Abstract
Endogenous thrombopoietin (eTPO) regulates platelet production by increasing the number, ploidy and maturation rate of bone marrow megakaryocytes. Early attempts to treat thrombocytopenia by the administration of recombinant TPO were successful but were complicated by the development of antibodies to one of the recombinant proteins. Two new TPO mimetics have recently been approved by the Food and Drug Administration for the treatment of immune thrombocytopenia (ITP). Romiplostim is a peptide TPO mimetic composed of an IgG Fc fragment to which are attached four 14-amino acid TPO peptides that activate the TPO receptor by binding to the extracytoplasmic domain just like eTPO. Romiplostim is administered as a weekly subcutaneous injection. Eltrombopag is a non-peptide TPO mimetic that is a 442 Da drug that binds to a transmembrane site on the TPO receptor and thereby activates it. It is administered daily as an oral tablet. Administration of both romiplostim and eltrombopag to healthy volunteers produced a dose-dependent rise in platelet count beginning on day 5 and peaking at days 12-15. Both have been highly effective in increasing the platelet count in patients with ITP and are currently being studied in the treatment of other thrombocytopenic conditions (MDS, chemotherapy, liver disease).
Introduction
In 1958 Kelemen proposed that a “thrombopoietin” must exist that would regulate platelet production just as erythropoietin was known to regulate red blood cell production.1 But it was not until 1994 that five laboratories reported the purification and/or cloning of thrombopoietin. While some called it Mpl ligand [after the Mpl (myeloproliferative leukemia) receptor,2, 3 correctly proposed as the thrombopoietin receptor in 19924], others called it megakaryocyte growth and development factor (MGDF),5 megapoietin,6 or thrombopoietin (TPO).7 The historical name for this molecule, TPO, has become widely accepted as has the name TPO receptor (instead of Mpl receptor).
After these discoveries, two recombinant thrombopoietins entered clinical trials in 1995: recombinant human TPO (rhTPO) and pegylated recombinant human MGDF (PEG-rhMGDF). rhTPO was a full-length, glycosylated recombinant protein identical to endogenous TPO (eTPO) and was produced in mammalian cells. PEG-rhMGDF was a non-glycosylated, recombinant protein comprising the first 163 amino acids of the native molecule, produced in bacteria, and then chemically coupled to a 20 kDa PEG (polyethylene glycol) moiety. Both recombinant thrombopoietins had a half-life of ∼40 hours and both markedly increased the platelet count in human volunteers, platelet apheresis donors, and patients undergoing non-myeloablative chemotherapy, as well as in patients with ITP or MDS (for review see reference8).
Unfortunately, a few patients who received PEG-rhMGDF (but not rhTPO) developed antibodies to the recombinant TPO that cross-reacted with eTPO causing thrombocytopenia;9 further development of recombinant thrombopoietic molecules ceased.
Given the perceived benefits of the recombinant TPO molecules, efforts were then made to create new TPO mimetics that were not antigenic and might have improved pharmacologic properties. A number of peptide and non-peptide TPO mimetics have been developed, and two, romiplostim and eltrombopag, have been approved by the US Food and Drug Administration (FDA). This article reviews the biology and chemistry of these TPO mimetics.
Structure and Physiology of eTPO
Endogenous human TPO is a 95 kDa protein that contains 332 amino acids of which the first 153 residues define the receptor-binding portion of the molecule. This region is not significantly glycosylated and is approximately 23 percent identical to (and 50 percent homologous with) erythropoietin.5 Despite its similarity to erythropoietin, it does not bind the erythropoietin receptor and erythropoietin does not bind the TPO receptor. Crystal structure analysis of this part of the TPO molecule has identified two important receptor-binding areas: a 13-amino acid high-affinity site (3.3×109 M-1) and an 11-amino acid low-affinity binding site (1.1×106 M-1) that allow one TPO to bind two TPO receptors.10 The remaining 179-residue C-terminal region has a large number of proline and glycine residues with 6 N-linked glycosylation sites and serves as a chaperone.
eTPO is made at a constant rate in the liver with no storage form and is released from hepatocytes into the circulation.11 Except for hepatic disease or resection, there is no known alteration of eTPO synthesis. There does not appear to be a “sensor” of the platelet count that regulates eTPO production.6 Rather, eTPO is made at a constant rate by the liver and enters the circulation and most is cleared by avid receptors on platelets and possibly megakaryocytes. Upon binding, eTPO is internalized and degraded.12 With a decrease in platelet production, eTPO clearance is reduced, and eTPO levels rise.
