Abstract
Platelets participate in a variety of responses of the blood to injury. An emerging body of evidence suggests that these cells express an intrinsic capacity to interact with and trigger both classical and alternative pathways of complement. This activity requires cell activation with biochemical agonists and/or shear stress, and is associated with the expression of P-selectin, gC1qR, and chondroitin sulfate. Platelet mediated complement activation measurably increases soluble inflammatory mediators (C3a and C5a). Platelets may also serve as targets of classical complement activation in autoimmune conditions such as antiphospholipid syndromes (APS) and immune thrombocytopenia purpura (ITP). Retrospective correlation with clinical data suggests that enhanced platelet associated complement activation correlates with increased arterial thrombotic events in patients with lupus erythematosus and APS, and evidence of enhanced platelet clearance from the circulation in patients with ITP. Taken together, these data support a role for platelet mediated complement activation in vascular inflammation and thrombosis.
Keywords: Platelets, complement, thrombosis, inflammation, systemic lupus erythematosus, antiphospholipid syndrome, immune thrombocytopenia purpura
Introduction
Complement activation is increasingly recognized as a major contributor to vascular inflammation (Makrides, 1998, Goldfarb, 2005). Complement deposition has been observed in atherosclerotic lesions (Niculescu et al., 2004, Niculescu and Rus 1999, Yasojima et al., 2001), and a growing body of evidence suggests that complement plays a significant role in ischemia/reperfusion injury (Arumugam et al., 2004). During complement activation, potent inflammatory mediators, C3a and C5a, are generated (Marceau and Hugli, 1984), which have cytokine like properties, enhance leukocyte recruitment, and support the host inflammatory response. Indeed, elevations in circulating C5a levels have been associated with increased cardiovascular risk in patients with advanced atherosclerosis (Speidl et al., 2005). Moreover, C1q, C3, and C4, as well as the generation of terminal complement complexes, C5b-9, have been described in human atherosclerotic lesions (Niculescu and Ruf, 2004), with the highest deposition of iC3b being reported in vulnerable and ruptured plaques (Niculescu and Rus 1999, Yasojima et al., 2001).
To better understand complement activation as a cause and/or consequence of vascular injury, this review will focus on the interaction between platelets and the complement system. The role of these hemostatic cells as mediators and also targets of classical and alternative pathway complement activation will be discussed, and pathophysiologic consequences considered. Under physiologic conditions, we propose that in situ complement activation may contribute to the clearance of activated platelets and platelet microparticles from the circulation, via deposition of C1q and generation of cell surface associated C3b (Makrides, 1998). Under pathologic conditions, dysregulated complement activation on/by platelets may contribute to vascular inflammation and thrombosis. Indeed, propagation of complement activation on/by platelets is reflected by deposition of C5b-9, the lytic terminal complement complex, which can activate platelets and induce expression of platelet membrane procoagulant activity (Wiedmer and Sims, 1985, Wiedmer et al., 1986).
Complement Activation on Platelets
Platelets play important roles in hemostasis, thrombosis, inflammation, and vascular injury (Wagner, 2005). Increasing experimental evidence supports the concept of direct classical (Peerschke et al., 2006, Hamad et al., 2008) and alternative (del Conde et al., 2005) pathway complement activation on/by platelets, producing measurable deposition of complement components, C1q, C4, C3b, and C5b-9 on the platelet surface, as well as generation of C3a and C5a inflammatory peptides (del Conde et al, 2005; Peerschke et al., 2006).
Complement activation requires platelet stimulation and is associated with the expression of P-selectin (del Conde et al., 2005) and gC1qR (Peerschke et al., 2006) on the platelet surface, as well as the secretion of chondroitin sulfate (Hamad et al., 2008) from internal platelet stores. P-selectin has been associated with activation of the alternative complement pathway, whereas gC1qR and chondroitin sulfate activate the classical pathway. Platelet mediated complement activation can be detected on adherent platelets and activated platelets in suspension, following in vitro exposure to purified complement components, normal plasma or serum, by flow cytometry or ELISA methods.
The intrinsic capacity of platelets to activate complement on /near their surface when exposed to plasma or serum is proportional to the extent of platelet activation (Peerschke et al., 2006). Platelets activated by weak agonists such as ADP and epinephrine support less complement activation than platelets activated by thrombin or arachidonic acid. In addition to chemical agonists, platelets exposed to shear stress (1800 sec-1 for 60 min) support complement activation. Interestingly, platelets activated by shear stress appear to preferentially activate the classical complement pathway. In contrast, platelets activated by agonists such as thrombin or its receptor activation peptide (TRAP6), which induce alpha granule secretion and P-selectin expression on the platelet surface, appear to support predominantly alternative pathway activation. This may reflect the secretion of C1 inhibitor (Schmaier et al., 1993), a potent inhibitor of C1s in the classical pathway, from platelet alpha granules. Indeed, an inverse correlation has been noted between C4 activation on/by platelets and P-selectin expression on their surface (Peerschke et al, 2006).
