SUMMARY
Despite substantial innovations in antiepileptic drug therapy over the past 15 years, the proportion of patients with uncontrolled epilepsy has not changed, highlighting the need for new treatments. New implantable antiepileptic devices, which are currently under development and in pivotal clinical trials, hold great promise for improving the quality of life for millions of people with epileptic seizures worldwide. A broad range of strategies is currently being investigated, using various modes of control and intervention in an attempt to stop seizures. The success of these devices rests upon collaboration between neuroengineers, physicians and industry to adapt new technologies for clinical use. The initial results are exciting, but considerable development and controlled clinical trials will be required before these treatments earn a place in our standard of clinical care.
Keywords: closed-loop devices, epilepsy, neuroengineering, open-loop devices, seizure control
INTRODUCTION
Epilepsy affects over 50 million people worldwide, and, for a quarter of those affected, no combination of standard therapy—primarily medications and surgery—can control their seizures. As the search for better medications and surgical approaches continues, another avenue of epilepsy treatment is now gaining momentum; namely, implantable devices designed to predict, detect, prevent, and abort seizures.
The relatively young field of neuroengineering uses engineering technology to investigate and treat neurological diseases. Epilepsy is one of its primary targets, along with movement disorders, stroke, affective disorders, head trauma and paralysis. Using the electrochemical properties of neurons as a foundation,1,2 neuroengineers seek to monitor and modulate abnormal brain function using several novel—and often nonpharmacological—methods.
There are two main approaches to neuroengineering research in epilepsy: first, monitoring and interpreting epileptic and potentially epileptic brain activity on multiple scales in brain networks to understand how seizures and epilepsy are generated over time, and second, using a creative array of approaches to model and manipulate the intrinsic properties of brain networks to modulate seizure generation and prevent clinical events. The ultimate goal of this research is to combine these approaches into ‘closed-loop’ devices that feed back brain signals to control interventions that stop seizures.
This Review discusses cutting-edge strategies for epilepsy control devices, and highlights promising areas that are under active investigation. First, we will discuss the milestones that have been achieved and some current research in seizure control devices. We will then focus on strategies for seizure prediction, a field that is less well developed and understood. Finally, we will address several central questions and challenges that remain in the field.
BACKGROUND
Principles of neuroengineering
On the basis of early neuroscience research showing that neural function can be recorded, manipulated and mathematically modeled,2,3 researchers are applying new technologies to the treatment of neurological disease. This goal closely follows the clinical success of similar approaches in cardiology, where analogous—albeit simpler—physiology responds dramatically to intravascular interventions, electrical pacing and closed-loop stimulation, involving pacemakers, automatic implantable cardiac defibrillators, and devices that ablate arrhythmia-producing foci. For many reasons, including the complexity of neural circuits, and the relative inaccessibility of the dysfunctional regions (usually necessitating a craniotomy or similar invasive procedure), clinical implementation of implantable brain devices continues to lag behind the cardiological applications. Nevertheless, as computing power, engineering capabilities and our knowledge of neurophysiology continue to expand, so too do the opportunities to develop clinical neurophysiological devices that can exploit this new understanding.
The field of neuroengineering is fairly new in name, although its roots extend back into the early twentieth century. The field encompasses projects such as brain–computer interfaces to control robots or other computerized devices to assist individuals with paralytic injuries,4-6 electrical stimulation of paralyzed limbs,7 and visual prostheses that translate digital pictures from cameras into signals that can be interpreted by the brain.8,9 Much of this research uses computational neuroscience, which involves both measuring and extracting quantitative features from neurophysiological data in order to localize, decode and predict the behavior of a system. Using mathematical models of neural function, investigators can test diagnostic and therapeutic technologies robustly before implementation in humans. Such computer models are particularly powerful because they can simulate neurological function on multiple scales simultaneously, ranging from individual ion channels and single cell function,10 through local networks of neurons,11-13 to complete systems.14,15
Applying neuroengineering to epilepsy
The mainstay of therapy for epilepsy is prophylactic treatment with antiepileptic drugs (AEDs) to prevent seizure onset. These medications work through a variety of mechanisms, often acting to suppress single or groups of ion channels. Although more new AEDs have come to market over the past 10 years than during any other time in history, their primary contribution has been to improve medication side effects, rather than to make more people seizure-free. The proportion of people with epilepsy worldwide whose seizures cannot be controlled by medical therapy has remained unchanged during this time, at around one-third. For this reason, researchers are investing increasing time and effort to develop novel approaches to treatment, such as gene therapy, ‘nano particles’ to target specific intracellular targets, and antiepileptic devices.
The central clinical problem in epilepsy is that a network of neurons in the brain becomes abnormally excitable and synchronized. Monitoring and localizing the resultant electrical discharge—the physiological signature of seizures—forms the basis of passive recording in electroencephalography (EEG).16 For over 50 years, EEG was the only method of monitoring functional activity in the brain. Over the past 20 years, new imaging techniques for measuring brain function have become available, including functional MRI, PET scanning, single-photon emission computed tomography, and magnetoencephalography, yet EEG has retained its place as the most important measure available to localize epileptic network function. EEG performed with scalp electrodes preferentially records activity from the largest or most superficial cortical networks, owing to spatial and high-frequency filtering from the skull, scalp, cerebrospinal fluid and dura.17 Consequently, intracranial electrodes are often required to map and track seizure generation and epileptic networks.
Since the 1970s, a growing group of researchers in neurology, neurosurgery and neuroscience has focused on actively modulating neuronal inputs and outputs to control system and network behaviors. Early attempts consisted of ablative therapy—focal brain resections or lesions—to treat movement disorders such as dystonia and tremor, as well as psychiatric disease. Several years later, armed with a knowledge of local circuitry in tremor and Parkinson’s disease in humans and primates, investigators found that focal brain stimulation could create longer lasting, reliable clinical effects without damaging tissue.18-20 More than 100,000 patients have received implanted stimulators to treat movement disorders over the past few years, and these same technologies are now being applied to a variety of CNS conditions, such as depression, eating disorders, addictive behaviors and epilepsy, in an attempt to modulate and abate abnormal network behavior. The same principles that guided these first-generation, implantable neurodevices and their cardiac predecessors form the foundation for the newer, more-intelligent antiepileptic systems that are currently under development.
