SUMMARY
The transcriptional regulation of neuroectoderm (NE) specification is unknown. Here we show that Pax6 is uniformly expressed in early NE cells of human fetuses and those differentiated from human embryonic stem cells (hESCs). This contrasts the later expression of Pax6 in restricted mouse brain regions. Knockdown of Pax6 blocks NE specification from hESCs. Overexpression of either Pax6a or Pax6b, but not Pax6 PD, triggers hESC differentiation. However, only Pax6a converts hESCs to NE. In contrast, neither loss nor gain of function of Pax6 affects mouse NE specification. Both Pax6a and Pax6b bind to pluripotent gene promoters but only Pax6a binds to NE genes during human NE specification. These findings indicate that Pax6 is a transcriptional determinant of the human NE and suggest that Pax6a and Pax6b coordinate with each other in determining the transition from pluripotency to the NE fate in human by differentially targeting pluripotent and NE genes.
INTRODUCTION
In mammals, the stepwise cell fate transition during early embryonic development is orchestrated by sequential activation/inactivation of lineage-determining transcription factors (Yamanaka et al., 2006). Oct4, Sox2, and Nanog are required for maintaining pluripotency of the inner cell mass (ICM) or the epiblast in a blastocyst embryo (Avilion et al., 2003; Chambers et al., 2003; Mitsui et al., 2003; Nichols et al., 1998). Differentiation of the ICM to extraembryonic tissues is governed by Cdx2 and Gata6, transcription factors that repress pluripotency while inducing genes of the trophectoderm and extraembryonic endoderm, respectively (Jedrusik et al., 2008; Koutsourakis et al., 1999; Niwa et al., 2005). After the formation of extraembryonic tissues, the pluripotent epiblasts are converted to three germ layers during gastrulation, but how these processes are regulated remains unknown.
One of the best studied processes during gastrulation, neuroectoderm (NE) specification, is at the center of developmental biology. Studies in lower vertebrates, including frogs and chicks, indicate that many transcription factors are involved in NE specification, including zinc finger proteins, Sox family, Otx family and helix-loop-helix transcription factors (Mizuseki et al., 1998; Nakata et al., 1997; Rex et al., 1997; Sheng et al., 2003). To date, it is unclear which transcription factor is responsible for the conversion from pluripotent cells to NE in mammals. The most promising factor is Sox1, since its expression pattern parallels NE formation in mouse (Bylund et al., 2003; Pevny et al., 1998). However, Sox1-knockout mice do not exhibit severe brain deficits, probably due to compensation by other Sox members (Nishiguchi et al., 1998). Similarly, the transcriptional determinant for human NE specification is unknown.
The failure in identifying mammalian transcriptional determinants underlying NE specification is at least partly due to the lack of model systems that permit easy genetic manipulation and direct observation of developmental processes. Embryonic stem cells (ESCs), derived from the ICM or epiblast, differentiate to cells/tissues of the three germ layers following developmental principles (Murry and Keller, 2008; Stern, 2005; Zhang, 2006). When human ESCs (hESCs) are differentiated toward the neural fate under a chemically defined medium in the absence of growth factors, NE cells appear around day 6–8 and form neural tube-like rosettes at day 14 with corresponding gene expression patterns (Li et al., 2005; Pankratz et al., 2007; Zhang et al., 2001; Zhang and Zhang, 2010). This differentiation process resembles in vivo development of the neural plate and neural tube, and it thus represents a useful tool for studying the molecular underpinnings of human NE specification (Zhang, 2006).
During hESC neural differentiation, the initial NE cells do not express Sox1, the earliest marker of NE in mouse embryos or in NE differentiated from mouse ESCs (mESCs) (Li et al., 2005; Pankratz et al., 2007; Pevny et al., 1998; Suter et al., 2008; Ying et al., 2003). Instead, Pax6, a paired box (Pax) transcription factor expressed in region-specific neural progenitors after neural tube closure in mouse (Schmahl et al., 1993; Walther and Gruss, 1991), is uniformly expressed in hESC-derived NE (Li et al., 2005; Pankratz et al., 2007). These observations raise an intriguing possibility that Pax6 may play a novel role in human NE specification. Three isoforms of Pax6 have been identified. The canonical Pax6a harbors two DNA binding domains, the paired domain (PD) and homeodomain (HD), and a proline-serine-threonine (PST)-rich transactivation domain. Pax6b is a spliced variant of Pax6, which is produced by insertion of 14 amino acids (exon5a) into the PD, thus conferring different DNA binding specificity (Epstein et al., 1994; Kozmik et al., 1997; Walther and Gruss, 1991). The third isoform of Pax6 (Pax6 PD) lacks the paired domain. Both Pax6a and Pax6b are expressed in the brain, while Pax6 PD is only identified in eye and olfactory bulb (Kim and Lauderdale, 2006). In rodents, Pax6 is essential for the development of several organ systems, including eye, pancreas, and cerebrum (Chi and Epstein, 2002). In the present study, by genetic manipulation of ESCs, we discovered that Pax6 is necessary and sufficient for NE specification from human but not mouse ESCs. We also found that cell lineage specification of ESCs not only requires repression of pluripotent genes but also depends on induction of the target lineage genes.