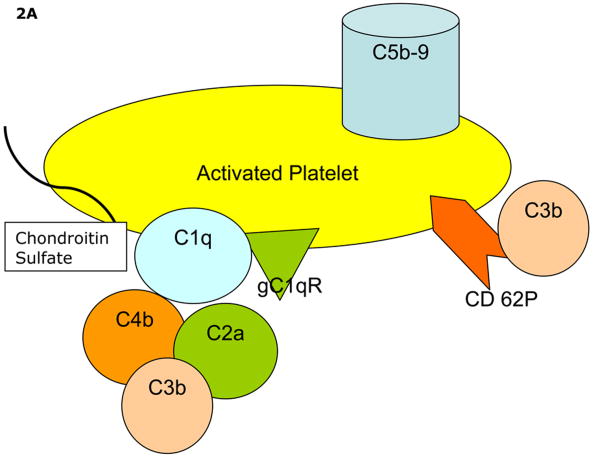
Classical pathway complement activation on/by platelets does not require immune complex formation at the platelet surface. Activation of C4 has been observed following exposure of activated platelets to purified C1 and C4 as well as to normal serum (Fig. 1A), and is significantly reduced or absent in C1 depleted serum (Peerschke et al., 2006). In contrast, both classical pathway and alternative pathways participate in C3b deposition on activated platelets. Thus, reductions in C3b deposition on platelets relative to normal serum are noted with either C1 or Factor B depleted serum (Peerschke et al., 2006). We believe that these findings reflect the intrinsic capacity of platelets to activate complement (Figure 2A). As described in subsequent sections, platelets also serve as targets of immune mediated complement activation.
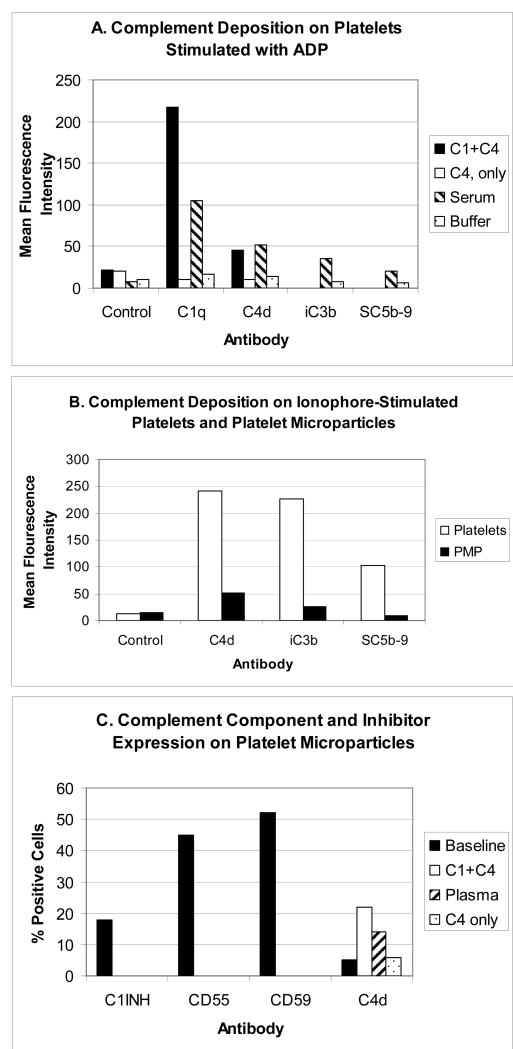
Figure 1.
Complement activation on/by platelets and platelet microparticles. Results are of typical experiments. Deposition of activated complement components on platelets and microparticles was evaluated by flow cytometry using monoclonal antibodies to C1q, C4d, iC3b, and SC5b-9, and an Alexa 488-conjugated secondary goat anti rabbit antibody (F(ab)'2). Platelet and microparticle suspensions were exposed to purified complement components, normal serum (diluted 1/10 in buffer containing 1 mM CaCl2 and 1 mM MgCl2) or buffer, as a control. Following incubation (60 min, 37o C), platelets and microparticles were washed by centrifugation and resuspension, and probed with anti complement antibodies. Detailed methods are provided in Peerschke et al., 2005, and Yin et al., 2007. Panel A illustrates the deposition of complement components on platelets stimulated with 10 μM ADP. Note that detection of C4d on platelets is dependent on the presence of C1. Panel B compares complement component deposition on platelets stimulated with 5 μM ionophore (A23187) and resulting microparticles. Although complement deposition on microparticles is low compared to platelets, when results are interpreted in the context of microparticle size, complement component deposition on microparticles exceeds that on platelets. Panel C depicts complement inhibitor expression on platelet microparticles, as well as classical pathway C4d detection on microparticles following incubation with either purified complement components C1 and C4, or normal plasma (reconstituted with 1 mM CaCl2 and 1 mM MgCl2 and a selective thrombin inhibitor, 500 μM D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone, to prevent thrombin generation and fibrin formation).
