Abstract
Natural hosts for simian immunodeficiency virus (SIV)can be, and are often naturally, infected with species-specific SIVs, but do not develop acquired immunodeficiency syndrome (AIDS). These natural hosts maintain high SIV viral loads, but avoid immunodeficiency. Elucidating the mechanisms that allow natural hosts to co-exist with SIV without overt disease may provide crucial information for understanding AIDS pathogenesis. Over the past few years, several key features of natural SIV infections have been described in studies conducted predominantly in sooty mangabeys (SMs), African green monkeys (AGMs), and mandrills. Natural SIV hosts are able to avoid the chronic, generalized immune system activation that is associated with disease progression in HIV-infected individuals and are known to down-modulate the expression of the receptors for SIV. In this perspective we propose that a critical factor that differentiates nonprogressive from progressive HIV or SIV infection is the maintenance of T cell immune competence in the face of a virus that infects and kills CD4+ T cells Elucidation of the mechanisms underlying the preservation of immune function during and after the acute phase of natural SIV infection may lead to the design of novel preventive and therapeutic interventions for treatment of chronic HIV infection.
The lentiviruses that cause immunodeficiency in humans and Asian macaques originated from cross-species transmission of viruses that naturally infect nonhuman primates in Africa (Hahn et al., 2000). These simian immunodeficiency viruses (SIV) belong to the group of lentiviruses that infect a wide range of non-human primate (NHP) species. Both SIV infection of Asian macaques and HIV-1 infection of humans result in a chronic infection, and the majority of individuals progress to acquired immunodeficiency syndrome (AIDS). In contrast, SIV-infected natural hosts generally do not progress to AIDS, with very limited reports of AIDS in natural hosts after almost two decades of SIV infection (Ling et al., 2004). It would seem likely that natural hosts of SIV have co-evolved with the virus to avoid disease progression, dissecting the mechanisms underlying the nonprogressive nature of natural SIV infection could lead to a better understanding of the aspects of HIV infection responsible for the progressive nature of the disease in humans (Hirsch, 2004; Pandrea et al., 2008b; Silvestri et al., 2007). Although there have been major strides in the unraveling of virological and immunological mechanisms underlying the nonpathogenic nature of SIV infections, a complete understanding is lacking. We propose a number of testable hypotheses that might account for the nonpathogenic nature of natural SIV infections.
Many studies have added to our understanding of key similarities and differences between natural non-progressive and progressive HIV and SIV infections. These studies have highlighted potential explanations for the lack of disease progression in SIV-infected natural hosts. Importantly, it is clear that immunological control, in terms of a virus-specific T cell and B cell response, does not account for the lack of disease progression. Indeed, SIV-infected natural hosts maintain high plasma viral loads (based on amounts of viral RNA) (Goldstein et al., 2006; Goldstein et al., 2000; Pandrea et al., 2006a; Pandrea et al., 2006b; Silvestri et al., 2003) and they do not exhibit superior cellular control of viremia compared to HIV-infected humans or SIV-infected rhesus macaques (RM)(Dunham et al., 2006).
The lentiviruses that infect natural hosts are themselves clearly pathogenic in certain contexts; indeed, SIV infection of African green monkeys (AGM) and Sooty mangabeys (SM) has been associated with more rapid death of infected cells in vivo (Gordon et al., 2008; Klatt et al., 2008; Pandrea et al., 2008a). Moreover, SIVagm, which naturally infects AGM, can be used to infect pigtail macaques who subsequently develop simian AIDS (Goldstein et al., 2005; Hirsch et al., 1995) and isolates of SIVsmm, which naturally infects SM, also cause progressive infection in RM (Fultz et al., 1989; Hirsch et al., 1997; Li et al., 1992; Watson et al., 1997). Therefore, the lack of disease progression in natural hosts is unlikely to be attributable to infection with nonpathogenic viruses. However, analysis of certain viral accessory proteins, such as nef, has led to the suggestion that differential down-regulation of certain key surface proteins on infected cells or differential recruitment of immune activation adapter proteins or binding to host restriction factors by SIV-specific nef variants could influence disease progression (Arhel and Kirchhoff, 2009).
