Abstract
Persistent postoperative pain is a very common phenomenon which severely affects the lives of patients who develop it following common surgical procedures. Opioid analgesics are of limited efficacy in the treatment of persistent pain states because of side effects including antinociceptive tolerance. We have previously shown that surgical incision injury and morphine tolerance share similar mechanisms, including a central nervous system (CNS) role of spinal cord glia. We therefore hypothesized that prior chronic morphine exposure would inhibit the resolution of postoperative allodynia through increased glial ionized calcium-binding adaptor protein 1 (Iba1) and glial fibrillary acidic protein (GFAP) protein expression and mitogen activated protein kinase (MAPK) activation. To test this hypothesis, rats were implanted with subcutaneous osmotic minipumps on day zero, releasing saline or morphine for seven days preceding or seven days preceding and following paw incision surgery, which was completed on day seven. Thermal hyperalgesia and mechanical allodynia were assessed postoperatively every three days. Chronic morphine attenuated the resolution of postoperative thermal hyperalgesia and mechanical allodynia through day twenty. However, no changes in Iba1 or GFAP expression were observed in the spinal cord dorsal horn between groups. Assessment of MAPK protein phosphorylation revealed that chronic morphine administration enhanced both p38 and extracellular receptor kinase (pERK) phosphorylation compared to saline on day twenty. p-p38 and pERK immunofluorescence were only observed to colocalize with a marker of microglial cells and not with markers of astrocytes or neurons. Together, these data demonstrate that chronic morphine administration attenuates the resolution of postoperative allodynia in association with microglial p38 and ERK phosphorylation, independent of changes in Iba1 and GFAP expression.
Keywords: Chronic pain, Microglial memory, MAPK, Opioids, Paw incision, Glia
Acute postoperative pain, caused by tissue and nerve damage, typically resolves following surgery. However this pain can become persistent in up to 50% of individuals depending upon the surgical procedure completed (Kehlet et al., 2006). Opioid analgesics, while effective in treating acute postoperative pain, have limited efficacy in treating persistent/ chronic pain due to side effects including antinociceptive tolerance. Opioid tolerance and chronic pain are recognized to share many of the same mechanisms (Mayer et al., 1999). However, only recently has the relationship between opioid tolerance and the transition from acute to chronic pain been studied (Joseph et al., 2010).
Previous studies regarding opioid priming effects on acute pain states or the transition from acute to persistent pain have been limited to the investigation of neuronal mechanisms (Joseph et al., 2010). The role of glial cells in opioid-induced priming and its effects on acute and persistent pain has yet to be investigated. Following insult to the CNS (injury or xenobiotic administration) spinal microglia undergo several alterations in functional state, which are characterized by morphological changes, increased expression of cytosolic and cell membrane proteins, proliferation and migration. We propose that, following insult, microglia become primed, i.e. they retain memory in the form of maintained functional changes, which alter responses to subsequent stimuli.
Central nervous system glial cells, specifically spinal microglia and astrocytes, have been shown to play a role in the development and maintenance of both morphine tolerance and postoperative allodynia. We have shown that chronic morphine administration enhances microglial CD11b and Iba1 expression and astrocytic GFAP expression both in vitro (Horvath and DeLeo, 2009) and in vivo (Raghavendra et al., 2002, Raghavendra et al., 2003, Raghavendra et al., 2004, Tawfik et al., 2005, Horvath et al., 2010). Treatment with the glial modulators propentofylline (Raghavendra et al., 2004) or minocycline (Cui et al., 2008), has been shown to reduce CD11b and Iba1 immunoreactivity and the development of morphine tolerance. Recently, we showed that inhibition of spinal microglial P2X4 receptor expression attenuated the development of morphine tolerance and inhibited morphine-induced increases in spinal Iba1 and GFAP expression (Horvath et al., 2010). We have also previously shown that paw incision surgery induced acute postoperative allodynia, which resolved over 7–9 days following injury (Romero-Sandoval et al., 2008). Microglial Iba1 and astrocytic GFAP expression were found to be increased during the period of allodynia and returned to baseline expression levels upon resolution of injury. Spinal cannabinoid receptor type 2 activation also reduced Iba1 and GFAP expression in association with reduced behavioral hypersensitivity following paw incision surgery (Romero-Sandoval and Eisenach, 2007).