RESULTS
Pax6 is uniformly expressed in early human, but not mouse NE
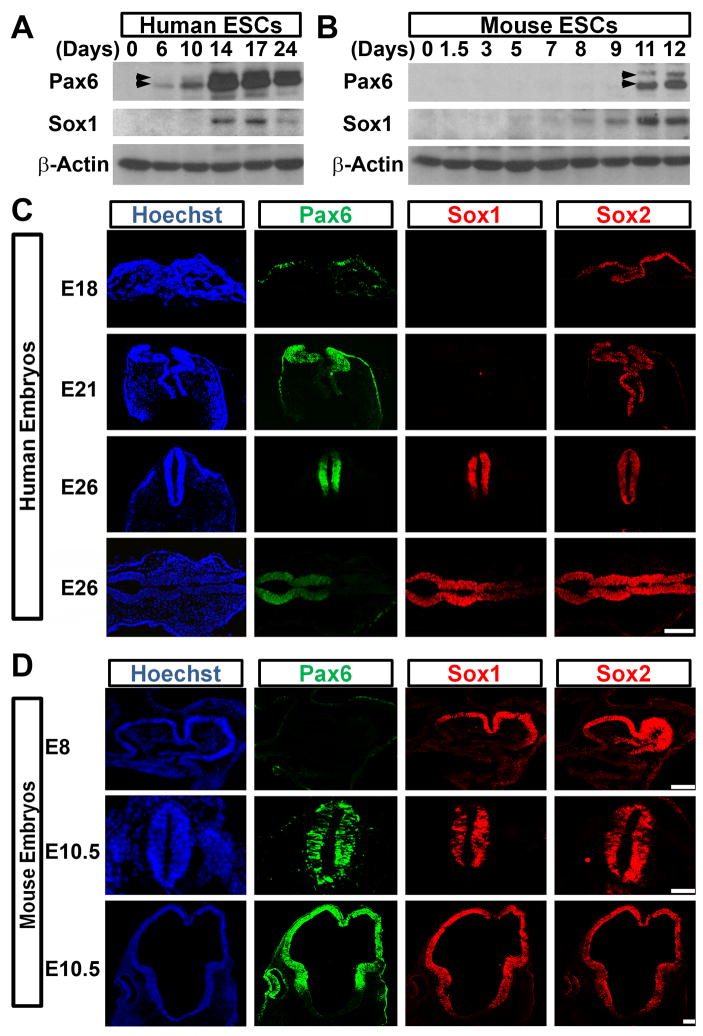
During mouse development, Pax6 is first detected in neural progenitors of the developing forebrain at E8.5–E9.5, one day after the formation of Sox1-expressing NE cells within the neural plate/tube (Bylund et al., 2003; Pevny et al., 1998; Walther and Gruss, 1991). However, NE cells differentiated from various hESC lines (H1, H9, H13, HSF1, HSF6) and induced pluripotent stem cells (iPSCs) under different conditions uniformly express Pax6 while Sox1 are still negative (Gerrard et al., 2005; Hu et al., 2010; Li et al., 2005; Pankratz et al., 2007; Wu et al., 2010; Yao et al., 2006). Importantly, the Pax6-expressing NE cells can be readily patterned to region-specific, Sox1-expressing neural progenitors, which will give rise to various neuronal subtypes, including dorsal and ventral forebrain, midbrain, spinal cord and retinal cells (Li et al., 2005; Li et al., 2009; Meyer et al., 2009; Pankratz et al., 2007; Yan et al., 2005; Zhang et al., 2001). This suggests that the early Pax6-expressing human NE cells represent a primitive state. We thus hypothesized that Pax6 may play a unique role in NE specification besides regional patterning in human. Western blotting analysis revealed that Pax6 was detectable 6 days after hESC differentiation, whereas Sox1 started to be detected around day 14 (Figure 1A). This was confirmed by immunostaining, showing that Pax6, but not Sox1, was expressed in NE cells at day 10 of differentiation from the H1 and H9 hESC lines as well as a human iPSC line (Figure S1A). In contrast, Pax6 was not detected until 2–3 days after Sox1 expression during mouse ESC neural differentiation (Figure 1B), consistent with previous reports (Bylund et al., 2003; Suter et al., 2008). It is also noteworthy that both Pax6a and Pax6b, but not Pax6 PD, were expressed in early human NE cells, as confirmed by an antibody recognizing the C-terminus of Pax6 (Kim and Lauderdale, 2006) (Figure S1B, S1C and S1D).
Figure 1.
Expression of neural transcription factors in fetuses and along ESC differentiation. A, B, Western blotting shows temporal expression of Pax6 and Sox1 along human and mouse ESC differentiation. Arrowheads, Pax6a (lower) and Pax6b (upper). C, Pax6 and Sox2, but not Sox1, are expressed in the neural plate of day-18 and day-21 human fetuses, and Sox1 is detected in the brain and neural tube of day-26 human fetus. D, Sox1 and Sox2 are expressed throughout the mouse neural plate and neural tube from day-8 to 10.5 whereas Pax6 is absent in day-8 embryos but present in the forebrain and neural tube at day-10.5. Bright field images are provided in supplementary Figure 1. Scale Bars, 100 μm (C) and 50 μm (D).
Validation analysis in human fetal tissues (Figure S1E) revealed that at E18 (Carnegie stage 8–9), when the neural plate begins to form, Pax6, but not Sox1, was detected in the single-layered NE cells that were also Sox2 positive (Figure 1C). This expression pattern was retained at E21 (Carnegie stage 10), in which the neural plate becomes pseudo-multiple layered. By the time that forebrain and midbrain have already been clearly demarcated at E26 (Carnegie stage 11–12), Pax6 was now restricted to the forebrain and part of the spinal cord but absent in the midbrain whereas both Sox1 and Sox2 were expressed in all NE cells (Figure 1C). Our previous study showed that NE cells differentiated from rhesus monkey ESCs also exhibited early Pax6 expression (Pankratz et al., 2007). Consistent with the in vitro observations, NE cells of rhesus monkey fetuses uniformly expressed Pax6, but not Sox1 (Figure S1F). In contrast to primates, Sox1 and Sox2 were highly expressed in the mouse neural plate at E8 whereas Pax6 was not expressed (Figure 1D). At E10.5, Pax6 was expressed in the dorsal forebrain and spinal cord, but not in the midbrain, whereas Sox1 and Sox2 were ubiquitously expressed in all NE cells (Figure 1D). Thus, Pax6 is expressed by early human but not mouse NE cells, suggesting a potential distinct role of Pax6 in human NE specification.
Pax6 is required for NE specification from hESCs
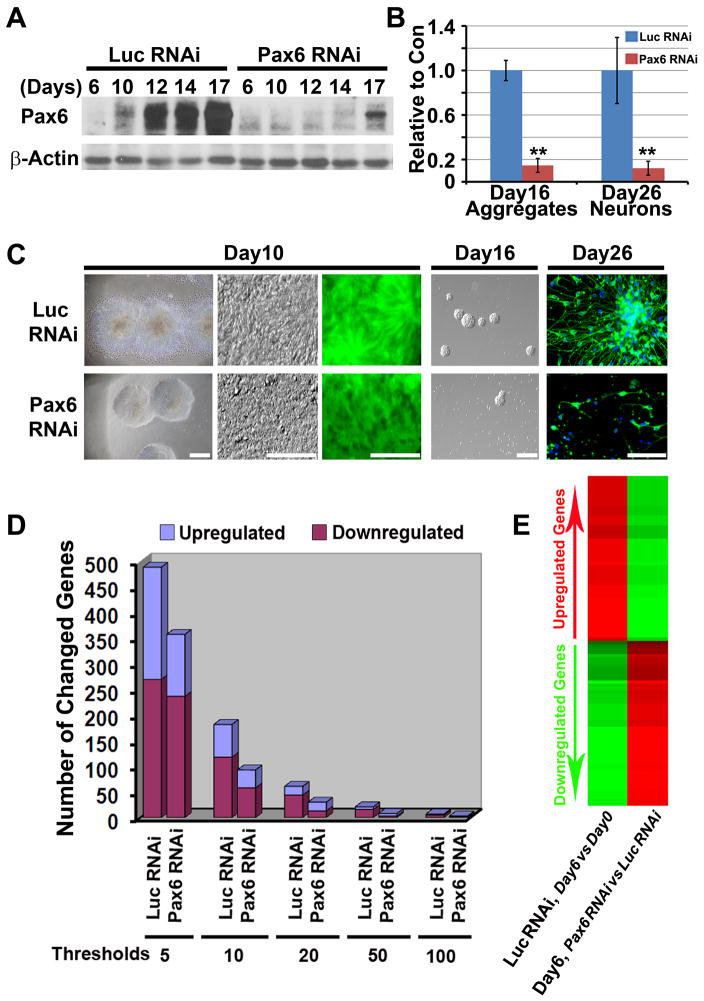
We then built ESC lines that constitutively express RNAi for Pax6 (targeting the homeodomain sequence and thus, all three isoforms) or luciferase (Luc, as a control) through lentiviral infection (Figure S2A), and the knockdown efficacy was confirmed by Western blotting (Figure 2A) and RT-PCR (Figure 7A and 7B). After 10 days of neural differentiation under our chemically defined conditions, hESC-derived NE cells with Luc RNAi presented typical columnar NE morphology and organized into rosettes (Pankratz et al., 2007; Zhang et al., 2001) (Figure 2C). Noticeably, differentiating hESCs with Pax6 RNAi remained as round aggregates formed by round cells but not migrating columnar cells (Figure 2C). Consistent results were obtained with different lines (with or without GFP, Figure S2A) and different batches of differentiation, indicating that the knockdown phenotype was not due to asynchronized differentiation or different viral integration.
