Summary
Feeding on high-calorie (HC) diets induces serious metabolic imbalances, including obesity. Understanding the mechanisms against excessive body weight gain is critical for developing effective anti-obesity strategies. Here, we show that lack of nicotinamide adenosine dinucleotide (NAD+)-dependent deacetylase SIRT1 in pro-opiomelanocortin (POMC) neurons causes hypersensitivity to diet-induced obesity due to reduced energy expenditure. The ability of leptin to properly engage the phosphoinositide 3-kinase (PI3K) signaling in POMC neurons and elicit remodeling of perigonadal white adipose tissue (WAT) is severely compromised in mutant mice. Also, electrophysiological and histomorphomolecular analyses indicate a selective reduction in sympathetic nerve activity and brown-fat-like characteristics in perigonadal WAT of mutant mice; suggesting a physiologically important role for POMC neurons in controlling this visceral fat depot. In summary, our results provide direct genetic evidence that SIRT1 in POMC neurons is required for normal autonomic adaptations against diet-induced obesity.
Introduction
Chronic feeding on diets rich in calories induces several serious metabolic defects, including obesity (Wisse et al., 2007). Homeostatic defense mechanisms against diet-induced obesity comprise behavioral and autonomic adaptations (e.g.: reduced food intake and increased energy expenditure) (Dhillon et al., 2006; Lowell and Spiegelman, 2000; Tong et al., 2008). The molecular mechanisms and the neuronal populations that coordinate these defense mechanisms are only partially known. These include actions of hormones (e.g.: leptin, insulin) on specialized central nervous system (CNS) neurons, as for example POMC neurons (Balthasar et al., 2004; Hill et al., 2008). POMC neurons reside in hypothalamic arcuate nucleus (ARH) and hindbrain nucleus of the solitary tract (NTS) (Bronstein et al., 1992; Elias et al., 1999). These neurons belong to the central melanocortin system, a family of diverse cells that comprise agouti-related peptide (AgRP)-, melanocortin-3-receptor (MC3R)-, and melanocortin-4-receptor (MC4R)-expressing neurons. POMC neurons secrete, along with other neuropeptides, α-melanocyte stimulating hormone (α-MSH), which suppresses food intake and increases energy expenditure by activating MC3Rs and MC4Rs (Coll et al., 2004; Fan et al., 1997). AgRP neurons secrete, along with other neuropeptides, AgRP that is the endogenous antagonist at the same melanocortin receptors (Cone et al., 1996; Ollmann et al., 1997). Because genetic defects impairing the function of brain melanocortin system result in obesity (in rodents and humans) (Butler et al., 2000; Butler et al., 2001; Farooqi et al., 2006; Farooqi et al., 2000), whereas increased α-MSH expression restrains diet-induced obesity (Lee et al., 2007), POMC neurons have served as prototypes for investigating the mechanisms underpinning normal energy homeostasis (Cone, 2005; O’Rahilly et al., 2003).
In addition to brain/peripheral feedback-loop pathways, cell-intrinsic, metabolic-sensing mechanisms in CNS neurons are also critical for body energy balance. For example, AMP-activated protein kinase and mammalian target of rapamycin in hypothalamic centers are both required for proper leptin-sensing and hence body weight homeostasis (Claret et al., 2007; Cota et al., 2006; Minokoshi et al., 2004). SIRT1 is also a metabolic-sensor protein as it uses oxidized nicotinamide adenine dinucleotide (NAD+) to deacetylate proteins (Imai et al., 2000). In peripheral tissues, SIRT1 regulates various metabolic processes. For example, deletion or reduction in the expression of SIRT1 in liver results in hypoglycemia and hypersensitivity to diet-induced hepatic steatosis (Erion et al., 2009; Li et al., 2007; Purushotham et al., 2009; Rodgers and Puigserver, 2007). Conversely, increased SIRT1 gene dosage in hepatocytes leads to deacetylation and thus activation of peroxisome proliferators activated receptor (PPAR)-γ coactivator 1α (PGC-1α), and forkhead box O1 (FoxO1) and hence enhanced expression of key genes of the gluconeogenic program (Banks et al., 2008). In skeletal muscle, SIRT1 activates PGC-1α but inhibits protein tyrosine phosphatase (PTP) 1B (a negative regulator of the insulin receptor signaling cascade) (Gerhart-Hines et al., 2007; Sun et al., 2007) and hence enhances mitochondrial activity, fatty acid β-oxidation and insulin sensitivity. In pancreatic β-cells, increased SIRT1 gene expression improves glucose stimulated insulin secretion and body glucose tolerance (Moynihan et al., 2005). In adipocytes, SIRT1 reduces lipogenesis and adipogenesis through a mechanisms involving inhibition of PPAR-γ (Picard et al., 2004).
In contrast to peripheral tissues, little is known about the relevance of SIRT1 in the brain on metabolic homeostasis. SIRT1 is expressed in the hypothalamus where its expression increases following fasting (Ramadori et al., 2008). Acute inhibition of hypothalamic SIRT1 dampens fasting-induced hyperphagia in rats (Cakir et al., 2009). Thus, because SIRT1 is expressed in POMC neurons (Ramadori et al., 2008), we reasoned that this metabolic-sensor protein in these metabolic-sensor neurons may play a critical role for coordinated body energy homeostasis. To directly test this hypothesis, we generated and characterized mice lacking SIRT1 only in POMC neurons.
