Abstract
Tinnitus, the most common auditory disorder, affects about 40 million people in the United States alone, and its incidence is rising due to an aging population and increasing noise exposure. Although several approaches for the alleviation of tinnitus exist, there is as of yet no cure. The present article proposes a testable model for tinnitus that is grounded in recent findings from human imaging and focuses on brain areas in cortex, thalamus, and ventral striatum. Limbic and auditory brain areas are thought to interact at the thalamic level. While a tinnitus signal originates from lesion-induced plasticity of the auditory pathways, it can be tuned out by feedback connections from limbic regions, which block the tinnitus signal from reaching auditory cortex. If the limbic regions are compromised, this “noise-cancellation” mechanism breaks down, and chronic tinnitus results. Hopefully, this model will ultimately enable the development of effective treatment.
Introduction
Since the landmark review by Eggermont & Roberts (2004) on the neuroscience of tinnitus, the level of interest in this often debilitating disorder has mounted steadily. This can be attributed to the rising average age of populations in Western countries and increased hearing loss in young people, partly due to the popularity of music players with in-ear loudspeakers. Tinnitus is also one of the most frequently reported problems among veterans returning from two recent armed conflicts, often co-occurring with traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD). Although the number of published articles on tinnitus is growing and more funding agencies are now supporting tinnitus research, our basic understanding of the disorder is stagnating (Adjamian et al., 2009; Langguth et al., 2007; Shulman et al., 2009).
It has been assumed for some time that most cases of tinnitus are caused by peripheral noise-induced hearing loss followed by changes in the central auditory pathways (Jastreboff, 1990). Animal models have corroborated this explanation (Irvine et al., 2001; Rauschecker, 1999b; Robertson and Irvine, 1989), but have not provided a conclusive answer as to the location and nature of these central changes (Eggermont and Roberts, 2004). Using a whole-brain approach, human neurophysiological and functional imaging studies have visualized various regions of hyperactivity in the auditory pathways of tinnitus patients (Arnold et al., 1996; Hoke et al., 1989; Lanting et al., 2009; Melcher et al., 2009) as well as cortical regions beyond classical auditory cortex, including prefrontal and temporo-parietal areas (Giraud et al., 1999; Mirz et al., 2000; Mirz et al., 1999; Schlee et al., 2009; Weisz et al., 2007). Imaging studies have also demonstrated activation of non-auditory, limbic brain structures, such as hippocampus and amygdala, in tinnitus patients (Eichhammer et al., 2007; Lockwood et al., 1998; Mirz et al., 2000; Shulman et al., 2009).
This limbic activation has been interpreted as a reflection of the emotional reaction of tinnitus patients to the tinnitus sound (Jastreboff, 2000). As the present article will argue, however, limbic and paralimbic structures may play a more extended role than previously proposed. In our model, efferents from structures in the subcallosal area, which includes the nucleus accumbens (NAc) of the ventral striatum and the ventral medial prefrontal cortex (vmPFC), are involved in the cancellation of the tinnitus signal at the thalamic level. Although the tinnitus signal may initially be generated in parts of the auditory system, it is the failure of the limbic regions to block this signal that leads to the tinnitus percept becoming chronic.
Lesion-Induced Reorganization of the Central Auditory System
Tinnitus, i.e. hearing a disturbing tone or noise in the absence of a physical sound source, is a phantom sensation (Jastreboff, 1990) comparable to phantom pain felt in an amputated limb (Ramachandran and Hirstein, 1998). In both cases, the firing of central neurons in the brain continues to convey perceptual experiences, even though the corresponding sensory receptor cells have been destroyed (Birbaumer et al., 1997; Rauschecker, 1999a). As such, chronic tinnitus is thought to originate from plastic reorganization of auditory cortex following peripheral deafferentation. According to this “remapping” hypothesis, the reorganization process usually begins with a loss of hair cells in the inner ear, a “sensorineural” hearing loss (SNHL). This cochlear lesion can result from acoustic trauma, i.e. loud-noise exposure within a certain frequency range, or age-related hair-cell degeneration (usually corresponding to high frequencies). Although the lesion causes elevated thresholds in the corresponding frequency range, neighboring frequencies become amplified because their central representations expand into the vacated frequency range. Indeed, preliminary findings from human PET and MEG studies indicate an expansion of the frequency representation in the auditory cortex that corresponds to the perceived tinnitus frequencies (Lockwood et al., 1998; Muhlnickel et al., 1998; Wienbruch et al., 2006). These observations, however, continue to await confirmation by high-resolution fMRI studies (Leaver et al., 2006).
Animal models of tinnitus also support the claim for a cortical origin of the tinnitus percept. Restricted cochlear lesions in cats and monkeys lead to a frequency-specific reorganization of auditory cortex and thalamus but not of more peripheral stations (Rajan and Irvine, 1998; Rajan et al., 1993; Schwaber et al., 1993). As detailed microelectrode mapping in these and other studies has shown, frequency regions adjacent to the lesioned area invade the vacated space and become “overrepresented” compared to other frequency regions. Additionally, the “lesion-edge frequencies” lose intracortical inhibitory input from the deafferented region. Thus, cortical neurons with input from frequency ranges next to the cut-off frequency display permanently elevated spontaneous activity (“hyperactivity”) as well as transiently enhanced burst-firing and increased synchronous activity (Norena and Eggermont, 2003; Weisz et al., 2006). Auditory brainstem hyperactivity has also been reported in human tinnitus patients (Melcher et al., 2000) as well as animal models of tinnitus (Bauer, 2003; Brozoski et al., 2002), but it seems to be the cortical remapping that ultimately forms the basis for a chronic tinnitus percept (Melcher et al., 2009).