A fundamental difference between progressive SIV or HIV infection and non-progressive SIV infection is the absence of immune activation during the chronic phase of infection in natural hosts (Chakrabarti et al., 2000; Pandrea et al., 2006a; Pandrea et al., 2003; Pandrea et al., 2007b; Silvestri et al., 2003; Sumpter et al., 2007). The importance of this observation rests on the findings that chronic systemic immune activation is associated with disease progression in HIV-infected individuals and is a better predictor of outcome than plasma viral load (Deeks et al., 2004; Giorgi et al., 1999; Rodriguez et al., 2006). The lack of immune activation during the chronic phase of natural SIV infection is therefore thought to be critical for the non-progressive nature of the infection (Silvestri, 2005; Silvestri et al., 2007) but might be somewhat surprising given that natural hosts mount a robust immune response, including production of type I interferons and increased frequencies of activated T cells, during the acute phase of SIV infection (Bosinger et al., 2009; Jacquelin et al., 2009). Moreover, in vitro production of type one interferons by SIVagm-stimulated AGM plasmacytoid dendritic cells are enhanced compared to SIVagm stimulation of RM plasmacytoid dendritic cells, while such innate immune responses to SIVmac are greater in RM compared to AGM (Jacquelin et al., 2009). However, in contrast to SIV or HIV infection of RM and humans, respectively, this immune activation resolves towards the end of the acute phase of infection in vivo in the natural hosts, and may be in part due to differential up-regulation of the membrane receptor programmed death 1 in lymph nodes in of acutely-infected natural hosts (Estes et al., 2008) and TNF related apoptosis-inducing ligand after in vitro infections (Kim et al., 2007).
Several studies have tried to elucidate the mechanisms underlying the differential immune activation in progressive and natural HIV and SIV infections. It has recently been shown that one cause of immune activation during the chronic phase of progressive infection is microbial translocation (Baroncelli et al., 2009; Brenchley et al., 2006b; Ciccone et al.; Funderburg et al.), which refers to the translocation of microbial products from the lumen of the intestinal tract into the systemic circulation. Microbial products can directly stimulate the immune system via interactions with pattern recognition receptors such as Toll-like receptors. Indeed, elevated amounts of soluble CD14, a protein produced by LPS-stimulated macrophages and monocytes, in the plasma of chronically HIV-infected individuals suggest that macrophages and monocytes are directly stimulated by LPS in vivo (Brenchley et al., 2006b). Moreover, elevated amounts of translocated microbial products are associated with immune activation of both the adaptive and innate arms of the immune system during the chronic phase of progressive HIV or SIV infection (Brenchley et al., 2006b). In stark contrast, microbial products are present at only very low amounts in chronically SIV-infected AGM (Pandrea et al., 2007b) and SM (Brenchley et al., 2006b) even in the presence of high viral loads. Thus, the lack of microbial translocation may in part account for the lack of systemic immune activation.
The mechanisms underlying microbial translocation during the chronic phase of progressive HIV or SIV infection and the lack thereof in non-progressive infection remain unclear but several plausible explanations exist. Massive damage to many components of the mucosal barrier of the GI tract, both immunologic and structural, occurs in the acute phase of the infection and persists through to the chronic phase in progressive HIV and SIV infection. Enteropathy with increased intestinal permeability has been routinely observed in chronically HIV-infected individuals (Bjarnason et al., 1996; Kapembwa et al., 1991; Kotler et al., 1984) and considerable enterocyte apoptosis is observed during the acute phase of SIV infection in RM (Li et al., 2008). In addition there is massive infection and loss of CD4+ T cells, particularly from the lamina propria, in acute infection of RM and humans(Brenchley et al., 2004; Guadalupe et al., 2003; Mehandru et al., 2004; Veazey et al., 1998). Thus, it has been proposed that the structural and immunologic damage to the gut in progressive HIV and SIV infection allows for microbial translocation to increase and contribute to persistent immune activation even though the high viral loads of the acute phase wane as the infected individual enters the chronic phase of the infection (Brenchley et al., 2006a; Pandrea et al., 2008b). Indeed, the consequences of such microbial translocation might offset any normal physiological attempts at immunological resolution of the acute immune activation that is likely driven in large part by high viral loads.
