Abstract
Diabetes mellitus is considered a relative contra-indication for implant therapy. However, the effect of glycemic level on implant integration in persons with diabetes remains poorly understood. The hypothesis of this research was that poor glycemic control is directly related to short-term-impairment implant stabilization. This prospective clinical study evaluated 10 non-diabetic individuals (12 implants) and 20 persons with type 2 diabetes (30 implants). Glycated hemoglobin (HbA1c) levels ranged from 4.7-12.6%. Implant stability was assessed by resonance frequency analysis over 4 months following placement. Minimum stability levels were observed 2-6 weeks following placement for all 42 implants. Persons with HbA1c ≥ 8.1% had a greater maximum decrease in stability from baseline and required a longer time for healing, as indicated by return of stability level to baseline. This study demonstrates alterations in implant stability consistent with impaired implant integration for persons with type 2 diabetes mellitus in direct relation to hyperglycemic conditions.
Keywords: implants, hyperglycemia, diabetes mellitus, resonance frequency analysis, implant stability
Introduction
Currently, diabetes mellitus is considered a relative contraindication for dental implant therapy, depending on levels of glycemic control (World Workshop in Periodontics, 1996; Blanchaert, 1998; Beikler and Flemmig, 2003). Poor glycemic control and hyperglycemia have been directly associated with an increased risk of co-morbidities for type 2 diabetes mellitus, including compromised dermal wound healing and immune responses.
Consistent with these associations, hyperglycemia has been shown to have an adverse effect on bone formation and implant integration in animal models, with levels of implant integration decreasing approximately 30% relative to those in control animals (Nevins et al., 1998; Gerritsen et al., 2000; McCracken et al., 2000). Clinical studies, however, have reported highly varied implant failure rates, ranging from 0-14.7% of implants and 0-31.3% of persons, leaving the consequences of diabetes on implant success in question (Shernoff et al., 1994; Garrett et al., 1998; Fiorellini et al., 2000; Morris et al., 2000; Olson et al., 2000; Abdulwassie and Dhanrajani, 2002; Moy et al., 2005). Furthermore, glycemic status of the persons defined as ‘well-controlled’ was not clearly reported in these studies. Taken together, the varied success rates and the lack of definition for glycemic control reinforce the need for a better understanding of the influences of diabetes and glycemic control on implant success in this population, critically dependent on dietary management of their condition.
Implant integration may be assessed by longitudinal measures of implant stability by resonance frequency analysis (Barewal et al., 2003). Implant stability has been shown to correlate with bone density, insertion torque, changes in supporting matrix, and bone-implant contact (Meredith et al., 1996, 1997a,b; Friberg et al., 1999; Barewal et al., 2003). Additionally, resonance frequency analysis has identified changes in implant stability consistent with resorptive and formative osseous healing following implant placement (Barewal et al., 2003).
We previously reported the clinical findings from this study showing no evidence of diminished clinical success or early healing complications of implant therapy associated with glycemic control in persons with type 2 diabetes (Dowell et al., 2007). In this paper, we test the hypothesis that poor glycemic control is directly related to short-term-impairment implant stabilization.
Materials & Methods
Study Design
Detailed descriptions of the study design and methods have been presented previously (Dowell et al., 2007). In brief, this prospective pilot study was designed to examine the effect of glycemic control on implant stabilization for persons with type 2 diabetes over 4 mos following implant placement. Participants were recruited from among individuals seeking dental treatment within the University of Texas Health Science Center at San Antonio Dental School. The study enrolled individuals missing one or more teeth and recognized as having the potential to benefit from dental implant therapy. Both healthy, non-diabetic individuals and persons with type 2 diabetes mellitus were enrolled. All persons with diabetes were under the care of a health care provider, and study participation did not alter their medical management. This study was approved by the Institutional Review Board; all participants gave written, informed consent.