Lesion-induced plasticity in the adult brain has also been documented in the somatosensory and visual systems (Calford et al., 2005; Florence and Kaas, 1995) (but see Smirnakis et al. (2005)). The cellular and synaptic mechanisms responsible for this process include the unmasking of hidden inputs (Buonomano and Merzenich, 1998) and the sprouting of new connections (Darian-Smith and Gilbert, 1994). Furthermore, changes at the cortical level have been found in combination with perceptual consequences of phantom sensations (Ramachandran et al., 1992) and a “filling-in” of the deprived region by neighboring representations (Gilbert et al., 2001). A similar line of work on the cortical contributions to focal dystonia (a somato-motor disorder affecting specific body parts, such as the hand in musicians or writers) has argued that this disorder shares the same signature (Breakefield et al., 2008; Elbert et al., 1998; Flor and Diers, 2009; Hirata et al., 2004). Taken together, these findings suggest that lesion-induced reorganization has similar consequences across modalities and that the same process in the auditory cortex may be an underlying cause of tinnitus.
Recently, high-resolution structural MRI studies with voxel-based morphometry (VBM) have indicated that tinnitus-related reorganization of auditory cortex in humans may be accompanied by structural changes at the thalamic level (Mühlau et al., 2006). The VBM technique can identify differences in volume and tissue density of brain regions by direct comparison between two groups. Using VBM on tinnitus patients, Mühlau et al. (2006) observed a significant increase of grey-matter density in the posterior thalamus, including the medial geniculate nucleus (MGN). Using individual morphological segmentation, which is better able to detect changes within small and anatomically variable areas, Schneider et al. (2009) also found volume loss in the medial portion of Heschl's gyrus (HG) in tinnitus patients. Additional evidence from the somatosensory system suggests that lesion-induced functional reorganization may be amplified at the thalamic level, and thus structural changes could manifest more readily there: Effects of cortical reorganization of the hand representation in macaque monkeys are enhanced in the ventroposterior nucleus of thalamus via descending projections (Ergenzinger et al., 1998).
In summary, as evidenced by the various approaches discussed, the long-term reorganization of central auditory pathways following sensorineural hearing loss appears to consist of changes at the cortical as well as thalamic level. It is entirely possible that tinnitus, like many apparently heterogeneous neurological syndromes, may be the end-result of various different causes. Different subtypes of tinnitus have indeed been described in cluster analyses, for instance cases in which tinnitus gets better with (i.e. is masked by) noise (a fundamental tenet for many treatment attempts; (Okamoto et al., 2010; Roberts, 2007)), but also cases in which tinnitus gets worse in noise (Stouffer and Tyler, 1990). Notably, tinnitus has been reported to occur in individuals with normal hearing (Heller and Bergman, 1953). In cases of tinnitus with intact hearing a model of lesion-induced plasticity would not apply, as a lesion in the auditory periphery may not exist. On the other hand, “normal hearing” is often determined on the basis of standard audiological examination, which is performed in octave-steps and only at frequencies below 8 kHz. Cases of mild notch-like hearing loss or hearing loss above 8 kHz could easily escape such testing. When audiological testing is performed at finer intervals and at frequencies above 8 kHz, cases of tinnitus with absolutely no hearing loss become more rare in our hands and in those of other investigators (Salvi et al., 2009). It is safe to say, therefore, that the great majority of tinnitus cases do involve SNHL, i.e. damage to the sensory periphery (Hoffman and Reed, 2004). Importantly, the reverse is not true, i.e. not everyone with SNHL develops tinnitus (see below). Thus, although universal models or treatments for tinnitus may not exist, we argue that central auditory system reorganization is a necessary prerequisite for chronic tinnitus.
Evidence of Paralimbic Involvement in Tinnitus
Although reorganization of auditory cortex and thalamus in response to peripheral deafferentation may be necessary for the tinnitus perception to arise, it is almost certainly not sufficient. First, if peripheral hearing loss results in central auditory reorganization, which leads to the tinnitus percept, why is it that only a subset of patients with noise-induced hearing loss (between 20 and 40%; (Hoffman and Reed, 2004)) develop chronic tinnitus? Shouldn't one expect that noise-induced hearing loss always leads to cortical reorganization and, thus, to tinnitus? Secondly, modulation of tinnitus strength by non-auditory factors is well known in chronic tinnitus. In cases of “somatic” tinnitus, the tinnitus percept can be modulated by movements of the eyes, neck or jaw (Fields, 2007; Levine et al., 2003). Significant changes in tinnitus level also occur as a result of stress or sleep deprivation (Alster et al., 1993; Folmer and Griest, 2000; Hallam, 1996). A sizeable portion of tinnitus patients suffer from what is known as “intermittent” tinnitus, in which the ringing sensation disappears completely for days or weeks, only to resume at full force. It appears that, in addition to changes in auditory pathways, a “switch” exists elsewhere in the brain that can turn the tinnitus sensation on or off. In neurophysiological terms, such a switch may be referred to as an inhibitory gating mechanism.