One might expect, therefore, that SIV-associated damage to the GI tract is minimal in natural hosts compared to hosts with progressive disease. Yet, recent studies actually demonstrated a substantial reduction in the frequency of CD4+ T cells at the mucosal surfaces of acutely SIV-infected SM (Gordon et al., 2007) and AGM (Pandrea et al., 2007b). However, more detailed analyses have revealed both quantitative and qualitative differences in CD4+ T cell depletion between natural and non-natural hosts that highlight the importance of target cell availability and depletion in determining disease progression. As mentioned above, several studies have shown that natural hosts differentially regulate the cell surface receptors for SIV. CD4+ T cells in all species of natural hosts that have been studied thus far have been shown to express very low amounts of CCR5 whereas CD8+ T cells appear to express CCR5 amounts comparable to those in humans and RM (Pandrea et al., 2007a). Whereas such low expression of CCR5 by CD4+ T cells has been suggested as a mechanism underlying the lack of vertical transmission in natural hosts (Pandrea et al., 2007a), one might also expect that low CCR5 expression would translate to reduced target cell availability, particularly in the GI tract. However, given the lack of disease progression in natural hosts the biological relevance of the lack of vertical transmission remains unclear and requires further studies of infection in infant natural hosts. Importantly, in addition to low amounts of CCR5, AGM down-regulate CD4 expression as naïve CD4+ T cells are activated to enter the memory T cell pool (Beaumier et al., 2009). The functional properties usually associated with memory CD4+ T cells are assumed by these CD4-negative T cells, thus ensuring the immunocompetence of AGM. The consequence of CD4 down-regulation in these natural hosts would be a reduction in the availability of target cells for SIV infection. Indeed, uninfected healthy adult AGM have few memory CD4+ T cells circulating or in the tissues (Beaumier et al., 2009; Pandrea et al., 2007b) and SM have less overall CD4+ T cell depletion upon SIV infection compared to either HIV-infected humans (Brenchley et al., 2004) or SIV-infected RM (Brenchley and Douek, 2008; Picker et al., 2004; Veazey et al., 1998). Thus, whereas depletion of CCR5+ CD4+ T cells in SIV-infected natural hosts may be important in terms of frequency within that subset (whatever CCR5+ CD4+ T cells targets exist are rapidly lost), their depletion in terms of absolute numbers, or frequency within the total T cell pool, may actually be so minimal that it is of no physiological or pathogenic consequence (Figure 1). However, natural or experimental SIVsmm-infection of sooty mangabeys with a rare CCR5-CXCR4-CCR8-tropic SIV results in severe depletion of mucosal and peripheral CD4+ T cells with no apparent susceptibility to opportunistic infections (Milush et al., 2007). The mechanisms by which these animals preserve immunological function and avoid disease progression remain unclear.
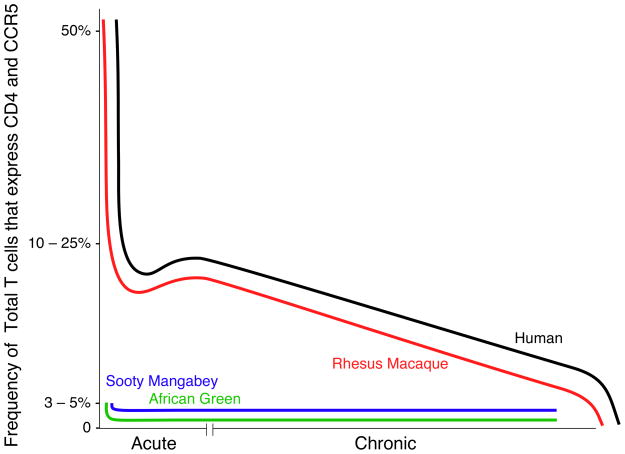
Figure 1. Frequencies of CCR5+CD4+ T cells in progressive and nonprogressive infections.