OPEN-LOOP DEVICES TO TREAT SEIZURES
The ability of an applied electric field to influence the excitability of a neuron has been known for over 40 years,21 and recognized as a potential treatment for epilepsy for over 20 years.22 Cerebellar electrical stimulation was used in patients in the early 1970s,23-25 with variable success. Later trials focused on stimulating specific regions in the thalamus, specifically the centromedian and anterior thalamic nuclei.26 Although limited in their statistical power, these studies supported the idea that focal brain stimulation can be effective for controlling seizures in some patients, and indicated that these types of interventions are relatively safe.27 These conclusions must be put into perspective, however. Many of the early trials of brain stimulation were empirical and uncontrolled, and the few early clinical trials that were performed resulted in non-robust and often controversial findings.25,28-31 There are many reasons for these contradictory results, not least of which was the lack of quality control, performance and manufacturing standards for devices, which lagged considerably behind similar government oversight and regulation for medications. For these reasons, reports of device efficacy must be treated as suggestive but inconclusive. The above studies are important, however, because they helped to pioneer the field of electrical stimulation for epilepsy, and demonstrated the need for strict research guidelines and blinded, controlled trials to test these interventions. In subsequent years, governing bodies such as the US FDA and its international counterparts have clarified the standards for demonstrating clinical safety and efficacy and the process for clinical approval for devices.32 These standards are a breakthrough for patient care and safety. The recent clinical studies involving stimulation devices have been carefully controlled and designed, with patient safety and measurable outcomes as primary goals,33 and the era of controlled trials in epileptic devices is now underway in earnest.34
Vagus nerve stimulation
The Vagus Nerve Stimulator (VNS, Cyberonics, Inc., Houston, TX, USA) is the first FDA-approved device for treating epilepsy. Approved by the US FDA in 1997 for adjunctive therapy in pharmacoresistant partial epilepsy, the VNS reduces the number of seizures by an average of 30–40%, though 10% or fewer patients are rendered seizure-free.35,36 The VNS functions through periodic electrical stimulation of the left vagus nerve by a contact wrapped around the nerve trunk in the neck (Figure 1). The right vagus nerve is generally avoided because stimulation on this side has the potential to cause bradyarrhythmias through stimulation of the heart’s atrioventricular node. It is unclear precisely how vagus nerve stimulation modulates seizures, but it can promote prophylaxis against seizure occurrence, and some patients report that it can abort seizures when manually triggered in response to an epileptic aura.37
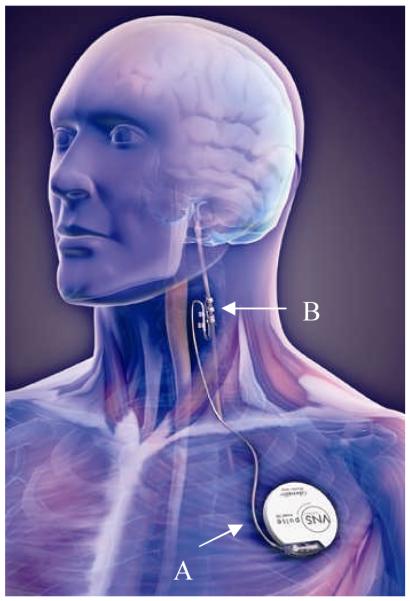
Figure 1.
The Vagus Nerve Stimulator manufactured by Cyberonics, Inc. The implantable pulse generator is implanted under the left clavicle (A), and the stimulation lead is wrapped around the left vagus nerve in the neck (B). Image courtesy of Cyberonics, Inc., Houston, TX, USA.
The VNS is an ‘open-loop’ antiepileptic device, meaning that there is no direct feedback to modulate therapy. To deliver therapy, this device stimulates the CNS through a cranial nerve in a repetitive ‘duty cycle’ (e.g. on for 30 seconds, then off for 5 minutes). Stimulation parameters are currently programmed by the physician to specify stimulation voltage, on-time, pulse-width, on–off cycle duration, and the stereotyped response when the device is triggered manually. Despite the relative simplicity of this design, which is similar to early models of cardiac pacemakers, these devices have been found to be quite effective in some patients.
The place of vagus nerve stimulation in the armamentarium of antiepileptic therapy remains in debate. Its ‘response rate’, meaning the proportion of patients with a 50% or greater reduction in seizures, is comparable to trying a new AED in a patient who has proven resistant to more than two medications. The device’s side-effect profile is favorable compared with that of many AEDs currently on the market, which raises the possibility of using this therapy earlier in the disease course in individuals with seizures. In view of the device’s greater upfront expense and greater invasiveness compared with medications, however, first-line or second-line treatment with the VNS has not been attempted in extensive clinical trials to date. These issues highlight the unspoken requirement that invasive therapies must have better response rates, side-effect profiles, or both, than less invasive or less costly treatments if market penetration and acceptance is to be high. This perception is unfortunate because it can discourage certain avenues of research, and researchers need to be careful not to neglect new ideas with true scientific merit simply on account of perceived market influences.
Clinical trial: deep brain stimulation
A promising open-loop device, currently in a pivotal multi-center clinical trial (the Stimulation of the Anterior Nucleus of the Thalamus in Epilepsy [SANTE] trial, Medtronic, Inc. Minneapolis, MN, USA) for treating partial-onset epilepsy, stimulates the anterior nucleus (AN) of the thalamus (Figure 2). In SANTE, essentially the same deep brain stimulation (DBS) device used for Parkinson’s disease (the first generation of which began as a spinal cord stimulator for chronic pain)18 is placed stereotaxically in the left and right AN. The device stimulates the AN with a protocol that differs slightly from those used to treat Parkinson’s disease and tremor, using intermittent rather than continuous stimulation.34,38 Initially requiring two separate implantable pulse generators (IPGs), one under each clavicle, the device being tested in SANTE now contains two IPGs in one device unit, implanted on only one side of the chest. The choice of the AN as a therapeutic target for this trial was made on the basis of studies in animal models of epilepsy, and two pilot trials in humans that supported the efficacy of AN stimulation for acute and chronic seizures.38-40 The study was allowed to proceed past its halfway point review by its unblinded steering committee, and results of the trial are expected by the end of 2008 (Graves N, personal communication).41,42
Figure 2.