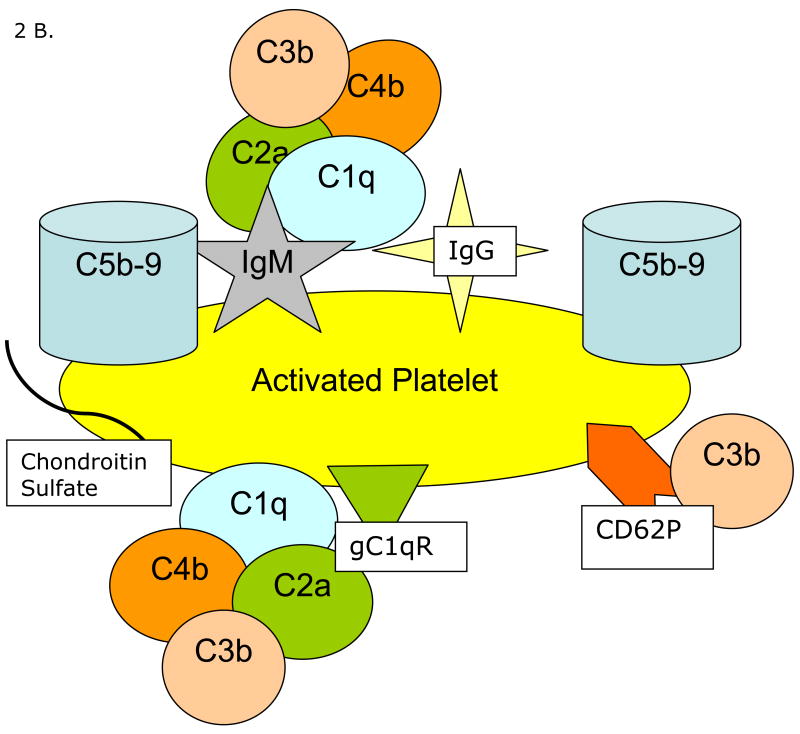
Figure 2.
Proposed mechanisms of complement activation on/by activated platelets. Panel A depicts the intrinsic capacity of platelets to activate the classical and alternative pathways of complement. Panel B demonstrates immune mediated enhanced complement activation requiring immune complex formation at the platelet surface (Panel B).
Although, studies of complement activation on/by platelets to date have been conducted exclusively in in vitro closed systems, evidence of in vivo complement deposition on platelets has been presented also. Platelet associated C4d has been reported in approximately 18% of patients with systemic lupus erythematosus (Navratil et al., 2006), and preliminary studies by the authors (EIBP, BG) demonstrate detectable levels of C4d, iC3b and C5b-9 on circulating platelets in approximately 14% of patients with coronary artery disease (n=5/35) (unpublished observations). These findings suggest complement activation on/by platelets in disorders associated with vascular inflammation and thrombosis, and lend strong support for the continued investigation of the physiologic and pathologic relevance of platelet mediated complement activation and its regulation.
Complement Activation on Platelet Microparticles
Platelet microparticles (PMP) are small vesicles that are released from activated platelets. They are encountered in a variety of clinical settings, including arterial thrombosis and peripheral vascular disease (Zeiger et al., 2000), hypertension, diabetes, stroke (Sinauridze et al., 2007; Piccin et al., 2007; Choudhury et al., 2007) and atherosclerosis (Tan and Lip, 2005). PMP enhance thrombus formation (Diamant et al., 2004; Tans et al., 1991) and contribute to platelet activation (VanWijk et al., 2003). PMP have been described as a storage pool for disseminating blood-borne bioactive effectors (Morel et al., 2004) such as tissue factor activity, procoagulant phospholipids, and inflammatory mediators.
We have recently demonstrated complement activation on/by PMP generated by platelets stimulated with the calcium ionophore A23187 (Yin et al., 2007). Results of a typical experiment are illustrated in Fig. 1B and 1C. PMP were distinguished from platelets by flow cytometry based on their light scattering properties/size. PMP were noted to express gC1qR and P-selectin, associated with classical (Peerschke et al., 2006) and alternative (delConde et al., 2005) complement pathway activation, respectively. Not surprisingly, therefore, complement components ranging from C1q to C5b-9 could be demonstrated on PMP (Yin et al., 2007), following incubation with plasma or serum.
Interestingly, C4 deposition on PMP exceeded that of C3b and C5b-9. Although direct quantitation of antibody binding was not performed, these findings may either reflect differences in antibody sensitivities and accessibility to their respective complement antigens on PMP, or, more interestingly, suggest potential regulation of C3 and C5 convertases, as well as assembly of C5b-9 on PMP by intrinsic platelet complement regulators. Indeed, our experiments have shown that PMP express complement regulatory proteins C1 INH, CD55 and CD59 (Yin et al., 2007), as shown in Fig. 1C. In addition, clusterin, a regulator of the assembly of the terminal complement complex, is packaged in platelet alpha granules (Tschopp et al, 1993), and is expressed on the surface of platelets (Gnatenko et al., 2003) and microparticles (Perez-Pujol et al., 2005).
