The past decade has seen rapid expansion in the area of homogeneous gold catalysis. The majority of reported methods rely on the π-acidic nature of cationic gold species to activate π-bonds toward nucleophilic attack.1 On the other hand, the two electron oxidation/reduction cycles typical of other late transition metal catalysts are not commonly encountered in gold catalysis.1e,2 Recently, a few reports of transformations best explained by Au(I)/(III) redox cycles, including Au-catalyzed oxidative intramolecular heteroarylation reactions, have appeared.3 Different modes of such heteroarylation reactions are accessible by combining two or all three reactant functional groups into a single substrate.4 Notably, examples of the fully intermolecular, three-component coupling variant have not previously been reported.5,6 Herein we disclose a protocol for the three-component gold-catalyzed oxidative oxyarylation of alkenes; alcohols, carboxylic acids and even water are viable nucleophiles in this context.
 |
(1) |
 |
(2) |
We initially observed this mode of reactivity while studying the related intramolecular aminoarylation.3b In an attempt to improve the solubility of the arylboronic acid, methanol was used as a co-solvent; however, the expected pyrrolidine 3a was not observed. Instead the alkoxyarylation product 2a was isolated in 38% yield (eq 1). The yield of 2a could be improved to 72% by increasing the temperature and employing 2 equivalents of phenylboronic acid.7 While previously reported gold-catalyzed additions of oxygen nucleophiles to alkenes have required temperatures greater than 85 °C,8 the oxidative alkoxyarylation proceeds efficiently at 50 °C. Under these conditions, various alkenylamines (1b and 4a-b) participated in the methoxyarylation reaction to afford the adducts of a three component coupling in good yields (eq 2). Moreover, gold-catalyzed reaction of 4b shows excellent chemoselectivity, leaving the more electron-deficient allylic amine unreacted.
In order to probe the scope of the reaction, 5-phthalimidopentene (6) was subjected to the optimized conditions for coupling with various alcohols and phenylboronic acid (Table 1). The expected ethers were obtained in high yield when using several primary alcohols (entries 1, 2, 4 and 6) and secondary alcohols (entries 3, 7 and 8). While neopentyl ether 10 was formed in 91% yield (entry 4), gold-catalyzed reaction with the more sterically encumbered tert-butyl alcohol gave only 33% of tert-butyl ether 11 (entry 5).9 Substituting carboxylic acids in place of alcohols afforded the corresponding esters, albeit in slightly lower yield (entries 9-12).
Table 1.
Alkoxy- and acyloxyarylation with various nucleophiles.a
 | |||
|---|---|---|---|
| entry | R | product | yield (%) |
| 1 | Me | 7 | 79 |
| 2 | Et | 8 | 85 |
| 3 | iPr | 9 | 90 |
| 4 | (Me)3CCH2 | 10 | 91c |
| 5 | tBu | 11 | 33b |
| 6 |
|
12 | 85 |
| 7 |
|
13 | 85 |
| 8 |

|
14 | 88 1:1 d.r. |
| 9 | Me(CO)- | 15 | 62 |
| 10 | Et(CO)- | 16 | 69 |
| 11 | iPr(CO)- | 17 | 50 |
| 12 | Ph(CO)- | 18 | 48c |
100 μmol of alkene, 0.1M in 9:1 MeCN:ROH at 50°C for 14h; 2 equiv PhB(OH)2, 2 equiv. Selectfluor®. The catalyst and PhB(OH)2 were added in two portions at t = 0h and 2h.
Yield determined by 1H-NMR versus an internal standard (nitrobenzene).
10 equiv. ROH used.
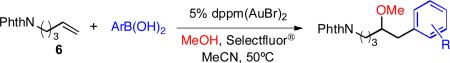
The arylboronic acid component of the reaction was also varied with similar success. The gold-catalyzed coupling of alkene 6, methanol and various arylboronic acids gave the expected methyl ethers in good yields (Table 2). The reaction performed well with alkyl-, halo-, carboxymethyl- and formyl-substituted arylboronic acids. Moreover, ortho- (eq 3), meta-(entries 3,6) and para-substituted (entries 1-2,4-5) arylboronic acids were tolerated in the methoxyarylation reaction.
Table 2.
Methoxyarylation with various ArB(OH)2.a
 | |||
|---|---|---|---|
| entry | R- | product | yield (%) |
| 1 | 4-Me | 19 | 88 |
| 2 | 4-Br | 20 | 90 |
| 3 | 3-F | 21 | 79 |
| 4 | 4-MeO2C | 22 | 83 |
| 5 | 4-H(CO) | 23 | 82 |
| 6 | 3,5-bis-CF3 | 24 | 77 |
100 μmol of alkene, 0.1 M in 9:1 MeCN:MeOH at 50 °C for 14h; 2 equiv. ArB(OH2), 2 equiv. Selectfluor®. The catalyst and ArB(OH)2 were added in 2 portions at t = 0 and 2h.