Figure 2.
Knockdown of Pax6 inhibits human NE specification in vitro. A, Western blotting confirms knockdown of Pax6 in hESCs along neural differentiation. B and C, Neural differentiation of hESCs with Pax6 knockdown shows a loss of migrating columnar NE cells and impairment in subsequent formation of NE aggregates and generation of βIII-tubulin positive neurons. Scale bars, 50 μm. **, p<0.01 vs Luc RNAi control. D, Microarray analyses show that fewer genes are up- or down-regulated when the Pax6-RNAi hESCs were differentiated toward the neural fate. E, Analysis of the 50 most up- and down-regulated genes along differentiation of the control ESCs showed that these genes were less changed in the Pax6 knockdown lines.
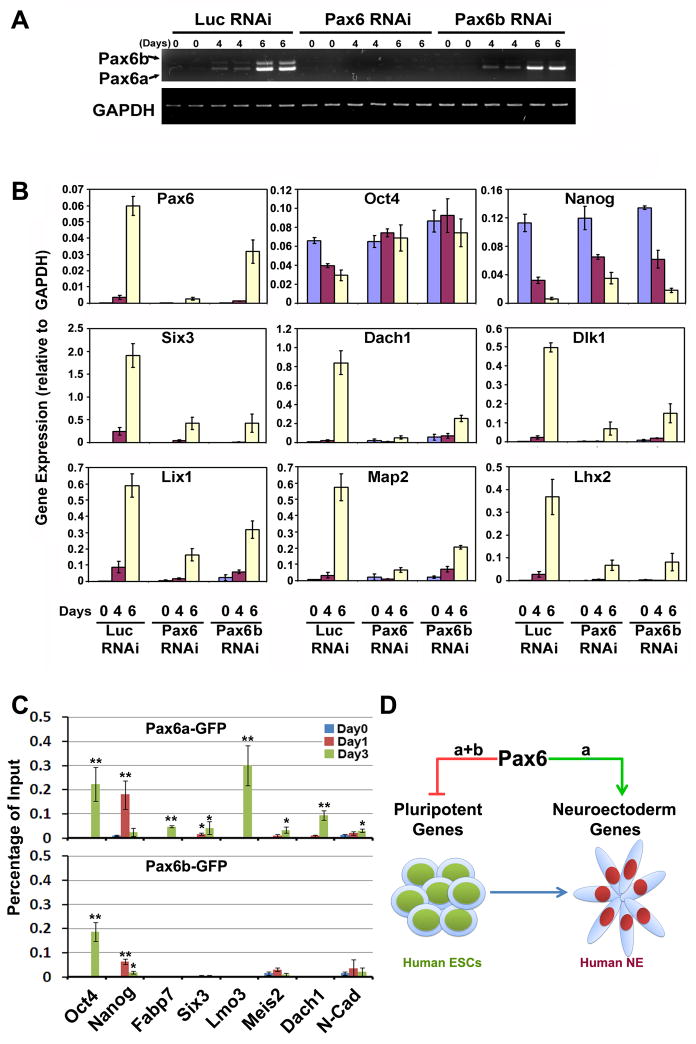
Figure 7.
Pax6a and Pax6b coordinate with each other in switching pluripotent cells to the NE fate. A, Pax6b RNAi downregulates Pax6b specifically in hESCs during neural differentiation. B, Knockdown of Pax6b diminishes the downregulation of pluripotent genes and upregulation of neural genes in a similar pattern to knockdown of both Pax6a and Pax6b. C, Both Pax6a and Pax6b can be recruited to the promoters of pluripotent genes, but only Pax6a binds to the promoters of neural genes. *, p<0.05; **, p<0.01 vs day 0. D, A model for Pax6 in neural specification from hESCs. Both Pax6a and Pax6b bind to the promoter of pluripotent genes (e.g., Oct4 and Nanog), repress the expression of pluripotent genes, and drive the cells out of the ESC state. However, loss of stem cell state does not automatically confer neural differentiation. Specification of the NE fate is dependent upon the neural inductive effect of Pax6a, which binds to a set of neural gene promoters and induces their expression.
The lack of columnar NE cells after Pax6 knockdown indicates failure of NE differentiation. Microarray analyses, using mRNA pooled from different transgenic lines, showed that about 500 genes were up- or down-regulated over 5 fold in the Luc RNAi control line after 6 days of differentiation (Figure 2D). Consistent with our previous report (Pankratz et al., 2007), the downregulated genes were related to ESC/epiblast (e.g., Oct4, Nanog, and Myc) and the upregulated genes (Lhx2, Six3, Six6, Lmo3, Meis2, N-cadherin, FGF8, FGF9, Delta like 1 homolog, and Wnt5b) were associated with the early NE (Table S1 and S2). In contrast, fewer genes were up- or down-regulated in the Pax6 knockdown cells no matter what threshold (fold change) was set (Figure 2D). The 50 most up- and down-regulated genes during differentiation of the control ESCs were less changed in the Pax6 knockdown lines (Figure 2E), which were confirmed by qRT-PCR (Figure 7B). Thus, cells with Pax6 knockdown largely retained pluripotent gene expression and had much less NE gene expression. Cell cycle analyses revealed no differential cell death or proliferation after Pax6 knockdown (Figure S6A, S6B and S6C). Therefore, Pax6 knockdown prevents hESCs from differentiation, thus trapping them in the pluripotent state.
After another 1–2 weeks of differentiation, NE cells from the Luc RNAi group readily formed NE aggregates and generated βIII-tubulin positive neurons. In contrast, cells with Pax6 knockdown under the same conditions rarely formed NE spheres and they failed to differentiate into neurons in adherent culture (Figure 2B and 2C). These data also suggest that cells derived from Pax6 RNAi lines are not properly developed to the NE stage.