Complement Activation on Platelets in Autoimmune Disorders
Antiphospholipid Syndrome (APS) and Systemic Lupus Erythematosus (SLE)
Complement plays a major role in inflammation and thrombotic complications associated with SLE and APS (Giannakopoulos et al., 2007; Levine et al, 2002). The risk of thrombosis is particularly high in patients with SLE and anti phospholipid antibodies (aPL) (Petri 2000). In vivo, complement activation is required for aPL-induced fetal loss and growth retardation (Salmon and Girardi, 2004). Moreover, an increase in complement products in the serum of patients with aPL has been associated with the development of stroke and transient ischemic events (Davis and Brey, 1992), and a high frequency of complement fixing aPL has been described in patients with APS (Shinzato et al., 2005).
In vivo and in vitro studies demonstrate that aPL activate endothelial cells and platelets and contribute to thrombosis and tissue injury (Pierangeli et al., 1999; DeJong et al., 2000). A major antigen recognized by aPL is beta2 glycoprotein 1, a member of the complement control protein superfamily (Giannakopoulos, 2007; Shi et al., 1993). Platelet associated C4d has been reported in 18% of patients with SLE, and is significantly associated with the presence of a lupus anticoagulant and anticardiolipin antibodies (Navratil et al., 2006).
Indeed, platelets have been described as targets of circulating aPL (Machin, 1996). The interaction of aPL with platelets has been reported to occur by several mechanisms, including direct recognition of platelet antigens, crosslinking of apolipoprotein ER2′ receptor (Pennings et al., 2007) and antibody interaction with platelet FcgRIIA receptors (Machin 1996). We propose that depending on antibody isotype, platelet associated aPL may play a role in the activation of the classical complement pathway.
To evaluate the role of platelets as targets of immune mediated complement activation in this setting, we designed an ELISA approach to investigate the capacity of patient serum to activate complement on fixed, immobilized test platelets (Peerschke et al., 2009b). Complement component deposition on test platelets was expressed as a ratio (CAC) relative to normal human serum, the latter representing the intrinsic capacity of platelets to activate complement described above. A ratio exceeding 1.0 reflects enhanced serum complement activation capacity relative to normal serum.
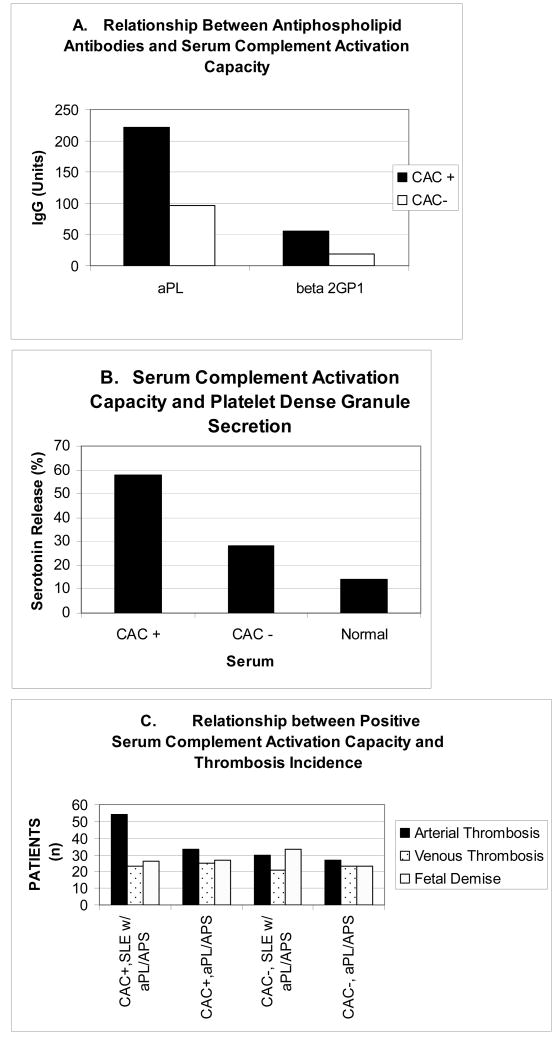
Using this approach, the complement activating capacity of sera from patients with SLE, SLE with aPL or APS, and primary aPL or APS were evaluated. Patient sera were designated complement positive (CAC +) if their complement activating capacity exceeded a ratio of 1.9 for C1q and/or C4 deposition on test platelets. Enhanced serum complement activation capacity correlated with antiphospholipid antibody titer (IgG), as well as antibody titers directed against β2 glycoprotein I (Fig. 3A). Interestingly, sera demonstrating enhanced complement activation capacity (CAC+) in the test system induced greater dense granule secretion from fresh platelets than sera designated CAC - (Fig 3B), suggesting a correlation between in situ complement activation on platelets and platelet stimulation. Indeed, a positive CAC was significantly associated with an increased incidence of arterial thrombosis in patients with SLE and aPL (Fig. 3C). However, a similar association with arterial thrombosis was not observed in patients with primary aPL/APS.