Results
Somatic deletion of SIRT1 in POMC neurons
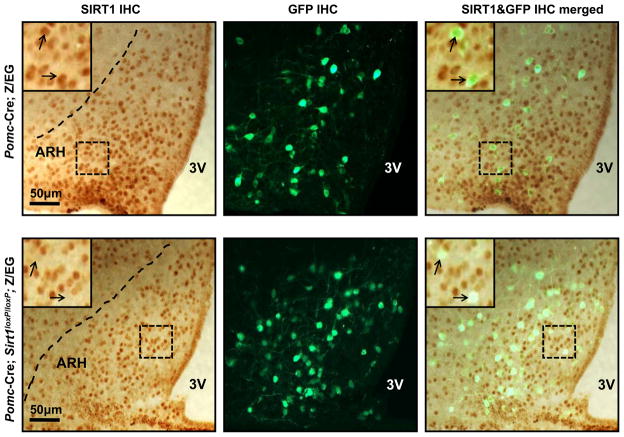
Cre-mediated deletion of a loxP-flanked Sirt1 allele (Sirt1loxP) results in a Sirt1 null allele (Cheng et al., 2003). Thus, to ablate SIRT1 selectively in POMC neurons, the Sirt1loxP allele was bred to the Pomc-Cre transgene that we and others have demonstrated expresses functional Cre-recombinase only in POMC cells (Balthasar et al., 2004; Belgardt et al., 2008; Plum et al., 2009; Plum et al., 2006). Genotyping PCR analyses of several tissues demonstrated the presence of the Cre-deleted, Sirt1 null allele in sites known to express POMC in Pomc-Cre mice homozygous for the Sirt1loxP allele (henceforward referred to as Pomc-Cre; Sirt1loxP/loxP mice) (Figure S1). The Cre-deleted, Sirt1 null allele in the pituitary gland is expected because POMC is also expressed in corticotrophs and melanotrophs (Belgardt et al., 2008) but without metabolic consequences as Pomc-Cre; Sirt1loxP/loxP mice have normal circadian fluctuations of serum corticosterone levels (Table S1). To directly investigate whether mutant mice lack SIRT1 in all POMC neurons, we performed double immunohistochemistry (IHC) for SIRT1 and GFP in Pomc-Cre; Sirt1loxP/loxP; Z/EG and Pomc-Cre; Z/EG (control) brains. The Z/EG allele was introduced to allow expression of GFP selectively in POMC neurons, as previously described (Balthasar et al., 2004). Virtually all of ARH POMC neurons expressed SIRT1 in control brains (176 out of 180 POMC cells (~98%) were positive for SIRT1 immunoreactivity; Figure 1, upper panel). Conversely, only ~7% of ARH POMC neurons expressed SIRT1 in Pomc-Cre; Sirt1loxP/loxP; Z/EG brains (14 out of 197 POMC neurons were positive for SIRT1 immunoreactivity; Figure 1, lower panel). Notably, SIRT1 expression was undisturbed in ARH non-POMC (GFP-negative) cells of mutant mice, a result that further supports the POMC-neuron specificity of SIRT1 deletion (Figure 1).
Figure 1. Deletion of SIRT1 is restricted to POMC neurons.
Representative photomicrographs of brain slices from Pomc-Cre; Z/EG (control) or Pomc-Cre; Sirt1loxP/loxP; Z/EG mice stained for SIRT1 and GFP (the Cre-conditional expression of GFP is restricted only to POMC neurons in these mice (Balthasar et al., 2004)). Dark-brown staining and green fluorescence represent SIRT1 and GFP immunoreactivity, respectively. Higher magnification of the boxed-region is in the top-left corner of the photomicrograph. Arrows indicate POMC neurons. Note the co-localization between SIRT1 and POMC in Pomc-Cre; Z/EG control brain. This co-localization is absent in almost all of POMC neurons in Pomc-Cre; Sirt1loxP/loxP; Z/EG brain. Dash lines indicate ARH boundaries. Abbreviations: third ventricle (3V), hypothalamic arcuate nucleus (ARH), immunohistochemistry (IHC). Scale bar = 50 μm.
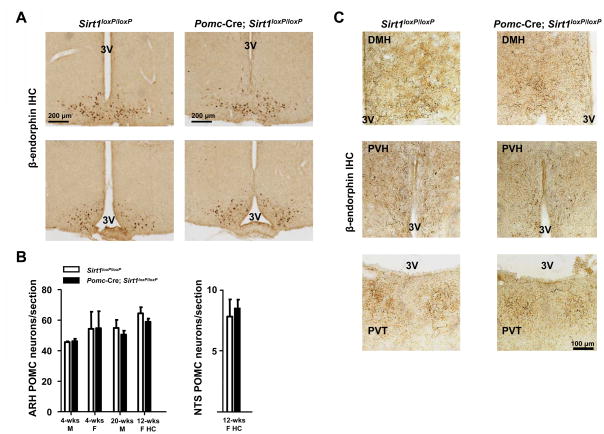
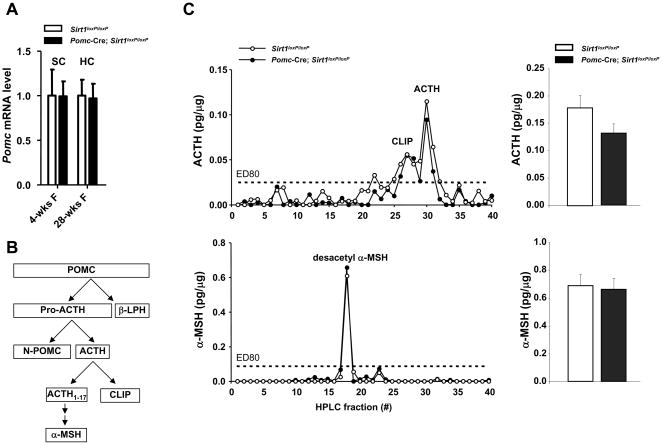
Because SIRT1 has been suggested to exert neuroprotective actions (Kim et al., 2007; Qin et al., 2006), we assessed if SIRT1 deficiency affects survival of POMC neurons. The anatomical distribution of β-endorphin (a product of POMC neurons) in the CNS of Pomc-Cre; Sirt1loxP/loxP mice was normal (Figure 2A and data not shown). The number of ARH and NTS POMC neurons as well as their projections to hypothalamic and extra-hypothalamic sites did not differ between mutants and controls (Figure 2B and C). Hypothalamic and hindbrain Pomc mRNA contents and the levels of POMC-derived neuropeptides adrenocorticotropic hormone (ACTH), corticotropin-like intermediate lobe peptide (CLIP) and α-MSH in hypothalamus were also unchanged (Figures 3A, 3B, 3C and data not shown). Altogether, these data demonstrate that Pomc-Cre; Sirt1loxP/loxP mice lack SIRT1 specifically in POMC neurons and that absence of SIRT1 does not alter POMC neurons survival.
Figure 2. SIRT1 is dispensable for POMC neuronal survival.