MAPKs are a family of kinases regulating intracellular signal transduction, leading to the downstream expression of several proinflammatory and pronociceptive molecules including chemokines and cytokines (Ji et al., 2009). Recently, the roles of several MAPKs, including p38 and ERK, have been investigated in animal models of morphine tolerance and postoperative allodynia in isolation. It is unknown however, whether prior morphine-induced MAPK activation affects the resolution of postoperative allodynia.
The present study was designed to investigate the effect of morphine-induced antinociceptive tolerance on the resolution of postoperative allodynia. We hypothesized that prior chronic morphine administration would inhibit or delay the resolution of postoperative allodynia via enhanced spinal glial Iba1 and GFAP protein expression and MAPK signaling. To test this hypothesis, rats were implanted with subcutaneous osmotic mini-pumps releasing continuous morphine for seven days to induce antinociceptive tolerance. Rats then underwent paw incision surgery, with some groups receiving another seven days of morphine administration, and were tested for behavioral sensitivity. Herein, we show that chronic morphine treatment attenuated the resolution of postoperative allodynia and enhanced microglial p38 and ERK phosphorylation, independent of changes in Iba1 or GFAP expression.
Experimental Procedures
Animals
All procedures used in these studies were approved by the Dartmouth College Institutional Animal Care and Use Committee, adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (Zimmermann, 1983) and were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996. All efforts were made to minimize the number of animals used and their suffering. Male Sprague-Dawley rats weighing 175–200 g at the beginning of the study were housed in 12:12 hr light: dark cycle with free access to food and water. All behavioral experiments were performed in the morning between the hours of 8:00 and 11:00 am.
Osmotic Mini-Pump Implantation
Osmotic mini-pumps (ALZET Osmotic Pumps, Durect Corporation, Cupertino, CA), model 2ML1 (10 µl/hr, 2 ml capacity, 7 days of compound release after priming) were filled with saline or morphine sulfate (kind gift of the National Institute on Drug Abuse) and primed in 0.9% saline at 37°C for 16 hr prior to implantation. All morphine pumps delivered 0.833 mg/kg/hr morphine, the equivalent of twice daily 10 mg/kg injections over the course of 24 hr. For pump implantation, a 3 cm incision was made between the scapulae under isoflurane anesthesia and the skin was separated from the underlying fascia to create a subcutaneous pocket. Pumps were inserted with the flow regulators facing caudally and the incision was closed with surgical staples.
Surgeries
Paw incision and L5 nerve transection surgeries have been previously described (Romero-Sandoval et al., 2008). Briefly, for the paw incision surgery, rats were anesthetized with 4% isoflurane in oxygen and maintained at 2% throughout surgery. The plantar surface of the left hind paw was sterilized with a 10% povidone-iodine solution and a 1 cm incision was made through the skin. The flexor tendon was elevated with small forceps, stimulated and the incision closed with two inverted 5.0 mattress sutures. For the L5 nerve transection surgery a small incision overlaying L5-S1 was made and the paravertebral muscles retracted. The L6 transverse process was removed and the L5 spinal nerve was identified and transected. The wound was irrigated with saline and closed in two layers with 3.0 sutures and skin staples respectively.
Thermal and Mechanical Allodynia
The antinociceptive efficacy of morphine, the development of antinociceptive tolerance to morphine and thermal and mechanical hypersensitivity induced by paw incision and L5 nerve transection injury were measured on ipsilateral and contralateral hind paws via testing of thermal hyperalgesia and mechanical allodynia. To test for thermal hyperalgesia, paw withdrawal latencies (s) to thermal stimulation were assessed via a Paw Thermal Stimulator with a radiant heat source (8 V halogen lamp) focused on the plantar surface of a hind paw (Hargreaves et al., 1988). Paw withdrawal latencies for the hind paws ipsilateral and contralateral to injury were measured with a timer that was started at the beginning of heat exposure and tripped when the paw was removed from the heat. A temperature of 54°C was used for all experiments with a cutoff of 24 s to avoid tissue damage. During each testing session, 5 trials were completed and the mean of these trials was taken as the withdrawal latency for the session. Rats were acclimatized to the testing chambers for at least 30 min prior to each testing session and baseline testing was completed daily for 4 days prior to osmotic pump implantation.