Implant sites were required to have at least 4 mos of healing following tooth extraction prior to implant placement, and adequate bone dimensions for implant placement without the need for bone grafting. Individuals with oral pathology, systemic disorders affecting surgical therapy protocols, history of bone grafting at the implant site, or currently smoking, were excluded. Type 2 diabetes mellitus status was self-reported and verified with physician report, glycemic level within 1 mo prior to implant surgery, and/or treatment record. Persons with diabetes and having a history of treatment for microvascular or macrovascular complications were excluded. Persons with type 2 diabetes could be on a modified diet, oral medication, insulin, or combination therapies.
Of 50 implants placed in 35 individuals, one non-diabetic individual (1 implant) and 1 implant (of 2) from a person with diabetes were excluded due to rotational movement during the 4 mos following implant placement. Additionally, 6 implants and two participants were excluded due to placement procedures inconsistent with the protocol. Data from 32 persons with 42 implants were analyzed in this study.
Glycemic Control
Glycemic control was assessed by glycated hemoglobin (HbA1c; Quest Diagnostics Laboratory, San Antonio, TX, USA). HbA1c level reflects average blood glucose levels over the preceding two- to three-month period (Derr et al., 2003). Persons with type 2 diabetes were stratified by HbA1c levels as well-controlled (HbA1c 6.1-8.0%), moderately controlled (HbA1c 8.1-10.0%), or poorly controlled (HbA1c ≥ 10.1%). All non-diabetic individuals had HbA1c ≤ 6.0%. HbA1c measurements taken 8 wks following implant placement were used for the assessment of glycemic control.
Surgical Implant Placement
Rough-surfaced implants (SP, SLA®; length, 10 or 12 mm; diameter, 4.1 mm) were placed consistent with the manufacturer’s (Institut Straumann AG, Basel, Switzerland) protocols, and covered with a transgingival healing cap. Non-diabetic individuals were prescribed post-operative antibiotics for 3 days, and persons with diabetes were prescribed antibiotics for 7-10 days. Implants were not restored during the four-month evaluation period, and prostheses were adjusted as needed to minimize inadvertent loading of the implant. The surgical visit was considered as the baseline (Week 0).
Clinical Measurements
Resonance frequency measurements as an assessment of implant stability (Implant Stability Quotient) were taken in triplicate by means of the Osstell® instrument (Integration Diagnostics Ltd., Oslo, Norway). The stability level was recorded at baseline, 2, 4, 6, 8, 12, and 16 wks following implant placement. The mean of 3 measurements was used in the statistical analysis. Subjective clinical assessments were made regarding bone type according to a four-tiered scale, based on mineral densities during osteotomy: high density (type I), moderate density (type II), low density (type III), and very low density (type IV) (Lekholm and Zarb, 1985).
Statistical Analysis
Implant Stability Quotient data were analyzed for associations with HbA1c level and time following implant placement (Baseline, 2, 4, 6, 8, 12, 16 wks), by analysis of variance for repeated measurements. The HbA1c group was a between-subjects factor, and follow-up time was a within-subjects factor. Multiple implants were considered nested within an individual. Contrasts among means following the analysis of variance were analyzed by Scheffé’s multiple-comparison procedure. The minimum stability level among the values at 2, 4, and 6 wks was identified. The follow-up time at which the minimum occurred was also determined. The follow-up time at which the stability returned to a level ≥ baseline value, defined as the “time to healing”, was also determined; if the stability value never returned to baseline, a “time to healing” of 16 wks was assigned. Differences in these responses (minimum stability, time of minimum, time to healing) among HbA1c classes measured at 8 wks were analyzed by analysis of variance for repeated measurements. For better assessment of the stabilization of implants over time, the stability data were normalized for each time (2, 4, 6, 8, 12, 16 wks) by calculation of the relative change from baseline for each individual and each implant site. The fraction of participants returning to baseline by 16 wks was analyzed with generalized linear models (Liang and Zeger, 1986), by the use of a binomial distribution with a logistic link function.