(A) Representative photomicrographs of rostral to caudal (top to bottom) brain sections from either Sirt1loxP/loxP (control) or Pomc-Cre, Sirt1loxP/loxP mice stained for β-endorphin (a product of POMC). Dark-brown staining represents β-endorphin immunoreactivity. Scale bar = 200 μm. (B) POMC neurons were counted in several sections that contained similar regions of the hypothalamus or hindbrain in both genotypes. No difference in the estimated number of POMC neurons per section was noted between genotypes in 4-week-old males (4-wks M), 4-week-old females (4-wks F), 20-week-old males (20-wks M), and 12-week-old females fed on the high-calorie diet for 4 weeks (12-wks F HC). (C), Representative photomicrographs of brain sections from either Sirt1loxP/loxP (control) or Pomc-Cre, Sirt1loxP/loxP mice stained for β-endorphin. Dark-brown staining represents β-endorphin immunoreactive fibers. Both genotypes display similar POMC neurons fibers density in dorsomedial hypothalamic nucleus (DMH), paraventricular hypothalamic nucleus (PVH), and paraventricular thalamic nucleus (PVT). Scale bar = 100 μm. Other abbreviations: third ventricle (3V); immunohistochemistry (IHC). n=2–3 in each group. Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student’s t test.
Figure 3. Normal hypothalamic Pomc mRNA and POMC-derived peptides levels in mice lacking SIRT1 in POMC neurons.
(A) Hypothalamic Pomc mRNA level in 4-week-old and in 28-week-old Sirt1loxP/loxP and Pomc-Cre; Sirt1loxP/loxP females fed on a high calorie (HC) diet for 20 weeks (n=9–11). Pomc mRNA levels were normalized to β-actin mRNA contents. (B) Schematic diagram depicting POMC processing in hypothalamus. (C) Hypothalamus was collected from 12-week-old Sirt1loxP/loxP and Pomc-Cre; Sirt1loxP/loxP females fed on a HC diet for 4 weeks (n=9–11). Acid-extracted peptides were HPLC-fractionated and quantified for ACTH or α-MSH using specific radioactive immunoassays. Representative HPLC profile for ACTH-related peptides and values of ACTH peptide for each group are shown (upper panel). Representative HPLC profile for α-MSH-related peptides and values of desacetyl-α-MSH peptide for each group are shown (lower panel). Each HPLC profile displays the elution position of respective synthetic standards. Values are the mean ± s.e.m. Statistical analyses were done using two-tailed unpaired Student’s t test and no differences were noted between groups. Abbreviations: ACTH (adrenocorticotropic hormone); CLIP (corticotropin-like intermediate lobe peptide); α-MSH (alpha melanocyte-stimulating hormone), and β-LPH (beta lipotropin hormone).
Body energy imbalance in mice lacking SIRT1 only in POMC neurons
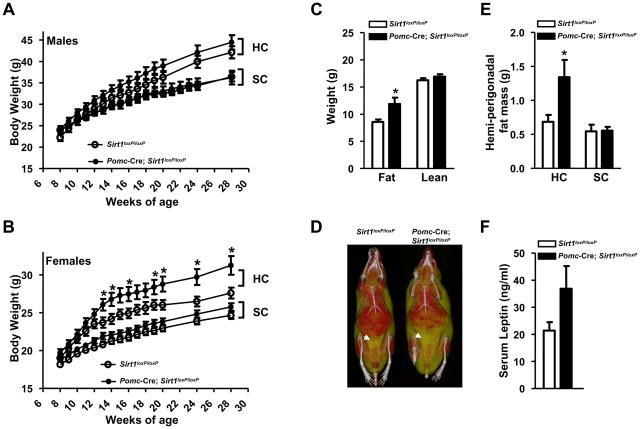
Pomc-Cre; Sirt1loxP/loxP mice were born at the expected Mendelian ratio and thrived into adulthood similarly to controls allowing us to investigate the physiological outcomes of POMC-neuron-specific SIRT1 deletion. Pomc-Cre; Sirt1loxP/loxP males and females fed ad libitum on a standard chow (SC) diet had body weights undistinguishable from their Sirt1loxP/loxP littermates (Figures 4A and 4B). Body fat and lean mass were assessed by magnetic resonance imaging (MRI) and found to be within the physiological range in SC-fed mutant mice (data not shown). Body length was also normal (Table S1) thus indicating that lack of SIRT1 in POMC neurons does not alter adiposity in mice fed on a regular diet. However, when animals were challenged with HC diet, body energy imbalance emerged in mutant mice. Pomc-Cre; Sirt1loxP/loxP males had a slight tendency to increased body weight (Figures 4A) whereas mutant females displayed a striking hypersensitivity to diet-induced obesity as their body weights were significantly elevated compared to controls fed on the same HC diet (Figure 4B). MRI analysis indicated that the increased body weight was solely due to increased fat mass (Figure 4C). Microcomputed tomography imaging suggested that visceral fat was enlarged (Figure 4D). In fact, mutant mice had almost double the mass of the major visceral fat depot (i.e.: perigonadal fat) compared to controls; a defect brought about by the HC diet as it was not seen in SC-fed mutants (Figure 4E). Circulating levels of the adipocyte-secreted hormone leptin also tended to be elevated (Figure 4F); an effect that we suggest is part of compensatory mechanisms aimed at restraining excessive body weight gain caused by SIRT1 deletion in POMC neurons. Energy imbalance was not secondary to hypercorticosteronemia (Table S1) or altered expression of key hypothalamic neuropeptides regulating energy balance (Figures 3A and S2). Although central melanocortins are critical for normal glucose and lipid homeostasis (Nogueiras et al., 2007; Parton et al., 2007), glycemia, insulinemia, insulin sensitivity, glucose tolerance, triglyceridemia and serum non-esterified fatty acids were unchanged in mutant mice (Table S1 and data not shown). Altogether, these results demonstrate that SIRT1 in POMC neurons is required for normal defenses against diet-induced obesity.
Figure 4. SIRT1 in POMC neurons is required for normal defenses against diet-induced obesity in females.