To test for mechanical allodynia, 50% paw withdrawal thresholds (g) to mechanical pressure were assessed via von Frey filaments, using the up-down statistical methodology (Chaplan et al., 1994). The maximal cutoff for behavioral responses was set at 19.59 g. The 50% paw withdrawal threshold was registered for five trials for the hind paws ipsilateral and contralateral to injury and the mean of these trials was taken as the withdrawal threshold for the session. Rats were acclimatized to the testing chambers for at least 30 min prior to each testing session. Baseline testing was completed daily for 4 days prior to osmotic pump implantations.
Western Blot Analysis
Lumbar spinal cord (5 mm, L4-L6) tissue was harvested from 6 rats per group, placed in PBS with protease inhibitor (1:1000, Sigma, St. Louis, MO), then sonicated and centrifuged. The protein in the supernatant was then quantified using the Lowry method (DC Assay, Bio-Rad, Hercules, CA). Forty micrograms of protein and a standard marker were subjected to SDS-PAGE (10% gels, Bio-Rad), transferred to PVDF membranes (Bio-Rad) and were blocked with 5% milk in TBS-Tween 20 (0.05%, Sigma). The membranes were probed with primary antibodies against Iba1 (1:1000, Wako Chemicals, Richmond, VA), GFAP (1:5000, DAKO, Carpinteria, CA), phospho-p38 (1:500, Cell Signaling, Danvers, MA), p38 (1:500, Cell Signaling), phospho-ERK (1:500, Cell Signaling) and ERK (1:500, Cell Signaling) for 16 hr at 4°C. Membranes were then incubated with either goat anti-rabbit or goat anti-mouse HRP-conjugated secondary antibody for 1 hr at 22°C and visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL) for 5 min and imaged using the Syngene G-Box (Syngene, Frederick, MD). Membranes probed for phosphorylated proteins were stripped and reprobed for the appropriate unphosphorylated protein. All membranes were then stripped and reprobed with mouse monoclonal anti-β-actin primary antibody (1:1000, Abcam, Cambridge, MA). Band intensities were quantified using the analysis software provided with the Syngene G-Box, as relative intensity of band of interest divided by the intensity of the β-actin band. For phosphorylated proteins, band intensities were quantified as relative intensity of the phosphorylated band, divided by the unphosphorylated band and the β-actin band. Data are expressed as fold change in band intensity normalized to naïve control ± SEM.
Immunofluorescence
Lumbar spinal cord (5mm, L4-L5) tissue from the remaining rats in each group (n=4–6) was harvested for sectioning and immunofluorescence analysis. Rats were anesthetized with isoflurane and transcardially perfused with PBS followed by 4% formaldehyde in PBS. The lumbar spinal cord was removed and cryoprotected with 30% sucrose for 48 hr. Tissue was imbedded in Optimal Cutting Temperature Compound (Sakura, Torrance, CA), frozen at −80°C and cut into 20 µm sections. Primary antibodies against, Iba1 (rabbit anti-rat, 1:1000, Wako) and GFAP (rabbit anti-rat, 1:5000, Dako) were incubated with tissue sections in 1% normal goat serum and 0.01% Triton-X-100 (Sigma) for 16 hr at 4°C. The appropriate fluorescent secondary antibodies (goat anti-rabbit 1:250, Alexa Fluor 488 or 555, Invitrogen) were used for each primary antibody. Non-specific staining was assessed by omitting primary antibody from the procedure. Sections for single antibody immunofluorescence were imaged with a Q-fired cooled CCD camera attached to an Olympus microscope. For quantification of Iba1 and GFAP immunofluorescence, an area encompassing the whole, superficial and deep layers of dorsal horn of the spinal cord ipsilateral to paw incision surgery were outlined and immunofluorescence intensities above background were quantified using SigmaScan Pro imaging analysis software as previously described (Romero-Sandoval and Eisenach, 2007, Romero-Sandoval et al., 2008). The total number of pixels above a predefined threshold for 5 tissue sections per animal was averaged to provide a mean fluorescence pixel value for each rat. All data are expressed as mean (n=4–6 animals per group) ± SEM. For dual antibody immunofluorescence, primary antibodies against Iba1 (1:1000, goat anti-rat), GFAP (mouse anti-rat, 1:400, Sigma), NeuN (mouse anti-rat, 1:10,000, Chemicon, Billerica, MA), phospho-p38 (goat anti-rabbit, 1:100, Cell Signaling) and phospho-ERK (goat anti-rabbit, 1:100, Cell Signaling) were incubated with tissue sections in 1% normal goat or donkey serum and 0.01% Triton-X-100 (Sigma) for 16 hr at 4°C. The appropriate fluorescent secondary antibodies (1:250, Alexa Fluor 488 or 555, Invitrogen) were used for each primary antibody. Non-specific staining was assessed by omitting primary antibodies from the procedure. Confocal microscopy of dual antibody immunofluorescence in ipsilateral spinal cord dorsal horns was performed with a Zeis LSM 510 Meta confocal microscope (Englert Cell Analysis Laboratory of Dartmouth Medical School).