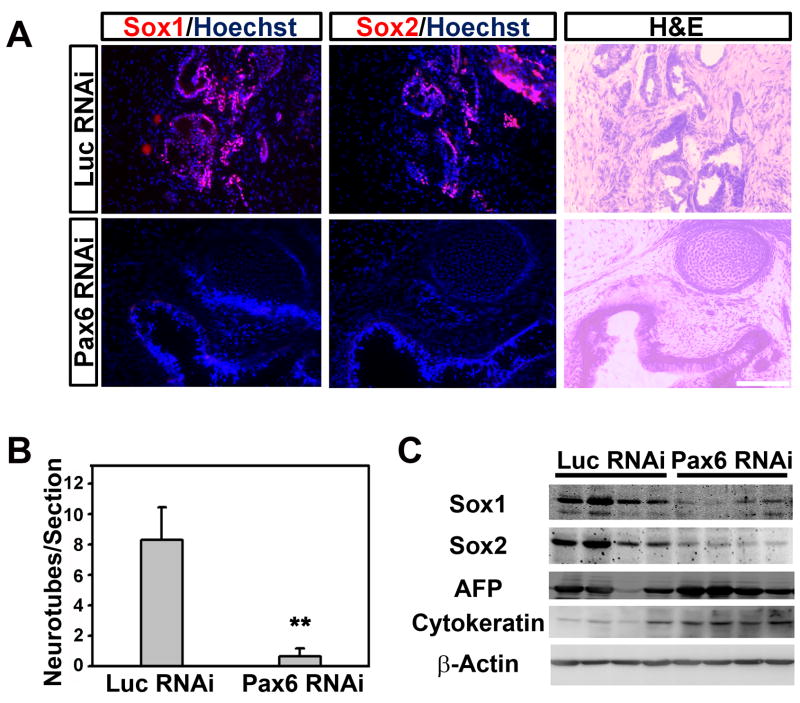
To exclude the possibility that the requirement of Pax6 in NE specification was due to our differentiation protocol, we adopted a new neural differentiation protocol through dual SMAD signaling inhibition (Chambers et al., 2009). Again, knockdown of Pax6 severely blocked pluripotent gene downregulation and NE gene upregulation even with the addition of BMP inhibitors (Figure S2B). To further exclude the possibility of cell culture artifact, undifferentiated hESCs were injected subcutaneously into severe combined immunodeficient (SCID) mice to produce teratomas, an in vivo system allowing ESC to differentiate into multi-lineages including neural tissues. Teratoma generation efficiency and size were comparable in both control and Pax6 knockdown groups. NE rosettes, revealed by hematoxylin and eosin (H&E) staining and confirmed by immunostaining for Sox1 and Sox2, were frequently observed in teratomas formed by hESCs with Luc RNAi but rarely in the Pax6 RNAi group (Figure 3A and 3B). Nevertheless, mesoderm (cartilage) and endoderm (gut epithelium) derivatives were observed in both Luc and Pax6 knockdown tumors (Figure 3A). Western blotting analyses of individual teratomas validated that the levels of neural transcription factors Sox1 and Sox2 drastically decreased in the Pax6 knockdown tumors, whereas the endodermal marker, alpha-fetoprotein (AFP), and epidermal marker, cytokeratin, were expressed at similar levels in both groups (Figure 3C). These data indicate that the requirement of Pax6 for human NE specification is not a culture artifact and Pax6 is likely a potential downstream factor of extracellular neural inducers during human NE specification.
Figure 3.
Knockdown of Pax6 inhibits human neural induction in vivo. A, B, Pax6 RNAi hESC-generated teratomas possess far fewer neural rosettes, confirmed by Sox1 and Sox2 staining, as compared to the luciferase RNAi lines. ** P<0.01 (t test); error bars, mean ± SEM. Scale bar, 100 μm. C, Teratomas from Pax6 RNAi lines express much lower amount of Sox1 and Sox2 proteins (28.3±12.2% for Sox1 and 18.4±5.8% for Sox2), but a similar level of α-fetoprotein and cytokeratin as compared to the luciferase RNAi lines.
Pax6 is not required for mouse NE specification
The opposite temporal expression pattern of Pax6 and Sox1 in human versus mouse suggests a differential role of Pax6 in NE specification in these two species. To test this hypothesis, we infected the D3 and Sox1/GFP reporter (Ying et al., 2003) mESCs with Pax6 or Luc RNAi lentiviruses (the RNAi targeting sequence is identical between human and mouse) and confirmed the knockdown efficiency by Western blotting (Figure S2E). Differentiation to Sox1-expressing mouse NE cells, indicated by GFP, was readily observable at day 6 and reached a peak at day 9–10, consistent with Western blotting analyses (Figure 1B). However, knockdown of Pax6 did not affect the Sox1 level as evaluated by fluorescent microscopy or FACS, suggesting that Pax6 is not necessary for mouse NE specification (Figure S2C and S2D). Western blotting using the naive mESCs (D3 line) confirmed that neither Pax6 nor Luc RNAi altered the expression of Sox1 (Figure S2E). The Pax6 RNAi-expressing mouse NE cells further differentiated to neurons with similar efficiency as the Luc RNAi control (Figure S2F). The side-by-side comparison of Pax6 RNAi effects on human versus mouse ESC neural differentiation strongly suggests that Pax6 is a crucial transcription factor for NE specification in human, but not mouse.
Overexpression of Pax6 in hESCs downregulates pluripotent gene expression
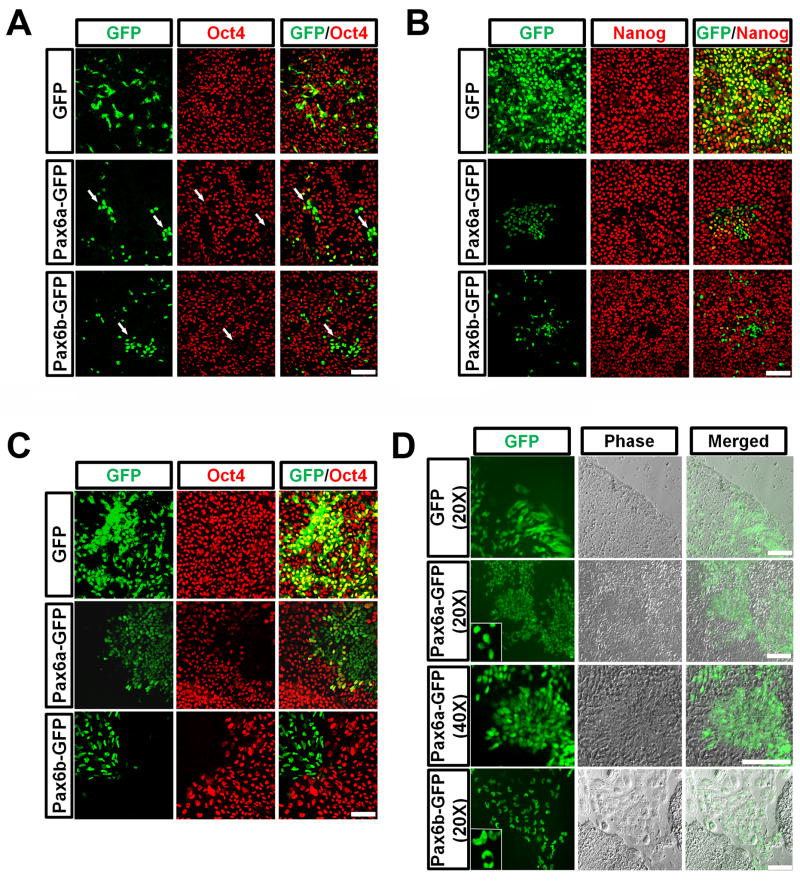
We next expressed Pax6a and Pax6b (with GFP fusion to the C-terminus) in hESCs under the elongation factor (EF) 1α promoter through lentiviral infection (Figure S1B). GFP expression was visible 30–40 hours after viral infection in both GFP and Pax6-GFP overexpressing cells with the highest GFP expression at day 4–5. Three days after infection, forced expression of GFP alone had no effect on Oct4 or Nanog expression, whereas overexpression of either Pax6a-GFP or Pax6b-GFP resulted in loss of Oct4 and Nanog expression even under the culture conditions that favored ESC maintenance (Figure 4A and 4B). Overexpression of Pax6 PD, however, did not affect Oct4 or Nanog expression (Figure S3), indicating the requirement of the paired domain in downregulating pluriptoent genes. Further experiments using Pax6 mutants indicated that deletion of the N-terminal PAI domain or the PST transactivation domain, but not the HD of Pax6 abrogated the effect of Pax6 in repressing Oct4 and Nanog (Figure S3). Therefore, except for the HD, all of the major parts of the Pax6 molecule, including the paired domain and the PST domain, are required for the effect of Pax6 on hESC differentiation.