Results
Participant Characteristics
Non-diabetic participants were significantly younger than those with type 2 diabetes (Table 1). Non-diabetic participants ranged in age from 29-61 yrs, with HbA1c levels ranging from 4.7-5.8%. Persons with diabetes ranged in age from 51-81 yrs, with HbA1c levels 6.3-12.6% at the eight-week follow-up visit. When the participants were classified into low (those with HbA1c ≤ 8.0) and high (those with HbA1c ≥ 8.1) HbA1c groups, independent of diabetes diagnosis, there was no significant difference in age distributions between the two groups.
Table 1.
Demographic Variables, Implant Location, and Bone Type by Glycemic Levels (HbA1c)
| Participants |
Implants |
Gender |
Age (yrs) |
Location |
Bone Type (no. of implants) |
||||
|---|---|---|---|---|---|---|---|---|---|
| HbA1c at 8 wks | n | n | Number female | Mean ± SE | Number mandible | Type 1 | Type 2 | Type 3 | Type 4 |
| ≤6.0 | 10 | 12 | 7 | 45.7 ± 3.1b | 7 | 4 | 3 | 5 | 0 |
| 6.1 to 8 | 12 | 18 | 6 | 66.0 ± 2.2 | 12 | 0 | 7 | 10 | 1 |
| 8.1 to 10 | 5 | 5 | 2 | 54.4 ± 1.7 | 3 | 0 | 2 | 1 | 2 |
| ≥10.1 | 5 | 7 | 2 | 64.0 ± 5.1 | 5 | 2 | 2 | 2 | 1 |
| Total number | 32 | 42 | 17c | 27d | 6 | 14e | 18e | 4 | |
| Significancea | p = 0.7094 | p = 0.0001 | p = 0.9277 | p = 0.0610 | |||||
Test of equality of HbA1c groups.
HbA1c ≤ 6.0 significantly (p ≤ 0.05, Scheffé’s multiple-comparison procedure) different from combined 6.1 to 8.0, 8.1 to 10.0, and ≥10.1.
53.1% of participants were female.
64.3% of implants were in the mandible.
76.2% of implants were in types II or III bone.
Implant Stability
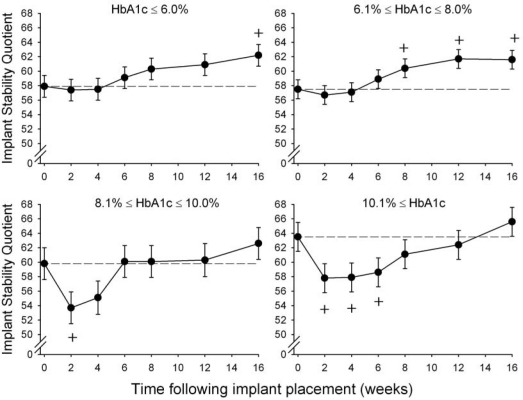
Implant stability was significantly affected by the combination of HbA1c level and the time following implant placement (Interaction of HbA1c and follow-up time; p = 0.0094; Fig. 1). The maximum decrease in implant stability relative to base-line was significantly greater for the HbA1c 8.1-10.0% and HbA1c ≥ 10.1% groups compared with the non-diabetic (HbA1c ≤ 6.0%) and well-controlled (HbA1c 6.1-8.0%) diabetic groups (Table 2).
Figure 1.
Implant Stability Quotient by HbA1c level and time following implant placement. Error bars represent SE. ‘+’ indicates significant (p ≤ 0.05) change from baseline for same HbA1c group. Dashed reference lines represent baseline stability level. HbA1c ≤ 6.0% n = 10 individuals; HbA1c 6.1-8.0%, n = 12 individuals; HbA1c 8.1-10.0%, n = 5 individuals; HbA1c ≥ 10.1%, n = 5 individuals.
Table 2.