Depletion of GI tract CCR5+ CD4+ T cells during the acute and chronic phases of HIV in humans (black line) and SIV in rhesus macaques (red line), sooty mangabeys (blue line), and African green monkeys (green line). Graphs are based upon published data. Neither African green monkeys nor sooty mangabeys (naturally infected animals that do not progress to AIDS) are as dramatically depleted of their initial GI tract CD4+ T cell populations during the acute phase of infection as SIV-infected rhesus macaques or HIV-infected humans. African green monkeys and sooty mangabeys have very low frequencies of CCR5+CD4+ T cells prior to infection, unlike humans and rhesus macaques. Moreover, progressive depletion of GI tract CCR5+CD4+ T cells does not seem to occur in SIV-infected sooty mangabeys and African green monkeys during the chronic phase of infection.
In addition to these quantitative differences, there may be qualitative differences in CD4+ T cell depletion that may contribute to the lack of disease progression in natural hosts. Indeed, recent studies have shown that CD4+ T cells producing IL-17 (Th17 cells) are preferentially depleted from gastrointestinal tracts of chronically HIV-infected humans (Brenchley et al., 2008) and SIV-infected RM (Cecchinato et al., 2008), but that Th17 cells are present at healthy frequencies in the GI tracts of chronically SIV-infected SM (Brenchley et al., 2008) and AGM (Favre et al., 2009). Th17 cells recruit neutrophils and myeloid cells to effector sites by inducing granulocyte-colony stimulating factor secretion (Ye et al., 2001), and are critical to epithelial cell regeneration in mucosal tissues (Brand et al., 2006) partly through the induction of expression of claudins, components of epithelial tight junctions. Th17 cells also induce epithelial cell production of defensins and mucin, both vital to the integrity of mucosal barrier (Chen et al., 2003; Sugimoto et al., 2008). Thus, preferential loss of Th17 cells from the GI tract during progressive HIV and SIV infections might lead to reduced structural and immunologic integrity of the mucosal barrier with a consequent increase in microbial translocation. Conversely, their preservation in SIV-infected natural hosts may partly explain the lack of microbial translocation observed in such animals. Therapeutic interventions such as IL-23 and retinoic acid that aim to increase Th17 cell frequencies in progressive infection and IL-1 receptor antagonists which aim to decrease Th17 cell frequencies in non-progressive infections could shed further light on the biological significance of Th17 cells in the maintenance of the tight epithelial barrier of the GI tract and microbial translocation. Epithelial damage could, in turn, also lead to decreased production of secretory IgA leading to decreased clearance of translocated microbial products.
While, recent studies have clearly demonstrated increased enteroycte apoptosis during the acute phase of SIV infection (Li et al., 2008), the mechanisms underlying this enterocyte apoptosis are unclear. Potential mechanisms underlying enterocyte apoptosis could involve lentiviral envelope proteins binding to oligosaccharide moieties present on a G coupled receptor GPR15 (Maresca et al., 2003) and recent studies have implicated TNF in the HIV-mediated enterocyte damage (Nazli et al.).
Thus, we propose that a critical factor that differentiates nonprogressive from progressive HIV or SIV infection is the maintenance of T cell immune competence during and after the acute phase of disease in the face of a virus that infects and kills CD4+ T cells. Hence natural hosts may have adopted mechanisms by which target cell availability for SIV is reduced while immunological function is maintained by subsets of T cells that are resistant to infection. Importantly, down regulation of CCR5 by all natural host species studied and down-regulation of CD4 by AGM occurs irrespective of SIV infection. Although the molecular mechanisms by which CD4 in AGM and CCR5 in all natural hosts are differentially expressed compared to other primate species remain unclear, these findings suggest the co-evolution of natural hosts with SIV.