The Medtronic Kinetra device. This device was used in the Stimulation of the Anterior Nucleus of the Thalamus in Epilepsy (SANTE) trial of open-loop brain stimulation for epilepsy. (A) A single device containing two pulse generators-one for each electrode-is implanted beneath the clavicle. (B) Intracranial electrodes are placed stereotaxically in the anterior thalamic nuclei bilaterally. Image courtesy of Medtronic. (C) Midsagittal and (D) transverse views demonstrating placement of stimulating electrodes in the thalamus.
More recently, a pilot study showed that seizure control might be achieved if the Medtronic DBS system was used to stimulate the hippocampus.43,44 Similar open-loop strategies have also been used in the subthalamic nucleus 45,46 or the centromedian nucleus in humans,47 and over the past 20 years many other targets have been stimulated.48,49 Research regarding these additional stimulation targets is still in the early stages of development and has not yet progressed to large-scale trials.
Other methods in development
Clinicians and researchers are currently developing a broad range of therapeutic antiepileptic technologies. Electric fields could prevent seizure onset in a variety of locations when applied to portions of epileptic networks.50-52 Focal cooling takes advantage of channel dynamics, slowing down their activity to make cells less excitable, and is being implemented in animal models of epilepsy and seizures using devices such as Peltier devices, which can rapidly cool focal brain regions.53-55 Transcranial magnetic stimulation has also been used to treat epilepsy. This technique has the benefit of allowing noninvasive, focal treatment, and is proposed to be safe and direct. To date, however, only preliminary clinical trials and case reports are available,56-59 some of which yielded disappointing results.58 Another intriguing avenue is the use of implanted devices to elute antiepileptic medications focally. This method has been successful experimentally, but has not yet been tested clinically.60-64 For many of these methods, the next stage is to determine not only how and where to administer the treatment, but also when. It is this last question that leads to the next frontier in epilepsy technology, namely the development of closed-loop anti-seizure devices.
CLOSED-LOOP DEVICES TO DETECT AND TREAT SEIZURES
Clinical trial:the Responsive Neurostimulator
An exciting new development in epilepsy therapy is the design and implementation of responsive, closed-loop devices to treat seizures. Analogous to the feedback control in automatic implantable cardiac defibrillators, these devices actively record biological signals (in this case EEG), process these signals in real time to detect evidence of imminent seizure onset, and then trigger an intervention. Many devices using various strategies are currently in development, but only one, the Responsive Neurostimulator (RNS, Neuropace Inc.; Figure 3), is in pivotal clinical trials. The RNS is a first-generation closed-loop device—it contains electrodes that record intracranial EEG as input to an algorithm that determines when a seizure has started or is imminent, and it triggers focal electrical stimulation to prevent or arrest clinical seizures.65 An important new feature of this technology is the use of an individual ‘training period’, in which the device is individually tuned to the patient after recording seizures. At the end of 2007, this study was still in the recruitment phase, but preliminary results were promising.66
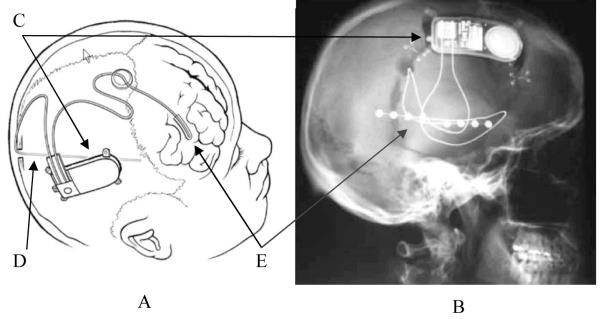
Figure 3.
The NeuroPace responsive neurostimulator. A schematic (A) and skull X-ray (B) of the NeuroPace responsive neurostimulator after implantation. The implantable device records, processes and transmits electroencephalographic signals, in addition to generating the electrical stimuli (C). The implantable depth (D) and strip (E) electrodes monitor brain signals and deliver electrical stimulation to stop seizures. Permission for panel A obtained from NeuroPace, Inc., Mountain View, CA, USA. Permission for panel B obtained from Nursing Spectrum Nurse Wire © 2004.
Current research: seizure prediction and second-generation closed-loop devices
It is worth pointing out that although there is great enthusiasm for closed-loop devices among some investigators and industry, perhaps spurred on by the success of similarly designed cardiac devices, no study has yet demonstrated greater efficacy of first-generation, seizure-detecting closed-loop systems compared with open-loop systems. The next phase in closed-loop systems—detecting abnormalities before the seizure begins (seizure prediction)—has been an area of active research for well over a decade.
To understand the significance and potential promise of closed-loop technologies, it is important to examine the history of their development over the past 10–15 years, and consider their relationship with the fields of seizure detection and prediction. The advent of digitized EEG recording opened up new possibilities for automated event detection for patients with epilepsy. Having a digital signal allows complex mathematical analyses, and currently available computer processors can rapidly analyze the large data streams generated by intracranial EEG recording. Although there have been improvements in the ability to detect spikes and seizures as they occur,67-70 seizure detection algorithms are still under development, and the uncertain accuracy of these methods has been a potential roadblock for responsive antiepileptic devices. When it is accurate, seizure detection appears to be an effective feedback mechanism for closed-loop devices, either to shorten or abort clinical seizures, but there is concern that an effective closed-loop device might require earlier warning. One concern about the first-generation systems is that an intervention might be too late once the seizure has started. The solution to this problem could lie in seizure prediction using algorithms that can detect seizure precursors much earlier than is currently possible.65,71
Neurologists have known for a long time that some patients can predict their own seizures well in advance, and recent evidence indicates that certain subsets of patients can do this quite reliably.72,73 These early predictions are not always associated with discernable changes on scalp EEG or even intracranial EEG. Studies conducted earlier this decade suggest that these ‘preictal’ changes are not detectable either because they consist of relatively small, intermittent changes in the EEG signal, or because they occur beyond the frequency or spatial resolution of the EEG systems currently used in clinical practice. By sampling intracranial EEG at a faster rate, objective EEG changes have been reported long before a seizure occurs in some patients.74
Although prospective seizure prediction has not yet been convincingly demonstrated, recent breakthroughs in the statistics of seizure prediction, and in our understanding of the probabilistic nature of these events, suggest that definitive evidence of statistically significant seizure prediction is imminent.75-78 The theoretical benefit of this technology with respect to antiepileptic devices is that if seizure generation can be identified long before it is manifested clinically, it is likely that the process is more spatially confined, and might be more amenable to abortive therapy, than when it involves many more neurons at the time of overt clinical onset. In addition, if seizures can be identified minutes or more before their clinical onset, there might be more opportunity to stop their progression using a variety of abortive and therapeutic strategies.