The nitrogen-containing functional groups in 1a-b, 4a-b, and 6 are not required for successful oxyarylation. Simple alkenes lacking these functionalities proved to be suitable substrates for alkoxy- and acyloxyarylation under our optimized conditions. The expected ethers were obtained in good yields when alkenes such as 1-octene and 1-bromo-2-(3-buten-1-yl)benzene were reacted with several combinations of alcohols and arylboronic acids (Table 3). Acetate esters were isolated in attenuated but synthetically useful yields from simple alkenes when using acetic acid as the nucleophile (entries 2, 7).
Table 3.
Alkoxy- and acyloxyarylation of simple alkenes.a
|
| ||||||
|---|---|---|---|---|---|---|
| entry | R1 | R2 | product | yield (%) | ||
| 1 |
|
|
4-Br | 25 | 69 | |
| 2 | Me | 2-CO2Me | 26 | 78 | ||
| 3 | Me(CO) | 4-Br | 27 | 51b | ||
| 4 |
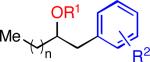
|
n = 5 |

|
4-Br | 28 | 76 |
| 5 | n = 5 | (Me)3CCH2 | 4-Br | 29 | 73 | |
| 6 | n = 9 | (Me)3CCH2 | H | 30 | 66 | |
| 7 | n = 5 | Me(CO) | 4-Br | 31 | 53b | |
100 μmol of alkene, 0.1 M in 9:1 MeCN:R1OH at 50 °C for 14h; 2 equiv. ArB(OH2), 2 equiv. Selectfluor®. The catalyst and ArB(OH)2 were added in 2 portions at t = 0 and 2h.
Only 5 equiv R1OH used, 0.1 M in MeCN.
Using water as a nucleophile would allow for the formation of alcohols directly via hydroxyarylation, circumventing the requirement for protective groups for installation of the alcohol functionality.10 We were pleased to find that simply replacing the alcohol in our protocol with water cleanly effected the formation of the desired hydroxyarylation products. A variety of terminal alkenes and arylboronic acids were subjected to these conditions, furnishing the expected products in good yield (Table 4). Various functional groups, including ether (entry 2), ester (entry 3), amide (entry 1), cyano (entry 2), nitro (entry 3) and arylhalide (entries 5-7), were well-tolerated.
Table 4.
Scope of hydroxyarylation reaction.a
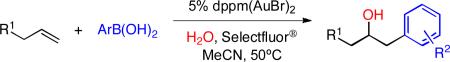 | |||
|---|---|---|---|
| entry | product | yield (%) | |
| 1 |
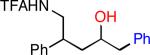
|
32 | 88b |
| 2 |

|
33 | 85c |
| 3 |
|
34 | 67d |
| 4 |
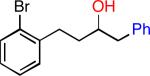
|
35 | 76 |
| 5 |
|
36 | 73 |
| 6 |
|
37 | 72 |
| 7 |
|
R = 3-F, 38 | 83 |
| 8 | R = 4-Me, 39 | 71 | |
At 0.1 M alkene in MeCN, 10 equiv. H2O, catalyst (2.5 mol%/addition) and ArB(OH)2 (1 equiv./addition) were added in 2 portions each, at t = 0h and 1h.
Isolated as a 1.2:1 mixture of diastereomers.
3 portions of catalyst (2.5 mol%/addition) and ArB(OH)2 (1 equiv./addition).
Using (4-CF3C6H4)3PAuBr as catalyst, 2 portions of 5 mol% each.
The ability to use either alcohols or water as nucleophiles in the gold-catalyzed three-component coupling provides access to a greater diversity of products. Moreover, the hydroxyarylation reaction offers the opportunity of forming an additional C-O bond to the resulting alcohol. For example, methoxyarylation of 6 with boronic acid 40 furnished methyl ether 41 in 85% yield. Using water as a nucleophile, 42 was formed in 87% yield from gold-catalyzed hydroxyarylation followed by in situ lactone formation of the resulting alcohol (eq 3).
 |
(3) |
At present, we proffer a catalytic cycle for the gold-catalyzed oxyarylation of alkenes that is analogous to the mechanism proposed for the related intramolecular aminoarylation reaction.3b Initial oxidation of the gold(I) bromide is thought to provide a cationic gold(III) species capable of activating the alkene toward nucleophilic attack. Oxyauration of the π-bond is then followed by C-C bond formation, without prior transmetallation of the aryl group from boron to gold (Figure 1).