A potential breakthrough in our understanding of such gating mechanisms occurred when, in their VBM study, Mühlau et al. (2006) found a highly significant volume loss in the “subcallosal area” of tinnitus patients (Fig. 1). This result was recently replicated by Hyde and colleagues (2009) and, in a more general form implicating paralimbic structures, by Landgrebe et al. (2009). Volume loss may point to atrophy of neurons or glia cells (i.e. changes in a region's intrinsic architecture) with characteristic functional consequences (May and Gaser, 2006). Though the subcallosal area is not sharply delineated as a homogeneous region and contains medial prefrontal, orbitofrontal and anterior cingulate areas, various findings suggest that it constitutes a major hub linking limbic-affective systems with thalamo-cortical perceptual systems. Activation of the subcallosal area, for instance, correlates by varying degrees with the unpleasant effects of dissonant music (Blood et al., 1999) and is modulated by the perception and anticipation of pain (Ploghaus et al., 2003). Furthermore, abnormal activity levels in the subcallosal (subgenual anterior cingulate and medial prefrontal) area are found in people with certain depressive disorders (Drevets et al., 1997; Mayberg et al., 2005).
Figure 1. Results of voxel-based morphometry (VBM) in the brains of tinnitus patients compared to normal controls.
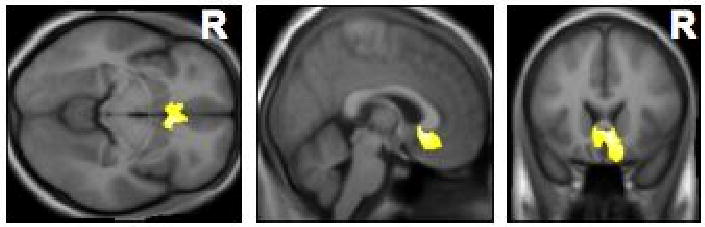
Whole-brain analysis identified a highly significant volume loss in the subcallosal area of tinnitus patients (from Mühlau et al., 2006). Horizontal, sagittal, and coronal slices are shown (from left to right).
In addition to encompassing the above cortical regions, the subcallosal area in its posterior portion overlaps with the nucleus accumbens (NAc), which is a major component of the ventral striatum (Blood and Zatorre, 2001; Gruber et al., 2009). The ventral striatum is intensely interconnected with the various cortical regions of the subcallosal area (Johansen-Berg et al., 2008; Ongur and Price, 2000). The NAc (and its associated network in the medial prefrontal cortex) contains dopaminergic and serotonergic neurons among other types. Whereas the dopaminergic system within the NAc is well known for its involvement in reward behavior and avoidance learning (McCullough et al., 1993), serotonergic neurons play a modulatory role in various emotion-related systems. Importantly, the NAc receives glutamatergic input from the amygdala, as well as projections from the hippocampus and the raphe nuclei (Ambroggi et al., 2008; Azmitia and Segal, 1978). The latter are the major origin of the serotonergic system and are responsible for the regulation of sleep.
Paralimbic Structures as Part of An Intrinsic Noise-Cancellation System
How do the limbic regions of the subcallosal area interact with the thalamo-cortical sensory/perceptual systems? In addition to corticofugal and transcortical glutamatergic projections from vmPFC, anatomical data indicate that serotonergic axons (from the dorsal raphe nucleus, the NAc, and other paralimbic regions) innervate the thalamic reticular nucleus (TRN) and the dorsal thalamus (Brown and Molliver, 2000; O'Donnell et al., 1997). Serotonin excites the GABAergic neurons of the TRN (McCormick and Wang, 1991; Pape and McCormick, 1989), which in turn exert a powerful inhibitory influence on sensory thalamic relay cells (Guillery and Sherman, 2002). TRN-mediated inhibition can also cause thalamic relay neurons to shift between tonic and burst-firing modes, the latter of which requires cells to be in a hyperpolarized state (Llinas and Jahnsen, 1982; Sherman, 2001). What role the TRN may play in the enhanced synchronous activity observed in animal models of tinnitus (Eggermont and Roberts, 2004; Norena and Eggermont, 2003) is an intriguing question. However, the TRN on the whole plays a pivotal role in the control of thalamo-cortical transmission and, with its strictly modality-specific and topographic organization (Jones, 2007; Sherman and Guillery, 2006), it can perform its gain-control function in a highly localized manner.
Much of the original evidence for the role of the TRN was accumulated by studies on the lateral geniculate nucleus (LGN) of the visual system (Crick, 1984; Llinas and Jahnsen, 1982). Recently, however, an analogous role of the TRN has been demonstrated for the medial geniculate nucleus (MGN) of the auditory system as well (Yu et al., 2009b). TRN input strongly inhibits MGN neurons in both anesthetized and conscious animals and does so in a highly frequency-specific manner (Yu et al., 2009a). Thus, serotonergic neurons in the subcallosal area, via their input to the TRN, can modulate transmission from the sensory periphery and brainstem to the cerebral cortex by interfering at the level of the thalamus. Since cortical activation is considered a prerequisite for conscious perception, the NAc-TRN system is in a position to cancel out the perception of persistent unpleasant noises, including phantom sensations such as tinnitus (Fig. 2). While the gain of the tinnitus frequency is selectively reduced, other frequencies remain unaffected.
Figure 2. Tinnitus as the result of a broken neural “noise-cancellation” mechanism.