Over the past 10 years, many strategies for analyzing and predicting seizures have been evaluated, including many nonlinear and chaos measurements, wavelet decompositions, machine learning, and other methods.69,75,76,79 The results have been somewhat inconsistent, and to date no method has been successfully tested prospectively. Many new methods are currently in development, both in private industry and through public grants. Perhaps the most important breakthrough has been the establishment of statistical methods to assess the success of any particular method.75,76 The field has progressed from the empirical, retrospective demonstration of principles, and is now evolving into probability-based, prospective trials. Our recent understanding that seizure ‘precursors’ are likely to fluctuate, with variable probability of triggering an epileptic event, combined with a new statistical understanding of what constitutes successful seizure prediction and how to measure it, are among the most important developments in this field over the past 5 years.
As seizure prediction technology improves, so too does our ability to deliver more-efficient closed-loop interventions. The benefits of closed-loop devices are twofold: first, feedback enables real-time correction if the intervention is insufficient, and second, there is likely to be a reduction in the overall treatment dose, thereby reducing side effects and system wear. A third theoretical benefit that is unique to epilepsy is that earlier interventions (i.e. in the prediction horizon rather than during the beginning of a clinical seizure) might prevent clinical seizures from ever occurring, rather than trying to abort a de facto seizure.
One dilemma in seizure prediction is how to set the threshold for false positives. Current opinion states that having a high sensitivity is preferable in order to ensure that no seizures are missed, even if stimulations are triggered for false positives. The rationale behind this approach is that responsive stimulations are selected to be harmless; that is, below the threshold for inducing tissue injury,47,80 and that the total treatment dose administered is lower than for open-loop devices. Therefore, the essence of a closed-loop device is to deliver less frequent—but hopefully more-effective—interventions. Several devices are currently in development, using treatment strategies such as electrical stimulation,81 a cooling device,53 the VNS,82 and localized drug delivery.60
FUTURE PROSPECTS
Research into the methods of predicting and treating seizures has benefited greatly from recent technological improvements. As this field moves forward, there are several important issues that need to be addressed.
Improving seizure prediction
Now that a statistical framework is available to assess prediction efficacy, it is possible to compare prediction methods, and later test their utility in anti-seizure devices. One popular current method focuses on extracting multiple quantitative features from the EEG signal. In this approach, various engineering methods borrowed from industry (e.g. algorithms controlling web search engines, credit card fraud detection, or pattern recognition in assembly lines) can be employed to analyze EEG in an automated fashion. These systems commonly use a ‘classifier’, such as a computational structure (e.g. knearest-neighbor or fuzzy clustering), neural network, or machine learning algorithm that can attempt to maximize predictability by comparing arrays of possible measures and weighted combinations of different features. In this way, the classifier removes human visualization and integration from the algorithm training process, and substitutes classification ‘rules’ that optimize some aspect of performance to find optimal solutions to the detection or prediction problem. Another method is to use wavelets to identify and detect patient-specific waveforms that are found to be important to seizure generation.83 Given the heterogeneity of causes of epilepsy, many investigators feel that it is likely that seizure detection and prediction methods will be improved if they are tuned to each individual patient. It is also likely that as the network dynamics and high-frequency data are further understood, new methods of seizure prediction will be discovered.
Electroencephalogram database
In addition to the statistical rigor necessary for predictive testing, one major hurdle to making better and more-effective antiepileptic devices is access to a well-documented, organized intracranial EEG database, particularly one that includes broadband EEG data (e.g. at least 0.1–2 kHz sampling rate). At recent NIH-sponsored Seizure Prediction Workshops in Bethesda, MD, USA (April 2006) and Freiburg, Germany (October 2007), sessions were dedicated to the creation of such a database, continuing from a similar discussion at the First International Collaborative Workshop on Seizure Prediction.79 The need for an EEG database has arisen because algorithms to detect and predict seizures must be developed and tested on real clinical data, which are expensive to acquire. In addition, it is extremely important to render standard clinical data usable for clinical research—this requires the careful conditioning of data to remove artifacts, erroneous markings and mislabeling of channels. It is necessary to have easily accessible digital storage for terabytes of data of increasing bandwidth, in a secure facility that protects patient privacy. The effort also must include a separate set of blinded ‘testing EEGs’ that can be used to verify methods in a quasi-prospective manner.
Defining and understanding seizures through basic research
What is a seizure? This is a fundamental—yet vital—question to seizure detection and prediction research, the answer to which is beyond the scope of this Review. Although seemingly simple on the surface, attempts to arrive at clinical, electrographic (i.e. EEG) or mechanistic definitions of this phenomenon have been elusive. This remains a crucial, active area of research for investigators studying a broad variety of epilepsies and related topics.
Although EEG has been used to detect seizures for over 50 years, little is known about the cellular and network processes that actually produce spontaneous seizures. Slightly more is known about reflex epilepsies that are evoked by certain stimuli, such as reading, calculating, photic stimulation or unexpected somatic sensations. By contrast, the events that initiate the more-common complex partial seizures are poorly understood, except at the level of risk factors such as sleep deprivation, alcohol or other medications. Research over the past 40 years has focused primarily on ion channels and electrophysiology in both individual cells and brain slices. More recently, a wealth of genetic research has linked several ion channel mutations to specific epilepsy syndromes.84 Comparatively little is known, however, about epilepsy on the network scale—the third spatial dimension that is eliminated in brain slice preparations. There is still much debate about even the models used to simulate epilepsy.85 Technology is now available to evaluate epilepsy in vivo: arrays of electrodes are being used to spatially map seizure activity,86 microwire electrode recordings can detect activity from single neurons (units) in patients with epilepsy (Worrell G, personal communication), and voltagesensitive dyes can monitor cortical seizure activity from millions of cells simultaneously, providing insights into functional networks.87 These and other studies are demonstrating seizure characteristics that are beyond the resolution of standard intracranial EEG grid and depth electrodes.