Effect of TPO Binding to the TPO Receptor
TPO receptors are found on a wide range of the hematopoietic cells, ranging from stem cells to mature platelets. They are not found on non-hematopoietic tissue or solid tumor cells.13 The TPO receptor is a typical hematopoietic receptor and contains two reduplicated cytokine receptor homology (CRH) domains (with the distinctive WSXWS sequence motif), a transmembrane region, and a cytoplasmic domain that interacts with a wide variety of intracellular signaling pathways. (Figure 1) It probably exists as a preformed but inactive dimer. Upon TPO binding to the distal CRH domain, steric inhibition is probably released, thereby activating the receptor; deletion of the distal CRH produces a receptor that is constitutively active in the absence of ligand binding.14
Figure 1.
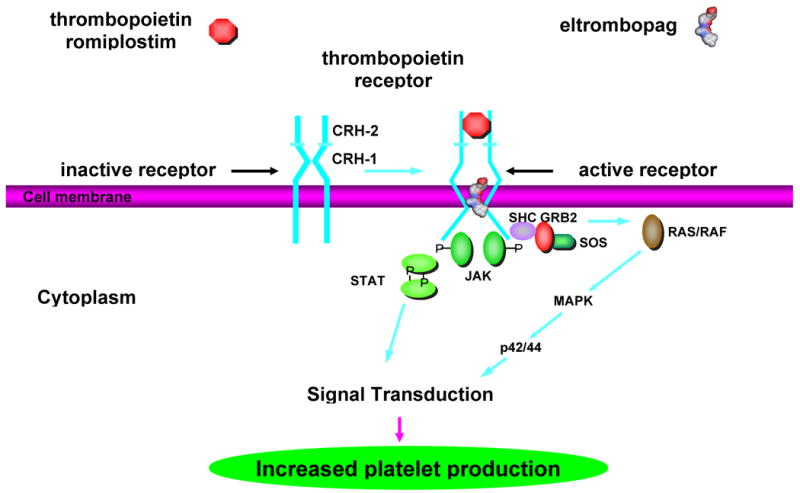
Mechanism of activation of TPO receptor by TPO, romiplostim, and eltrombopag. The TPO receptor has been proposed to exist as an inactive preformed dimer (left) with proximal (CRH-1) and distal (CRH-2) cytokine receptor homology (CRH) domains. Upon binding of thrombopoietin of ?or? romiplostim to the distal CRH-2 domain or binding of eltrombopag to the transmembrane region, the receptor (right) conformation changes and a number of signal transduction pathways are activated that increase platelet production.
TPO receptor activation leads to phosphorylation of the cytoplasmic domain of the receptor as well as downstream activation of JAK2, STAT5, MAP kinase, and PI-3 kinase as well as a number of anti-apoptotic pathways.15 (see article by Geddis in this issue) This increases the viability of stem cells and precursors of all lineages.16 For megakaryocyte precursors, TPO increases their number, endomitosis, and maturation, prevents apoptosis, and thereby increases platelet production.6 The anti-apoptotic effect of TPO is probably important in patients with ITP: bone marrow megakaryocytes and megakaryocyte precursors are already increased but these cells are undergoing apoptosis mediated by anti-platelet IgG and T cells, thereby limiting platelet production. TPO treatment probably reduces the apoptotic rate of these megakaryocyte precursors and permits full platelet production.17 Once produced, platelets are still affected by TPO. High (pharmacological, not physiological) TPO concentrations sensitize platelets to weak agonists such as ADP but do not cause spontaneous aggregation.18
Romiplostim
In 1997, a 14-amino-acid peptide was identified that had no sequence homology with TPO but bound and activated the TPO receptor.19 Chemically linking two 14-amino acid peptides together increased its activity 10,000-fold, comparable to that of recombinant TPO. Unlike the monomeric peptide, with two different receptor binding sites just like eTPO, the dimeric peptide could now more efficiently bind and activate the TPO receptor. However, peptides have very short half-lives, making them inconvenient drugs.