Figure 3.
Serum complement activating capacity (CAC) in patients with antiphospholipid antibodies (aPL), anti phospholipid syndrome (APS) and systemic lupus erythematosus (SLE). Panel A illustrates the correlation between antiphospholipid (aPL) and anti beta 2 glycoprotein 1 antibody titers expressed in GPL and SG units, respectively, and serum CAC. Sera were classified as CAC positive, if they produced a C1q and/or C4d deposition ratio on test platelets that was greater than 1.9 when compared to the assay normal serum standard. Panel B shows results of a typical experiment demonstrating the ability of CAC positive sera to activate platelets, as reflected by serotonin release from platelet dense bodies (Peerschke and Wainer, 1985). Panel C summarizes the relationship between serum complement CAC and incidence of arterial thrombosis in patients with SLE and aPL/APS, and patients with primary aPL or APS.
A proposed mechanism of aPL mediated complement activation on platelets is shown in Figure 2B. Circulating aPL antibodies bind to the platelet surface, forming immune complexes, which, depending on antibody isotype, are recognized by C1q, and activate the classical complement pathway, as demonstrated by C4d deposition on test platelets in the assay. In vivo, this may result in complement mediated platelet activation via generation of C5b-9 (Wiedmer et al., 1986), and the generation of complement derived inflammatory mediators (Markiewski and Lambris, 2007), conspiring to promote arterial thrombosis (Ikeda et al., 1997; Oksjoki et al., 2007). In the setting of SLE, immune mediated vascular injury may create an environment that predisposes to platelet accumulation and supports subsequent in situ complement activation.
Immune Thrombocytopenic Purpura (ITP)
ITP is an autoimmune disorder which manifests clinically as mucocutaneous bleeding in the setting of a low platelet count (Cines et al., 2009). Platelet destruction in ITP occurs by a variety of immune mediated mechanisms (Cines et al., 2009). Both humoral and cell mediated mechanisms have been described, but the role of the complement system has not been well defined (McMillan 2000). Limited studies of plasma complement deficiencies in patients with ITP (Trent et al., 1980; Ohali et al., 2005) and elevated levels of platelet associated complement, particularly C3 (Kayser et al., 1983; Foster et al., 1989; Panzer et al., 1986) have been reported. The observed association between platelet associated IgG/IgM and C3 suggests the potential for classical pathway complement activation on the platelet surface.
Using our in vitro assay (Peerschke et al., 2009b) to measure serum complement activation capacity on immobilized test platelets, we compared the complement activating capacity (CAC) of plasma from healthy volunteers and patients with ITP, as well as patients with non-immune mediated thrombocytopenia (Peerschke et al, 2009a). To prevent thrombin generation while supporting complement activation in plasma anticoagulated with 0.32% sodium citrate, these studies were performed in the presence of 1 mM MgCl2, 1 mM CaCl2, and 500 μM D-phenylalanyl-L-prolyl-L-arginine chloromethylketone, a selective thrombin inhibitor. Plasma complement activation capacity on test platelets exceeding a ratio of 1.9 relative to normal plasma was observed with 58% of plasma from patients with ITP, but none of the plasma samples from patients with non-immune mediated thrombocytopenia, who were predominantly patients with malignancies treated with chemotherapy. These findings are consistent with the presence of complement fixing anti platelet antibodies in the plasma from patients with ITP. These antibodies recognize platelets in the test system and, depending on their isotype, increase complement activation above baseline (Fig. 2 B).
