI-labeled PrV efferents are found in both wild-type (C) and mutant (D) VPMs, but the extent of DiI labeling in the mutant is reduced and more sporadic (D). Scale bar: 100 μm.
Absence of whisker-related patterning in the Lmx1b−/− PrV
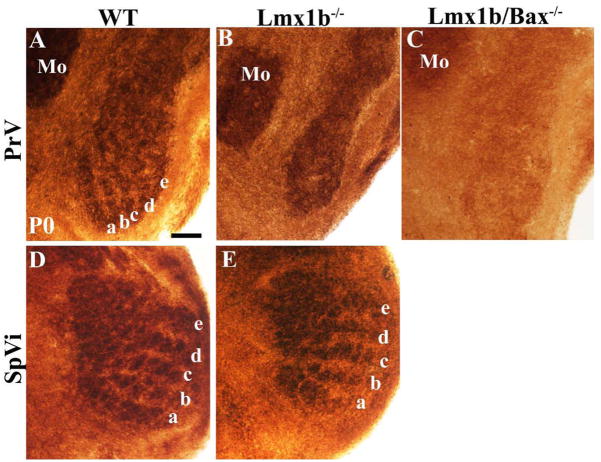
In the newborn PrV and SpVi, wild-type cases exhibited very clear whisker-related patterning, as revealed by cytochrome oxidase staining (Fig. 9A,D), such that 5 rows of whiskers, as well as individual whiskers, were represented. However, such staining patterns were not observed in the Lmx1b single null or Lmx1b/Bax double null cases (Fig. 9B,C), whereas the mutant SpVi was normally patterned (Fig. 9E). Shrinkage of the PrV in the single null mutants is particularly obvious in this material (Fig. 9B), whereas a normally sized PrV was observed in the Lmx1b/Bax double null mutants (Fig. 9C), although cytochrome oxidase staining intensity in their PrV was unusually low for reasons that are currently unclear.
Figure 9.
CO staining of the whisker-like patterns in wild-type and Lmx1b−/− PrV and SpVi at P0. Note that the absence of whisker-like patterns in the mutant PrV (B) and Lmx1b−/−/Bax−/− PrV (C), compared to the patterns in wild-type PrV (A). The whisker-related patterns are present in both wild-type (D) and Lmx1b−/− SpVi (E). Scale bar: 100 μm.
Lmx1b−/− effects upon expression of other transcription factors in the PrV
An array of molecular markers was examined in the mutant PrV on E15.5 as a first step in revealing the mechanism(s) through which Lmx1b impacts pattern formation in the PrV. Drg11 expression was absent in the Lmx1b−/− PrV (Fig. 10A,B); conversely, Drg11 expression in the V ganglion was indistinguishable from wild-type controls (Fig. 10I,J). Tlx3 and Ebf1 gene expression were downregulated in the mutants (Figs. 10C–F), whereas Ebf3 expression (Fig. 10G,H) was unaffected.
Figure 10.

Expression of molecular markers in the PrV detected by in situ hybridization. Coronal sections of E15.5 PrV of wild-type (A,C,E,G,), TG (I), and Lmx1b−/− mice (B, D, F, H,) and TG (J). Compared with wild-type, expression of Drg11, Tlx3 and Ebf1 is lost or markedly downregulated in Lmx1b−/− mice (B,D,F). Expression of Ebf3 is also down-regulated in Lmx1b−/− mice (H) compared with wild-type PrV (G). No significant change is found in the expression pattern of Drg11 in TG between wild-type (I) and Lmx1b−/− mice (J) at E15.5. Scale bar: 100 μm.