Study Design
For the paw incision injury study, animals were randomly divided into one of six treatment groups (n=10): naïve, non-injured morphine 7d , non-injured morphine 14d, saline with paw incision, morphine 7d with paw incision or morphine 14d with paw incision. Beginning on day zero, rats in the morphine 7d and morphine 14d treatment groups were implanted with primed subcutaneous osmotic minipumps releasing 0.833 mg/kg/hr morphine (2× 10 mg/kg injections per day equivalent) for seven days. On day seven, paw incision surgeries were completed and rats in the morphine 14d group were implanted with a fresh osmotic minipump. Rats were tested at least every three days (days −1 to 20) in a blinded manner for thermal and mechanical allodynia behavior and were euthanized on day 14 or 20 for western blot and immunofluorescence analysis. For the L5 nerve transection study, animals were randomly divided into one of four treatment groups (n=10): naïve, saline with nerve transection, morphine 7d with nerve transection and morphine 14d with nerve transection. The same study design as described for the paw incision injury study was followed with the exception that rats were euthanized on day 26.
Statistical Analysis
All values are expressed as mean ± SEM. Statistical analyses were performed with GraphPad Prism 4 (GraphPad Software Inc., La Jolla, CA) with the significance level set at p<0.05. Two-way ANOVA analyses were used for all behavioral experiments and one-way ANOVA analyses were used for all western blot and immunofluorescence experiments and group differences assessed by Bonferroni post-hoc analyses.
Results
Paw Incision Surgery Behavior
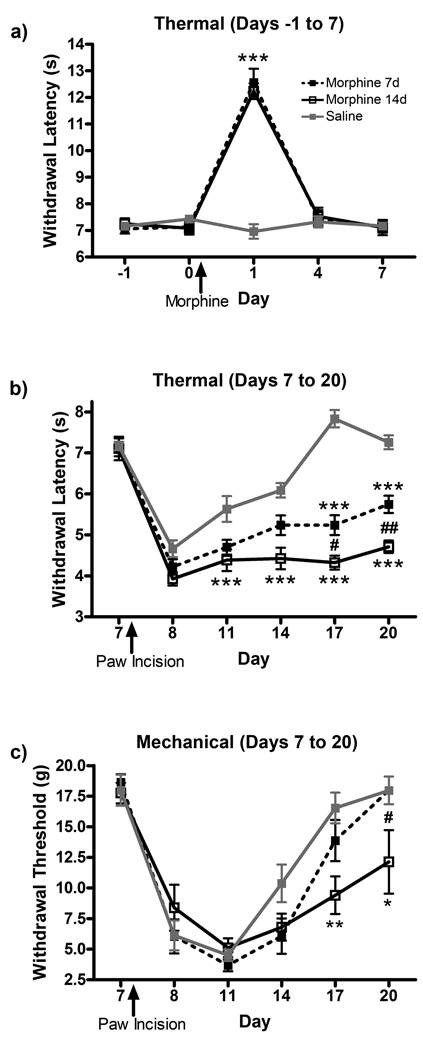
To assess the acute antinociceptive effect of continuous subcutaneous morphine administration, thermal paw withdrawal latencies were measured. Continuous morphine administration significantly increased paw withdrawal latencies to thermal stimulation one day after pump implantation, with loss of antinociceptive efficacy at day four (Fig 1a). Morphine efficacy peaked one day after pump implantation in the morphine 7d (12.6 ± 0.56 s, p<0.001) and morphine 14d (12.2 ± 0.32 s, p<0.001) groups compared to saline (7.0 ± 0.29 s). A reduction in morphine-induced antinociceptive efficacy to baseline levels was observed in both the morphine 7d (7.4 ± 0.33 s, p<0.001 compared to day 1, p>0.05 compared to day 0) and morphine 14d (7.5 ± 0.35 s, p<0.001 compared to day 1, p>0.05 compared to day 0) groups after four days of morphine administration.
Figure 1.