In addition to new techniques for studying seizures spatially, a wealth of new information has been obtained from sampling electrophysiological activity at higher frequency.74 This new recording technology could potentially allow the spatial and temporal topology of seizures to be characterized, although spatial sampling is limited in humans by safety concerns and clinical necessity. It is important to establish what recording bandwidth will be necessary and sufficient for accurate seizure detection and prediction, and on what spatial scale more-effective, second-generation implantable devices need to operate.
Investigators are now approaching epilepsy on many levels, from genes and proteins up to intact networks, and from clinical investigations using broad field potentials down to analysis of functional networks. The synergy of these new parallel efforts is now becoming apparent, and it is envisaged that the ‘bottom-up’ and ‘top-down’ approaches will meet somewhere in the middle, in the area of systems neuroscience.
What intervention can prevent a seizure?
The success of cardiac defibrillators, exploiting cardiac physiology to eliminate deadly arrhythmias, is admirable. Is such a calculated method possible in epilepsy? The network physiology is clearly much more complex, and little is known of the network timing and topology that produces seizures. Furthermore, the brain is not amenable to the large-scale ‘defibrillation’ pulses that are used in the heart. Nevertheless, clear examples of effective antiepileptic stimulation to arrest seizures are becoming easier to find, as clinical trials of closed-loop stimulation continue.65,66
One strategy used by cardiac defibrillators is that of tiered intervention, in which progressively stronger electric shocks are given as the heart rhythm becomes more pathologic, or as abnormalities persist in time. Such a strategy would lend itself well to epilepsy control, but to date has not been implemented in any of the devices under investigation. It is possible to imagine a scenario in which a small, asymptomatic seizurelike burst on the EEG might trigger a mild localized intervention, such as an electrical stimulus. As abnormal activity becomes more widespread or prolonged, the intervention could become much broader spatially or more prolonged, or the amplitude could be increased. An additional step could perhaps be another intervention, such as flooding the affected region with an AED infusion, as failure of stimulation to abort the seizure becomes clear.
There are some potential drawbacks to tiered interventions. One major issue is that more-aggressive interventions are likely to produce more side effects. A challenge of this research will be to evaluate the limits of each therapeutic intervention, and its threshold for generating adverse effects. Another difficulty with tiered intervention, as with all seizure intervention strategies, is how to assess overall effectiveness. Will it be sufficient simply to count the number of seizures and auras with and without intervention, as is common clinical practice for medications? It is likely that many subclinical events will be recorded by an implanted device, and this could potentially confound statistics for clinical efficacy if these events are not symptomatic. Further research will be required to establish the relationship between subclinical seizure data and clinical seizure control.
The currently proposed strategies for intervention in implantable antiepileptic devices are electrical stimulation, magnetic stimulation,44 localized drug delivery,51 and focal cooling.71 Electrical stimulation is further divided into continuous, responsive and controlling paradigms. All three of the electrical devices currently in use or in clinical trials use periodic pulse trains: the VNS and Medtronic SANTE-trial devices are both continuous open-loop devices, whereas the RNS is responsive and administers a series of up to five pulse trains, on the basis of its automated interpretation of intracranial EEG activity. Pulse trains are simple to implement, and have been remarkably successful in the DBS devices used to treat Parkinson’s disease, but they are not necessarily the best stimulation method. Investigations are currently underway, by our group and others, on continuous control paradigms in which therapy is yoked to measures of preictal or ictal activity on the EEG, and therapy is continuously adjusted in response to the error signals between injected and recorded signals. These types of continuous control approaches, which are similar to those used in airplane autopilots and other automated systems, hold considerable theoretical promise, though it is too early to assess their effectiveness for preventing clinical seizures.
New strategies will no doubt be developed as we expand our knowledge about the complex interplay of excitation, inhibition and coupling. Understanding this interplay will help define the cutting edge of novel antiepileptic therapy, beyond standard drug delivery. This Review only hints at the exciting and rapid developments that are underway in epilepsy research.
Personalized control
One of the primary hurdles that separates a seizure control device from a cardiac defibrillator is the heterogeneity of pathology and clinical manifestations in epilepsy. A staggering number of different pathologies can produce intractable epilepsy, including genetic causes, trauma, infection, brain malformations (such as cortical dysplasia), and medications. As a result, there is no standard, identifiable brain equivalent of basic cardiac arrhythmias such as ventricular tachycardia or atrial fibrillation. Rather, seizures are associated with an extensive range of EEG patterns. Current research with high-frequency recordings is now finding that many different onset patterns are also seen in the preseizure period (Worrell G, personal communication). Consequently, both seizure detection and prediction, as defined in an individual patient, are difficult to generalize to all patients. As noted above, the solution might be to tune, or ‘train’, an antiseizure device to a particular patient.88 Devices capable of ‘learning’ patterns from individuals, such as those based on machine learning or other artificial intelligence techniques might also hold promise. To date, no clinical devices have incorporated either individual patient training or machine learning in an automated form, beyond the algorithms used in the NeuroPace RNS device, which are manually trained to individual patient patterns and updated as necessary at subsequent doctor visits. One serious limitation for clinical use has been the memory and processing speed available in current implantable device platforms. Advances in technology are making these strategies more feasible. Modern storage devices and processors are increasingly capable of handling the data from EEG recording. Wireless technology also affords new opportunities, as does the potential to download data from implantable devices and transmit it over the Internet for remote processing and algorithm training. One possible solution is to have wireless-capable implanted devices that can transmit prolonged data streams for offline processing and data storage.
CONCLUSIONS
First-generation antiepileptic devices are currently in pivotal clinical trials, and are showing considerable promise. Motivated by the success of similarly conceived therapeutic cardiac devices, the field is poised to produce more-advanced second-generation devices that can track seizure generation in epileptic networks, with the aim of arresting or preventing clinical events. Much of this progress has been motivated by recent technological advances at a variety of temporal and spatial levels, from molecular processes, through single cells, to functional or dysfunctional neuronal networks and broader neural systems.
The evolution of engineering technology as applied to epilepsy presents renewed promise to potentially identify periods of time when the probability of seizure onset is increased, and to deliver responsive therapy to prevent epileptic events from occurring. For medication-resistant epilepsy, devices such as those discussed above present an exciting new avenue to help patients in an era when new AEDs have not markedly reduced seizure burden significantly, despite having considerable positive impact on patient quality of life. Through multidisciplinary, multiscale research and collaboration, implantable devices hold promising, exciting possibilities for diagnosis, mapping epileptic networks, and dramatically improving epilepsy therapy. In addition, discoveries along the way promise to greatly improve our knowledge of the mechanisms underlying seizure generation.