Discussion
Conclusions
The present study suggests a number of conclusions that bear upon the function of Lmx1b in the development of the V system. First, Lmx1b is abundantly expressed in the postmitotic cells destined to become the PrV, but it is not expressed in the V ganglion. Second, generation, migration and differentiation of PrV cells is not affected by Lmx1b gene deletion indicating that this gene functions in later embryonic aspects of PrV development. Third, PrV cell morphogenesis is dramatically affected in Lmx1b−/− embryos to the extent that about half of the normal complement of PrV cells are absent on the day of birth. Fourth, apoptosis is a mechanism of PrV cell death insofar as PrV cells are rescued in Lmx1b/Bax double null mutants. Fifth, the peripheral and central projections of V ganglion cells that survive this mutation are normal to the extent that can be ascertained by bulk anterograde labeling techniques. Sixth, other transcription factors are down regulated in the Lmx1b−/− PrV, most notably Drg11, which previously has been shown to be necessary for PrV pattern formation (Jacquin et al., 2008). Seventh, consequently, Lmx1b single null mice and Lmx1b/Bax double null mice lack whisker-related patterns in the PrV neuropil on the day of birth, whereas such patterns develop normally in the SpVi. Eighth, PrV projections to the thalamus develop later than normal and they are abnormally sparse. All of the above provides strong support for our overarching hypothesis that Lmx1b is a transcriptional regulator acting within a molecular signaling cascade inclusive of Drg11 that is a true ‘pattern generator’ in the developing PrV. Somatotopic patterning anomalies in the PrV of Lmx1b mutants are not reflective of an altered V primary afferent projection; rather, this truly does appear to be a CNS-restricted phenotype.
Multiple roles for Lmx1b in PrV development
The persistent expression of Lmx1b in developing PrV cells from the time of their migration to the nascent PrV and through the day of birth, a time span of more than a week, suggests that Lmx1b may have multiple roles in PrV development. First, target-derived signals are known to be important in guiding the growth of projecting axons. Thus, one possible function for Lmx1b is to regulate the expression of downstream axonal guidance molecules in the PrV. In the PrV, only a few axonal guidance molecules have been identified (Ding et al., 2003; Erzurumulu et al., 2006, and Fig. 10 here). Whether such molecules, such as slit and Robo receptors, are regulated by Lmx1b is unknown.
Second, while we don’t have a good handle on exactly when excess PrV cell death begins in earnest, there is no question that Lmx1b has a PrV cell survival function. The fact that about 50% of these cells die in the absence of Lmx1b attests to it being a robust one. The impression gained from extensive Nissl staining is that the major epoch for such cell death is the latter third of the embryonic period. This now expands the list of transcription factors that are known to sustain PrV cells. Qian et al. (2002) reported that PrV neurons depend on two related homeobox genes, Tlx-3 and Tlx-1, for proper development and that Tlx-3 and Tlx-1 maintain expression of Drg11, which we (Jacquin et al., 2008) have shown to have a similarly robust cell survival function to that of Lmx1b. Whether these transcription factors serve redundant functions in the same signaling pathway is currently unclear. What is clear is that a mechanism mediating Lmx1b−/−-induced PrV cell death is apoptosis. This conclusion is based upon the rescue of PrV cells in Lmx1b/Bax double null mutants. Given the current observation that Lmx1b is not expressed in V ganglion cells, it is somewhat puzzling that there occurred a significant loss of V ganglion cells in Lmx1b null mutants. Because the majority of V ganglion cells project to the PrV, indeed virtually all such ganglion cells destined to be myelinated do so (Shortland et al., 1996), perhaps these cells die by a process of retrograde transneuronal degeneration in the light of the extensive PrV cell death. One would think, however, that the collaterals of these same primary afferents in all of the spinal V subnuclei, which are shown here to be intact (Fig. 7), would sustain the ganglion cells. This remains enigmatic.
Third, the present study’s observation that the thalamic projection of the PrV is delayed and diminished in Lmx1b mutants suggests that Lmx1b contributes to a mechanism that times the outgrowth of surviving V-thalamic axons. Because Lmx1b is not expressed in the VPm thalamus, the primary target of PrV cell axons, such a mechanism must exist within the PrV cell themselves or along the lemniscal pathway en route to the thalamus. It is likely that the diminished projection to the VPM thalamus shown here is a direct result of the death of about half of the PrV cells that would normally come to project to thalamus. The extent to which thalamic-projecting PrV cells are disproportionately targeted by the Lmx1b mutation, as opposed to PrV’s local circuit neurons, is currently unclear.