(A) Body weight curves of standard chow (SC)-fed Sirt1loxP/loxP (n=17) and Pomc-Cre, Sirt1loxP/loxP (n=20) males, high-calorie (HC)-fed Sirt1loxP/loxP (n=15) and Pomc-Cre, Sirt1loxP/loxP (n=20) males, (B) SC-fed Sirt1loxP/loxP (n=33) and Pomc-Cre, Sirt1loxP/loxP (n=37) females, HC-fed Sirt1loxP/loxP (n=14) and Pomc-Cre, Sirt1loxP/loxP (n=19) females. (C) Body composition, (D) representative microcomputed tomography images, (E) mass of hemi-perigonadal fat, and (F) serum leptin levels of Sirt1loxP/loxP and Pomc-Cre, Sirt1loxP/loxP females after 20 weeks on the HC diet (n=10–19). Mass of hemi-perigonadal fat of 28-week-old SC-fed Sirt1loxP/loxP (n=12) and Pomc-Cre, Sirt1loxP/loxP (n=12) females is shown in (E). In all figures, HC-fed mice were fed on a SC diet up to 8 weeks of age and then switched and maintained on a HC diet. In (D) red and yellow/green colors represent lean and fat mass, respectively. Arrows indicate visceral adipose tissue. Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student’s t test. *P<0.05.
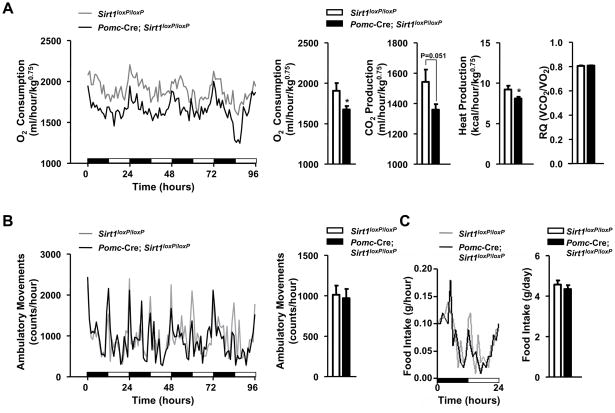
Energy homeostasis is kept in equilibrium when energy intake equals energy expenditure over time (Spiegelman and Flier, 2001). Autonomic and behavioral adaptations to prevent excessive body weight gain in mammals fed on a hypercaloric diet are i) increased energy expenditure (a phenomenon referred to as diet-induced thermogenesis) and ii) reduced food intake (Dhillon et al., 2006; Lowell and Spiegelman, 2000), respectively. To investigate the mechanisms underlying hypersensitivity to diet-induced obesity in Pomc-Cre; Sirt1loxP/loxP mice, we measured acute and chronic metabolic adjustments to the HC diet. Mutant mice increased energy expenditure and decreased food intake during the four days that immediately followed the switch from SC to HC diet similarly to their controls (Figures S3A and S3B). However, after 4 weeks on the HC diet, O2 consumption, CO2 and heat production were reduced in Pomc-Cre; Sirt1loxP/loxP mice (Figure 5A). Importantly, at the time these metabolic parameters were assessed, body weight and composition as well as leptinemia were still normal in mutant mice (Figure S3C); thus suggesting that reduced energy expenditure is causing the increased body adiposity rather than be secondary to it. Fuel type utilization (carbohydrates vs. lipids) was not affected by SIRT1 deletion in POMC neurons as the average respiratory quotient did not differ between genotypes (Figure 5A). Of note, the reduced metabolic rate was not the result of hypoactivity because ambulatory movements were unaltered in Pomc-Cre; Sirt1loxP/loxP mice (Figure 5B). Despite established anorectic roles for POMC neurons (Cone, 2005), food intake before and during the dynamic phase of excessive body weight gain was normal in mutant mice (Figures 5C, S3D). Collectively, our data demonstrate that SIRT1 in POMC neurons is required for normal energy expenditure and body weight homeostasis after chronic hypercaloric feeding.
Figure 5. Normophagia but reduced energy expenditure following chronic hypercaloric feeding in females lacking SIRT1 in POMC neurons. (A).
O2 consumption, CO2 and heat production, respiratory quotient (RQ), (B) ambulatory movements, and (C) food intake were measured before body weight diverged in 12-week-old Sirt1loxP/loxP and Pomc-Cre, Sirt1loxP/loxP females fed on a high-calorie diet for 4 weeks (n=12 in each group). Data were collected using the Columbus Instruments Comprehensive Lab Animal Monitoring System (CLAMS). Time 0 represents the beginning of the dark cycle in a 12-hour dark/light cycle environment. Black and white boxes represent dark and light cycles, respectively. Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student’s t test. *P<0.05.
Impaired BAT-like remodeling of perigonadal WAT in Pomc-Cre; Sirt1loxP/loxP mice
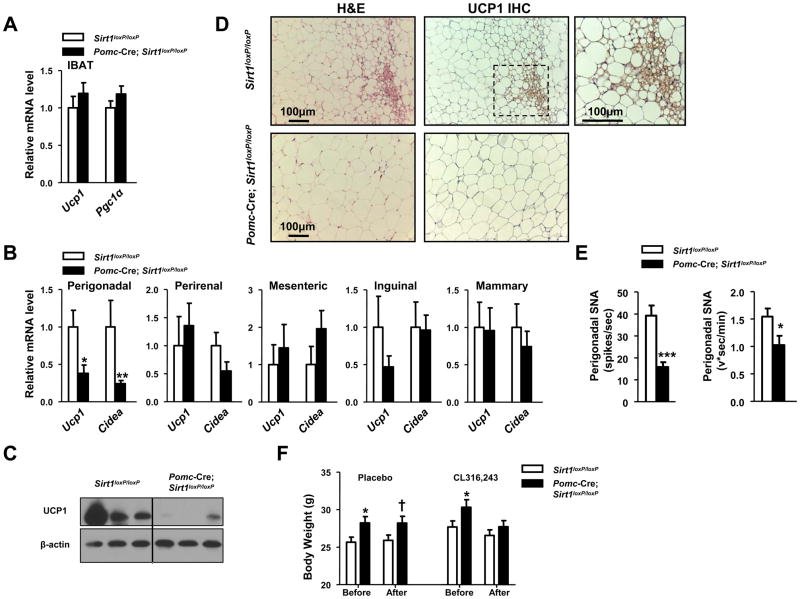
To identify the defective tissue/s underlying the impaired energy expenditure, we focused on brown adipose tissue (BAT) because of its established roles in mediating diet-induced thermogenesis (Lowell and Spiegelman, 2000). Through stimulation of the sympathetic nervous system activity (SNA), HC diet enhances dissipation of chemical energy in the form of heat in brown adipocytes. This physiological adjustment is achieved, at least in part, through activation of PGC1α-dependent pathways that lead to mitochondriogenesis and increased expression of BAT-selective genes (e.g.: uncoupling protein 1 (UCP1)) (Seale et al., 2009). To gather insights into BAT functions, we assessed Ucp1 and Pgc1α mRNA contents and the weight of interscapular BAT but found these parameters to be normal in Pomc-Cre; Sirt1loxP/loxP HC-fed mice (Figure 6A and data not shown). Next, we focused on characterizing brown adipocytes’ function in other tissues in which these cells are known to exert important anti-obesity actions as well (Kopecky et al., 1995; Plum et al., 2007; Seale et al., 2009). We first assessed UCP1 expression in several visceral (perigonadal, perirenal, and mesenteric) and subcutaneous (inguinal and mammary) WAT depots and in skeletal muscle. Ucp1 mRNA and protein contents were significantly and selectively reduced in the perigonadal fat depot of Pomc-Cre; Sirt1loxP/loxP females (Figures 6B, 6C and data not shown). Similarly, mRNA contents of the BAT-specific gene Cidea were also selectively reduced in this fat depot (Figure 6B). Noteworthy, impaired expression of BAT-specific genes as well as of key genes for mitochondrial biogenesis and function preceded changes in body adiposity (Figure S4A), suggesting that these defects contribute to the development of energy imbalance rather than be secondary to it. To further investigate these visceral BAT abnormalities, we performed histomorphological analyses. Several multilocular/UCP1-expressing brown adipocytes were present in perigonadal WAT of Sirt1loxP/loxP HC-fed females (Figure 6D). In contrast, significantly fewer brown adipocytes were found embedded in this visceral fat depot of Pomc-Cre; Sirt1loxP/loxP HC-fed mice (Figure 6D). Altogether, these data indicate that POMC neurons selectively regulate BAT-like remodeling of perigonadal WAT.