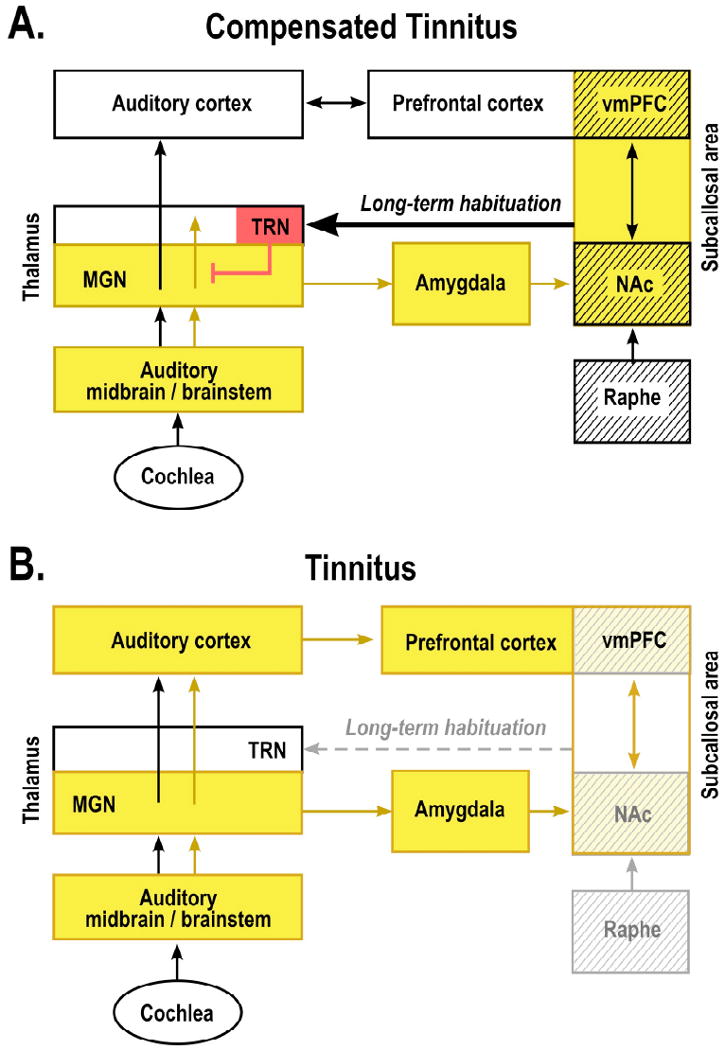
According to the theory proposed here, the nucleus accumbens (NAc) and its associated paralimbic networks in the ventromedial prefrontal cortex (vmPFC) play an important role in long-term habituation to continuous unpleasant sounds. Sound-evoked neural activity is relayed from the auditory periphery via the brainstem and thalamus (MGN) to the auditory cortex for conscious perception. The same signal is directed in parallel via the amygdala to the subcallosal area (which includes the NAc region of the ventral, “limbic” striatum and the vmPFC) for evaluation of the sound's emotional content. From the subcallosal area, an excitatory projection feeds back to the thalamic reticular nucleus (TRN), which in turn inhibits selectively the sections of the MGN corresponding to the unpleasant sound frequencies. This gain-control mechanism leads to a highly specific filtering (“tuning out”) of repetitive unwanted noises, which, as a consequence, do not reach conscious perception in the auditory cortex. The initial tinnitus signal results from peripheral deafferentation (loss of hair cells through injury, aging, or loud-noise exposure) and subsequent lesion-induced reorganization of central auditory structures. As long as the NAc-system is intact (A: “Compensated Tinnitus”), the tinnitus signal is filtered out and will not be relayed to the auditory cortex. If, however, the NAc-system becomes compromised (B: “Tinnitus”), cancellation of the tinnitus signal at the thalamic level is no longer possible, tinnitus perception results, and long-term reorganization of auditory cortex sets in to render the tinnitus chronic. Cases of intermittent tinnitus may occur during a stage at which damage to the subcallosal area is still temporary: fluctuating activity (and corresponding neurotransmitter) levels allow transient filtering of the tinnitus signal. The raphe nuclei, which control serotonin levels and sleep cycles, provide a major input to the NAc and may thus contribute to the correlation between tinnitus strength and insomnia. Structures with serotonergic innervation are shown by hatching; inhibitory structures are shown in red; yellow indicates presence of tinnitus signal.
In our view, cochlear lesions will inevitably begin to induce a process of plastic reorganization, which leads to a perceptual filling-in of the deafferented frequency range but also generates hyperactivity (and thus an initial tinnitus signal) in the ascending auditory pathways. Normally, this unwanted noise signal is identified by the limbic system and eliminated from perception (“tuned out”) by feeding it back to the (inhibitory) TRN, which subtracts it from the afferent auditory signal. Thus, this circuit serves as an active neural “noise-cancellation” mechanism (Fig. 2A). In cases in which the pertinent limbic regions become dysfunctional, noise-cancellation breaks down and the tinnitus signal permeates to the auditory cortex, where it enters into consciousness and eventually leads to permanent auditory cortical reorganization (Fig. 2B).
What Breaks the Noise-Cancellation System?
There are various ways in which the subcallosal area could become dysfunctional. First, it could be a consequence of overload (and resulting excitotoxicity) from chronic firing of NAc neurons trying to compensate for the tinnitus signal. Evidence for abnormal activity in the NAc of tinnitus patients is indeed forthcoming (Leaver et al., 2010). Why neurons in some individuals (i.e., those with SNHL but no tinnitus) are better protected from damage than in others (i.e., individuals with tinnitus) remains unclear. However, the use of neuroprotective drugs, such as the NMDA antagonist neramexane (Merz Pharmaceuticals), might aid with the prevention (or even reversal) of chronic tinnitus.