Failed whisker-related PrV pattern formation in Lmx1b−/− mice
A fourth and possibly most compelling feature of the Lmx1b mutant is its failure to develop barrelettes in the whisker-related lemniscal pathway, whereas these same animals display normal barrelettes in the SpVi. The restriction of a patterning anomaly to the PrV suggests that the Lmx1b gene functions specifically in the development of the PrV-based lemniscal pathway. This conclusion is strengthened by the fact that the very same primary afferent axons provide collaterals to both the PrV and the SpVi, yet in the former they are not sufficient to make a barrelette, but in the latter they are sufficient.
In the V system, the precise segregation of primary afferent terminals in the brainstem replicates the patterned organization in the whisker follicles (Jacquin et al., 1993; Henderson and Jacquin, 1995), and this somatotopic segregation relays the whisker map to the PrV. Why does the whisker-related pattern fail to form in the PrV of Lmx1b−/− mice? At least two possibilities exist. First, extensive cell death in the mutant PrV could singularly account for failed PrV pattern formation. This is unlikely to be explanatory because Bax−/− induced rescue of Lmx1b-deficient PrV cells fails to rescue PrV pattern formation (Fig. 9). This finding is similar to that of our recent studies showing that prevention of excess cell death in the V ganglion and PrV of Drg11−/− mutants also failed to rescue whisker-related pattern formation (Jacquin et al, 2008). The second possible explanation, the one we favor, is that Lmx1b is part of a transcriptional signaling pathway that makes PrV barrelettes. How this occurs is not clear at present, nor is it clear whether Lmx1b and consequent Drg11 activity engage other known PrV patterning factors. The latter include the transcription factor Hoxa2 (Oury et al., 2006) and various ephrin-associated axon guidance molecules (North et al., 2010).
And, a final comment with respect to failed PrV patterning and cell death in the Lmx1b mutant. This is yet another example of these two effects occurring in the same mutant, although we have shown here that cell death is not causal to failed patterning. Indeed, all known mutations that preclude PrV patterning display a PrV that is smaller than normal. As discussed in detail in Jacquin et al. (2008) with respect to the Drg11 mutant phenotype, it is worth pointing out again that, conversely, failed patterning in PrV may be what causes excessive PrV cell death.
Lmx1b-Drg11: a key molecular pathway that specifies the PrV-based lemniscal pathway
An important conclusion to be derived from the present study is that the phenotype of Lmx1b−/− mice mimics that of Drg11−/− mice. Briefly (see Jacquin et al., 2008, for details), both mutants show qualitatively normal innervation of the PrV and whiskerpad, and their PrV-thalamic projections are delayed and sparse. Moreover, whisker-related patterns fail to develop in the PrV of both preparations, neither of which can be attributed to excess apoptotic cell death. These similarities lead to our belief that Lmx1b and Drg11 are linked in a molecular signaling pathway that is necessary for normal development of the PrV-based lemniscal pathway. This conclusion is supported by the present finding that Drg11 expression is completely eliminated in the PrV of Lmx1b mutants. Thus, Lmx1b regulates Drg11, either directly or indirectly, in the developing PrV.
However, there are potentially instructive differences in the Lmx1b and Drg11 mutant phenotypes. First, the former is lethal at birth, whereas the latter is not, possibly reflecting Lmx1b’s role in the development of other life sustaining organs. Second, PrV cell death is more extensive than that seen in the Drg11 mutant; consequently, the Lmx1b−/− PrV appears smaller. Third, our cursory examinations to date indicate that more transcription factors are downregulated or eliminated (e.g. the Ebf gene and Tlx3) in Lmx1b−/− mice, as compared with Drg11 mutants. Thus, the greater number of additional transcription factors that are affected by the Lmx1b−/− mutation may underlie its greater phenotypic severity, such as the case for PrV cell death. However, whether other transcription factors contribute to the transcriptional signaling machinery that specifies PrV pattern formation has yet to be determined. Fourth, unlike Lmx1b, Drg11 is expressed in both V ganglion and PrV neurons. However, as previously argued (Jacquin et al., 2008), the findings that other brainstem targets of V ganglion cell collaterals develop normal whisker-related patterns, e.g. the SpVi, provide strong support for the notion that the primary locus of action of Lmx1b and Drg11 is the PrV. Thus, we contend that Lmx1b acts through Drg11 to specify somatotopic patterning in the developing PrV. That latter is then conveyed to the thalamus and cortex to produce barrels via yet-to-be-determined mechanisms. It must be emphasized that a completely different set of mechanisms must impart patterns upon the Spinal V nucleus in the light of their normal development in Lmx1b and Drg11 mutants. Identification of the varied transcriptional networks and their effectors acting to specify separate components of the developing V system, along with their mechanisms of action, remain worthy topics for future study.