The mitigation of lentivirus infection of CD4+ T cells through downregulation of the receptors for the virus raises an interesting paradox that clearly needs some explanation, namely: how are high plasma virus loads maintained in natural hosts if target cell availability has been reduced? In both natural hosts and hosts with progressive infection an equilibrium is set up between the availability of target cells, the depletion of target cells and the production of infectious virions. In progressive disease, this equilibrium is unstable and decays towards a state of loss of immune competence related to the reduced provision of effector memory CD4+ T cells that protect from pathogens that define AIDS. In contrast, this equilibrium appears to be maintained for the normal lifespan of natural hosts with no apparent detriment to their health. We propose that such a stable equilibrium may be achieved through the “uncoupling” of virus production from the processes that lead to disease progression. To understand how this might occur we should first consider the maturation of CD4+ T cells into their different subsets. Upon stimulation through the TCR, naïve CD4+ T cells may mature into different memory T cell subsets as shown in Figure 2 top. Central memory T cells are thought to have stem cell-like qualities in that they may be regarded as a self-renewing source of effector memory and activated T cells (van Grevenynghe et al., 2008). Central memory T cells tend to be localized to organized lymphoid tissues such as lymph nodes and gastrointestinal Peyer’s patches, whereas effector memory T cells tend to be localized to peripheral tissues such as the broncoalveolar fluid and gastrointestinal lamina propria. Both of these T-cell subsets are in a “resting” state and thus their infection with HIV or SIV would result in the production of small numbers of virions. However, activated memory T cells, defined by the expression of Ki67, may produce up to six-fold more virions per cell (Reilly et al., 2007). Thus the target cells responsible for the production of the majority of virions in a chronically-infected individual would be contained within the activated memory T cell subset (Figure 2 center, bottom). Indeed, it has been shown that the availability of activated CD4+ T cells in naturally SIV-infected sooty mangabeys dictates the degree of viremia (Klatt et al., 2008) (Figure 2 bottom). When compared to central memory T cells, both effector memory and activated memory T cells have a far greater tendency to activation-induced cell death; thus, theoretically, their death from viral infection should not have a great impact on their steady state numbers while enabling the production of great quantities of virions. By contrast, the loss of T cells from central memory pool would, over time, severely limit the numbers of effector memory and activated memory T cells available to control pathogens in the tissues. Thus it is the loss of central memory CD4+ T cells which introduces instability into the equilibrium between the availability of target cells, the depletion of target cells and the production of infectious virions, and progression to AIDS occurs because of the eventual failure of the provision of effector memory CD4+ T cells to the tissues (Okoye et al., 2007).
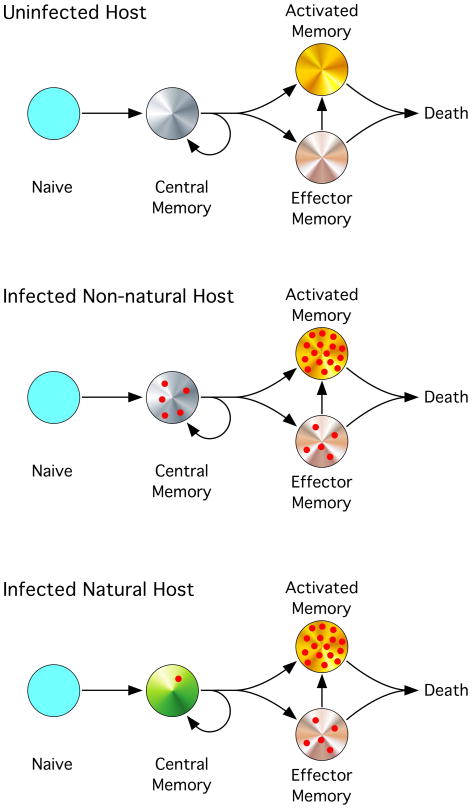
Figure 2. Uncoupling of Disease Progression from Virus Production.