Second, it is possible that tinnitus patients have an independent, systemic vulnerability in one or more limbic-relevant transmitter systems, such as serotonin (5-HT) (see below), making these individuals more susceptible to tinnitus as well as other disorders, like chronic pain or depression. In affected individuals, underlying transmitter levels may decline faster over time or with age than in unaffected individuals. Although mechanisms driving such “system dysfunctions” (e.g., dopaminergic cells in the basal ganglia are known to fail over time in Parkinsonism) are not yet characterized, they are likely to be multifactorial, with genetic vulnerability, developmental insults, and environmental stressors all considered important and synergistic contributors (Insel, 2007; Mayberg et al., 2005).
Previous theories of tinnitus have assumed a largely “reactive” role for limbic structures that reflects a mostly learned distress response (Jastreboff, 1990; Jastreboff, 2000). Tinnitus was thought to cause insomnia, depression, or anxiety; limbic activation found in prior PET imaging studies was seen largely as a reflection of these emotional effects of tinnitus and the suffering associated with this condition. The present model assigns a more central role to limbic and paralimbic structures in and around the subcallosal area, in that they participate in a self-regulating gating process that can actually prevent the tinnitus signal from being perceived. More generally, therefore, limbic activity does not only color our perception, it is even in a position to completely suppress or tune out the signal if it is deemed unpleasant and/or redundant. Such limbic influences on thalamo-cortical transmission may prove to be more fundamental in health and disease than hitherto thought and may be realized by a relatively simple feedback circuit, as proposed here.
Open Questions and Future Directions
How can the model laid out in the present article be tested? Direct study of the relevant networks in animal models would be most compelling. Electrical or chemical stimulation of the NAc or surrounding areas and simultaneous recording from the TRN, MGN or auditory cortex would be feasible (Fig. 3). Animals could be trained to signal the presence of a tone or narrow-band noise; restricted cochlear lesions could be induced in these animals via loud-noise exposure, and they could subsequently be tested for the presence of tinnitus (Jastreboff, 1990; Lobarinas et al., 2008; Turner et al., 2006). However, even in intact animals, information regarding the effectiveness of NAc stimulation for inhibiting sensory transmission in the MGN would be useful. In severely debilitating cases of tinnitus in human patients, deep-brain stimulation of the NAc, which has proven to be an effective treatment for obsessive-compulsive disorder and depression (Mayberg et al., 2005; McCracken and Grace, 2009; Schlaepfer et al., 2008), may be considered for approval.
Figure 3. Possible animal model of tinnitus testing the validity of the NAc-TRN hypothesis.
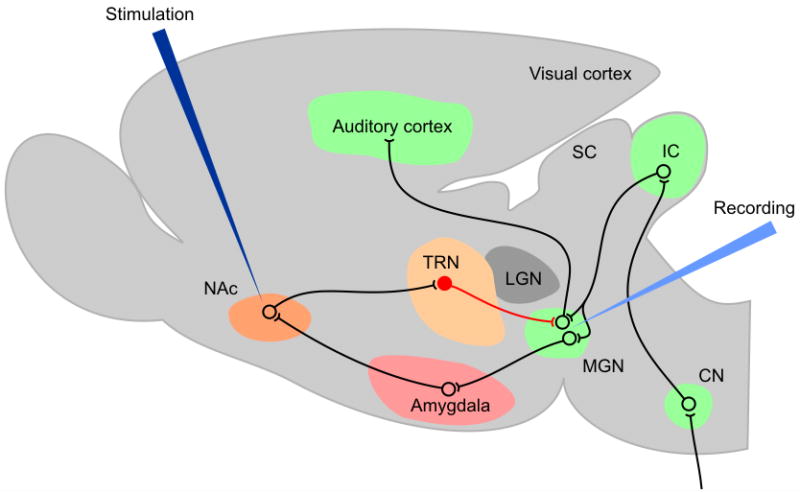
Electrical or chemical stimulation of the NAc is proposed while recording from MGN or auditory cortex. Similar studies of the TRN have been performed by Yu et al. (2009b) and could be extended to testing the modulatory influence from paralimbic structures, such as the NAc, on auditory transmission. Inhibitory connection from TRN to MGN is shown in red. Auditory nuclei in green (CN: cochlear nuclei; IC: inferior colliculus; SC: superior colliculus).
If the sensory “gating” mechanism we propose is indeed mediated by serotonergic neurons in the NAc/subcallosal network, the present model would re-invoke a “serotonin hypothesis of tinnitus” (Dobie, 2003; Simpson and Davies, 2000). This hypothesis was derived from the correlates of serotonin depletion, such as hypersensitivity to noise, reduced REM sleep, and depression/anhedonia (Geyer and Vollenweider, 2008; Marriage and Barnes, 1995), all of which co-occur with tinnitus to varying degrees. Thus, tinnitus may be another symptom of this overall depletion, and, accordingly, patients with intermittent tinnitus could simply have fluctuating levels of serotonin. However, the effectiveness of drugs that modulate serotonin is largely untested in tinnitus patients. Thus far, two studies of selective serotonin reuptake inhibitors (SSRIs) report mixed results (Baldo et al., 2006; Robinson, 2007), and the success of noradrenergic and specific serotonergic antidepressants (NaSSAs), such as mirtazapine, remains anecdotal. There is a need for rigorous and systematic trials using double-blind, placebo-controlled and randomized designs, and effects on different 5-HT receptor subtypes need to be separated. Tinnitus may also respond to treatment with serotonin agonists (triptanes), which have been shown to be an effective treatment for migraine headaches.