Experimental Methods
Generation, maintenance and genotyping of mutant mice
Lmx1b+/+ and Lmx1b−/− mice were generated from Lmx1b+/− mice as described previously (Chen et al., 1998; Zhao et al., 2004). Lmx1b/bax double null mice were generated by breeding Lmx1b+/− and Bax−/− mice (Jacquin et al., 2008); Bax mutants are on a C57BL/6 background. All mice and embryos were genotyped by PCR as described previously (Chen et al., 1998; Ding et al., 2004). Maintenance of Lmx1b+/− mice, Bax−/− and Lmx1b/bax+/− and all animal procedures described were performed according to NIH mandated protocols approved by the Division of Comparative Medicine at the Washington University School of Medicine.
Nissl staining, immunocytochemistry and histochemical staining
Embryos from embryonic days (E) 12 to 19 were removed from timed-pregnant female mice and fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for at least 4 hours. Following cryoprotection with 15 to 30% sucrose in PBS, 18-μm-thick consecutive brain sections were cut with a cryostat and collected on slides. Nissl staining and immunocytochemistry were performed as described (Chen et al., 2001; Ding et al., 2003; Ding et al., 2004). Sections were incubated with rabbit anti-Lmx1b (1:1500, gift from Dr. Ding), mouse anti-NeuN antibody (1:200), or rabbit anti-GFAP (1:1000) in PBS containing 2% normal donkey serum and 0.3% Triton X-100 overnight, biotinylated donkey anti-mouse or anti-rabbit (Jackson Immuno Research, West Grove, PA; 1:200) for 2 hr, and FITC-conjugated streptavidin (Molecular Probes, Eugene, OR; 1:1000) for 1 hr. After washing with PBS, slides were observed under an Olympus fluorescent microscope (BX51). For cytochrome oxidase (CO) staining, because the Lmx1b mutation is lethal at birth, newborn pups were kept alive as long as possible on the day of birth on a 37°C heating pad in order to facilitate staining of whisker-related patterns in the brainstem. Brains were removed after perfusion with 4% PFA, postfixed in 4% PFA for 2 to 4 hours, then cryoprotected with 30% sucrose in 0.1M PBS, pH 7.4. Coronal sections through the hindbrain at a thickness of 50 to 60 μm were incubated with CO staining solution [0.24 mg cytochrome C (type III, Sigma, St. Louis, MO), 0.5 mg DAB (Sigma), and 44 mg sucrose (Sigma) in 1 ml PBS] under gentle agitation (Wong-Riley and Welt, 1980). After 12–24 hr incubation at room temperature, sections were mounted onto gelatin-coated glass slides and observed under a light microscope.
Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining
Consecutive sections were directly mounted onto 5 to 6 serial glass slides. At least two slides from each embryo were subjected to TUNEL examination (Gavrieli et al., 1992; Ding et al., 2003). Sections were incubated in a permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 10 min, preincubated with terminal deoxynucleotidyl tranferase (TDT) buffer (1x; Promega, Madison, WI) for 5 min and then were incubated with the TDT buffer containing 0.5 mM TDT (Promega) and 40 μm biotin-dUTP (Roche, Indianapolis, IN) for 1 hr at 37°C. After washing with PBS, sections were incubated with Cy3 –conjugated streptavidin for 1 hour, then counterstained with Hoechst (Acros Organics, Morris Plains, NJ) and observed with a fluoresent microscope. To distinguish regions of the V brainstem complex and to designate subnuclei, tissues were also viewed under phase contrast and darkfield optics or the tissue was counterstained for Nissl substance. Previously described cytoarchitectonic criteria were followed (Henderson and Jacquin, 1995).