Effects of Glycemic Levels (HbA1c) on Implant Stability Quotient
| Baseline |
Minimum |
Time to Minimum (wks) |
Maximum Change Relative to Baseline (%) |
16 wks |
Change at 16 wks Relative to Baseline (%) |
Time to Healing (wks) |
Fraction Returning to Baseline (%) |
|
|---|---|---|---|---|---|---|---|---|
| HbA1c at 8 wks | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| ≤6.0 | 58.0 ± 1.5 | 55.3 ± 1.5 | 3.6 ± 0.4 | −4.4 ± 1.8 | 62.2 ± 1.5 | 7.4 ± 2.3 | 5.1 ± 1.6 | 91.7 |
| 6.1 to 8 | 57.5 ± 1.3 | 55.7 ± 1.4 | 3.4 ± 0.3 | −3.0 ± 1.5 | 61.6 ± 1.3 | 7.6 ± 2.0 | 6.4 ± 1.4 | 83.3 |
| 8.1 to 10 | 59.8 ± 2.1 | 53.5 ± 2.1 | 2.4 ± 0.6 | −10.4 ± 2.5b | 62.6 ± 2.2 | 4.8 ± 3.3 | 11.6 ± 2.4b | 80.0 |
| ≥10.1 | 63.8 ± 2.0c | 53.9 ± 2.1 | 3.2 ± 0.5 | −14.8 ± 2.4b | 65.6 ± 2.0 | 3.9 ± 3.1 | 12.5 ± 2.2b | 57.1 |
| Significancea | p = 0.0673 | p = 0.7781 | p = 0.4216 | p = 0.0014 | p = 0.3356 | p = 0.7453 | p = 0.0248 | p = 0.6279 |
Test of equality of HbA1c groups.
Combined HbA1c 8.1 to 10 and 10.1 significantly (p ≤ 0.05, Scheffé’s multiple-comparison procedure) different from combined ≤ 6.0 and 6.1 to 8.0.
The higher initial stability for the ≥10.1% group appears to be largely due to an elevated initial stability for 2 implants (Implant Stability Quotients of 71 and 78) in one person. Without this person, the mean initial stability was 60.5 (SE = 1.9).
The time required for stability to return to baseline level (time to healing) for the moderately and poorly controlled diabetic groups was approximately double that required for the non-diabetic and well-controlled diabetic groups (Table 2). In the poorly controlled group (HbA1c ≥ 10.1%), only 57.1% of the implants returned to or exceeded baseline stability levels after 16 wks, compared with 80% or more for each of the other HbA1c groups. Although there was not a significant difference between HbA1c groups in the changes in stability from baseline to 16 wks, the two HbA1c ≥ 8.1% groups tended to show less improvement in stability from baseline.
Regardless of HbA1c, a decrease in implant stability was evident at 2 wks, with progressively increasing stability beginning at 4 to 8 wks following implant placement. After adjustment for HbA1c level, there were no significant associations of implant stability with gender, age, bone type, or implant site (maxilla or mandible).
Primary stability—that is, baseline stability obtained at the time of implant placement—was greater, but not significantly greater, for the HbA1c 8.1-10.0% and HbA1c ≥ 10.1% groups compared with the non-diabetic (HbA1c ≤ 6.0%) and well-controlled (HbA1c 6.1-8.0%) diabetic groups (Table 2).
There were no significant differences among HbA1c groups in minimum stability levels measured between 2 and 6 wks following implant placement or in 16-week stability level (Table 2). The average time at which the minimum stability occurred in all Hb A1c groups ranged from 2.4 to 3.6 wks and did not differ significantly among HbA1c groups.
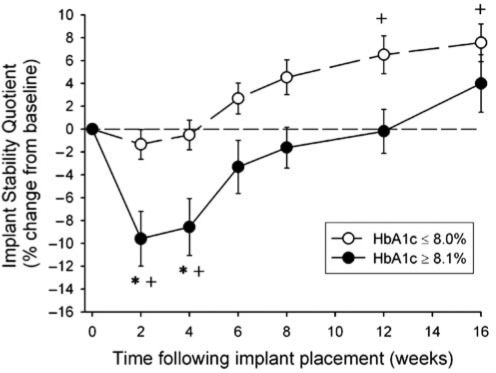
The results indicate similar responses for the two groups with HbA1c ≤ 8.0% (low group) and for the two groups with HbA1c ≥ 8.1% (high group), and show that the changes in implant stability for the high-HbA1c group were significantly different from those of the low-HbA1c group (Table 2). This dichotomous classification into low- and high-HbA1c groups (Fig. 2) shows the decreases in stability at wks 2 and 4 and longer time to healing for the high-HbA1c group (Table 2) relative to the low-HbA1c group. The differences in stability between low and high groups, apparent from wk 6 through wk 12, were not statistically significant. In the low-HbA1c group, there were no significant decreases in stability from baseline, and there were significant increases in stability from baseline after 12 and 16 wks following implant placement that were not evident for the high-HbA1c group.