In this context, analogies between tinnitus and chronic pain may be informative (Flor et al., 2006; King et al., 2009; Moller, 2007). Many forms of chronic pain are modulated by stress, emotions and fatigue and are co-morbid with anxiety and depression (Folmer et al., 2001). Chronic pain often results from previous bodily injury, which could lead to long-term central reorganization. It is quite possible, therefore, that a limbic gating system also exists for the somatosensory system and is responsible not only for creating the unpleasantness of pain but also for modulating its intensity. Indeed, neuroimaging data do implicate the NAc/vmPFC system in several forms of chronic pain, e.g. fibromyalgia (Becerra et al., 2001; Kuchinad et al., 2007; Schweinhardt et al., 2009). Combined selective serotonin-norepinephrine reuptake inhibitors (SNRIs) have been approved for treatment of neuropathic pain; however, dopamine also mediates some analgesia, including the astounding effects of placebos (Scott et al., 2007). How these effects co-vary in individuals that suffer from both tinnitus and chronic pain would be of particular interest.
It will be important to further investigate other comorbidities observed in tinnitus patients as well, including depression and insomnia. Arguably, insomnia is the single most important complaint associated with tinnitus (Alster et al., 1993), and it has become clear that the tinnitus sound itself is not necessarily what keeps the patients awake (McKenna and Daniel, 2006). Instead, the opposite may be true: insomnia may cause tinnitus, and both may be associated with serotonin depletion. It appears, therefore, that tinnitus is the auditory symptom of an underlying syndrome, which becomes evident in patients who happen to have a hearing loss. Similarly, Parkinsonism was long considered primarily a motor disorder because motor symptoms are most apparent, yet cognitive deficits are revealed after more refined testing and with better hypotheses.
Summary and Conclusions
According to the model laid out in this article, tinnitus most likely results from the following factors:
In most, if not all cases, the process leading to tinnitus is triggered by a lesion to the auditory periphery, e.g. a loss of hair cells in the inner ear resulting from acoustic trauma or aging.
Loss of input in the lesioned frequency range leads to an overrepresentation of lesion-edge frequencies, which causes hyperactivity and possible burst-firing in central auditory pathways, constituting the initial tinnitus signal.
Under normal circumstances, the tinnitus signal is cancelled out at the level of the thalamus by an inhibitory feedback loop originating in paralimbic structures: activity from these structures reaches the TRN, which in turn inhibits the MGN. If, however, paralimbic regions are compromised, inhibition of the tinnitus signal at the thalamic gate is lost, and the signal is relayed all the way to the auditory cortex, where it leads to permanent reorganization and chronic tinnitus.
Finally, identification of the transmitter systems involved in the brain's intrinsic NAc/vmPFC-TRN noise cancellation system could open avenues for drug treatment of tinnitus.
Acknowledgments
Grant support was provided by the National Institutes of Health (grants R01 NS052494, RC1 DC010720), the Tinnitus Research Consortium (TRC), the Tinnitus Research Initiative (TRI), and the Skirball Foundation. We thank P. Chablani and A. Seydell for critical comments on the manuscript, and D. Klemm, P. Kusmierek and X. Zhan for help with the graphics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjamian P, Sereda M, Hall DA. The mechanisms of tinnitus: perspectives from human functional neuroimaging. Hear Res. 2009;253:15–31. doi: 10.1016/j.heares.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Alster J, Shemesh Z, Oman M, Attias J. Sleep disturbance associated with chronic tinnitus. Biol Psychiatry. 1993;34:84–90. doi: 10.1016/0006-3223(93)90260-k. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec. 1996;58:195–199. doi: 10.1159/000276835. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Baldo P, Doree C, Lazzarini R, Molin P, McFerran DJ. Antidepressants for patients with tinnitus. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003853.pub2. CD003853. [DOI] [PubMed] [Google Scholar]
- Bauer CA. Animal models of tinnitus. Otolaryngol Clin North Am. 2003;36:267–285. vi. doi: 10.1016/s0030-6665(02)00171-8. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Lutzenberger W, Montoya P, Larbig W, Unertl K, Topfner S, Grodd W, Taub E, Flor H. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci. 1997;17:5503–5508. doi: 10.1523/JNEUROSCI.17-14-05503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2:382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Brown P, Molliver ME. Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J Neurosci. 2000;20:1952–1963. doi: 10.1523/JNEUROSCI.20-05-01952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Calford MB, Chino YM, Das A, Eysel UT, Gilbert CD, Heinen SJ, Kaas JH, Ullman S. Neuroscience: rewiring the adult brain. Nature. 2005;438:E3. doi: 10.1038/nature04359. discussion E3-4. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- Dobie RA. Depression and tinnitus. Otolaryngol Clin North Am. 2003;36:383–388. doi: 10.1016/s0030-6665(02)00168-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Hajak G, Kleinjung T, Landgrebe M, Langguth B. Functional imaging of chronic tinnitus: the use of positron emission tomography. Prog Brain Res. 2007;166:83–88. doi: 10.1016/S0079-6123(07)66008-7. [DOI] [PubMed] [Google Scholar]