In situ hybridization
Embryos were fixed in 4% PFA overnight, sunk in 15–30% sucrose, frozen, sectioned and mounted on frosted slides. In situ hybridization was performed as described previously (Birren et al., 1993; Ding et al., 2003; Zhao et al., 2004). RNA probes were labeled with digoxygenin. Sections were hybridized with probes in hybridization solution at 60°C for 12–16 hr, then incubated with anti-digoxygenin-AP (1:2000, Roche) at 4°C overnight. Hybridization signals were visualized by the use of blue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) staining.
DiI and DiI/DiA labeling
Embryos from E12.5 to E19.5 (day of birth) were fixed with 4% PFA in PBS. To transganglionically label components of the V infraorbital nerve projections to the PrV, a small amount of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) or 4-[4-(dihexadecylamino)styryl]-N-methylpyridium iodide (DiA) (Molecular Probes) crystals were placed in individual whisker follicles. In E12.5–E16.5 cases, DiA was placed in B-row follicles; a few days later DiI was also placed in the D-row follicles. For other older embryos at E16.5 or later, DiI was placed in the B3 and C3 whisker follicles. Tissues were incubated in fixative at 37°C for 3–4 d (E12.5), 2–3 weeks (E13.5–E16.5), or 4–5 weeks (E17.5–E19.5). To anterogradely label all of the infraorbital nerve projections to the brainstem on the day of birth, newborns were perfused with 4% PFA and DiI was applied to the transected infraorbital nerve at its exit from the infraorbital foramen. Tissues were stored as above for at least one month before processing. To anterogradely label projections from the V ganglion to the whiskerpad in E12.5–E15.5 cases, DiI crystals were placed into the V ganglion and stored for 5–7 days as above. Projections from the PrV to the ventrobasal thalamus were also studied in fixed E15.5 to E19.5 embryos. Brainstems were transversely severed at the level of the caudal pole of the PrV. DiI crystals were inserted into the PrV and tissues stored as above for 1 week (E15.5) or 3–4 weeks (E17.5, E19.5). Brains were then removed and coronal sections were taken at 100 μm on a vibratome. For whiskerpad viewing, heads were cut either transversely or longitudinally at 100 μm on a vibratome. Labeling was observed under epifluorescent optics.
Data analyses
For all estimates of total neuron number in the mutant or wildtype V ganglion or PrV, the optical dissector probe was used via the Stereo Investigator software package (MicroBrightField, Williston, VT). All procedures were performed identically to those previously described for the same cell types (see Jacquin et al., 2008, for details). In other embryonic cases, TUNEL-positive PrV cell profiles were counted in a series of sections. Consecutive sections were cut through the PrV and collected on five glass slides. Two slides from each of 4–6 animals were TUNEL stained and labeled PrV cell profiles were counted. Whisker-related patterning in CO-stained sections through the brainstem was qualitatively evaluated by the use of previously described methods (Henderson et al., 1994). Cell number estimates in the V ganglia and PrV were subjected to statistical analyses by students T-tests and a p < .05 significance cutoff.