Figure 2.
Changes in Implant Stability Quotient (%) from baseline by HbA1c level and time following implant placement. ◯ HbA1c ≤ 8.0% (n = 22 individuals), ● HbA1c ≥ 8.1% (n = 10 individuals). Error bars represent SE. ‘*’ indicates HbA1c ≥ 8.1% significantly (p ≤ 0.05) different from HbA1c ≤ 8.0% at same follow-up time. ‘+’ indicates significant (p ≤ 0.05) change from baseline for same HbA1c group.
Discussion
The importance of maintaining stringent glycemic control to minimize diabetic co-morbidities is becoming increasingly appreciated (UKPDS, 2000). However, a majority of persons with diabetes still struggle with an inability to maintain adequate glycemic control, with HbA1c levels for individuals frequently averaging between 8.5 and 9% (Kirk et al., 2005). Since diabetes mellitus remains a relative contraindication for dental implant therapy, depending upon the person’s level of glycemic control, many persons with diabetes may be denied the benefits of implant therapy.
The detrimental effects on implant integration with HbA1c levels ≥ 8.1% found in this study are consistent with the results of several diabetes co-morbidity studies which suggested that the risk of microvascular complications does not rise dramatically until HbA1c levels are greater than 8%, and that the most prominent threshold for glycemic damage leading to renal and retinal complications is between 8 and 8.5% (DCCT, 1993). Our findings show that persons with HbA1c levels ≥ 8.1% have compromises in implant stabilization that suggest alterations in the biologic integration of the implants in direct relation to glycemic control.
The findings of the current study are consistent with reports from previous studies that have demonstrated that hyperglycemic conditions lead to alterations in bone physiology (Funk et al., 2000; Amir et al., 2002; Lu et al., 2003). Impaired osseous healing in association with hyperglycemia has been demonstrated in several cross-sectional and retrospective studies (Loder, 1988; White et al., 2003). Animal and in vitro studies have extended these findings to include the effect of blood glucose control on fracture healing and bone turnover, with decreased consequences of the hyperglycemic state in animals receiving insulin treatment to reduce the hyperglycemia (Funk et al., 2000; Beam et al., 2002; Gebauer et al., 2002; Follak et al., 2004). Consistent with these findings, an investigation in a murine model reported that the reduced expression of 2 genetic markers of osteoblastic differentiation, Cbfa1 and Dlx5, found in response to hyperglycemia, was reversed with insulin treatment controlling the hyperglycemia (Lu et al., 2003).
The effects of a hyperglycemic state have been shown to include inhibition of osteoblastic cell proliferation and collagen production during the early stages of callus development, resulting in reduced bone formation, as well as diminished mechanical properties of the newly formed bone (Gooch et al., 2000; Amir et al., 2002; Beam et al., 2002; Gebauer et al., 2002; Lu et al., 2003). The diminished bone formation may be exacerbated further by increased apoptosis of bone-lining cells in a hyperglycemic state (He et al., 2004). More recently, several animal studies have demonstrated a more persistent inflammatory response that may also lead to increased osteoclastic activity in a hyperglycemic state (Liu et al., 2006; Kayal et al., 2007).
Thus, the potential for alterations in bone metabolism in association with hyperglycemia is consistent with the longitudinal assessments of implant stabilization found in this study. It is noteworthy that the differences in implant stability change relative to low and high HbA1c levels are consistent with clinically relevant differences in bone density found between type 1 and type 4 bone (Lekholm and Zarb, 1985; Barewal et al., 2003). The implications for clinical treatment based on these stability changes remain to be determined.