Chronic morphine administration attenuates the resolution of allodynia following paw incision surgery. (a) Thermal hyperalgesia measurement of acute analgesia and tolerance to continuous subcutaneous morphine administration. Thermal hyperalgesia (b) and mechanical allodynia (c) were measured in saline, morphine 7d and morphine 14d treatment groups following paw incision surgery on day 7. *p<0.05, **p<0.01, ***p<0.001 compared to saline. #p<0.05, ##p<0.01 compared to morphine 7d.
To assess the effect of chronic morphine on resolution of allodynia following paw incision surgery, both thermal withdrawal latencies and mechanical withdrawal thresholds were measured. In thermal testing, paw withdrawal latencies reached a minimum one day after injury in all treatment groups (Fig 1b). In the saline group, recovery from injury, as measured by decreased paw sensitivity and increased withdrawal latencies to baseline levels, was complete by day 17 of testing (7.8 ± 0.23 s compared to 7.3 ± 0.12 s on day 7, prior to injury). However, rats in the morphine 7d and morphine 14d treatment group remained hypersensitive through day 20 (5.7 ± 0.22 s, p<0.001 and 4.7 ± 0.16 s, p<0.001 respectively) compared to saline (7.3 ± 0.18 s). In mechanical testing, paw withdrawal thresholds reached a minimum four days after injury in all treatment groups (Fig 1c). In the saline and morphine 7d groups, recovery from injury, as measured by decreased paw sensitivity and increased withdrawal thresholds to baseline levels, was complete by day 20 of testing (18.0 ± 1.21 g and 18.0 ± 1.21 g respectively compared to 18.0 ± 1.32 g for the saline group on day 7, prior to injury). Rats in the morphine 14d treatment group remained hypersensitive through day 20 (12.1 ± 2.78 g, p<0.05 compared to saline and morphine 7d groups).
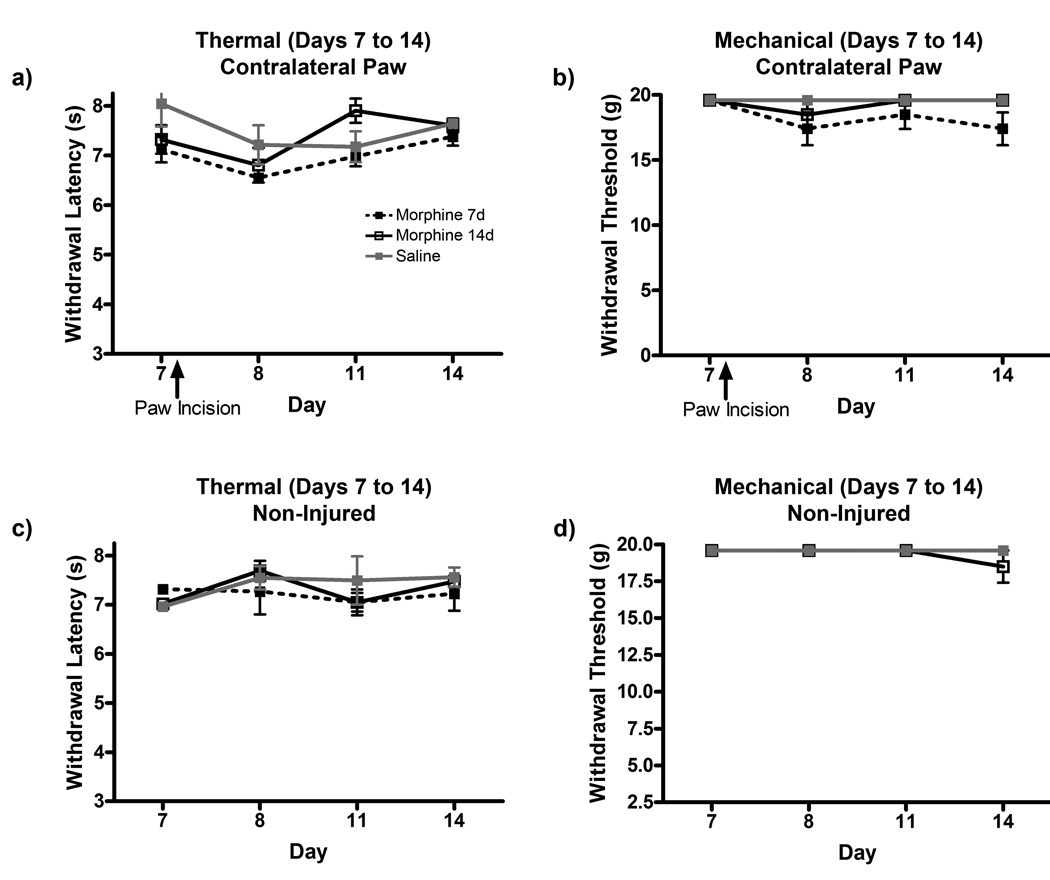
The contralateral paw withdrawal latencies and thresholds were also measured to assess the possible central sensitizing effects of chronic morphine treatment following paw incision surgery (Fig 2a, b). Rats in the saline group did not show any contralateral sensitivity after surgery. Morphine treatment for 7 or 14 days did not enhance contralateral paw sensitivity in either testing paradigm. Two additional groups of animals receiving morphine for 7 or 14 days without any injury were also tested to determine if morphine withdrawal following pump exhaustion had sensitizing effects on behavioral hypersensitivities (Fig 2c, d). No changes in withdrawal latencies or thresholds were observed in the non-injured morphine 7 and 14d groups through 14 days of testing.