Figure 4.
Overexpression of Pax6a or Pax6b results in differentiation to NE and trophectoderm, respectively. A, B, Overexpression of Pax6a or Pax6b in hESCs for 3 days causes Oct4 and Nanog downregulation. C, 5 days after lentiviral infection, Pax6+/Oct4− cells start to aggregate. Pax6b cells are present primarily outside of the hESC colony. D, 8 days after infection, Pax6a+ cells form columnar NE and organize into rosettes whereas Pax6b+ cells stay outside of the hESC colony and possess kidney- or horseshoe-shape nuclei. Insets show the different nuclear morphology of Pax6a+ and Pax6b+ cells. Scale bars, 50 μm.
Overexpression of Pax6a but not Pax6b directs hESCs to NE
Although both Pax6a and Pax6b downregulated pluripotent genes, it was not known whether the two Pax6 isoforms acted similarly on NE specification. By monitoring the hESC cultures daily we discovered that unlike the GFP control cells, the initially scattered Pax6a-GFP cells gradually aggregated in the hESC colonies (Figure 4C). Similar aggregation was observed in Pax6a HD mutant (Figure S3). 8 days after lentiviral infection, Pax6a positive cells exhibited an elongated columnar morphology and formed rosettes (Figure 4D), indicative of their neural identity. Interestingly, we found that Pax6b-GFP expressing cells migrated to the edge of the hESC colonies and eventually they became large flat cells, giving a membranous appearance outside of the hESC colonies (Figure 4C and Figure 4D). By fluorescent microscopy, we noticed kidney-like or horseshoe-shape large nuclei with two or more lobes in most Pax6b-GFP positive cells (Figure 4D). The migration property, cell morphology and multiploid nuclei suggest that the Pax6b expressing cells have adopted a trophoblast-like fate.
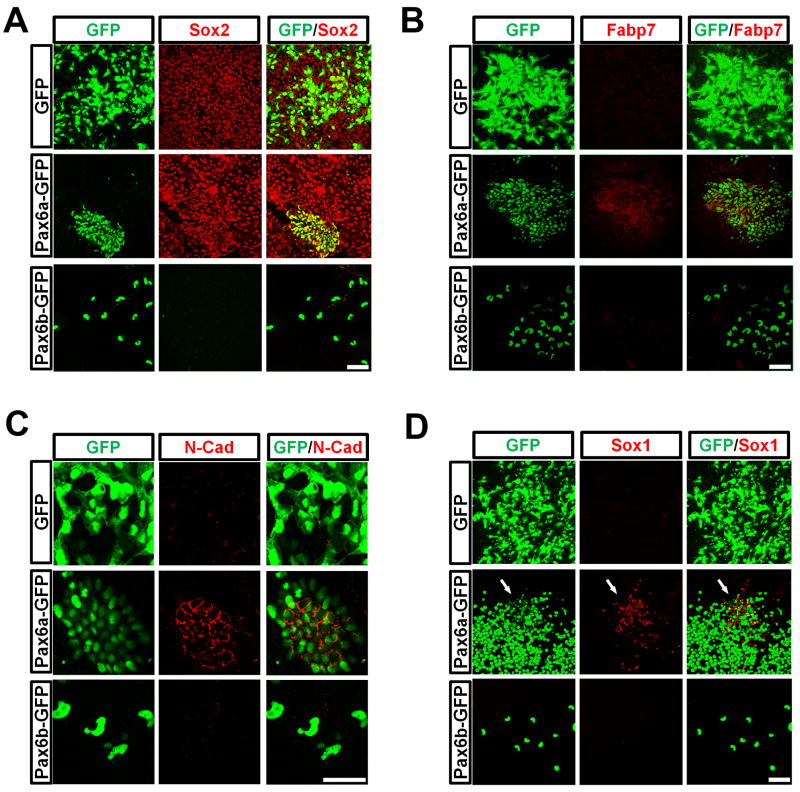
Although forced expression of Pax6a downregulated Oct4 and Nanog quickly, expression of another pluripotent factor, Sox2 (also a NE transcription factor) was retained (Figure 5A). The Pax6a-overexpressing cells also expressed fatty acid binding protein 7 (Fabp7) and N-cadherin (Figure 5B and 5C), which are specifically expressed in NE cells. It should be noted that N-cadherin was distributed evenly on the membrane of the Pax6a-expressing cells. We have previously shown that the primitive NE cells express N-cadherin evenly on the cell membrane whereas regional neural progenitors that express Sox1 and are polarized, express N-cadherin on the lumen side (Pankratz et al., 2007). Hence, the specific expression pattern of N-cadherin in Pax6a-overexpressing cells indicates their primitive NE state, which coincides with our finding that most Pax6a positive cells were negative for Sox1 (Figure 5D). Occasionally, Sox1 was found in the Pax6a positive cells. Interestingly, the Sox1-expressing cells always had lower Pax6a expression (Figure 5D). In contrast to Pax6a, Pax6b-overexpressing cells showed no expression of any neural marker tested, confirming their non-neural identity. Furthermore, both Pax6a and Pax6b cells lacked expression of Brachyury and AFP, mesodermal and endodermal markers, respectively, or Gata6, an extraembryonic endodermal maker (data not shown). Thus, while both Pax6a and Pax6b triggered hESC differentiation through downregulation of pluripotent genes, only Pax6a directed the cells to a neural fate.
Figure 5.
Overexpression of Pax6a induces neural genes. A, Overexpression of Pax6a, but not Pax6b in hESCs, maintains Sox2 expression. B, C, Pax6a positive cells express pan neural markers, Fabp7 and N-cadherin. D, Most Pax6a-overexpressing cells do not express Sox1. However, some cells with a lower Pax6a level (weaker GFP) are positive for Sox1 (arrows). Scale bars, 50 μm.
In contrast to the results seen with hESCs, overexpression of either Pax6a or Pax6b in mESCs neither changed the ESC morphology nor induced the formation of neural rosettes. Overexpression of Pax6a or Pax6b in mESCs did not decrease Oct4 expression and the mESCs could be passaged continuously as normal ESCs (Figure S4A and S4B). Therefore, the prominent ESC-differentiation and neural-inducing effects of Pax6 are unique to human ESCs.