In conclusion, the results of the current study justify the continued investigation of the effects of diabetes and glycemic control on bone metabolism, as well as the longer-term effects of glycemic control on implant integration, success, and complications for persons with type 2 diabetes. Findings from this study and future studies must be considered in light of the potential increased risk for long-term complications, such as peri-implant inflammation and bone loss.
Acknowledgments
The authors also thank the Institut Straumann AG (Basel, Switzerland) for providing the implants used in this study.
Footnotes
This study was supported by NIH/NIDCR grant R01 DE017882 (T.W.O.), the ITI Foundation, and the San Antonio Area Foundation.
References
- Abdulwassie H, Dhanrajani PJ. (2002). Diabetes mellitus and dental implants: a clinical study. Implant Dent 11:83-86 [DOI] [PubMed] [Google Scholar]
- Amir G, Rosenmann E, Sherman Y, Greenfeld Z, Ne’eman Z, Cohen AM. (2002). Osteoporosis in the Cohen diabetic rat: correlation between histomorphometric changes in bone and microangiopathy. Lab Invest 82:1399-1405 [DOI] [PubMed] [Google Scholar]
- Barewal RM, Oates TW, Meredith N, Cochran DL. (2003). Resonance frequency measurement of implant stability in vivo on implants with a sandblasted and acid-etched surface. Int J Oral Maxillofac Implants 18:641-651 [PubMed] [Google Scholar]
- Beam HA, Parsons JR, Lin SS. (2002). The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res 20:1210-1216 [DOI] [PubMed] [Google Scholar]
- Beikler T, Flemmig TF. (2003). Implants in the medically compromised patient. Crit Rev Oral Biol Med 14:305-316 [DOI] [PubMed] [Google Scholar]
- Blanchaert RH. (1998). Implants in the medically challenged patient. Dent Clin North Am 42:35-45 [PubMed] [Google Scholar]
- Derr R, Garrett E, Stacy GA, Saudek CD. (2003). Is HbA(1c) affected by glycemic instability? Diabetes Care 26: 2728-2733 [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial (DCCT) Research Group (1993). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977-986 [DOI] [PubMed] [Google Scholar]
- Dowell S, Oates TW, Robinson M. (2007). A pilot study of implant success in individuals with type 2 diabetes mellitus with varying glycemic control. J Am Dent Assoc 138:355-361 [DOI] [PubMed] [Google Scholar]
- Fiorellini JP, Chen PK, Nevins M, Nevins ML. (2000). A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent 20:366-373 [PubMed] [Google Scholar]
- Follak N, Kloting L, Wolf E, Merk H. (2004). Delayed remodeling in the early period of fracture healing in spontaneously diabetic BB/OK rats depending on the diabetic metabolic state. Histol Histopathol 19:473-486 [DOI] [PubMed] [Google Scholar]
- Friberg B, Sennerby L, Meredith N, Lekholm U. (1999). A comparison between cutting torque and resonance frequency measurements of maxillary implants. Int J Oral Maxillofac Surg 28:297-303 [PubMed] [Google Scholar]
- Funk JR, Hale JE, Carmines D, Gooch HL, Hurwitz SR. (2000). Biomechanical evaluation of early fracture healing in normal and diabetic rats. J Orthop Res 18:126-132 [DOI] [PubMed] [Google Scholar]
- Garrett NR, Kapur KK, Hamada MO, Roumanas ED, Freymiller E, Han T, et al. (1998). A randomized clinical trial comparing the efficacy of mandibular implant-supported overdentures and conventional dentures in diabetic patients: Part II. Comparisons of masticatory performance. J Prosthet Dent 79:632-640 [DOI] [PubMed] [Google Scholar]
- Gebauer GP, Lin SS, Beam HA, Vieira P, Parsons JR. (2002). Low-intensity pulsed ultrasound increases the fracture callus strength in diabetic BB Wistar rats but does not affect cellular proliferation J Orthop Res 20:587-592 [DOI] [PubMed] [Google Scholar]
- Gerritsen M, Lutterman JA, Jansen JA. (2000). Wound healing around bone-anchored percutaneous devices in experimental diabetes mellitus. J Biomed Mater Res 53:702-709 [DOI] [PubMed] [Google Scholar]
- Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. (2000). Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connect Tissue Res 41:81-85 [DOI] [PubMed] [Google Scholar]
- He H, Liu R, Desta T, Leone C, Gerstenfeld LC, Graves DT. (2004). Diabetes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology 145:447-452 [DOI] [PubMed] [Google Scholar]
- Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, et al. (2007). Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res 22:560-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JK, Bell RA, Bertoni AG, Arcury TA, Quandt SA, Goff DC, Jr, et al. (2005). Ethnic disparities: control of glycemia, blood pressure, and LDL cholesterol among US adults with Type 2 diabetes. Ann Pharmacother 39:1489-1501 [DOI] [PubMed] [Google Scholar]
- Lekholm U, Zarb G. (1985). Patient selection and preparation. In: Tissue integrated prostheses: osseointegration in clinical dentistry. Brånemark PI, Albrektsson T, Zarb G, editors. Chicago, IL: Quintessence, pp. 199-210 [Google Scholar]
- Liang KY, Zeger SL. (1986). Longitudinal data analysis using generalized linear models. Biometrika 73:13-22 [Google Scholar]
- Liu R, Bal HS, Desta T, Behl Y, Graves DT. (2006). Tumor necrosis factor-alpha mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am J Pathol 168:757-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder RT. (1988). The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop Relat Res 232:210-216 [PubMed] [Google Scholar]
- Lu H, Kraut D, Gerstenfeld LC, Graves DT. (2003). Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 144:346-352 [DOI] [PubMed] [Google Scholar]
- McCracken M, Lemons JE, Rahemtulla F, Prince CW, Feldman D. (2000). Bone response to titanium alloy implants placed in diabetic rats. Int J Oral Maxillofac Implants 15:345-351 [PubMed] [Google Scholar]
- Meredith N, Alleyne D, Cawley P. (1996). Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin Oral Implants Res 7:261-267 [DOI] [PubMed] [Google Scholar]
- Meredith N, Book K, Friberg B, Jemt T, Sennerby L. (1997a). Resonance frequency measurements of implant stability in vivo. Clin Oral Implants Res 8:226-233 [DOI] [PubMed] [Google Scholar]
- Meredith N, Shagaldi F, Alleyne D, Sennerby L., Cawley P. (1997b). The application of resonance frequency measurements to study the stability of titanium implants during healing in the rabbit tibia. Clin Oral Implants Res 8:234-243 [DOI] [PubMed] [Google Scholar]
- Morris HF, Ochi S, Winkler S. (2000). Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol 5:157-165 [DOI] [PubMed] [Google Scholar]
- Moy P, Medina D, Shetty V, Aghaloo T. (2005). Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants 20:569-577 [PubMed] [Google Scholar]
- Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. (1998). Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants 13:620-629 [PubMed] [Google Scholar]
- Olson JW, Shernoff AF, Tarlow JL, Colwell JA, Scheetz JP, Bingham SF. (2000). Dental endosseous implant assessments in a type 2 diabetic population: a prospective study. Int J Oral Maxillofac Implants 15:811-818 [PubMed] [Google Scholar]
- Shernoff AF, Colwell JA, Bingham SF. (1994). Implants for type 2 diabetic patients: interim report. Implant Dent 3:183-189 [DOI] [PubMed] [Google Scholar]
- United Kingdom Prospective Diabetes Study (UKPDS) Group (2000). Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35). BMJ 321:405-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CB, Turner NS, Lee GC, Haidukewych GJ. (2003). Open ankle fractures in patients with diabetes mellitus. Clin Orthop Relat Res 414:37-44 [DOI] [PubMed] [Google Scholar]
- World Workshop in Periodontics (1996). Consensus report. Implant therapy II. Ann Periodontol 1:816-820 [DOI] [PubMed] [Google Scholar]