Key Points
Up to 25% of the 50 million people with epilepsy worldwide are unable to control their seizures with currently available medications
Implantable devices are being developed to help control seizures in patients with medically refractory epilepsy
Open-loop electrical stimulation devices, which lack intrinsic feedback control, are currently being used to treat medically refractory epilepsy
A closed-loop device with real-time surface and depth electroencephalograhic monitoring is currently in clinical trials; second-generation closed-loop devices will use earlier seizure markers as feedback
Other anti-seizure devices currently in development use techniques such as drug delivery, focal cooling and magnetic stimulation
Future research must address the questions of how to define a seizure, what causes a seizure, and how a seizure can be stopped
Review criteria PubMed and Google were searched online on 21 January 2007 using the terms “epilepsy device” and “epilepsy control device”. The abstracts and web content retrieved were reviewed and prioritized. Full articles were obtained and additional references reviewed when appropriate. In addition, personal communications from and knowledge of several groups throughout the world were used for directed PubMed searches.
Biography
WC Stacey is a Postdoctoral Fellow and B Litt is an Associate Professor in the Departments of Epilepsy and Bioengineering, University of Pennsylvania, Philadelphia, PA, USA.
Footnotes
Competing interests B Litt has declared associations with the following companies: NeuroPace, Neuro Vista. See the article online for full details of the relationship. WC Stacey declared no competing interests.
References
- 1.Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Little Brown and Co; Boston: 1954. [Google Scholar]
- 2.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamill OP, et al. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 4.Pfurtscheller G, et al. 15 years of BCI research at Graz University of Technology: current projects. IEEE Trans Neural Syst Rehabil Eng. 2006;14:205–210. doi: 10.1109/TNSRE.2006.875528. [DOI] [PubMed] [Google Scholar]
- 5.Isaacs RE, et al. Work toward real-time control of a cortical neural prothesis. IEEE Trans Rehabil Eng. 2000;8:196–198. doi: 10.1109/86.847814. [DOI] [PubMed] [Google Scholar]
- 6.Donoghue JP, et al. Assistive technology and robotic control using motor cortex ensemble-based neural interface systems in humans with tetraplegia. J Physiol. 2007;579:603–611. doi: 10.1113/jphysiol.2006.127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–360. doi: 10.1146/annurev.bioeng.6.040803.140103. [DOI] [PubMed] [Google Scholar]
- 8.Brelen ME, et al. Intraorbital implantation of a stimulating electrode for an optic nerve visual prosthesis. Case report. J Neurosurg. 2006;104:593–597. doi: 10.3171/jns.2006.104.4.593. [DOI] [PubMed] [Google Scholar]
- 9.Normann RA. Technology insight: future neuroprosthetic therapies for disorders of the nervous system. Nat Clin Pract Neurol. 2007;3:444–452. doi: 10.1038/ncpneuro0556. [DOI] [PubMed] [Google Scholar]
- 10.Contreras DA, et al. Intracellular and computational characterization of the intracortical inhibitory control of synchronized thalamic inputs in vivo. J Neurophysiol. 1997;78:335–350. doi: 10.1152/jn.1997.78.1.335. [DOI] [PubMed] [Google Scholar]
- 11.Traub RD, Bibbig A. A model of high-frequency ripples in the hippocampus based on synaptic coupling plus axon-axon gap junctions between pyramidal neurons. J Neurosci. 2000;20:2086–2093. doi: 10.1523/JNEUROSCI.20-06-02086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyhrfjeld-Johnsen J, et al. Topological determinants of epileptogenesis in large-scale structural and functional models of the dentate gyrus derived from experimental data. J Neurophysiol. 2007;97:1566–1587. doi: 10.1152/jn.00950.2006. [DOI] [PubMed] [Google Scholar]
- 13.Netoff TI, et al. Synchronization in hybrid neuronal networks of the hippocampal formation. J Neurophysiol. 2005;93:1197–1208. doi: 10.1152/jn.00982.2004. [DOI] [PubMed] [Google Scholar]
- 14.Traub RD, et al. Combined experimental/simulation studies of cellular and network mechanisms of epileptogenesis in vitro and in vivo. J Clin Neurophysiol. 2005;22:330–342. [PubMed] [Google Scholar]
- 15.Traub RD, et al. Single-column thalamocortical network model exhibiting gamma oscillations, sleep spindles, and epileptogenic bursts. J Neurophysiol. 2005;93:2194–2232. doi: 10.1152/jn.00983.2004. [DOI] [PubMed] [Google Scholar]
- 16.Ebersole JS, Pedley TA. Current Practice of Clinical Electroencephalography. 3rd Ed Lippincott, Williams and Wilkins; Philadelphia: 2003. [Google Scholar]
- 17.Benbadis S. Invasive EEG. In: Luders HO, Noachtar S, editors. In Epileptic Seizures: Pathophysiology and Clinical Semiology. Churchill Livingstone; New York: 2000. pp. 32–53. [Google Scholar]
- 18.Benabid AL, et al. Deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: methodologic aspects and clinical criteria. Neurology. 2000;55(12 Suppl 6):S40–S44. [PubMed] [Google Scholar]
- 19.Uc EY, Follett KA. Deep brain stimulation in movement disorders. Semin Neurol. 2007;27:170–182. doi: 10.1055/s-2007-971175. [DOI] [PubMed] [Google Scholar]
- 20.Wichmann T, DeLong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 21.Creutzfeldt OD, et al. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- 22.Durand D. Electrical stimulation can inhibit synchronized neuronal activity. Brain Res. 1986;382:139–144. doi: 10.1016/0006-8993(86)90121-6. [DOI] [PubMed] [Google Scholar]
- 23.Cooper IS, et al. Chronic cerebellar stimulation in epilepsy. Clinical and anatomical studies. Arch Neurol. 1976;33:559–570. doi: 10.1001/archneur.1976.00500080037006. [DOI] [PubMed] [Google Scholar]
- 24.Grabow JD, et al. Cerebellar stimulation for the control of seizures. Mayo Clin Proc. 1974;49:759–774. [PubMed] [Google Scholar]