Figure 6. Altered BAT and sympathetic nerve activity (SNA) in perigonadal fat of females lacking SIRT1 in POMC neurons.
(A) Ucp1 and Pgc1α mRNA levels in interscapular brown adipose tissue (IBAT) of 28-week-old Sirt1loxP/loxP and Pomc-Cre; Sirt1loxP/loxP females fed on a high calorie (HC) diet for 20 weeks. (B) Ucp1 and Cidea mRNA levels in visceral (perigonadal, perirenal, mesenteric) and subcutaneous (inguinal and mammary) white adipose tissue (WAT) of same mice as in A (n=7–11). Individual mRNA levels were normalized to β-actin mRNA contents. (C) UCP1 and β-actin (used as loading control) protein levels were assessed in the perigonadal fat of same mice as in in A (UCP1/β-actin content is reduced in mutants; P=0.021; n=3–4) by western blot. (D) Representative photomicrographs of paraffin-embedded perigonadal WAT sections stained with hematoxylin and eosin (H&E) or treated for UCP1 immunohistochemistry (IHC). Tissues were collected from same mice as in A. Dark-brown staining represents UCP1-expressing brown adipocytes. Higher magnification of the boxed-region is in the top-right corner. Scale bar = 100 μm. (E) Quantification of SNA in perigonadal WAT of 12-week-old Sirt1loxP/loxP and Pomc-Cre; Sirt1loxP/loxP females fed on a HC diet for 4 weeks (n=8). (F) Body weights before and after one week of treatment with either placebo or the selective β3-adrenergic receptor agonist CL316,243 (n=15–16). Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student’s t test. † P=0.05; *P<0.05; **P<0.01; **P<0.001.
Reduced SNA in perigonadal WAT of Pomc-Cre; Sirt1loxP/loxP mice
HC-induced changes in hormonal levels lead to increased energy expenditure in part via enhanced SNA in diverse peripheral tissues (Plum et al., 2007; Rahmouni et al., 2004). To explore whether the hypersensitivity to diet-induced obesity was due to altered sympathetic nervous system function, we directly assessed the electrophysiological properties of nerves subserving different fat depots (of note, only sympathetic nerves are found within fat tissues (Bartness and Song, 2007)). To rule out that eventual difference in SNA may have been secondary to dissimilarities in body energy statuses, these measurements were performed before body weights and adiposity differed between mutants and controls. As shown in Figures 6E and S4B, these assays indicated a selective impairment in SNA in perigonadal WAT as nerve activity was normal in interscapular BAT and inguinal WAT but greatly reduced in perigonadal fat of mutant mice. In agreement with the electrophysiological data, the amounts of UCP1 and tyrosine hydroxylase (TH; an enzyme required for synthesis of catecholamines), that are known to positively correlate with SNA in fat tissues, were also normal in interscapular BAT but reduced in perigonadal WAT of mutant mice (Figures 6C and S4C). Collectively, these results pinpoint to a selective reduction in SNA in perigonadal WAT of Pomc-Cre; Sirt1loxP/loxP HC-fed mice.
To test if reversal of the defect in BAT contents in perigonadal WAT would rescue body energy imbalance, we treated mice with the selective β3-adrenergic receptor agonist CL316,243 that is known to potently stimulate accumulation of BAT in perigonadal WAT (Seale et al., 2008). As shown in Figure 6F, before CL316,243 or placebo treatment began, Pomc-Cre; Sirt1loxP/loxP HC-fed mice were heavier than controls. Remarkably, treatment with β3-adrenoreceptor agonist completely rescued this body weight phenotype in a food intake-independent fashion (Figure 6F and data not shown); thus suggesting an underlying energy expenditure component. In agreement with this hypothesis, CL316,243 treatment increased BAT-specific gene expression and the abundance of multilocular/UCP1-expressing brown adipocytes in perigonadal WAT to levels that were indistinguishable between genotypes (Figure S4D). These results suggest that defects upstream of β3-adrenergic receptor signaling (e.g.: reduced SNA) underlie the impaired perigonadal WAT to BAT remodeling and body weight imbalance in Pomc-Cre; Sirt1loxP/loxP HC-fed mice.