A stable and effective peptide TPO mimetic was achieved by attaching four of these 14-amino-acid peptides to the C-terminus of an IgG1 Fc fragment, creating a new biologic platform called a “peptibody”. (Figure 2) Two of these 14-amino-acid peptides were attached to each arm of the Fc γ heavy chain using polyglycine linkers. The peptibody contains functional CH2 and CH3 regions, allowing it to be bound by the FcRn receptors but not to fix complement. Since it contains four identical peptides that have no sequence homology with TPO, if antibodies formed against these peptides, they would not cross-react with and neutralize eTPO as had occurred with PEG-rhMGDF. Developed under the names AMP-2 and AMG-531, it is now called romiplostim (Nplate).20
Figure 2.
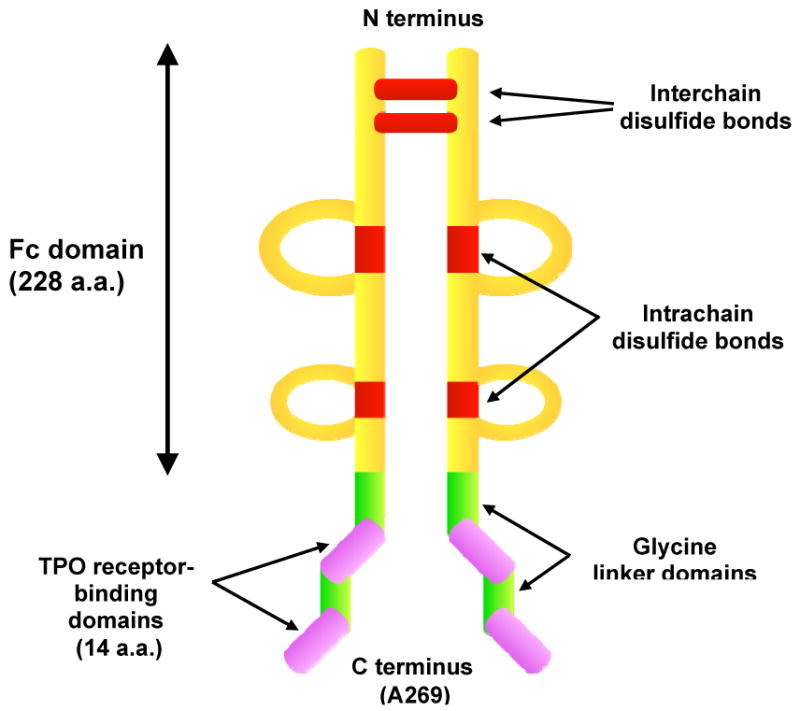
Structure of romiplostim. Romiplostim is composed of the Fc (fragment “crystallized”) portion of IgG1 to which two 14-amino acid TPO peptides (purple) are coupled via glycine bridges (green) at the carboxy-terminus of each γ heavy chain. Interchain (at cysteines C7 and C10) and intrachain (cysteines C42-C102, C148-C206) disulfide bridges are indicated in red. Heavy chain constant domains 2 (CH2) and 3 (CH3) are also shown. Romiplostim has a molecular weight of ∼60 kDa.
Romiplostim competes with TPO for binding to the TPO receptor but with a 15-fold lower-binding affinity (D. Kuter, unpublished data, 2002). Nonetheless, once bound, romiplostim rapidly produces phosphorylation of the TPO receptor and activation of the JAK2 and STAT5 pathways like eTPO. (Figure 1) It stimulated dose-dependent CFU-Mk growth and increased megakaryocyte ploidy in culture.21
In mice and rhesus monkeys romiplostim produced a dose-dependent rise in platelet count with no major toxicities even after prolonged exposure. In 40 healthy volunteers, the platelet count response to single subcutaneous or intravenous doses of 0.1-10 mcg/kg was studied.22 The platelet count did not start to rise until approximately Day 5, followed by a dose-dependent rise in platelet count that peaked on Days 12-16. (Figure 3) At the highest dose of 10 mcg/kg, a 6-fold increase in platelets occurred with a mean platelet count of 1380×109/L (range: 923-1790×109/L). There was a nearly linear relationship between romiplostim dose and platelet count rise (r2 = 0.815). (Figure 4) By Day 28, platelet counts had returned to their baseline level with no rebound thrombocytopenia. There was no effect upon white blood cell or red blood cell counts. Intravenous and subcutaneous doses produced the same response. Except for mild headache, subjects reported no serious adverse events; none developed antibodies to romiplostim. Romiplostim did not cause spontaneous platelet aggregation but did sensitize platelets to weak agonists. This is probably not a clinically relevant effect because extensive prior studies showed no increased thrombosis in animal models or in cancer chemotherapy patients treated with recombinant TPO (for review see 8).