- 25.Levy LF, Auchterlonie WC. Chronic cerebellar stimulation in the treatment of epilepsy. Epilepsia. 1979;20:235–245. doi: 10.1111/j.1528-1157.1979.tb04800.x. [DOI] [PubMed] [Google Scholar]
- 26.Krauss GL, Fisher RS. Cerebellar and thalamic stimulation for epilepsy. Adv Neurol. 1993;63:231–245. [PubMed] [Google Scholar]
- 27.Velasco AL, et al. Subacute and chronic electrical stimulation of the hippocampus on intractable temporal lobe seizures: preliminary report. Arch Med Res. 2000;31:316–328. doi: 10.1016/s0188-4409(00)00064-3. [DOI] [PubMed] [Google Scholar]
- 28.Kellinghaus C, Loddenkemper T. Double-blind, randomized controlled study of bilateral cerebellar stimulation. Epilepsia. 2006;47:1247. doi: 10.1111/j.1528-1167.2006.00598_4.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Buren JM, et al. Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg. 1978;48:407–416. doi: 10.3171/jns.1978.48.3.0407. [DOI] [PubMed] [Google Scholar]
- 30.Wright GD, et al. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. 1984;47:769–774. doi: 10.1136/jnnp.47.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velasco F, et al. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia. 2005;46:1071–1081. doi: 10.1111/j.1528-1167.2005.70504.x. [DOI] [PubMed] [Google Scholar]
- 32.Litt B. Evaluating devices for treating epilepsy. Epilepsia. 2003;44(Suppl 7):30–37. doi: 10.1046/j.1528-1157.44.s7.3.x. [DOI] [PubMed] [Google Scholar]
- 33.Oommen J, et al. Experimental electrical stimulation therapy for epilepsy. Curr Treat Options Neurol. 2005;7:261–271. doi: 10.1007/s11940-005-0036-9. [DOI] [PubMed] [Google Scholar]
- 34.Graves NM, Fisher RS. Neurostimulation for epilepsy, including a pilot study of anterior nucleus stimulation. Clin Neurosurg. 2005;52:127–134. [PubMed] [Google Scholar]
- 35.Cyberonics . Physician’s Manual, VNS Therapy Pulse Model 102 Generator. vol. June. Cyberonics, Inc; Houston, Texas: 2002. [Google Scholar]
- 36.Fisher RS, et al. Assessment of vagus nerve stimulation for epilepsy: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1997;49:293–297. doi: 10.1212/wnl.49.1.293. [DOI] [PubMed] [Google Scholar]
- 37.Cyberonics VNS Therapy—Mechanism of Action. [ http://www.vnstherapy.com/epilepsy/hcp/vnstherapy/mechanismofaction.aspx]
- 38.Kerrigan JF, et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004;45:346–354. doi: 10.1111/j.0013-9580.2004.01304.x. [DOI] [PubMed] [Google Scholar]
- 39.Hodaie M, et al. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002;43:603–608. doi: 10.1046/j.1528-1157.2002.26001.x. [DOI] [PubMed] [Google Scholar]
- 40.Mirski MA, et al. Anterior thalamus and substantia nigra: two distinct structures mediating experimental generalized seizures. Brain Res. 1986;397:377–380. doi: 10.1016/0006-8993(86)90642-6. [DOI] [PubMed] [Google Scholar]
- 41.NIH Clinical Trials.gov SANTE— Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy. [ http://clinicaltrials.gov/ct/show/NCT00101933]
- 42.Medtronic Intercept™ Epilepsy Control System Clinical Trial. [ http://www.epilepsycontrol.com]
- 43.Vonck K, et al. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol. 2002;52:556–565. doi: 10.1002/ana.10323. [DOI] [PubMed] [Google Scholar]
- 44.Tellez-Zenteno JF, et al. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology. 2006;66:1490–1494. doi: 10.1212/01.wnl.0000209300.49308.8f. [DOI] [PubMed] [Google Scholar]
- 45.Dinner DS, et al. EEG and evoked potential recording from the subthalamic nucleus for deep brain stimulation of intractable epilepsy. Clin Neurophysiol. 2002;113:1391–1402. doi: 10.1016/s1388-2457(02)00185-2. [DOI] [PubMed] [Google Scholar]
- 46.Benabid AL, et al. Antiepileptic effect of high-frequency stimulation of the subthalamic nucleus (corpus luysi) in a case of medically intractable epilepsy caused by focal dysplasia: a 30-month follow-up: technical case report. Neurosurgery. 2002;50:1385–1391. doi: 10.1097/00006123-200206000-00037. [DOI] [PubMed] [Google Scholar]
- 47.Fisher RS, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841–851. doi: 10.1111/j.1528-1157.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 48.Pollo C, Villemure JG. Rationale, mechanisms of efficacy, anatomical targets and future prospects of electrical deep brain stimulation for epilepsy. Acta Neurochir Suppl. 2007;97:311–320. doi: 10.1007/978-3-211-33081-4_34. [DOI] [PubMed] [Google Scholar]
- 49.Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. 2004;3:111–118. doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]
- 50.Franaszczuk PJ, et al. External excitatory stimuli can terminate bursting in neural network models. Epilepsy Res. 2003;53:65–80. doi: 10.1016/s0920-1211(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 51.Lian J, et al. Local suppression of epileptiform activity by electrical stimulation in rat hippocampus in vitro. J Physiol. 2003;547:427–434. doi: 10.1113/jphysiol.2002.033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson KA, et al. In vivo modulation of hippocampal epileptiform activity with radial electric fields. Epilepsia. 2003;44:768–777. doi: 10.1046/j.1528-1157.2003.35402.x. [DOI] [PubMed] [Google Scholar]
- 53.Rothman SM, et al. Focal cooling for epilepsy: an alternative therapy that might actually work. Epilepsy Behav. 2005;7:214–221. doi: 10.1016/j.yebeh.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Yang XF, et al. Neocortical seizure termination by focal cooling: temperature dependence and automated seizure detection. Epilepsia. 2002;43:240–245. doi: 10.1046/j.1528-1157.2002.33301.x. [DOI] [PubMed] [Google Scholar]
- 55.Imoto H, et al. Use of a Peltier chip with a newly devised local brain-cooling system for neocortical seizures in the rat. Technical note. J Neurosurg. 2006;104:150–156. doi: 10.3171/jns.2006.104.1.150. [DOI] [PubMed] [Google Scholar]
- 56.Fregni F, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006;60:447–455. doi: 10.1002/ana.20950. [DOI] [PubMed] [Google Scholar]