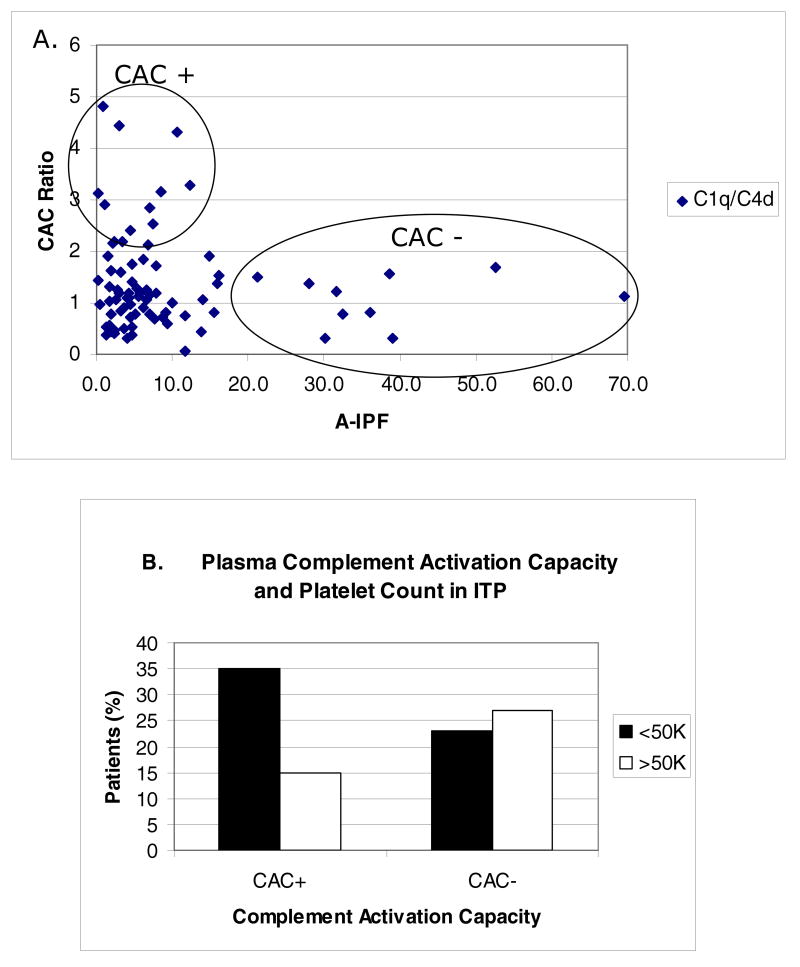
Figure 4 demonstrates that despite significant overlap, patients with CAC+ plasma had a lower peripheral blood absolute immature platelet fraction (A-IPF) than patients with CAC – plasma (p<0.05). The A-IPF is widely regarded as a measure of effective thrombopoiesis (Rinder et al., 1993, Richards and Baglin, 1995). In addition, a higher incidence of thrombocytopenia (Fig. 4B) was observed in patients with positive plasma CAC. These findings suggest that enhanced complement fixation may be associated with decreased platelet survival in vivo due to enhanced clearance of opsonized platelets by the reticuloendothelial system. Consistent with this interpretation is the observation that patients with positive plasma complement activation appear to have a higher response rate to splenectomy (Peerschke et al, 2009a). Moreover, since enhanced C5b-9 deposition on target cells is associated with cytolysis, positive plasma CAC may further contribute to thrombocytopenia by direct platelet damage and damage to megakaryocytes, leading to ineffective thrombopoiesis.
Figure 4.
Plasma complement activating capacity (CAC) in patients with ITP. Panel A correlates CAC and the absolute immature platelet fraction (A-IPF) in peripheral blood. A-IPF values reflect number × 109/L. A low A-IPF suggests decreased or ineffective thrombopoiesis. Note that patients with the highest plasma CAC ratios had the lowest A-IPF, and that none of the patients with A-IPF >15 × 109/L had a positive plasma CAC. Panel B shows the relationship between CAC and thrombocytopenia, defined as circulating peripheral blood platelet counts less than 50K/μl. Plasma was classified as CAC positive, if it produced a C1q and/or C4d deposition ratio on test platelets that was greater than 1.9 when compared to the assay normal plasma standard.
Conclusions and Future Directions
In vitro evidence is accumulating to support direct complement activation on stimulated platelets. Under physiologic conditions, complement activation may contribute to the clearance of spent platelets and microparticles from the circulation, in an effort to regulate prothrombotic effects. Under pathologic conditions, complement activation on/by platelets may contribute to thrombosis and thrombocytopenia. Further studies are required to characterize the molecular mechanisms contributing to complement activation on/by platelets, particularly as these may represent novel therapeutic targets. Clinical studies are required to further evaluate pathologic and physiologic sequelae associated with in vivo platelet mediated complement activation, and to evaluate the effect of anti platelet therapies and complement inhibitors.
Acknowledgments
This work was supported in part by grants HL67211 (EIBP) and AI060866 (BG) from the National Institutes of Health, and an American Heart Association Heritage Affiliate postdoctoral award # 0625900T (WY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Choudhury A, Chung I, Blann AD, Lip GY. Elevated platelet microparticle levels in nonvalvular atrial fibrillation: relationship to p-selectin and antithrombotic therapy. Chest. 2007;131:809–815. doi: 10.1378/chest.06-2039. [DOI] [PubMed] [Google Scholar]
- Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WD, Brey RL. Antiphospholipid antibodies and complement activation in patients with cerebral ischemia. Clin Exp Immunol. 1992;10:445–460. [PubMed] [Google Scholar]
- DeJong A, Siboh V, Robbins D. Antiphospholipid antibodies and platelets. Curr Rheum Rep. 2000;2:238–245. doi: 10.1007/s11926-000-0085-8. [DOI] [PubMed] [Google Scholar]
- del Conde I, Cruz MA, Zhang H, Lopez JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201:871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34:392–401. doi: 10.1111/j.1365-2362.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- Forster J, Katzikadamos Z, Zinn P. Platelet-associated IgG, IgM, and C3 in paediatric infectious disease. Helv Paediatr Acta. 1989;43:415–422. [PubMed] [Google Scholar]
- Giannakopoulos B, Passam F, Rahgozar S, Krillis SA. Current concepts on the pathogenesis of the antiphospholipid syndrome. Blood. 2007;109:422–430. doi: 10.1182/blood-2006-04-001206. [DOI] [PubMed] [Google Scholar]
- Gnatenko DV, Dunn JJ, McCorkel SR, Weissmann D, Oerritta PL, Bahou WF. Blood. 2003;101:2285–2293. doi: 10.1182/blood-2002-09-2797. [DOI] [PubMed] [Google Scholar]