Figure 3.
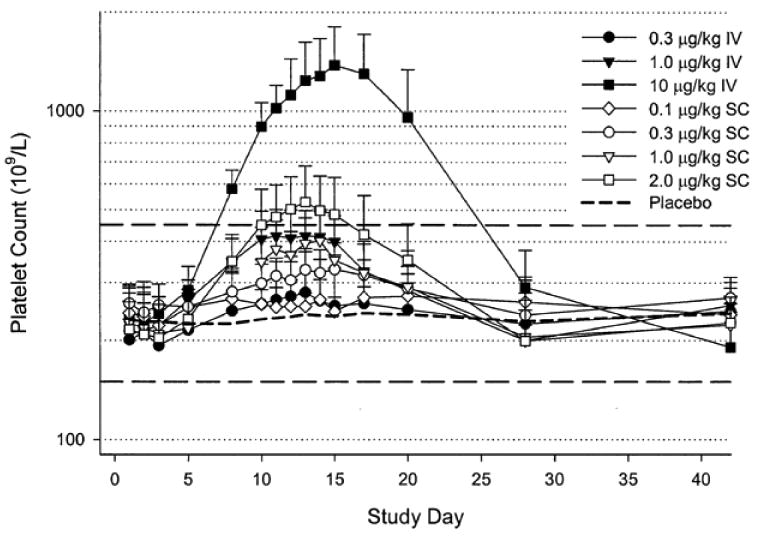
Romiplostim increased the platelet count in healthy human volunteers. Mean (+/- SD) platelet counts after single subcutaneous (SC, open symbols) or intravenous (IV, closed symbols) administration of various doses of romiplostim or placebo. Dashed lines indicate normal platelet count ranges (150-450×109/L). Note logarithmic scale for platelet count.
Figure 4.
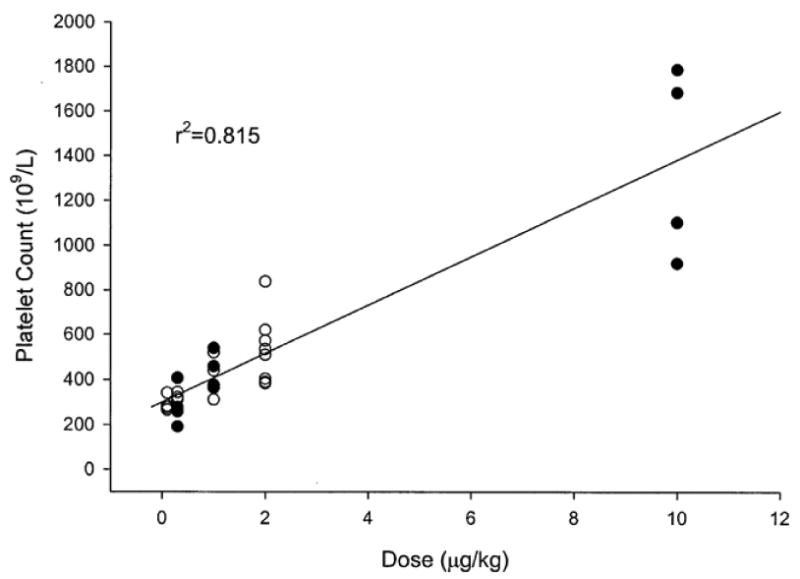
Dose-response effect of romiplostim on peak platelet counts in healthy human volunteers after single intravenous (solid symbols) or subcutaneous (open symbols) dose of romiplostim or placebo (see Figure 3 for more details).
After single doses of 0.3-10 mcg/kg, the pharmacokinetics of romiplostim is non-linear with a half-life of 120-140 hours. The long half-life is largely due to the binding of romiplostim by the FcRn receptors which internalize it and then release it back into the circulation, just like normal IgG. It is ultimately cleared from the body by the reticuloendothelial system. There have been few studies in patients with renal or hepatic impairment, but no dose adjustments would seem to be required in this setting given its structure and metabolism.
Romiplostim has been shown to be highly effective in increasing the platelet counts in patients with ITP (see article by Ghanima and Bussel in this issue) and is currently approved by the FDA for the treatment of thrombocytopenia in patients with chronic ITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.20 It is not to be given to pregnant or nursing women because it is likely to be transported into the fetus or milk by FcRn receptor.