Pax6a but not Pax6b induces NE gene expression
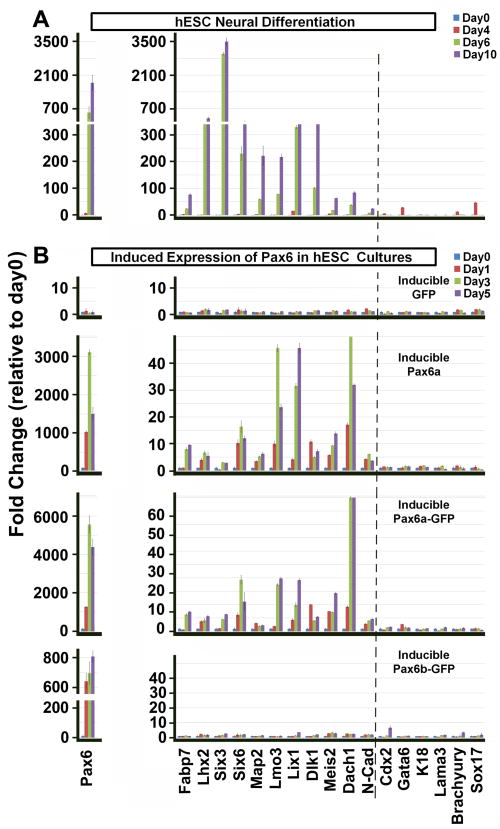
Expression of either Pax6a or Pax6b differentiates hESCs rapidly and this prevented us from establishing stable transgenic lines for biochemical studies. We therefore built inducible Pax6a, Pax6a-GFP, Pax6b-GFP and GFP clonal hESC lines using a lentivirus-based inducible system (Xia et al., 2008). As shown by the inducible GFP line, doxycycline treatment or induction of GFP expression did not alter the morphology and growth of hESCs. In contrast, induction of Pax6a-GFP expression in hESCs for 3–4 days trigged neural rosette formation in the ESC colony (Movie S1, and S2). We again found that Pax6b-GFP overexpressing cells tended to localize in the periphery of the colony and they possessed the same kidney-like or horseshoe-shape nuclei as seen previously (data not shown). These results confirmed the observations made using constitutive Pax6-expressing cells that Pax6a, but not Pax6b promotes NE specification.
To examine the dynamics of Pax6 effects, we performed qRT-PCR analyses after Pax6 was induced for 1, 3 or 5 days in ESC culture conditions. Consistent with microarray data (Figure 2E and Table S1), neural differentiation of normal hESCs was accompanied by upregulation of neural transcription factors including Lhx2, Six3, Six6, Lmo3 and Meis2 as well as neural related signaling molecules, such as Fabp7, Lix1, Dlk1, Dach1 and N-cadherin at day 6 and 10 (Figure 6A). Induction of GFP expression did not alter the gene expression pattern in hESCs (Figure 6B). Pax6a or Pax6a-GFP expression greatly induced those neural genes within 1–3 days, but not genes of extraembryonic lineages, mesoderm, endoderm or epidermal tissues (Figure 6B). These results suggest that Pax6a induced neural gene expression and the fusion of GFP to Pax6 did not interfere with its function. In animal studies, Pax6 is important for eye and pancreas development and brain patterning. RT-PCR analysis indicated that retinal genes (Crx, Chx10, and RPE65), mesoendodermal (Brachyury) and pancreatic genes (Sox17, Hnf1b and Pdx1) or regional patterning genes (FoxG1, En1, Hoxb4, and Nkx2.1) were not induced by Pax6a (Figure S5A, S5B and S5C), further supporting the NE specification effect of Pax6a. In contrast, overexpression of Pax6b-GFP did not induce NE gene expression or characteristic genes from other germ layers except Cdx2 (Figure 6B), a key factor for trophectoderm development. In this case, Cdx2 was not increased until 5 days after induction of Pax6b.
Figure 6.
Overexpression of Pax6a converts hESCs to the neural fate in inducible lines. A, Native hESCs were differentiated for 4, 6 and 10 days to the neural lineage and gene expression was analyzed by qRT-PCR. Pax6 along with other NE genes are significantly transcribed during differentiation. In contrast, extraembryonic markers Cdx2 and Gata6; epidermal markers K18 and Lama3; mesodermal and endodermal markers Brachyury and Sox17 are not expressed when the cells are differentiated to NE. B, Induction of GFP or Pax6b-GFP expression does not alter the expression of any of the neural genes. Expression of Pax6a or Pax6a-GFP in hESCs causes expression of neural specific genes, but not other lineage markers. The trophectoderm marker Cdx2 is slightly transcribed but only after 5 days of Pax6b induction.
It is noteworthy that the NE inducing effect of Pax6a is quick and robust. Even in the presence of Activin A and Bio (a GSK3β inhibitor), a condition that favors mesoendoderm differentiation (Kroon et al., 2008), Pax6a overexpression induced neural rosette formation within hESC colonies (Figure S5D) with concomitant elevated expression of NE genes and repressed mesoendodermal transcripts (Figure S5E and S5F). These data suggest that Pax6 is an intrinsic regulator of human NE specification.
Pax6a and Pax6b coordinate with each other to specify the NE fate
Since both Pax6a and Pax6b were expressed during hESC NE differentiation (Figure 1A and Figure 7A) but overexpression of Pax6a alone was sufficient to convert hESCs to NE, we asked whether Pax6b was needed for NE specification. We selected one RNAi sequence targeting exon5a which can specifically knock down Pax6b (Figure 7A and Figure S2A). qRT-PCR showed that similar to knockdown of both isoforms, specific knockdown of Pax6b reduced pluripotent gene downregulation and neural gene upregulation during normal NE differentiation, although at a modest level (Figure 7B). These results suggest that Pax6b is also required for human NE specification. Since overexpression of Pax6b cannot induce neural genes, this result suggests that the way Pax6b functions in human NE specification is through coordinating with Pax6a in downregulation of pluripotent genes, which is a prerequisite for subsequent upregulation of neural genes. In addition, the neural blocking effect was reproduced with two Pax6 RNAi constructs, ensuring that the phenotype was due to knockdown of Pax6, but not off-target effects.
We then asked whether Pax6 can regulate lineage genes directly. Pax6a-GFP, Pax6b-GFP and GFP lines were induced with doxycycline for 1 and 3 days, and chromatin immunoprecipitation (ChIP) analysis was performed to examine the binding of Pax6 to promoters of lineage-specific genes. GFP protein did not show any binding to the pluripotent genes or neural genes (data not shown). Both Pax6a and Pax6b were found to localize to the Oct4 and Nanog promoters (Figure 7C). Pax6 bound to the Nanog promoter 1 day after Pax6 was induced, earlier than it bound to the Oct4 promoter. This is consistent with the observation that Nanog was downregulated earlier than Oct4 in normally differentiated cells (Figure 7B). As expected, only Pax6a bound to the promoters of neural genes that were upregulated following Pax6a expression, mostly at day 3 (Figure 7C). In summary, both Pax6a and Pax6b bound to the promoters of pluripotent genes, corresponding to the downregulation of Oct4 and Nanog. Pax6a, but not Pax6b, occupied the promoters of neural genes, coinciding with the NE fate mediated by Pax6a.
DISCUSSION
Since the groundbreaking work by Spemann and Mangold, signaling pathways that lead to NE induction, including BMP inhibition and FGF activation, are now well established (Levine and Brivanlou, 2007; Munoz-Sanjuan and Brivanlou, 2002; Stern, 2005, 2006). However, transcriptional networks that control NE specification are not well defined. Our present study provides evidence for the first time that Pax6 is both necessary and sufficient for NE specification from human, but not mouse ESCs. This finding raises a question of how such a well-conserved protein acquired the novel function in human brain development over evolution. Furthermore, we discovered that the neural inductive function of Pax6 is achieved by its repression of pluripotent genes and activation of NE genes. Taken together with the unique differential effects of Pax6a and Pax6b, we propose that specification of epiblast or ESCs to an embryonic germ layer depends upon induction of the target germ layer genes and repression of pluripotent genes and possibly also genes of other germ layers (Figure 7D). This proposition opens the possibility for the existence of a determinant gene(s) for mesoderm and endoderm.