Altered biological actions of leptin in Pomc-Cre; Sirt1loxP/loxP mice
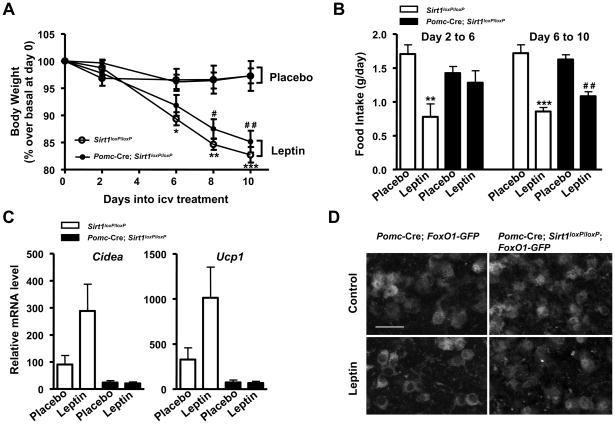
Leptin exerts pleiotropic actions including the concurrent inhibition and activation of diverse hypothalamic neurons that leads to reduced food intake and increased energy expenditure in part via enhanced SNA and BAT in perigonadal WAT (Hill et al., 2008; Plum et al., 2007). Thus, to determine whether SIRT1 in POMC neurons is required for leptin’s biological actions, mutants and controls were challenged with intracerebroventricular (icv) administration of leptin. As shown in Figures 7A and 7B, icv leptin treatment decreased body weight in mutants and controls to similar extents; however leptin’s acute anorectic effects (i.e.: from day 2 to 6 after treatment began) were impaired in mutant mice. A blunted acute anorectic response to exogenously-administered leptin in animals that are otherwise normophagic (Figures 5C, 7B and S3D) strongly resembles the metabolic outcomes of impaired PI3K signaling in POMC neurons (Hill et al., 2008). Next, we assessed the ability of central leptin administration to enhance BAT in perigonadal WAT; an effect also known to be mediated by hypothalamic PI3K signaling (Plum et al., 2007). Leptin treatment almost tripled the amount of Ucp1 and Cidea mRNA in perigonadal WAT of control mice (P values = 0.05, leptin vs. placebo); an effect that was virtually absent in Pomc-Cre; Sirt1loxP/loxP mice (Figure 7C). Histomorphological analyses confirmed the impaired perigonadal WAT to BAT remodeling induced by central leptin administration in mutants (Figure S5).
Figure 7. SIRT1 in POMC neurons is required for normal biological actions of leptin.
(A) Relative body weights of high-calorie (HC)-fed Sirt1loxP/loxP and Pomc-Cre, Sirt1loxP/loxP mice during the 10 days of intracerebroventricular delivery of either placebo or leptin and (B) food intake from the second to the sixth and from the sixth to the tenth day into the treatment. (C) Ucp1 and Cidea mRNA levels in perigonadal WAT at the end of the treatment (n=8–10). Individual mRNA levels were normalized to β-actin mRNA contents. (D) Representative photomicrographs of brain slices from Pomc-Cre; FoxO1-GFP (control) or Pomc-Cre; Sirt1loxP/loxP; FoxO1-GFP mice stained for GFP (the Cre-conditional expression of FoxO1-GFP is restricted only to POMC neurons in these mice). Scale bar = 20 μm. Error bars represent s.e.m. Statistical analyses were done using one-way ANOVA (Tukey’s post test). *P<0.05; **P<0.01; **P<0.001 Sirt1loxP/loxP leptin vs. Sirt1loxP/loxP placebo. #P<0.05; # #P<0.01; Pomc-Cre, Sirt1loxP/loxP leptin vs. Pomc-Cre, Sirt1loxP/loxP placebo.
As mentioned above, the plasticity of this visceral WAT is under the control of leptin-induced PI3K signaling in hypothalamic neurons (Plum et al., 2007). Thus, we investigated if lack of SIRT1 diminishes the ability of leptin to properly engage the PI3K pathway in POMC neurons. To directly test this hypothesis, we introduced the Cre-conditional FoxO1-GFP allele (Fukuda et al., 2008) in either Pomc-Cre or Pomc-Cre; Sirt1loxP/lox mice. By exploiting the robustness of FoxO1 nuclear exclusion upon PI3K activation, the nucleocytoplasmic shuttling of FoxO1-GFP has been successfully used for monitoring PI3K signaling in hypothalamic neurons (Fukuda et al., 2008). In basal conditions, FoxO1-GFP was localized in nucleus and cytoplasm of control POMC neurons whereas it was significantly more abundantly localized in the nucleus of POMC neurons lacking SIRT1 (Figure 7D). Also, leptin-induced nuclear exclusion of FoxO1-GFP was severely blunted in mutant POMC neurons (Figure 7D) demonstrating that SIRT1 is required for normal leptin-induced activation of PI3K signaling in these neurons. Altogether, these in vivo and in vitro data demonstrate that SIRT1 in POMC neurons is necessary for normal leptin’s physiological (i.e.: reduced food intake and increased BAT in perigonadal WAT) and molecular (i.e.: activation of PI3K signaling) actions; thus, lack of SIRT1 in POMC neurons causes leptin resistance.
Discussion
SIRT1 is a protein deacetylase whose targets include histones, transcription factors, and cofactors (Imai et al., 2000; Michan and Sinclair, 2007). The long-term effects of CNS SIRT1 on body weight homeostasis are however controversial as reduced food intake has been observed following knockdown of hypothalamic SIRT1 (Cakir et al., 2009) but also in mice with enhanced SIRT1 activity (Banks et al., 2008). Our findings establish SIRT1 in the brain, and more specifically in POMC neurons, as an important molecular component of long-term control of body weight homeostasis. Indeed, we found that SIRT1 in POMC neurons is required for normal energy expenditure adaptations and hence body weight homeostasis following HC diet feeding. Interestingly, our results indicate that the outcomes of SIRT1 deletion in POMC neurons on body weight balance are more pronounced in females than in males (Figure 4). To better understand the underlying causes of this effect, we first assessed if SIRT1 deletion in POMC neurons alters TH and BAT-like contents in perigonadal WAT in males as it does in females. Interestingly, TH and UCP1 protein levels in perigonadal WAT of mutant males were also reduced compared to controls (Figure S4E) suggesting that SIRT1 in POMC neurons is required for normal diet-induced remodeling of this visceral fat depot in both genders. The sexual dimorphic body weight phenotype may then be due to the fact that the contribution of BAT in perigonadal WAT on body energy balance differs between genders. This hypothesis seems to be plausible as males have far fewer brown-like adipocytes and ~10 fold less BAT-specific gene expression in this tissue compared to age- and diet-matched females (Figure S4E and data not shown). Thus, we speculate that the reduced BAT in perigonadal WAT is a defect sufficient to cause overt body energy imbalance in females but not in males.