- Goldfarb RD. Crit Care Med. 2005;33:S482–484. doi: 10.1097/01.ccm.0000186789.27047.84. [DOI] [PubMed] [Google Scholar]
- Hamad OA, Ekdahl KN, Nilsson PH, Andersson J, Magotti P, Lambris JD, Nilsson B. Complement activation triggered by chondroitin sulfate released by thrombin receptor activated platelets. J Thromb Haemos. 2008;6:1413–1421. doi: 10.1111/j.1538-7836.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997;77:294–398. [PubMed] [Google Scholar]
- Kayser W, Mueller-Eckhardt C, Bhakdi S, Ebert K. Platelet associated complement C3 in thrombocytopenic states. Br J Haematol. 1983;54:353–363. doi: 10.1111/j.1365-2141.1983.tb02110.x. [DOI] [PubMed] [Google Scholar]
- Levine JS, Branch W, Rauch J. The antiphospholipid syndrome. N Eng J Med. 2002;346:752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- Machin SJ. Platelets and antiphospholipid antibodies. Lupus. 1996;5:386–387. doi: 10.1177/096120339600500510. [DOI] [PubMed] [Google Scholar]
- Makrides SC. Therapeutic inhibition of the complement system. Pharmacol Rev. 1998;50:59–87. [PubMed] [Google Scholar]
- Marceau F, Hugli TE. Effect of C3a and C5a anaphylatoxins on guinea pig isolated blood vessels. J Pharmacol Exp Ther. 1984;230:749–754. [PubMed] [Google Scholar]
- Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan R. Autoantibodies and autoantigens in chronic immune thrombocytopenic purpura. Semin Hematol. 2000;37:239–248. doi: 10.1016/s0037-1963(00)90102-1. [DOI] [PubMed] [Google Scholar]
- Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- Navratil JS, Manzi S, Kao AH, Krishnaswami S, Liu CC, Ruffing MJ, Shaw PS, Nilson AC, Dryden ER, Johnson JJ, Ahearn JM. Platelet C4d is highly specific for systemic lupus erythematosus. Arth Rheum. 2006;54:670–674. doi: 10.1002/art.21627. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Niculescu T, Rus H. C5b-9 terminal complement complex assembly on apoptotic cells in human arterial wall with atherosclerosis. Exper Molec Pathol. 2004;76:17–23. doi: 10.1016/j.yexmp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus H. Complement activation and atherosclerosis. Molec Immunol. 1999;36:949–955. doi: 10.1016/s0161-5890(99)00117-0. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus H. The role of complement activation in atherosclerosis. Immunol Res. 2004;30:73–80. doi: 10.1385/IR:30:1:073. [DOI] [PubMed] [Google Scholar]
- Ohali M, Maizlish Y, Abramov H, Schlesinger M, Bransky D, Lugassy G. Complement profile in childhood immune thrombocytopenic purpura: a prospective pilot study. Ann Hematol. 2005;84:812–815. doi: 10.1007/s00277-005-1085-6. [DOI] [PubMed] [Google Scholar]
- Oksjoki R, Laine P, Helske S, Vehmaan-Kreula P, Mikko I, Gasque P, Kovanen PT, Markku O. Receptors for the anaphylatoxins C3a and C5a are expressed in human atherosclerotic coronary plaques. Atherosclerosis. 2007;195:90–99. doi: 10.1016/j.atherosclerosis.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Panzer S, Szamait S, Boedeker RH, Haas OA, Haubenstock A, Mueller-Eckhardt C. Platelet associated IgG, IgM, IgA and complement C3 in immune thrombocytopenic disorders. Am J Hematol. 1986;23:89–99. doi: 10.1002/ajh.2830230203. [DOI] [PubMed] [Google Scholar]
- Peerschke EIB, Andemariam B, Yin W, Bussel JB. Complement deposition on platelets in immune thrombocytopenic purpura correlates with a decrease in circulating immature platelets. Brit J Haematol. 2009a;148:638–645. doi: 10.1111/j.1365-2141.2009.07995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke EI, Wainer JA. Examination of irreversible platelet fibrinogen interactions. Am J Physiol Cell Physiol. 1985;248:C466–472. doi: 10.1152/ajpcell.1985.248.5.C466. [DOI] [PubMed] [Google Scholar]
- Peerschke EIB, Yin W, Alpert DR, Roubey RAS, Salmon JE, Ghebrehiwet B. Serum complement activation on heterologous platelets is associated with arterial thrombosis in patients with systemic lupus erythematosus and antiphospholipid antibodies. Lupus. 2009b;18:530–538. doi: 10.1177/0961203308099974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke EIB, Yin W, Grigg SE, Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J Thromb Haemost. 2006;4:2035–2042. doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- Pennings MT, Derksen RH, van Lummel M, Adelmeijer J, VanHoorelbeke K, Urbanus RT, Lisman T, deGroot PG. Platelet adhesion to dimeric beta-glycoprotein 1 under conditions of flow is mediated by at least two receptors: glycoprotein IB alpha and apolipoprotein E receptor 2′. J Thromb Haemost. 2007;5:369–377. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- Perez-Pujol S, Martines MB, Higgins LA, Lesestuen GL, Key NS. Proteomic analysis of platelets and platelet-derived microparticles by iTRAQ Laser spectrometry. J Thromb Haemost. 2005;3:371. [Google Scholar]
- Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145–151. doi: 10.1006/jaut.2000.0409. [DOI] [PubMed] [Google Scholar]
- Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Pierangeli SS, Colden-Stanfield M, Liu X, Barker JH, Anderson GL, Harris EN. Antiphospholipid antibodies from antiphospholipid syndrome patients: activation of endothelial cells in vitro and in vivo. Circulation. 1999;99:1007–2002. doi: 10.1161/01.cir.99.15.1997. [DOI] [PubMed] [Google Scholar]
- Richards EM, Baglin TP. Quantitation of reticulated platelets: methodology and clinical application. Br J Haematol. 1995;91:445–451. doi: 10.1111/j.1365-2141.1995.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Rinder HM, Munz UJ, Ault KA, Bonan JL, Smith BR. Reticulated platelets in the evaluation of thrombopoietic disorders. Arch Pathol Lab Med. 1993;117:606–610. [PubMed] [Google Scholar]
- Salmon JE, Girardi G. The role of complement in antiphospholipid syndrome. Curr Dir Autoimmun. 2004;7:133–148. doi: 10.1159/000075690. [DOI] [PubMed] [Google Scholar]
- Schmaier AH, Amenta S, Xiong T, Heda GD, Gewirtz AM. Expression of platelet C1 inhibitor. Blood. 1993;82:465–474. [PubMed] [Google Scholar]
- Shi W, Chong BH, Chesterman CN. β2-glycoprotein 1 is a requirement for anticardiolipin antibodies binding to activated platelets: differences with lupus anticoagulants. Blood. 1993;81:1255–1262. [PubMed] [Google Scholar]
- Shinzato MM, Bueno C, Trindade-Viana VS, Borba EF, Goncalves CR, Bonfa E. Complement fixing activity of anticardiolipin antibodies in patients with and without thrombosis. Lupus. 2005;14:953–958. doi: 10.1191/0961203305lu2252oa. [DOI] [PubMed] [Google Scholar]
- Sinauridze EI, Kireeve DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membranes have 50 -100 fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–434. [PubMed] [Google Scholar]
- Speidl WS, Exner M, Amighi J, Kastl SP, Zorn G, Maurer G, Wagner G, Huber K, Minar E, Wojta J, Schillinger M. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. Eur Heart J. 2005;26:2294–2299. doi: 10.1093/eurheartj/ehi339. [DOI] [PubMed] [Google Scholar]
- Tan KT, Lip GY. The potential role of platelet microparticles in atherosclerosis. Thromb Haemost. 2005;94:488–492. doi: 10.1160/TH05-03-0201. [DOI] [PubMed] [Google Scholar]
- Tans G, Rosing J, Thomassen MC, Heeb MJ, Zwaal RF, Griffin JH. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood. 1991;77:2641–2648. [PubMed] [Google Scholar]
- Trent RJ, Clancy RL, Danis V, Basten A. Immune complexes in thrombocytopenic patients: cause and effect? Br J Haematol. 1980;44:645–654. doi: 10.1111/j.1365-2141.1980.tb08719.x. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Jemme DE, Hertig S, Preissner KT, Morgenstern H, Sapino AP, French L. Human megakaryocytes express clusterin and package it without apolipoprotein A-1 into alpha granules. Blood. 1993;82:118–125. [PubMed] [Google Scholar]
- VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277–287. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;24:1321–1324. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68:875–880. [PubMed] [Google Scholar]
- Wiedmer T, Sims PJ. Effect of complement proteins C5b-9 on blood platelets. Evidence for reversible depolarization of membrane potential. J Biol Chem. 1985;260:8014–8019. [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Complement components, but not complement inhibitors, are upregulated in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1214–1219. doi: 10.1161/hq0701.092160. [DOI] [PubMed] [Google Scholar]
- Yin W, Ghebrehiwet B, Peerschke EIB. Expression of complement components and inhibitors on platelet microparticles. Platelets. 2007;19:225–233. doi: 10.1080/09537100701777311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger F, Stephan S, Hoheisel G, Pfeiffer D, Ruehlmann C, Koksch M. P-selectin expression, platelet aggregates, and platelet derived microparticle formation are increased in peripheral arterial disease. Blood Coag Fibrinol. 2000;11:723–728. doi: 10.1097/00001721-200012000-00005. [DOI] [PubMed] [Google Scholar]