Pax6 is necessary and sufficient for human NE specification
In this study, we have demonstrated that overexpression of Pax6, either constitutively or conditionally, converts hESCs to NE, even under conditions that favor hESC maintenance or mesoendoderm differentiation. The NE identity was verified by the characteristic columnar cells that organize into rosettes, loss of pluripotent gene expression, upregulation of NE genes, and lack of other germ layer markers. Knockdown of Pax6 blocks NE specification from hESCs not only in the teratoma assay, which allows spontaneous three-germ-layer differentiation in vivo, but also in our chemically-defined NE differentiation system and a newly developed dual SMAD inhibition culture, both of which strongly promote hESC neural differentiation. These results, gathered from both gain-of-function and loss-of-function of Pax6 under opposing conditions, strongly indicate that Pax6 is an intrinsic determinant for the human NE fate. The fact that overexpression of Pax6 does not induce mesoendoderm and that knockdown of Pax6 does not inhibit mesoendodermal lineage differentiation excludes the possibility that Pax6 first promotes mesoendodermal differentiation which in turn induces neural differentiation. This is further supported by the result that dual SMAD inhibition by Noggin and SB431542 does not rescue the neural blocking effect when Pax6 is knocked down. Therefore, Pax6 is most likely a crucial downstream effector of neural inducers, such as BMP inhibitors.
Pax6-mediated NE specification depends on both repression of pluripotent genes and induction of NE genes
It is quite remarkable that a single transcription factor, Pax6, can act as a switch from proliferating hESCs to differentiating NE. This is a direct cell fate conversion rather than an indirect process through promoting cell proliferation or survival of existing NE in the hESCs (Schroeder, 2008). First, hESCs, maintained under standard culture conditions, do not express Pax6, an early marker of human NE cells now widely used. Second, overexpression or knockdown of Pax6 does not alter cell proliferation or survival (Figure S6). Third, time-lapse tracking reveals that once Pax6 is turned on the cells become columnar NE, migrate, and aggregate to form rosettes (Movie S1 and S2). Furthermore, at the molecular level, Pax6 binds to pluripotent genes and NE genes directly.
Both Pax6a and Pax6b bind to promoters of pluriptoent genes, including Oct4 and Nanog, and repress their expression whereas only Pax6a binds to NE gene promoters and activates NE genes. Therefore, the NE fate determining role of Pax6 is achieved through coordination of Pax6a and Pax6b in preventing hESC self-renewal, thus initiating their differentiation, and inducing the cells toward the NE fate by Pax6a. Suppression of pluripotent factors alone is not sufficient for differentiating ESC/epiblast to NE. This is demonstrated by the fact that overexpression of Pax6b, which does not possess neural inducing activity, drives hESCs out of the stem cell state but these cells turn into trophoblast. This phenomenon is reminiscent of the extraembryonic outcome of ESCs with knockdown of Oct4, Nanog or Sox2 (Chew et al., 2005; Fong et al., 2008; Hay et al., 2004; Hyslop et al., 2005; Matin et al., 2004; Zaehres et al., 2005). Thus, repression of pluripotent genes initiates the differentiation process but it alone is not sufficient for embryonic germ layer differentiation. Pax6a is likely the key inductive signal for the NE fate. Indeed, Pax6a binds to a set of downstream neural genes, which corresponds to the neural phenotypes. Pax6b, though by itself not a direct neural inducer, potentiates the neural inductive effect of Pax6a through collaboration with Pax6a for sufficient repression of pluripotent genes, which is a prerequisite for induction of neural genes (Figure 7D).
The NE specification role of Pax6 is unique to primates
The Pax6 protein is highly conserved. It plays critical roles in the development of eyes and pancreas and patterning of neural progenitors across species (Chi and Epstein, 2002). Indeed, the expression pattern of Pax6 in the developing human nervous system (after brain regions are formed) is very similar to that in other model systems, including mouse, frog, chick, and fish (Amirthalingam et al., 1995; Goulding et al., 1993; Schlosser and Ahrens, 2004; Walther and Gruss, 1991). We have also confirmed that Pax6 is essential for patterning human NE cells to ventral spinal progenitors and dorsal telencephalic progenitors (Li et al., 2005; Li et al., 2009). Our side-by-side comparison of Pax6 expression and function between mouse and human revealed a novel role of Pax6 in early human, but not mouse, NE specification. Considering the similar expression pattern of Pax6 in early rhesus monkey fetuses, this NE specification role of Pax6 probably is unique to primates. This finding raises a question as to why the classical transcription factor, with 100% amino acid sequence homology between mouse and human, acquires a new role in human brain development. The brain, especially the forebrain, is the most highly evolved structure in either size or complexity among species (Dorus et al., 2004; Kaas, 2006; Rakic, 2009). Corresponding to the increasing size of the forebrain, some neural transcription factors, especially anterior transcription factors Sox2 and Otx2 whose expression is restricted to the neural lineage in lower vertebrates, are now found at earlier developmental stages in mammals, even in the inner cell mass and the epiblast of the embryo (Avilion et al., 2003; Simeone et al., 1993). The cerebrum in primates, especially in human, is proportionally larger and more complex in neural circuitry than in rodents (Dorus et al., 2004; Kaas, 2006; Rakic, 2009). We and others have also found that under similar culture conditions without exogenous morphogens, hESC-derived NE cells tend to generate cortical glutamatergic neurons whereas mouse NE are inclined to generate ventral GABAergic neurons (Gaspard et al., 2008; Li et al., 2009). We speculate that early Pax6 expression might be the first step to ensure a large cerebrum in primates. Further studies to identify target genes of Pax6 during NE specification may well shed light on the evolutionary complexities of our human brain. Our finding also raises the question of what would be the determinant gene for the NE fate in mouse or other animals. Comparison of our gene profiles with available database of mouse NE (Aiba et al., 2006) revealed profound differences in gene expression between human and mouse NE, some of which are presented in Figure S1G. While this comparison corroborates our present finding it indicates a need of uncovering the long-sought NE determinant in animals.
EXPERIMENTAL PROCEDURES
Culture and maintenance of mouse and human ESCs
Human ESCs (H9 and H1 lines, passages 18–35) were provided by the WiCell Institute and were cultured on irradiated mouse embryonic fibroblasts (MEFs) as previously described (Zhang et al., 2001; Zhang and Zhang, 2010). Human iPSCs were generated from skin fibroblasts by overexpressing Oct4, Sox2, Klf4 and c-Myc through retroviral infection (Hu et al., 2010). The standard protocol can be found at www.wicell.org.
Mouse ESCs (D3 line and Sox1/GFP reporter line 46C) were cultured on MEF supplemented with 50% medium conditioned by Buffalo rat liver cells (BRL-CM).
Neural differentiation from human and mouse ESCs
Neural differentiation of hESCs was performed following a published protocol (Zhang et al., 2001; Zhang and Zhang, 2010). For mESC neural differentiation, half a million cells were suspended in DMEM-F12/neurobasal medium (1:1 DMEM-F12/neurobasal medium, 1X N2 neural supplement, 1X lipid concentrate, 1mM L-glutamine, 0.1mM β-mercaptoethanol and 40 μg/ml N-acetyl cysteine). For the first two days, 2 ng/ml of LIF was supplied. After another 7 days of culture in suspension without LIF, neruoepithelial aggregates were dissociated and plated in the same way as for human ESCs.