Romiplostim is available only through a restricted distribution program called the Nplate NEXUS (Network of Experts Understanding and Supporting Nplate and Patients) Program (1-877-Nplate1). It is supplied as 250 or 500 mcg vials of a sterile, preservative-free, lyophilized, solid white powder for subcutaneous injection.20 For chronic ITP patients, romiplostim is administered as a weekly subcutaneous injection at a dose of 1-10 mcg/kg. Although an initial dose of 1 mcg/kg is suggested in the prescribing information,20 the mean therapeutic dose in several trials was 3-4 mcg/kg;23 recently an initial dose of 3 mcg/kg was demonstrated to be effective.24 Subsequent dosing is based on the platelet count with a usual target of 50-200×109/L.
Known adverse effects of romiplostim include worsening of thrombocytopenia upon discontinuation (“rebound thrombocytopenia”) and increased bone marrow reticulin (but without consequent cytopenias). (See article by Cuker in this issue)
Eltrombopag
A second approach to the identification of TPO mimetics was to screen libraries of chemical structures for the ability to activate the TPO receptor (using a TPO-dependent luciferase reporter construct). A surprisingly large number of non-peptide TPO mimetics have been so identified. Initial hydrazone compounds were subsequently modified to create structures with favorable pharmacokinetic properties (eg, solubility, oral bioavailability, and metabolism) that were as potent as recombinant thrombopoietin.25, 26
Developed under the name SB497115, eltrombopag (Promacta, Revolade) is a member of the bioarylhydrazone class of compounds with an empirical formula of C25H22N4O4 and a molecular weight of 442.5 d. It has an acidic (COOH) group at one end, lipophilic (CH3) groups at the other end, and a metal chelate group in the center.27 (Figure 5)
Figure 5.
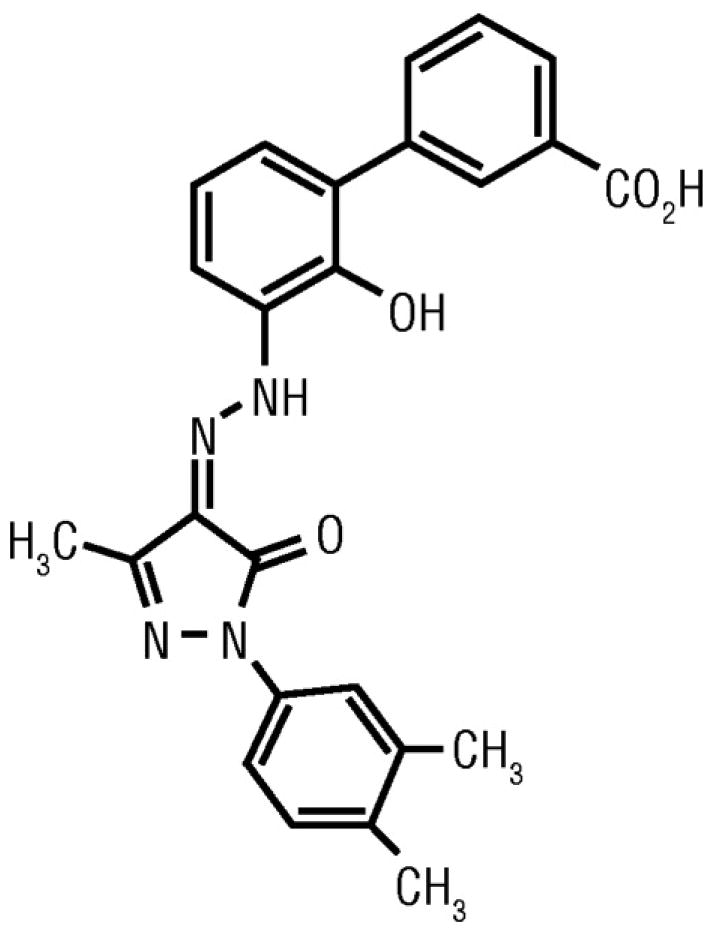
Structure of eltrombopag [3′-{(2Z)-2-[1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene]hydrazino}-2′-hydroxy-3-biphenylcarboxylic acid].
Eltrombopag has a number of properties which it shares with almost all of the other non-peptide TPO mimetics.
It is orally available and amenable to daily, possibly weekly, administration.26
It binds only to the human and chimp TPO receptor.28 While minimizing off-target effects, this has certainly limited effectiveness studies in preclinical models.