- Elbert T, Candia V, Altenmuller E, Rau H, Sterr A, Rockstroh B, Pantev C, Taub E. Alteration of digital representations in somatosensory cortex in focal hand dystonia. Neuroreport. 1998;9:3571–3575. doi: 10.1097/00001756-199811160-00006. [DOI] [PubMed] [Google Scholar]
- Ergenzinger ER, Glasier MM, Hahm JO, Pons TP. Cortically induced thalamic plasticity in the primate somatosensory system. Nat Neurosci. 1998;1:226–229. doi: 10.1038/673. [DOI] [PubMed] [Google Scholar]
- Fields RD. The case of the loud eyeballs. Sci Am Mind 2007 [Google Scholar]
- Flor H, Diers M. Sensorimotor training and cortical reorganization. NeuroRehabilitation. 2009;25:19–27. doi: 10.3233/NRE-2009-0496. [DOI] [PubMed] [Google Scholar]
- Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–881. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- Florence SL, Kaas JH. Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J Neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer RL, Griest SE. Tinnitus and insomnia. Am J Otolaryngol. 2000;21:287–293. doi: 10.1053/ajot.2000.9871. [DOI] [PubMed] [Google Scholar]
- Folmer RL, Griest SE, Martin WH. Chronic tinnitus as phantom auditory pain. Otolaryngol Head Neck Surg. 2001;124:394–400. doi: 10.1067/mhn.2001.114673. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Chery-Croze S, Fischer G, Fischer C, Vighetto A, Gregoire MC, Lavenne F, Collet L. A selective imaging of tinnitus. Neuroreport. 1999;10:1–5. doi: 10.1097/00001756-199901180-00001. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Hussain RJ, O'Donnell P. The nucleus accumbens: a switchboard for goal-directed behaviors. PLoS One. 2009;4:e5062. doi: 10.1371/journal.pone.0005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Hallam RS. Correlates of sleep disturbance in chronic distressing tinnitus. Scand Audiol. 1996;25:263–266. doi: 10.3109/01050399609074965. [DOI] [PubMed] [Google Scholar]
- Heller MF, Bergman M. Tinnitus aurium in normally hearing persons. Ann Otol Rhinol Laryngol. 1953;62:73–83. doi: 10.1177/000348945306200107. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Schulz M, Altenmuller E, Elbert T, Pantev C. Sensory mapping of lip representation in brass musicians with embouchure dystonia. Neuroreport. 2004;15:815–818. doi: 10.1097/00001756-200404090-00015. [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB, editor. Tinnitus: Theory and Management. Hamilton, Ont: BC Decker Inc.; 2004. [Google Scholar]
- Hoke M, Feldmann H, Pantev C, Lutkenhoner B, Lehnertz K. Objective evidence of tinnitus in auditory evoked magnetic fields. Hear Res. 1989;37:281–286. doi: 10.1016/0378-5955(89)90028-2. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Zatorre RJ, Evans AC, Hebert S. Structural brain differences in unilateral tinnitus. Organization Human Brain Mapping (San Francisco) 2009 [Google Scholar]
- Insel TR. Neuroscience. Shining light on depression. Science. 2007;317:757–758. doi: 10.1126/science.1147565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine DR, Rajan R, Brown M. Injury- and use-related plasticity in adult auditory cortex. Audiol Neurootol. 2001;6:192–195. doi: 10.1159/000046831. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ. Tinnitus Habituation Therapy (THT) and Tinnitus Retraining Therapy (TRT) In: Tyler R, editor. Handbook on Tinnitus. San Diego, CA: Singular Publishing Group; 2000. pp. 357–376. [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. 2nd. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, de Ridder D, Hajak G. Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage. 2009;46:213–218. doi: 10.1016/j.neuroimage.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Langguth B, Goodey R, Azevedo A, Bjorne A, Cacace A, Crocetti A, Del Bo L, De Ridder D, Diges I, Elbert T, et al. Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Prog Brain Res. 2007;166:525–536. doi: 10.1016/S0079-6123(07)66050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet M, Morgan S, Kim HJ, Rauschecker JP. Functional and structural abnormalities in limbic and auditory areas contributing to tinnitus. Organization of Human Brain Mapping (Barcelona) 2010 [Google Scholar]
- Leaver AM, Renier L, Purcell J, Costanzo M, Fieger A, Morgan S, Kim HJ, Rauschecker JP. Auditory cortical map plasticity in tinnitus. Soc Neurosci. 2006;32:800–802. [Google Scholar]
- Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153:643–648. doi: 10.1007/s00221-003-1747-3. [DOI] [PubMed] [Google Scholar]
- Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Stolzberg D, Lu J, Salvi R. Human brain imaging of tinnitus and animal models. Semin Hear. 2008;29:333–349. doi: 10.1055/s-0028-1095893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- Marriage J, Barnes NM. Is central hyperacusis a symptom of 5-hydroxytryptamine (5-HT) dysfunction? J Laryngol Otol. 1995;109:915–921. doi: 10.1017/s0022215100131676. [DOI] [PubMed] [Google Scholar]
- May A, Gaser C. Magnetic resonance-based morphometry: a window into structural plasticity of the brain. Curr Opin Neurol. 2006;19:407–411. doi: 10.1097/01.wco.0000236622.91495.21. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Wang Z. Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J Physiol. 1991;442:235–255. doi: 10.1113/jphysiol.1991.sp018791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–5363. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Sokolowski JD, Salamone JD. A neurochemical and behavioral investigation of the involvement of nucleus accumbens dopamine in instrumental avoidance. Neuroscience. 1993;52:919–925. doi: 10.1016/0306-4522(93)90538-q. [DOI] [PubMed] [Google Scholar]
- McKenna L, Daniel HC. Tinnitus-related insomnia treatment. In: Tyler RS, editor. Tinnitus Treatment. New York: Thieme; 2006. [Google Scholar]