Tissue collection
The human fetal tissues used in this study were from patients requesting termination of pregnancy. All the procedures were approved by the institutional review board (Ethics Committee) of Fudan University Shanghai Medical School and the Shanghai Institute of Biological Sciences, Chinese Academy of Science, Shanghai and with the informed consent of the patients. Fetal tissues were obtained within 4 hours after abortion and the developmental stages of fetus specimens were identified according to the anatomy established by Carnegie Institute in Washington, USA. Fetal monkey tissues were obtained from animals at the Wisconsin National Primate Research Center in early pregnancy as previously described (Bondarenko et al., 2007). The tissues were cut into 15–20 μm frozen sections for immunostaining. (if you remove this part, you need to include it in supplemental materials as this is an important issue)
Generation and analysis of teratomas
Human ESCs were injected subcutaneously into the backs of severe combined immunodeficient (SCID) mice (Jackson Laboratory) (Xia et al., 2008). All animal experiments were performed following the protocols approved by the Institutional Animal Care and Use Committee, University of Wisconsin.
Statistical Analyses
Data are presented as mean ± SEM. Student’s t tests were used for statistical analysis. P<0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Qilong Ying and Austin Smith for the Sox1 reporter mouse ESCs and Y. Sassai for the FoxG1 antibody. We also thank Elizabeth E. Capowski for reading our manuscript and helpful discussion. This study was supported by the National Institutes of Neurological Diseases and Stroke (NS045926 to SCZ), partly by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352), Ministry of Science and Technology, China (2006CB94700, 2006AA02A101), and Shanghai Municipality (06dj14001). The Wisconsin National Primate Research Center is supported by NIH grant RR00167, with support from Research Facilities Improvement Progress grant RR15459 and RR020141.
Footnotes
Competing Interests Statement
The authors state that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiba K, Sharov AA, Carter MG, Foroni C, Vescovi AL, Ko MS. Defining a developmental path to neural fate by global expression profiling of mouse embryonic stem cells and adult neural stem/progenitor cells. Stem Cells. 2006;24:889–895. doi: 10.1634/stemcells.2005-0332. [DOI] [PubMed] [Google Scholar]
- Amirthalingam K, Lorens JB, Saetre BO, Salaneck E, Fjose A. Embryonic expression and DNA-binding properties of zebrafish pax-6. Biochem Biophys Res Commun. 1995;215:122–128. doi: 10.1006/bbrc.1995.2441. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko GI, Burleigh DW, Durning M, Breburda EE, Grendell RL, Golos TG. Passive immunization against the MHC class I molecule Mamu-AG disrupts rhesus placental development and endometrial responses. J Immunol. 2007;179:8042–8050. doi: 10.4049/jimmunol.179.12.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi N, Epstein JA. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002;18:41–47. doi: 10.1016/s0168-9525(01)02594-x. [DOI] [PubMed] [Google Scholar]
- Dorus S, Vallender EJ, Evans PD, Anderson JR, Gilbert SL, Mahowald M, Wyckoff GJ, Malcom CM, Lahn BT. Accelerated evolution of nervous system genes in the origin of Homo sapiens. Cell. 2004;119:1027–1040. doi: 10.1016/j.cell.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Glaser T, Cai J, Jepeal L, Walton DS, Maas RL. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev. 1994;8:2022–2034. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26:1931–1938. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23:1234–1241. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Lumsden A, Gruss P. Signals from the notochord and floor plate regulate the region-specific expression of two Pax genes in the developing spinal cord. Development. 1993;117:1001–1016. doi: 10.1242/dev.117.3.1001. [DOI] [PubMed] [Google Scholar]
- Hay DC, Sutherland L, Clark J, Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop L, Stojkovic M, Armstrong L, Walter T, Stojkovic P, Przyborski S, Herbert M, Murdoch A, Strachan T, Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- Jedrusik A, Parfitt DE, Guo G, Skamagki M, Grabarek JB, Johnson MH, Robson P, Zernicka-Goetz M. Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev. 2008;22:2692–2706. doi: 10.1101/gad.486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Evolution of the neocortex. Curr Biol. 2006;16:R910–914. doi: 10.1016/j.cub.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Kim J, Lauderdale JD. Analysis of Pax6 expression using a BAC transgene reveals the presence of a paired-less isoform of Pax6 in the eye and olfactory bulb. Dev Biol. 2006;292:486–505. doi: 10.1016/j.ydbio.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]
- Kozmik Z, Czerny T, Busslinger M. Alternatively spliced insertions in the paired domain restrict the DNA sequence specificity of Pax6 and Pax8. EMBO J. 1997;16:6793–6803. doi: 10.1093/emboj/16.22.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH. Proposal of a model of mammalian neural induction. Dev Biol. 2007;308:247–256. doi: 10.1016/j.ydbio.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin MM, Walsh JR, Gokhale PJ, Draper JS, Bahrami AR, Morton I, Moore HD, Andrews PW. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004;22:659–668. doi: 10.1634/stemcells.22-5-659. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc Natl Acad Sci U S A. 1997;94:11980–11985. doi: 10.1073/pnas.94.22.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–781. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex M, Orme A, Uwanogho D, Tointon K, Wigmore PM, Sharpe PT, Scotting PJ. Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn. 1997;209:323–332. doi: 10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev Biol. 2004;271:439–466. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Schmahl W, Knoedlseder M, Favor J, Davidson D. Defects of neuronal migration and the pathogenesis of cortical malformations are associated with Small eye (Sey) in the mouse, a point mutation at the Pax-6-locus. Acta Neuropathol. 1993;86:126–135. doi: 10.1007/BF00334879. [DOI] [PubMed] [Google Scholar]
- Schroeder T. Imaging stem-cell-driven regeneration in mammals. Nature. 2008;453:345–351. doi: 10.1038/nature07043. [DOI] [PubMed] [Google Scholar]
- Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–613. doi: 10.1016/s0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D’Apice MR, Nigro V, Boncinelli E. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 1993;12:2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction: 10 years on since the ‘default model’. Curr Opin Cell Biol. 2006;18:692–697. doi: 10.1016/j.ceb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Suter DM, Tirefort D, Julien S, Krause KH. A Sox1 to Pax6 switch drives neuroectoderm to radial glia progression during differentiation of mouse embryonic stem cells. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0319. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Habegger L, Noisa P, Szekely A, Qiu C, Hutchison S, Raha D, Egholm M, Lin H, Weissman S, et al. Dynamic transcriptomes during neural differentiation of human embryonic stem cells revealed by short, long, and paired-end sequencing. Proc Natl Acad Sci U S A. 2010;107:5254–5259. doi: 10.1073/pnas.0914114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Ayala M, Thiede BR, Zhang SC. In vitro- and in vivo-induced transgene expression in human embryonic stem cells and derivatives. Stem Cells. 2008;26:525–533. doi: 10.1634/stemcells.2007-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Ralston A, Stephenson RO, Rossant J. Cell and molecular regulation of the mouse blastocyst. Dev Dyn. 2006;235:2301–2314. doi: 10.1002/dvdy.20844. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005;23:299–305. doi: 10.1634/stemcells.2004-0252. [DOI] [PubMed] [Google Scholar]
- Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol. 2006;16:132–142. doi: 10.1111/j.1750-3639.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Zhang SC. Differentiation of neural precursors and dopaminergic neurons from human embryonic stem cells. Methods Mol Biol. 2010;584:355–366. doi: 10.1007/978-1-60761-369-5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.