It probably binds to the transmembrane region of the TPO receptor and histidine 499 in this region appears to be vital. (Figure 1) This residue is a leucine in all species but humans and chimpanzees and explains the strict species restriction. In studies where the human TPO receptor was changed at residue 499 from histidine to leucine, eltrombopag was no longer active. Changing residue 499 from a leucine to a histidine in the murine TPO receptor allowed eltrombopag to be active.29
Since eltrombopag does not compete with TPO for receptor binding it has effects that are at least additive to TPO both in tissue culture and in preclinical animal models.28
Eltrombopag binds to the TPO receptor probably by association with metal ions (eg, zinc) and induces phosphorylation of the TPO receptor with subsequent activation of the JAK2, STAT5, PI-3 kinase, and MAP kinase pathways, but with less intensity than TPO. It promoted the growth of TPO-dependent cell lines. It produced a dose-dependent increase in CFU-Mk, megakaryocyte number, ploidy and maturation but only for cells from chimps and humans.26, 28
Extensive toxicology studies were performed in mice, rats and dogs and showed no immunotoxicity, teratogenicity or carcinogenicity. The major toxicities were increased cataracts (juvenile rodents) and hepatotoxicity (rodents and dogs); increased cataracts have not been seen in subsequent human studies.27 However, due to the species-specificity of this drug, preclinical efficacy studies were limited to three chimpanzees. Daily doses of 10 mg to chimps were well tolerated with no clinical or laboratory abnormalities noted. One chimp increased the platelet count 2-fold and the other two increased 1.5 fold.28
In healthy humans, single doses of eltrombopag did not produce a rise in platelet count. However daily doses for 10 days did show a modest dose-dependent rise in platelets; as with recombinant TPO, the platelet count started to rise on Day 8 and peaked at Day 16 (after 10 days of treatment).30 (Figure 6) By Day 22 (12 days after the last treatment) platelets had returned to baseline without a rebound thrombocytopenia. Doses below 30 mg a day had little effect. The mean increase in platelet count was 24% (258×109/L to 320×109/L), 43% (254×109/L to 363×109/L) and 50% (235×109/L to 355×109/L) for the 30, 50 and 75 mg doses, respectively.a Platelet aggregation and activation were not affected by eltrombopag.31 None of the 73 subjects had any adverse effects.
Figure 6.
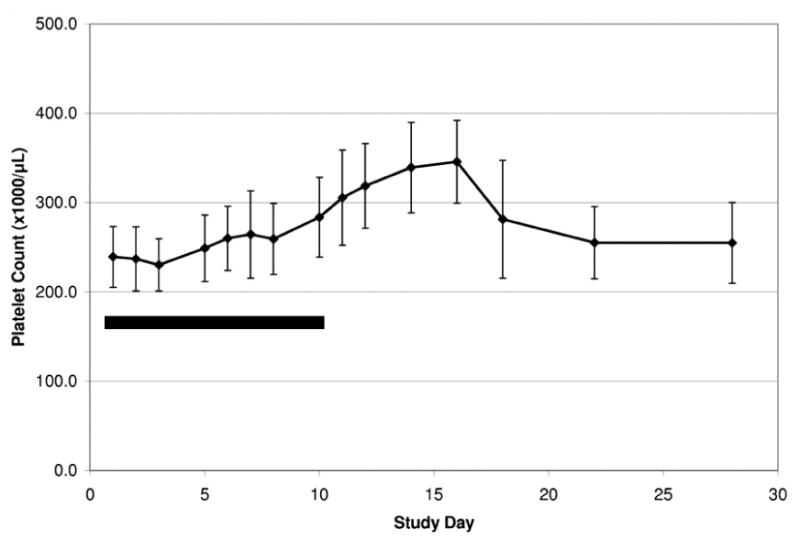
Eltrombopag increased the platelet count in healthy human volunteers. Platelet count (+/- SD) after administration of the maximal dose of 75 mg daily for ten days (solid bar) to 9 healthy volunteers.
The pharmacokinetics of eltrombopag was linear and dose-dependent with an elimination half-life of 21-32 h. Additional studies have demonstrated the following pharmacological properties of eltrombopag:27
It is an inhibitor of the OATB1B1 transporter and may increase the exposure of patients to drugs (eg, rosuvastatin) that are substrates of OATB1B1.
Polyvalent cations (iron, calcium, aluminum, magnesium, selenium, zinc) reduce the absorption of eltrombopag (probably by binding to its metal chelate site).