(Top) In an uninfected host, naïve CD4+ T cells mature into central memory (CCR7+CD62L+, markers not shown) CD4+ T cells which may be regarded as having self-renewing potential. (CCR7−CD62L−) effector memory CD4+ T cells and activated CD4+ T cells are more prone to activation induced cell death. (center) In an HIV or SIV-infected host that progresses to AIDS, the infection and death of central memory CD4+ T cells eventually depletes this important cellular source and results in disease progression to AIDS. The infection and death of effector memory and activated CD4+ T cells produces the bulk of virus but makes little impact on these cellular pools as they are nonetheless destined to die by AICD. (bottom) In non-progressive SIV infection of natural hosts, virus load is maintained by the infection of effector memory and activated CD4+ T cells. The resistance of the + central memory CD4+ T cells to infection, by co-receptor down-regulation, prevents their depletion thus ensuring immune competence and precludes disease progression. Please label in figure as “Uninfected, etc.” Top to bottom.
Thus one might consider HIV and SIV infection as having two separable components: disease progression which is dependent on infection and depletion of central memory CD4+ T cells, and virus production which is dependent on infection of activated memory T cells. A survival mechanism could therefore have evolved in natural hosts to uncouple disease progression from virus production by reducing infection and depletion of central memory CD4+ T cells while maintaining viral loads through infection of activated CD4+ T cells (Figure 2 bottom) (Paiardini et al., 2009). The critical aspect of such uncoupling is to alter target cell availability and we have described above how this has been achieved in AGM and SM by two different mechanisms. In progressive disease of RM and humans any activating stimuli generate target cells within the central memory T cell population by increasing CCR5 expression whereas in natural hosts any activating stimuli remove target cells from central memory CD4+ T cells by reducing CCR5 in SM and CD4 in AGM, thus preserving this critical T cell subset while maintaining effector cells that produce virus and thus maintain plasma viral loads. However, the amount of virus production by individual subsets of CD4+ T cells in progressive and nonprogressive infections is not well understood. Thus, one critical question that must be further investigated is how much virus is produced from given infected CD4+ T cell subsets in order to maintain particular viral loads in different hosts.
Although it is unlikely that humans will be afforded the same experiment of nature that has allowed natural hosts to avoid disease progression (nor should they), we can nevertheless apply the lessons learned from the study of these animals to novel therapeutic avenues. Clearly, reduction of target cell availability is one such goal and CCR5 blockers such as maraviroc could be viewed as approximating the consequences of CCR5 down-regulation in SM. Moreover, understanding the molecular mechanisms underlying target cell availability, down-regulation of immune activation after the acute phase of infection, differential expression of SIV and HIV receptors and co-receptors and maintenance of immunological function by natural hosts will inevitably lead to a better understanding of the lack of disease progression in natural hosts and to the development of novel adjunctive therapeutic interventions for HIV-infected humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arhel NJ, Kirchhoff F. Implications of Nef: host cell interactions in viral persistence and progression to AIDS. Curr Top Microbiol Immunol. 2009;339:147–175. doi: 10.1007/978-3-642-02175-6_8. [DOI] [PubMed] [Google Scholar]
- Baroncelli S, Galluzzo CM, Pirillo MF, Mancini MG, Weimer LE, Andreotti M, Amici R, Vella S, Giuliano M, Palmisano L. Microbial translocation is associated with residual viral replication in HAART-treated HIV+ subjects with <50copies/ml HIV-1 RNA. J Clin Virol. 2009;46:367–370. doi: 10.1016/j.jcv.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat Med. 2009;15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I, Sharpstone DR, Francis N, Marker A, Taylor C, Barrett M, Macpherson A, Baldwin C, Menzies IS, Crane RC, et al. Intestinal inflammation, ileal structure and function in HIV. Aids. 1996;10:1385–1391. doi: 10.1097/00002030-199610000-00011. [DOI] [PubMed] [Google Scholar]
- Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Current Opinion in HIV and AIDS. 2008;3:356–361. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006a;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006b;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchinato V, Trindade C, Laurence A, Heraud J, Brenchley J, Ferrari M, Zaffiri L, Tryniszewska E, Tsai W, Vaccari M, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficienty virus-infected macaques. Mucosal Immunology. 2008;1:279–288. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti LA, Lewin SR, Zhang L, Gettie A, Luckay A, Martin LN, Skulsky E, Ho DD, Cheng-Mayer C, Marx PA. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J Virol. 2000;74:1209–1223. doi: 10.1128/jvi.74.3.1209-1223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- Ciccone EJ, Read SW, Mannon PJ, Yao MD, Hodge JN, Dewar R, Chairez CL, Proschan MA, Kovacs JA, Sereti I. Cycling of gut mucosal CD4+ T cells decreases after prolonged anti-retroviral therapy and is associated with plasma LPS levels. Mucosal Immunol. 3:172–181. doi: 10.1038/mi.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- Dunham R, Pagliardini P, Gordon S, Sumpter B, Engram J, Moanna A, Paiardini M, Mandl JN, Lawson B, Garg S, et al. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood. 2006;108:209–217. doi: 10.1182/blood-2005-12-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz PN, McClure HM, Anderson DC, Switzer WM. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM) AIDS Res Hum Retroviruses. 1989;5:397–409. doi: 10.1089/aid.1989.5.397. [DOI] [PubMed] [Google Scholar]
- Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, Luciano AA, Stevens W, Rodriguez B, Brenchley JM, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 115:161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Brown CR, Ourmanov I, Pandrea I, Buckler-White A, Erb C, Nandi JS, Foster GJ, Autissier P, Schmitz JE, Hirsch VM. Comparison of simian immunodeficiency virus SIVagmVer replication and CD4+ T-cell dynamics in vervet and sabaeus African green monkeys. J Virol. 2006;80:4868–4877. doi: 10.1128/JVI.80.10.4868-4877.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Ourmanov I, Brown CR, Beer BE, Elkins WR, Plishka R, Buckler- White A, Hirsch VM. Wide range of viral load in healthy african green monkeys naturally infected with simian immunodeficiency virus. J Virol. 2000;74:11744–11753. doi: 10.1128/jvi.74.24.11744-11753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Ourmanov I, Brown CR, Plishka R, Buckler-White A, Byrum R, Hirsch VM. Plateau levels of viremia correlate with the degree of CD4+-Tcell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J Virol. 2005;79:5153–5162. doi: 10.1128/JVI.79.8.5153-5162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SN, Dunham RM, Engram JC, Estes J, Wang Z, Klatt NR, Paiardini M, Pandrea IV, Apetrei C, Sodora DL, et al. Short-lived infected cells support virus replication in sooty mangabeys naturally infected with simian immunodeficiency virus: implications for AIDS pathogenesis. J Virol. 2008;82:3725–3735. doi: 10.1128/JVI.02408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, Dunham RM, Paiardini M, Klucking S, Danesh A, et al. Severe Depletion of Mucosal CD4+ T Cells in AIDS-Free Simian Immunodeficiency Virus-Infected Sooty Mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- Hirsch V, Adger-Johnson D, Campbell B, Goldstein S, Brown C, Elkins WR, Montefiori DC. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543–3. J Virol. 1997;71:1608–1620. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch VM. What can natural infection of African monkeys with simian immunodeficiency virus tell us about the pathogenesis of AIDS? AIDS Rev. 2004;6:40–53. [PubMed] [Google Scholar]
- Hirsch VM, Dapolito G, Johnson PR, Elkins WR, London WT, Montali RJ, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapembwa MS, Fleming SC, Sewankambo N, Serwadda D, Lucas S, Moody A, Griffin GE. Altered small-intestinal permeability associated with diarrhoea in human-immunodeficiency-virus-infected Caucasian and African subjects. Clin Sci (Lond) 1991;81:327–334. doi: 10.1042/cs0810327. [DOI] [PubMed] [Google Scholar]