- Melcher JR, Levine RA, Bergevin C, Norris B. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res. 2009;257:63–74. doi: 10.1016/j.heares.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher JR, Sigalovsky IS, Guinan JJ, Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol. 2000;83:1058–1072. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- Mirz F, Gjedde A, Ishizu K, Pedersen CB. Cortical networks subserving the perception of tinnitus--a PET study. Acta Otolaryngol Suppl. 2000;543:241–243. doi: 10.1080/000164800454503. [DOI] [PubMed] [Google Scholar]
- Mirz F, Pedersen B, Ishizu K, Johannsen P, Ovesen T, Stodkilde-Jorgensen H, Gjedde A. Positron emission tomography of cortical centers of tinnitus. Hear Res. 1999;134:133–144. doi: 10.1016/s0378-5955(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Moller AR. Tinnitus and pain. Prog Brain Res. 2007;166:47–53. doi: 10.1016/S0079-6123(07)66004-X. [DOI] [PubMed] [Google Scholar]
- Mühlau M, Rauschecker JP, Oestreicher E, Gaser C, Röttinger M, Wohlschläger AM, Simon F, Etgen T, Conrad B, Sander D. Structural brain changes in tinnitus. Cereb Cortex. 2006;16:1283–1288. doi: 10.1093/cercor/bhj070. [DOI] [PubMed] [Google Scholar]
- Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183:137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Lavin A, Enquist LW, Grace AA, Card JP. Interconnected parallel circuits between rat nucleus accumbens and thalamus revealed by retrograde transynaptic transport of pseudorabies virus. J Neurosci. 1997;17:2143–2167. doi: 10.1523/JNEUROSCI.17-06-02143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Stracke H, Stoll W, Pantev C. Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. Proc Natl Acad Sci U S A. 2010;107:1207–1210. doi: 10.1073/pnas.0911268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn Sci. 2003;7:197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine DR. Absence of plasticity of the frequency map in dorsal cochlear nucleus of adult cats after unilateral partial cochlear lesions. J Comp Neurol. 1998;399:35–46. [PubMed] [Google Scholar]
- Rajan R, Irvine DR, Wise LZ, Heil P. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol. 1993;338:17–49. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science. 1992;258:1159–1160. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends in Neurosciences. 1999a;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Making brain circuits listen. Science. 1999b;285:1686–1687. doi: 10.1126/science.285.5434.1686. [DOI] [PubMed] [Google Scholar]
- Roberts LE. Residual inhibition. Prog Brain Res. 2007;166:487–495. doi: 10.1016/S0079-6123(07)66047-6. [DOI] [PubMed] [Google Scholar]
- Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Robinson S. Antidepressants for treatment of tinnitus. Prog Brain Res. 2007;166:263–271. doi: 10.1016/S0079-6123(07)66024-5. [DOI] [PubMed] [Google Scholar]
- Salvi R, Lobarinas E, Sun W. Pharmacological treatments for tinnitus: new and old. Drugs of the Future. 2009;34:381–400. doi: 10.1358/dof.2009.034.05.1362442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N. Mapping cortical hubs in tinnitus. BMC Biol. 2009;7:80. doi: 10.1186/1741-7007-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Andermann M, Wengenroth M, Goebel R, Flor H, Rupp A, Diesch E. Reduced volume of Heschl's gyrus in tinnitus. Neuroimage. 2009;45:927–939. doi: 10.1016/j.neuroimage.2008.12.045. [DOI] [PubMed] [Google Scholar]
- Schwaber MK, Garraghty PE, Kaas JH. Neuroplasticity of the adult primate auditory cortex following cochlear hearing loss. Am J Otol. 1993;14:252–258. [PubMed] [Google Scholar]
- Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci. 2009;29:4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–126. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus and Its Role in Cortical Function. 2nd. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- Shulman A, Goldstein B, Strashun AM. Final common pathway for tinnitus: theoretical and clinical implications of neuroanatomical substrates. Int Tinnitus J. 2009;15:5–50. [PubMed] [Google Scholar]
- Simpson JJ, Davies WE. A review of evidence in support of a role for 5-HT in the perception of tinnitus. Hear Res. 2000;145:1–7. doi: 10.1016/s0378-5955(00)00093-9. [DOI] [PubMed] [Google Scholar]
- Smirnakis SM, Brewer AA, Schmid MC, Tolias AS, Schuz A, Augath M, Inhoffen W, Wandell BA, Logothetis NK. Lack of long-term cortical reorganization after macaque retinal lesions. Nature. 2005;435:300–307. doi: 10.1038/nature03495. [DOI] [PubMed] [Google Scholar]
- Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Disord. 1990;55:439–453. doi: 10.1044/jshd.5503.439. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Weisz N, Hartmann T, Dohrmann K, Schlee W, Norena A. High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hear Res. 2006;222:108–114. doi: 10.1016/j.heares.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, Elbert T. The neural code of auditory phantom perception. J Neurosci. 2007;27:1479–1484. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienbruch C, Paul I, Weisz N, Elbert T, Roberts LE. Frequency organization of the 40-Hz auditory steady-state response in normal hearing and in tinnitus. Neuroimage. 2006;33:180–194. doi: 10.1016/j.neuroimage.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Xu XX, Chen X, He S, He J. Slow recovery from excitation of thalamic reticular nucleus neurons. J Neurophysiol. 2009a;101:980–987. doi: 10.1152/jn.91130.2008. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Xu XX, He S, He J. Change detection by thalamic reticular neurons. Nat Neurosci. 2009b;72:1165–1170. doi: 10.1038/nn.2373. [DOI] [PubMed] [Google Scholar]