AUC is 70-80% higher in East Asian than Caucasian patients.
Eltrombopag is highly protein bound in the circulation.
Oral absorption of the 75 mg dose is >52% with peak serum concentrations occurring at 2-6 h.
Once absorbed, eltrombopag is highly metabolized and eliminated in the feces (59%) and urine (31%).
Eltrombopag plasma levels increase in proportion to the extent of hepatic dysfunction.
The effect of renal impairment has not been studied.
Eltrombopag is supplied as 25 and 50 mg tablets and is currently FDA-approved for the treatment of thrombocytopenia in patients with chronic ITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.27 It is available through a restricted distribution program (PROMACTA CARES, 1-877-9-PROMACTA). The initial dose in ITP is usually 50 mg daily (25 mg if East Asian or if moderate or severe hepatic insufficiency is present) with subsequent doses (up to 75 mg) determined by the platelet count. It is taken on an empty stomach 1 h before or 2 h after a meal. It should not be taken within 4 h of any meal or products containing polyvalent cations (antacids, dairy products). Other drugs (eg, rosuvustatin) that are substrates of OATB1B1 should have their dose reduced. Liver function tests should be checked before starting and at intervals thereafter. Eltrombopag is not to be given to pregnant or nursing women.
Known adverse effects of eltrombopag are the same as romiplostim but also include a 13% rate of abnormal liver function tests (See article by Cuker in this issue).
Conclusions
Romiplostim and eltrombopag are new FDA-approved TPO mimetics that are highly effective in raising the platelet count in ITP. When compared with other chronic ITP therapies (eg, corticosteroids), they have minimal adverse effects even after years of administration. Both continue to be studied in other thrombocytopenic conditions including MDS (see article by Bryan et al. in this issue), chemotherapy (see article by Vadhan-Raj in this issue), and chronic hepatitis (see article by Tillmann and McHutchinson in this issue).
Acknowledgments
Supported in part by Grants HL82889 and HL072299 from the National Institutes of Health.
Footnotes
It should be noted that at the maximal FDA-approved doses, romiplostim is ∼8 times more potent in raising the platelet count in healthy volunteers than is eltrombopag. It remains to be demonstrated whether this difference in potency is clinically important.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelemen E, Cserhati I, Tanos B. Demonstration and some properties of human thrombopoietin in thrombocythaemic sera. Acta Haematol. 1958;20:350–355. doi: 10.1159/000205503. [DOI] [PubMed] [Google Scholar]
- 2.Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–71. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- 3.de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369:533–8. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 4.Vigon I, Mornon JP, Cocault L, Mitjavila MT, Tambourin P, Gisselbrecht S, et al. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci USA. 1992;89:5640–4. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartley TD, Bogenberger J, Hunt P, Li YS, Lu HS, Martin F, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77:1117–24. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 6.Kuter DJ, Beeler DL, Rosenberg RD. The purification of megapoietin: a physiological regulator of megakaryocyte growth and platelet production. Proc Natl Acad Sci USA. 1994;91:11104–8. doi: 10.1073/pnas.91.23.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato T, Ogami K, Shimada Y, Iwamatsu A, Sohma Y, Akahori H, et al. Purification and characterization of thrombopoietin. J Biochem. 1995;118:229–36. doi: 10.1093/oxfordjournals.jbchem.a124883. [DOI] [PubMed] [Google Scholar]
- 8.Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002;100:3457–69. doi: 10.1182/blood.V100.10.3457. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–8. doi: 10.1182/blood.v98.12.3241. [DOI] [PubMed] [Google Scholar]
- 10.Feese MD, Tamada T, Kato Y, Maeda Y, Hirose M, Matsukura Y, et al. Structure of the receptor-binding domain of human thrombopoietin determined by complexation with a neutralizing antibody fragment. Proc Natl Acad Sci USA. 2004;101:1816–21. doi: 10.1073/pnas.0308530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoffel R, Wiestner A, Skoda RC. Thrombopoietin in thrombocytopenic mice: evidence against regulation at the mRNA level and for a direct regulatory role of platelets. Blood. 1996;87:567–73. [PubMed] [Google Scholar]