- Kim N, Dabrowska A, Jenner RG, Aldovini A. Human and simian immunodeficiency virus-mediated upregulation of the apoptotic factor TRAIL occurs in antigen-presenting cells from AIDS-susceptible but not from AIDSresistant species. J Virol. 2007;81:7584–7597. doi: 10.1128/JVI.02616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Villinger F, Bostik P, Gordon SN, Pereira L, Engram JC, Mayne A, Dunham RM, Lawson B, Ratcliffe SJ, et al. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest. 2008;118:2039–2049. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler DP, Gaetz HP, Lange M, Klein EB, Holt PR. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- Li Q, Estes JD, Duan L, Jessurun J, Pambuccian S, Forster C, Wietgrefe S, Zupancic M, Schacker T, Reilly C, et al. Simian immunodeficiency virusinduced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J Infect Dis. 2008;197:420–429. doi: 10.1086/525046. [DOI] [PubMed] [Google Scholar]
- Li Y, Hui H, Burgess CJ, Price RW, Sharp PM, Hahn BH, Shaw GM. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J Virol. 1992;66:6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B, Apetrei C, Pandrea I, Veazey RS, Lackner AA, Gormus B, Marx PA. Classic AIDS in a sooty mangabey after an 18-year natural infection. J Virol. 2004;78:8902–8908. doi: 10.1128/JVI.78.16.8902-8908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca M, Mahfoud R, Garmy N, Kotler DP, Fantini J, Clayton F. The virotoxin model of HIV-1 enteropathy: involvement of GPR15/Bob and galactosylceramide in the cytopathic effects induced by HIV-1 gp120 in the HT-29- D4 intestinal cell line. J Biomed Sci. 2003;10:156–166. doi: 10.1007/BF02256007. [DOI] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milush JM, Reeves JD, Gordon SN, Zhou D, Muthukumar A, Kosub DA, Chacko E, Giavedoni LD, Ibegbu CC, Cole KS, et al. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol. 2007;179:3047–3056. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiardini M, Pandrea I, Apetrei C, Silvestri G. Lessons learned from the natural hosts of HIV-related viruses. Annu Rev Med. 2009;60:485–495. doi: 10.1146/annurev.med.60.041807.123753. [DOI] [PubMed] [Google Scholar]
- Pandrea I, Apetrei C, Dufour J, Dillon N, Barbercheck J, Metzger M, Jacquelin B, Bohm R, Marx PA, Barre-Sinoussi F, et al. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J Virol. 2006a;80:4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007a;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Onanga R, Kornfeld C, Rouquet P, Bourry O, Clifford S, Telfer PT, Abernethy K, White LT, Ngari P, et al. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virology. 2003;317:119–127. doi: 10.1016/j.virol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Pandrea I, Onanga R, Souquiere S, Mouinga-Ondeme A, Bourry O, Makuwa M, Rouquet P, Silvestri G, Simon F, Roques P, Apetrei C. Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J Virol. 2008a;82:5501–5509. doi: 10.1128/JVI.02555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Silvestri G, Onanga R, Veazey RS, Marx PA, Hirsch V, Apetrei C. Simian immunodeficiency viruses replication dynamics in African nonhuman primate hosts: common patterns and species-specific differences. J Med Primatol. 2006b;35:194–201. doi: 10.1111/j.1600-0684.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008b;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007b;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr, Wang C, et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly C, Wietgrefe S, Sedgewick G, Haase A. Determination of simian immunodeficiency virus production by infected activated and resting cells. AIDS. 2007;21:163–168. doi: 10.1097/QAD.0b013e328012565b. [DOI] [PubMed] [Google Scholar]
- Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, Boswell SL, Mathews WC, Bangsberg DR, Martin J, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. Jama. 2006;296:1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- Silvestri G. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J Med Primatol. 2005;34:243–252. doi: 10.1111/j.1600-0684.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. The Journal of Clinical Investigation. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter B, Dunham R, Gordon S, Engram J, Hennessy M, Kinter A, Paiardini M, Cervasi B, Klatt N, McClure H, et al. Correlates of preserved CD4(+) T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J Immunol. 2007;178:1680–1691. doi: 10.4049/jimmunol.178.3.1680. [DOI] [PubMed] [Google Scholar]
- van Grevenynghe J, Procopio FA, He Z, Chomont N, Riou C, Zhang Y, Gimmig S, Boucher G, Wilkinson P, Shi Y, et al. Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat Med. 2008;14:266–274. doi: 10.1038/nm1728. [DOI] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson PR, Hu SL, Haigwood NL. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]