Figure 2.
Chronic morphine administration does not affect contralateral hind paw sensitivity following paw incision surgery or upon withdrawal of morphine treatment. Thermal hyperalgesia (a) and mechanical allodynia (b) were measured in the contralateral hind paw of rats in saline, morphine 7d and morphine 14d treatment groups following paw incision surgery on day 7. Thermal (c) and mechanical (d) allodynia was also measured in the hindpaw of non-injured rats from day 7 (last day of morphine 7d pump output) to day 14 (last day of morphine 14d pump output).
L5 Nerve Transection Behavior
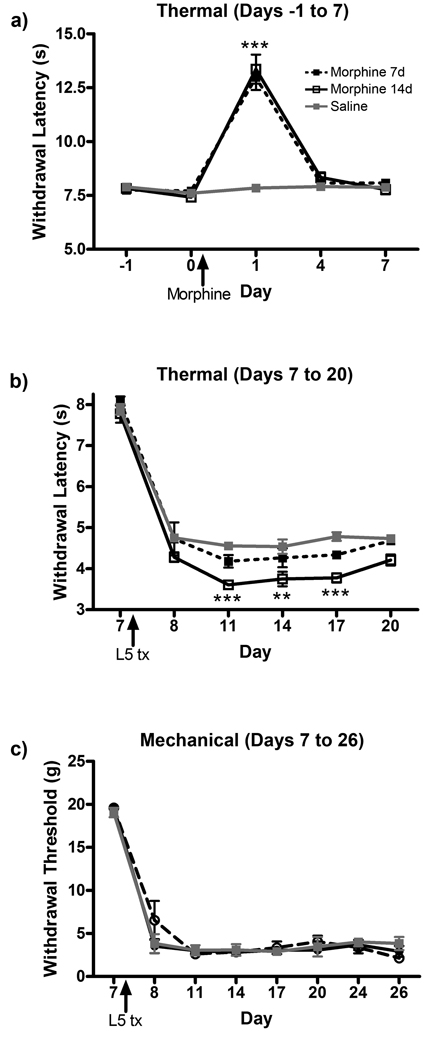
Chronic morphine treatment impaired the resolution of allodynia and recovery from paw incision surgery in an acute pain model. To determine if morphine treatment affects allodynia after injury in a model of chronic pain, L5 nerve transection surgeries were performed on animals receiving saline or morphine for 7 or 14 days. Morphine provided acute analgesia on day 1 after pump implantation in the morphine 7 and 14d groups (Fig 3a). Tolerance to the antinociceptive effect was observed by day 4. Subsequent L5 nerve transection surgery produced robust allodynia one day after surgery, with no later recovery observed (Fig 3 b, c). Although statistical significance was achieved between treatment groups because of the consistency of behavioral data, only very small differences in postoperative hypersensitivity following L5 nerve transection surgery were observed between the saline, morphine 7d and morphine 14d groups in measurements of thermal hyperalgesia. No differences in the treatment groups were observed in mechanical allodynia testing.
Figure 3.