- 57.Joo EY, et al. Antiepileptic effects of low-frequency repetitive transcranial magnetic stimulation by different stimulation durations and locations. Clin Neurophysiol. 2007;118:702–708. doi: 10.1016/j.clinph.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Theodore WH, et al. Transcranial magnetic stimulation for the treatment of seizures: a controlled study. Neurology. 2002;59:560–562. doi: 10.1212/wnl.59.4.560. [DOI] [PubMed] [Google Scholar]
- 59.Bae EH, et al. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav. 2007;10:521–528. doi: 10.1016/j.yebeh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Fisher RS, Chen DK. New routes for delivery of anti-epileptic medications. Acta Neurol Taiwan. 2006;15:225–231. [PubMed] [Google Scholar]
- 61.Lohman RJ, et al. Validation of a method for localised microinjection of drugs into thalamic subregions in rats for epilepsy pharmacological studies. J Neurosci Methods. 2005;146:191–197. doi: 10.1016/j.jneumeth.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Tamargo RJ, et al. The intracerebral administration of phenytoin using controlled-release polymers reduces experimental seizures in rats. Epilepsy Res. 2002;48:145–155. doi: 10.1016/s0920-1211(01)00330-8. [DOI] [PubMed] [Google Scholar]
- 63.Stein AG, et al. An automated drug delivery system for focal epilepsy. Epilepsy Res. 2000;39:103–114. doi: 10.1016/s0920-1211(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 64.Ludvig N, et al. Epidural pentobarbital delivery can prevent locally induced neocortical seizures in rats: the prospect of transmeningeal pharmacotherapy for intractable focal epilepsy. Epilepsia. 2006;47:1792–1802. doi: 10.1111/j.1528-1167.2006.00642.x. [DOI] [PubMed] [Google Scholar]
- 65.Kossoff EH, et al. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45:1560–1567. doi: 10.1111/j.0013-9580.2004.26104.x. [DOI] [PubMed] [Google Scholar]
- 66.Barkley GL, et al. Safety and preliminary efficacy of the RNS™ Responsive Neurostimulator for the treatment of intractable epilepsy in adults [abstract #A.12]. Presented at the Annual Meeting of the American Epilepsy Society; San Diego, CA, USA. 2006 December.2006. pp. 1–5. [Google Scholar]
- 67.Firpi H, et al. Epileptic seizure detection using genetically programmed artificial features. IEEE Trans Biomed Eng. 2007;54:212–224. doi: 10.1109/TBME.2006.886936. [DOI] [PubMed] [Google Scholar]
- 68.Saab ME, Gotman J. A system to detect the onset of epileptic seizures in scalp EEG. Clin Neurophysiol. 2005;116:427–442. doi: 10.1016/j.clinph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Gardner AB, et al. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin Neurophysiol. 2007;118:1134–1143. doi: 10.1016/j.clinph.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee HC, et al. Comparison of seizure detection algorithms in continuously monitored pediatric patients. J Clin Neurophysiol. 2007;24:137–146. doi: 10.1097/WNP.0b013e318033715b. [DOI] [PubMed] [Google Scholar]
- 71.Litt B, Echauz J. Prediction of epileptic seizures. Lancet Neurol. 2002;1:22–30. doi: 10.1016/s1474-4422(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 72.Haut SR, et al. Can patients with epilepsy predict their seizures? Neurology. 2007;68:262–266. doi: 10.1212/01.wnl.0000252352.26421.13. [DOI] [PubMed] [Google Scholar]
- 73.Litt B, Krieger A. Of seizure prediction, statistics, and dogs: a cautionary tail. Neurology. 2007;68:250–251. doi: 10.1212/01.wnl.0000255912.43452.12. [DOI] [PubMed] [Google Scholar]
- 74.Litt B, et al. Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron. 2001;30:51–64. doi: 10.1016/s0896-6273(01)00262-8. [DOI] [PubMed] [Google Scholar]
- 75.Wong S, et al. A stochastic framework for evaluating seizure prediction algorithms using hidden Markov models. J Neurophysiol. 2007;97:2525–2532. doi: 10.1152/jn.00190.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mormann F, et al. Seizure prediction: the long and winding road. Brain. 2007;130:314–333. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- 77.Lehnertz K, et al. State-of-the-art of seizure prediction. J Clin Neurophysiol. 2007;24:147–153. doi: 10.1097/WNP.0b013e3180336f16. [DOI] [PubMed] [Google Scholar]
- 78.Schelter B, et al. Seizure prediction: the impact of long prediction horizons. Epilepsy Res. 2007;73:213–217. doi: 10.1016/j.eplepsyres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Lehnertz K, Litt B. The First International Collaborative Workshop on Seizure Prediction: summary and data description. Clin Neurophysiol. 2005;116:493–505. doi: 10.1016/j.clinph.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 80.Worrell G, et al. Safety and evidence for efficacy of an implantable Responsive Neurostimulator (RNS) for the treatment of medically intractable partial onset epilepsy in adults [abstract #2.397] Epilepsia. 2005;46(Suppl 8):226. [Google Scholar]
- 81.Osorio I, et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57:258–268. doi: 10.1002/ana.20377. [DOI] [PubMed] [Google Scholar]
- 82.Trafton A. Epilepsy breakthrough on horizon. 2006 [ http://web.mit.edu/newsoffice/2006/epilepsy.html]
- 83.Shoeb A, et al. Patient-specific seizure onset detection. Epilepsy Behav. 2004;5:483–498. doi: 10.1016/j.yebeh.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 84.Hirose S, et al. Genetics of idiopathic epilepsies. Epilepsia. 2005;46(Suppl 1):38–43. doi: 10.1111/j.0013-9580.2005.461011.x. [DOI] [PubMed] [Google Scholar]
- 85.Stables JP, et al. Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia. 2002;43:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- 86.Akiyama T, et al. Topographic movie of ictal high-frequency oscillations on the brain surface using subdural EEG in neocortical epilepsy. Epilepsia. 2006;47:1953–1957. doi: 10.1111/j.1528-1167.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 87.Ang CW, et al. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.D’Alessandro M, et al. A multi-feature and multi-channel univariate selection process for seizure prediction. Clin Neurophysiol. 2005;116:506–516. doi: 10.1016/j.clinph.2004.11.014. [DOI] [PubMed] [Google Scholar]