Figure 1.
Proposed catalytic cycle for alkene hydroxyarylation.
Reaction of monodeuterated alkene d1-6 stereospecifically produced d1-42 with stereochemistry consistent with initial syn-hydroxyauration followed by invertive C-C bond formation. The stereochemical course of the hydroxyarylation is consistent with our earlier observations from the gold-catalyzed intramolecular aminoarylation (eq 4).3b
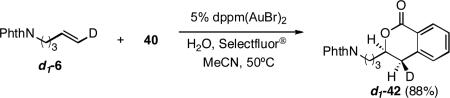 |
(4) |
In conclusion, we have demonstrated the gold-catalyzed three-component coupling reaction of alkenes, arylboronic acids and several types of oxygen nucleophiles, including alcohols, carboxylic acids and water. It is notable that the latter effectively participates as a nucleophile in these reactions, especially given difficulties associated with transition metal-catalyzed hydration of alkenes.11 This method represents the first fully intermolecular alkene heteroarylation reaction and stands as one of the few examples of gold-catalyzed multicomponent couplings.12
Supplementary Material
Acknowledgement
We gratefully acknowledge NIHGMS (RO1 GM073932), Amgen, and Novartis for financial support and thank Johnson Matthey for a gift of AuCl3.
Footnotes
Supporting Information Available: Experimental procedures and compound characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews on gold-catalyzed reactions see: Shapiro ND, Toste FD. Synlett. 2010:675. doi: 10.1055/s-0029-1219369. Fürstner A. Chem. Soc. Rev. 2009;38:3208. doi: 10.1039/b816696j. Shen HC. Tetrahedron. 2008;64:7847. Gorin DJ, Sherry BD, Toste FD. Chem. Rev. 2008;108:3351. doi: 10.1021/cr068430g. Li Z, Brouwer C, He C. Chem. Rev. 2008;108:3239. doi: 10.1021/cr068434l.
- 2.Garcia P, Malacria M, Aubert C, Gandon V, Fensterback L. ChemCatChem. 2010;2:493. [Google Scholar]
- 3.For Au-catalyzed amino- and oxyarylation of alkenes: Zhang G, Cui L, Wang Y, Zhang L. J. Am. Chem. Soc. 2010;132:1474. doi: 10.1021/ja909555d. Brenzovich WE, Jr., Benitez D, Lackner AD, Shunatona HP, Tkatchouk E, Goddard WA, III, Toste FD. Angew. Chem., Int. Ed. 2010 doi: 10.1002/anie.201002739. DOI: 10.1002/anie.201002739. For other oxidative Au-catalyzed reactions see: Hopkinson MN, Tessier A, Salisbury A, Giuffredi GT, Combettes LE, Gee AD, Gouverneur V. Chem. Eur. J. 2010;16:4739. doi: 10.1002/chem.201000322. Ye L, Cui L, Zhang G, Zhang L. J. Am. Chem. Soc. 2010;132:3258. doi: 10.1021/ja100041e. Iglesias A, Muñiz K. Chem. Eur. J. 2009;15:10563. doi: 10.1002/chem.200901199. Zhang G, Peng Y, Cui L, Zhang L. Angew. Chem., Int. Ed. 2009;48:3112. doi: 10.1002/anie.200900585. Kar A, Mangu N, Kaiser HM, Beller M, Tse MK. Chem. Commun. 2008;3:386. doi: 10.1039/b714928j. Hashmi ASK, Ramamurthi TD, Rominger F. J. Organomet. Chem. 2009;694:592.
- 4.For selected reports of Pd-catalyzed amino- and oxyarylation see: Schultz DM, Wolfe JP. Org. Lett. 2010;12:1028. doi: 10.1021/ol100033s. Lemen GS, Giampietro NC, Hay MB, Wolfe JP. J. Org. Chem. 2009;74:2533. doi: 10.1021/jo8027399. Nakhla JS, Kampf JW, Wolfe JP. J. Am. Chem. Soc. 2006;128:2893. doi: 10.1021/ja057489m. Yang Q, Ney JE, Wolfe JP. Org. Lett. 2005;7:2575. doi: 10.1021/ol050647u. Hay MB;, Wolfe JP. J. Am. Chem. Soc. 2005;127:16468. doi: 10.1021/ja054754v. Lira R, Wolfe JP. J. Am. Chem. Soc. 2004;126:13906. doi: 10.1021/ja0460920. Wolfe JP, Rossi MA. J. Am. Chem. Soc. 2004;126:1620. doi: 10.1021/ja0394838. Sibbald PA, Rosewall CF, Swartz RD, Michael FE. J. Am. Chem. Soc. 2009;131:15945. doi: 10.1021/ja906915w. Sibbald PA, Michael FE. Org. Lett. 2009;131:9488. doi: 10.1021/ol9000087. Hayashi S, Yorimitsu H, Oshima K. Angew. Chem., Int. Ed. 2009;48:7224. doi: 10.1002/anie.200903178. For Cu-catalysis see: Sherman ES, Chemler SR. Adv. Synth. Catal. 2009;351:467. doi: 10.1002/adsc.200800705. Fuller PH, Kim JW, Chemler SR. J. Am. Chem. Soc. 2008;130:17638. doi: 10.1021/ja806585m.