Chronic morphine administration does not increase behavioral hypersensitivity following L5 nerve transection surgery. (a) Thermal hyperalgesia measurement of acute analgesia and tolerance to continuous subcutaneous morphine administration. Thermal hyperalgesia (b) and mechanical allodynia (c) were measured in saline, morphine 7d and morphine 14d treatment groups following L5 nerve transection surgery on day 7. **p<0.01, ***p<0.001 compared to saline.
Iba1 and GFAP Expression
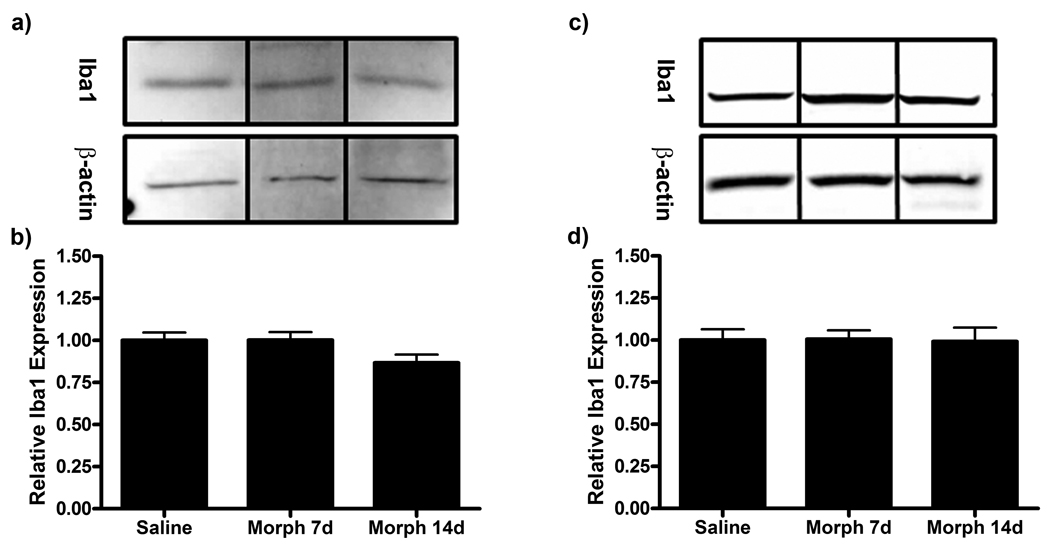
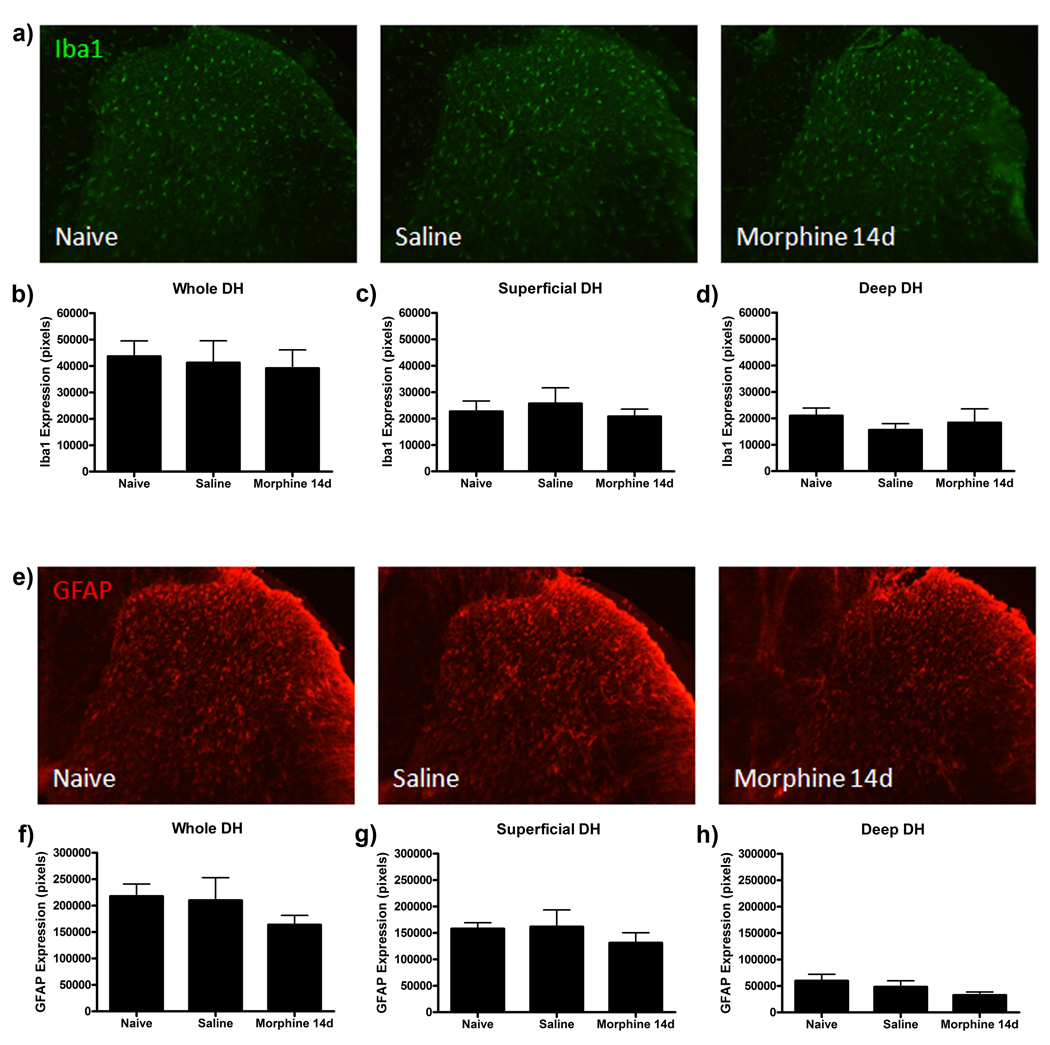
Our laboratory has previously shown that enhanced expression of the microglial marker Iba1 and the astrocytic marker GFAP in the dorsal horn of the lumbar spinal cord is associated with behavioral allodynia following paw incision surgery (Romero-Sandoval et al., 2008). Following resolution of behavioral hypersensitivity, Iba1 and GFAP expression returned to baseline expression levels. Therefore, in this study, protein for western blot analysis was harvested at day 20 from saline, morphine 7d and morphine 14d treatment groups to assess changes in Iba1 and GFAP expression. No changes in Iba1 and GFAP expression were observed in lumbar spinal cord protein from saline and morphine treated animals (Fig 4).
Figure 4.
Iba1 and GFAP expression is unaltered in morphine treated groups following paw incision injury. Representative western blot membranes of day 20 lumbar spinal cord protein from rats receiving saline or chronic subcutaneous morphine for 7 or 14 days probed for Iba1 (a) or GFAP (c) and β-actin protein expression. Quantification of fold changes of Iba1 (b) or GFAP (d) expression normalized to β-actin loading control ± SEM (n=6).