- 12.Fielder PJ, Gurney AL, Stefanich E, Marian M, Moore MW, Carver-Moore K, et al. Regulation of thrombopoietin levels by c-mpl-mediated binding to platelets. Blood. 1996;87:2154–61. [PubMed] [Google Scholar]
- 13.Columbyova L, Loda M, Scadden DT. Thrombopoietin receptor expression in human cancer cell lines and primary tissues. Cancer Res. 1995;55:3509–3512. [PubMed] [Google Scholar]
- 14.Sabath DF, Kaushansky K, Broudy VC. Deletion of the extracellular membrane-distal cytokine receptor homology module of Mpl results in constitutive cell growth and loss of thrombopoietin binding. Blood. 1999;94:365–7. [PubMed] [Google Scholar]
- 15.Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–45. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- 16.Carver-Moore K, Broxmeyer HE, Luoh SM, Cooper S, Peng J, Burstein SA, et al. Low levels of erythroid and myeloid progenitors in thrombopoietin- and c-mpl-deficient mice. Blood. 1996;88:803–8. [PubMed] [Google Scholar]
- 17.Harker LA, Carter RA, Marzec UM, Cherry JK, Gunthel CJ, Lennox JL, et al. Correction of thrombocytopenia and ineffective platelet production in patients infected with human immunodeficiency virus (HIV) by PEG-rHuMGDF therapy. Blood. 1998;92:707a. [PubMed] [Google Scholar]
- 18.Harker LA, Marzec UM, Hunt P, Kelly AB, Tomer A, Cheung E, et al. Dose-response effects of pegylated human megakaryocyte growth and development factor on platelet production and function in nonhuman primates. Blood. 1996;88:511–21. [PubMed] [Google Scholar]
- 19.Cwirla SE, Balasubramanian P, Duffin DJ, Wagstrom CR, Gates CM, Singer SC, et al. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science. 1997;276:1696–9. doi: 10.1126/science.276.5319.1696. [DOI] [PubMed] [Google Scholar]
- 20.Nplate prescribing information. Amgen, Inc; 2008. [Google Scholar]
- 21.Broudy VC, Lin NL. AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl. Cytokine. 2004;25:52–60. doi: 10.1016/j.cyto.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharm Therapeut. 2004;76:628–38. doi: 10.1016/j.clpt.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 24.Kuter D, Rummel M, Boccia R, Macik G, Pabinger I, Selleslag D, et al. Comparison of splenectomy and treatment failure incidence in nonsplenectomized patients with immune thrombocytopenia (ITP) receiving romiplostim or medical standard of care: 1-year treatment and 6-month safety follow-up. Blood. 2009 [Google Scholar]
- 25.Duffy KJ, Darcy MG, Delorme E, Dillon SB, Eppley DF, Erickson-Miller C, et al. Hydrazinonaphthalene and azonaphthalene thrombopoietin mimics are nonpeptidyl promoters of megakaryocytopoiesis. J Med Chem. 2001;44:3730–45. doi: 10.1021/jm010283l. [DOI] [PubMed] [Google Scholar]
- 26.Erickson-Miller CL, DeLorme E, Tian SS, Hopson CB, Stark K, Giampa L, et al. Discovery and characterization of a selective, nonpeptidyl thrombopoietin receptor agonist. Exp Hematol. 2005;33:85–93. doi: 10.1016/j.exphem.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Promacta prescribing information. GlaxoSmithKline, Inc; 2008. [Google Scholar]
- 28.Erickson-Miller CL, Delorme E, Tian SS, Hopson CB, Landis AJ, Valoret EI, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27:424–30. doi: 10.1634/stemcells.2008-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamane N, Tanaka Y, Ohyabu N, Yamane S, Maekawa K, Ishizaki J, et al. Characterization of novel non-peptide thrombopoietin mimetics, their species specificity and the activation mechanism of the thrombopoietin receptor. Eur J Pharmacol. 2008;586:44–51. doi: 10.1016/j.ejphar.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins JM, Williams D, Deng Y, Uhl J, Kitchen V, Collins D, et al. Phase 1 clinical study of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist. Blood. 2007;109:4739–41. doi: 10.1182/blood-2006-11-057968. [DOI] [PubMed] [Google Scholar]
- 31.Erhardt JA, Erickson-Miller CL, Aivado M, Abboud M, Pillarisetti K, Toomey JR. Comparative analyses of the small molecule thrombopoietin receptor agonist eltrombopag and thrombopoietin on in vitro platelet function. Exp Hematol. 2009;37:1030–7. doi: 10.1016/j.exphem.2009.06.011. [DOI] [PubMed] [Google Scholar]