Acknowledgments
This work was supported by National Institutes of Health (NIH) R01-NS046036 and P01-NS049048 grants to M.F.J., R.S.E and Z.-F.C. We thank Y. Ding, C. Wang, A Kim, and Z.Q. Zhao for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birren SJ, Lo LC, Anderson DJ. Sympathetic neurons undergo a developmental switch in trophic dependence. Development. 1993;119:597–610. doi: 10.1242/dev.119.3.597. [DOI] [PubMed] [Google Scholar]
- Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51–55. doi: 10.1038/ng0598-51. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Rebelo S, White F, Malmberg AB, Baba H, Lima D, Woolf CJ, Basbaum AI, Anderson DJ. The paired homeodomain protein DRG11 is required for the projection of cutaneous sensory afferent fibers to the dorsal spinal cord. Neuron. 2001;31:59–73. doi: 10.1016/s0896-6273(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Yin J, Xu HM, Jacquin MF, Chen ZF. Formation of whisker-related principal sensory nucleus-based lemniscal pathway requires a paired homeodomain transcription factor, Drg11. J Neurosci. 2003;23:7246–7254. doi: 10.1523/JNEUROSCI.23-19-07246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Chen ZF, Jacquin MF. Molecular determinants of the face map development in the trigeminal brainstem. Anat Rec. 2006;288:121–134. doi: 10.1002/ar.a.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson TA, Jacquin MF. What makes subcortical barrels? Requisite trigeminal circuitry and developmental mechanisms. In: Jones EG, Diamond IT, editors. Cerebral Cortex. Vol. 11. 1995. pp. 123–187. [Google Scholar]
- Henderson TA, Johnson EM, Osborne PA, Jacquin MF. Fetal NGF augmentation preserves excess trigeminal ganglion cells and interrupts whisker-related pattern formation. J Neurosci. 1994;14:3389–3403. doi: 10.1523/JNEUROSCI.14-05-03389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson TA, Rhoades RW, Bennett-Clarke CA, Osborne PA, Johnson EM, Jacquin MF. NGF augmentation rescues trigeminal ganglion and principalis neurons, but not brainstem or cortical whisker patterns, after infraorbital nerve injury at birth. J Comp Neurol. 1993;336:243–260. doi: 10.1002/cne.903360207. [DOI] [PubMed] [Google Scholar]
- Iwasoto T, Erzurumlu RS, Huerta PT, Chen DF, Sasaoka T, Ulupinar E, Tonegawa S. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19:1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Arends JJA, Xiang C, Shapiro LA, Ribak CE, Chen ZF. In Drg11 knockout mice, trigeminal cell death is extensive and does not account for failed brainstem patterning. J Neurosci. 2008;28:3577–3585. doi: 10.1523/JNEUROSCI.4203-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin MF, Zahm DS, Henderson TA, Golden JP, Johnson EM, Renehan WE, Klein BG. Structure-function relationships in rat brainstem subnucleus interpolaris: X. Mechanisms underlying enlarged spared whisker projections after infraorbital nerve injury at birth. J Neurosci. 1993;13:2946–2964. doi: 10.1523/JNEUROSCI.13-07-02946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Diamond IT. Cerebral Cortex. Vol. 11. Plenum Press; NY: 1995. Barrel Cortex. [Google Scholar]
- Killackey HP, Fleming K. The role of the principal sensory nucleus in central trigeminal pattern formation. Brain Res. 1985;354:141–145. doi: 10.1016/0165-3806(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Ma PM. The barrelettes—Architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. I. Normal structural organization. J Comp Neurol. 1991;309:161–199. doi: 10.1002/cne.903090202. [DOI] [PubMed] [Google Scholar]
- North HA, Karim A, Jacquin MF, Donoghue MJ. EphA4 is necessary for spatially selective peripheral somatosensory topography. Dev Dyn. 2010;239:630–638. doi: 10.1002/dvdy.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury F, Murakami Y, Renaud JS, Pasqualetti M, Charnay P, Ren SY, Rijli FM. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science. 2006;313:1408–1413. doi: 10.1126/science.1130042. [DOI] [PubMed] [Google Scholar]
- Qian Y, Shirasawa S, Chen CL, Cheng L, Ma Q. Proper development of relay somatic sensory neurons and D2/D4 interneurons requires homeobox genes Rnx/Tlx-3 and Tlx-1. Genes Dev. 2002;16:1220–1233. doi: 10.1101/gad.982802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Lichtman JW. Principles of Neural Development. Sinauer Associates; Sunderland, MA: 1985. [Google Scholar]
- Shortland PJ, DeMaro JA, Shang F, Waite PME, Jacquin MF. Peripheral and central predictors of whisker afferent morphology in the rat brainstem. J Comp Neurol. 1996;375:481–501. doi: 10.1002/(SICI)1096-9861(19961118)375:3<481::AID-CNE10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci U S A. 1980;77:2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MN, Zahm DS, Jacquin MF. Differential foci and synaptic organization of the principal and spinal trigeminal projections to thalamus in rat. Europ J Neurosci. 1994;6:429–453. doi: 10.1111/j.1460-9568.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of the mouse cerebral cortex. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiecio S, Wang JS, Renner JR, Gereau RW, IV, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]