The normophagia in mutant mice is surprising considering the fact that POMC neurons govern both arms of the energy balance (Cone, 2005). The anorectic effects of POMC neurons are mediated, at least in part, by α-MSH and its actions on downstream MC4R-expressing neurons in paraventricular hypothalamic nucleus (Balthasar et al., 2005; Coll et al., 2004; Fan et al., 1997; Plum et al., 2009). But, as shown in Figures 2 and 3, POMC projections to this brain area as well as hypothalamic contents of α-MSH and Pomc mRNA are normal in mutant mice. Furthermore, phenotypes found in mammals bearing Pomc or Mc4r null mutations, as for example increased body length, hyperinsulinemia, glucose intolerance and abnormal lipid metabolism (Farooqi et al., 2006; Huszar et al., 1997; Nogueiras et al., 2007), were not seen in Pomc-Cre; Sirt1loxP/loxP mice (Table S1 and data not shown); suggesting that SIRT1 deletion in POMC neurons does not lead to overt dysfunctions of the CNS melanocortin system. Thus, the reduced energy expenditure without concurrent changes in food intake in mutant mice pinpoints to a divergence in CNS SIRT1-dependent mechanisms of body weight control. Our aforementioned results however may seem to be in conflict with a previous study reporting reduced food intake and body weight in rats in which ARH SIRT1 expression was reduced by small interfering RNAs delivery (Cakir et al., 2009). These discrepancies may be explained by the following not mutually exclusive possibilities: i) because our experiments were done in mice whereas Cakir and colleagues employed rats, it may be possible that the roles of hypothalamic SIRT1 on energy balance are different in these two species, and/or ii) the acute (Cakir et al., 2009) and chronic (as reported here) loss of SIRT1 in POMC neurons have opposite effects on body weight, and/or iii) because Cakir and colleagues undertook an approach that was not POMC-neuron-specific, it may be possible that the acute anorectic consequences of diminished ARH SIRT1 they reported were mediated by non-POMC cells (e.g.: AgRP neurons). Future studies employing animal models bearing AgRP-neuron-specific SIRT1 loss- or gain-of-function mutations will be required to directly test this latter hypothesis.
Leptin-induced activation of PI3K signaling in hypothalamic neurons has been previously reported to selectively enhance SNA and BAT in perigonadal and pararenal WAT (Plum et al., 2007). Our study shows a selective and physiological relevant connection from POMC neurons to perigonadal WAT. Also, we identified SIRT1 as a critical molecular component of leptin-sensing mechanisms in CNS neurons. Indeed, our in vitro and in vivo experiments shown in Figure 7 indicate that lack of SIRT1 in POMC neurons causes impaired responsiveness to leptin: at the molecular level, leptin-induced activation of the PI3K-FoxO1 signaling cascade is impaired in POMC neurons lacking SIRT1; at the organismal level, the ability of icv leptin administration to acutely reduce food intake and enhance BAT in perigonadal WAT is blunted in mutant mice. Although it will require additional experiments to totally understand the modalities by which SIRT1 regulates the PI3K signaling in central neurons, one candidate pathway may involve PTP1B-dependent mechanisms as SIRT1 suppresses PTP1B that is a negative regulator of the PI3K signaling cascade (Sun et al., 2007). Another possibility is that SIRT1 directly deacetylates molecular components of the leptin-receptor-PI3K-FoxO1 signaling cascade as suggested by the fact that FoxO1 itself is deacetylated (i.e.: activated) by SIRT1. Because it has been previously postulated that FoxO1 regulates Pomc gene expression and POMC-derived peptides levels (Kitamura et al., 2006; Plum et al., 2009), the increased FoxO1 nuclear retention in POMC neurons with concurrent unaltered hypothalamic Pomc gene expression and POMC-derived peptides levels in mutant mice (Figures 3 and 7D) may seem to be at odds with those previous results. However, one explanation for this conundrum may be that lack of SIRT1 leads to FoxO1 hyperacetylation and hence inhibition. Future studies will be required to determine the intracellular consequences of SIRT1 deletion in POMC neurons on the acetylation statuses of FoxO1 and other SIRT1’s targets.
As mentioned above, another interesting aspect unveiled by our study is the very selective and physiological relevant connection between POMC neurons and perigonadal WAT. The rationale for this network may rest on the fact that this circuitry is in place to allow the mammalian body to finely tune its energy expenditure following changes in food availability. This idea is in line with other CNS-mediated selective regulations of homeostatic pathways. For example, baroreflex activation triggers SNA subserving cardiovascular system but not interscapular BAT (Rahmouni and Haynes, 2004). Also, neurons in the hypothalamic median preoptic area selectively increase SNA in interscapular BAT to elevate body temperature after immune challenges (Lazarus et al., 2007). Moreover, neurons within the ventromedial hypothalamic nucleus convey hormonal signals into enhanced SNA in skeletal muscle to increase glucose uptake at times it is most needed (e.g.: during wakefulness) (Minokoshi et al., 1999; Toda et al., 2009). Furthermore, leptin-receptor-expressing hypothalamic neurons have been reported to regulate SNA in a fat-selective fashion (Plum et al., 2007). Collectively, our and previously reported results strongly indicate that specific branches of the sympathetic nervous system are activated by specific stimuli to allow only selective responses (i.e.: the ones required to cope with that particular stimulus) to be triggered.
Along these lines, enhanced BAT function has been suggested as an alternative anti-obesity strategy (Kajimura et al., 2009; Seale et al., 2009). This concept has been recently galvanized due to the fact that BAT, a tissue previously thought to be present only in infants, is functionally active in adult humans as well (Cypess et al., 2009). Yet, available means to activate/expand BAT in humans are limited as they may include chronic administrations of adrenergic receptors’ agonists that, unfortunately, have well-characterized deleterious actions. Here, we report that SIRT1 in POMC neurons selectively coordinate BAT in perigonadal WAT via the sympathetic nervous system. There are several neuronal networks by which POMC neurons can regulate SNA in this fat depot: one may involve a direct control of sympathetic preganglionic neurons in the spinal cord as POMC neurons project to these sites (Elias et al., 1998); another possibility is via POMC-neuron-mediated control of neurons within paraventricular hypothalamic nucleus (a well-known site for autonomic regulation). Regardless to the circuitry, the specificity of POMC-neurons-to-perigonadal WAT control indicate that this network may represent a novel route to finely tune SNA and BAT function in a tissue-selective fashion, and hence avoiding deleterious effects of systemic and tissue-unspecific adrenergic receptors stimulation. Human clinical trials to test the anti-diabetic efficacy of putative SIRT1 activators are currently underway (Elliott and Jirousek, 2008). We suggest that pharmacological manipulations of SIRT1 in CNS neurons should also be contemplated for the treatment of diet-induced obesity.