- 5.For a Pd-catalyzed intermolecular aminofluorination of styrenes, see: Qui S, Xu T, Zhou J, Guo Y, Liu G. J. Am. Chem. Soc. 2010;132:2956. doi: 10.1021/ja909716k.
- 6.For reviews of on multi-component coupling see: Touré B,B, Hall DG. Chem. Rev. 2009;109:4439. doi: 10.1021/cr800296p. Domling A. Chem. Rev. 2006;106:17. doi: 10.1021/cr0505728.
- 7.The arylboronic acids are known to dimerize under similar conditions, see reference 3f and Carrettin S, Guzman J, Corma A. Angew. Chem., Int. Ed. 2005;44:2242. doi: 10.1002/anie.200462560.
- 8.Yang C-G, He C. J. Am. Chem. Soc. 2005;127:6966. doi: 10.1021/ja050392f. Zhang X, Corma A. Chem. Commun. 2007:3080. doi: 10.1039/b706961h. Hirai T, Hamasaki A, Nakamura A, Tokunaga M. Org. Lett. 2009;11:5510. doi: 10.1021/ol9023166. For gold-catalyzed hydroamination of simple alkenes see: Giner X, Nájera C. Org. Lett. 2008;10:2929. doi: 10.1021/ol801104w. Zhang J, Yang C-G, He C. J. Am. Chem. Soc. 2006;128:1798. doi: 10.1021/ja053864z. Han X, Widenhoefer RA. Angew. Chem., Int. Ed. 2006;45:1747. doi: 10.1002/anie.200600052. Liu X-Y, Li C-H, Che C-M. Org. Lett. 2006;8:2707. doi: 10.1021/ol060719x. Bender CF, Widenhoefer RA. Org. Lett. 2006;8:5303. doi: 10.1021/ol062107i. Brouwer C, He C. Angew. Chem. Int. Ed. 2006;45:1744. doi: 10.1002/anie.200504495.
- 9.Readily oxidized (benzyl, allyl) alcohols do not participate in the reaction.
- 10.For gold-catalyzed hydration of allenes, see: Zhang Z, Lee SD, Fisher AS, Widenhoefer RA. Tetrahedron. 2009;65:1794. doi: 10.1016/j.tet.2008.10.113.
- 11.For relevant discussion, see: Koch HF, Girard LA, Roundhill DM. Polyhedron. 1999;18:2275.
- 12.For examples of gold-catalyzed 3-component (alkyne, aldehyde, amine) coupling, see: Wei C, Li C-J. J. Am. Chem. Soc. 2003;125:9584. doi: 10.1021/ja0359299. Lo VK-Y, Liu Y, Wong M-K, Che C-M. Org. lett. 2006;8:1529. doi: 10.1021/ol0528641. Yan B, Liu Y. Org. Lett. 2007;9:4323. doi: 10.1021/ol701886e. Zhang X, Corma A. Angew. Chem., Int. Ed. 2008;47:4358. doi: 10.1002/anie.200800098. Zhang Q, Cheng M, Hu X, Li B-G, Ji J-X. J. Am. Chem. Soc. 2010;132:725. doi: 10.1021/ja101804p. For gold/Brønsted acid co-catalysis see: Wang C, Han Z-Y, Luo H-W, Gong L-Z. Org. Lett. 2010;12:2269. doi: 10.1021/ol1006086. For a related reaction (alkyne, alkyne, amine) see: Zeng X, Frey GD, Kinjo R, Donnadieu B, Bertrand G. J. Am. Chem. Soc. 2009;131:8690. doi: 10.1021/ja902051m. For alcohol nucleophiles see: Tian G-Q, Shi M. Org. Lett. 2007;9:4917. doi: 10.1021/ol702341a.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