Experimental Procedures
Generation of Pomc-Cre; Sirt1loxP/loxP mice
Mice were housed in groups of 4–5 with food (either a standard chow rodent diet or the high-calorie (HC) diet D12331 from Research Diets, New Brunswick, NJ, USA) and water available ad libitum in light- and temperature-controlled environments unless otherwise specified. Care of mice was within the Institutional Animal Care and Use Committee (IACUC) guidelines, and all the procedures were approved by the University of Texas Southwestern Medical Center IACUC. Pomc-Cre mice (Balthasar et al., 2004) were mated with mice carrying a loxP-flanked Sirt1 allele (Sirt1loxP) (Cheng et al., 2003). Breeding colonies were maintained by mating Pomc-Cre; Sirt1loxP/loxP and Sirt1loxP/loxP mice. Only animals on same mixed background strain generation were compared to each other. Tail DNA was collected from each animal to determine the presence of the Pomc-Cre transgene, the loxP-flanked, and/or the Cre-deleted Sirt1 allele by PCR analyses as previously described (Balthasar et al., 2004; Cheng et al., 2003). The Pomc-Cre transgene is sporadically turned on during gametogenesis, before the first meiotic division (Balthasar et al., 2004). Thus, all mice positive for the Cre-deleted Sirt1 allele in tail DNA samples were excluded from our studies. Tissues PCR genotyping analyses for determining the presence of the loxP-flanked and/or the Cre-deleted Sirt1 allele were done as previously described (Cheng et al., 2003). Sterile saline solution (placebo) or the selective β3-adrenergic receptor agonist CL316,243 at 1 mg kg−1, was injected intraperitoneally (IP) into age-matched, 7–8 months old, HC-fed females daily for 7 days.
Highlights
SIRT1 in POMC neurons is required for normal energy expenditure adaptations against diet-induced obesity
SIRT1 in POMC neurons is required for normal leptin’s molecular (e.g.: activation of PI3K signaling) and physiological (e.g.: suppression of food intake and stimulation of BAT-like remodeling of perigonadal WAT) actions
POMC neurons selectively govern sympathoactivation in perigonadal WAT
Supplementary Material
Acknowledgments
We thank Wenhao Li and Kristen Wertz for technical assistance, Dr. Joyce Repa (UTSW Medical Center, USA) for quantitative real time PCR primers, Dr. Yoshiyuki Horio (Sapporo Medical School, Japan) for the SIRT1 antirsera, Dr. Frederick Alt (Harvard Medical School, USA) for the Cre-conditional Sirt1 null mice, Dr. Aktar Ali and Laura Brule for metabolic assessments at the Mouse Metabolic Phenotyping Core at UTSW Medical Center (supported by PL1 DK081182-01 and 1UL1RR024923-01), and Drs. Joel K. Elmquist (UTSW Medical Center, USA) and Bradford B. Lowell (Harvard Medical School, USA) for helpful discussions and careful reading of the manuscript. This work was supported by the Department of Internal Medicine/Division of Hypothalamic Research, UTSW Medical Center (start-up to R.C.), the American Heart Association (Scientist Development Grant to R.C. and Postdoctoral Fellowship to G.R.), and by National Institute of Health Grants (DK080836 to R.C. and DK58148 to E.A.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Song CK. Brain-adipose tissue neural crosstalk. Physiology & behavior. 2007;91:343–351. doi: 10.1016/j.physbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 Deficiency in POMC-Expressing Cells Reveals FOXO1-Dependent and -Independent Pathways in Control of Energy Homeostasis and Stress Response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Schafer MK, Watson SJ, Akil H. Evidence that beta-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res. 1992;587:269–275. doi: 10.1016/0006-8993(92)91007-2. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nature neuroscience. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS ONE. 2009;4:e8322. doi: 10.1371/journal.pone.0008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. The Journal of clinical investigation. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, Challis BG, Yeo GS, O’Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. The Journal of clinical endocrinology and metabolism. 2004;89:2557–2562. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nature neuroscience. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent progress in hormone research. 1996;51:287–317. discussion 318. [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science (New York, NY. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9:371–378. [PubMed] [Google Scholar]
- Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ, Iwasaki T, Stark R, Weismann D, Yu XX, et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11288–11293. doi: 10.1073/pnas.0812931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Drop S, Clements A, Keogh JM, Biernacka J, Lowenbein S, Challis BG, O’Rahilly S. Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes. 2006;55:2549–2553. doi: 10.2337/db06-0214. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O’Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. The Journal of clinical investigation. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Jones JE, Olson D, Hill J, Lee CE, Gautron L, Choi M, Zigman JM, Lowell BB, Elmquist JK. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci. 2008;28:13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. The EMBO journal. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. The Journal of clinical investigation. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. The EMBO journal. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nature medicine. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. The Journal of clinical investigation. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nature neuroscience. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Lee M, Kim A, Chua SC, Jr, Obici S, Wardlaw SL. Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab. 2007;293:E121–131. doi: 10.1152/ajpendo.00555.2006. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Molecular cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. The Biochemical journal. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, et al. The central melanocortin system directly controls peripheral lipid metabolism. The Journal of clinical investigation. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly S, Farooqi IS, Yeo GS, Challis BG. Minireview: human obesity-lessons from monogenic disorders. Endocrinology. 2003;144:3757–3764. doi: 10.1210/en.2003-0373. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science (New York, NY. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nature medicine. 2009 doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. The Journal of clinical investigation. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. The Journal of biological chemistry. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG. Leptin and the cardiovascular system. Recent progress in hormone research. 2004;59:225–244. doi: 10.1210/rp.59.1.225. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. The Journal of clinical investigation. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes & development. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes. 2009;58:2757–2765. doi: 10.2337/db09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature neuroscience. 2008 doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse BE, Kim F, Schwartz MW. Physiology. An integrative view of obesity. Science (New York, NY) 2007;318:928–929. doi: 10.1126/science.1148032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.