Our previous study showed that significant increases in Iba1 and GFAP expression were evident in the whole dorsal horn, or solely superficial or deep laminae depending upon the time after paw incision surgery (Romero-Sandoval et al., 2008). To assess the possible differences in anatomical expression of these markers, a series of immunofluorescence studies were completed to assess Iba1 and GFAP expression in the whole, superficial and deep laminae of the dorsal horn. On day 20, when differences between morphine treated and saline groups were observed in behavioral testing, no differences in Iba1 or GFAP immunofluorescence were observed in the whole, superficial or deep laminae of the spinal cord dorsal horn between saline and morphine 14d groups (Fig 5). To determine if the amount of Iba1 and GFAP expression in either group remained elevated following paw incision surgery, tissue from the naïve group was also analyzed. No differences in Iba1 or GFAP expression in dorsal horn regions were found between naïve, saline and morphine 14d groups, indicating that Iba1 and GFAP expression returned to baseline expression by day 20 in the injured groups.
Figure 5.
Chronic morphine treatment does not alter Iba1 and GFAP immunofluorescence in ipsilateral spinal cord dorsal horn regions following paw incision surgery on day 20. (a) Representative Iba1 immunofluorescence images of ipsilateral spinal cord dorsal horn sections from naïve, saline and morphine 14d treated animals on day 20. Quantification of Iba1 immunofluorescence in the whole dorsal horn (b), superficial dorsal horn (c) and deep dorsal horn (d) represented as number of pixels (n=6, 4 images per rat). (e) Representative GFAP immunofluorescence images of ipsilateral spinal cord dorsal horn sections from naïve, saline and morphine 14d treated animals on day 20. Quantification of GFAP immunofluorescence in the whole dorsal horn (b), superficial dorsal horn (c) and deep dorsal horn (d) represented as number of pixels (n=6, 4 images per rat).
p38 and ERK Phosphorylation
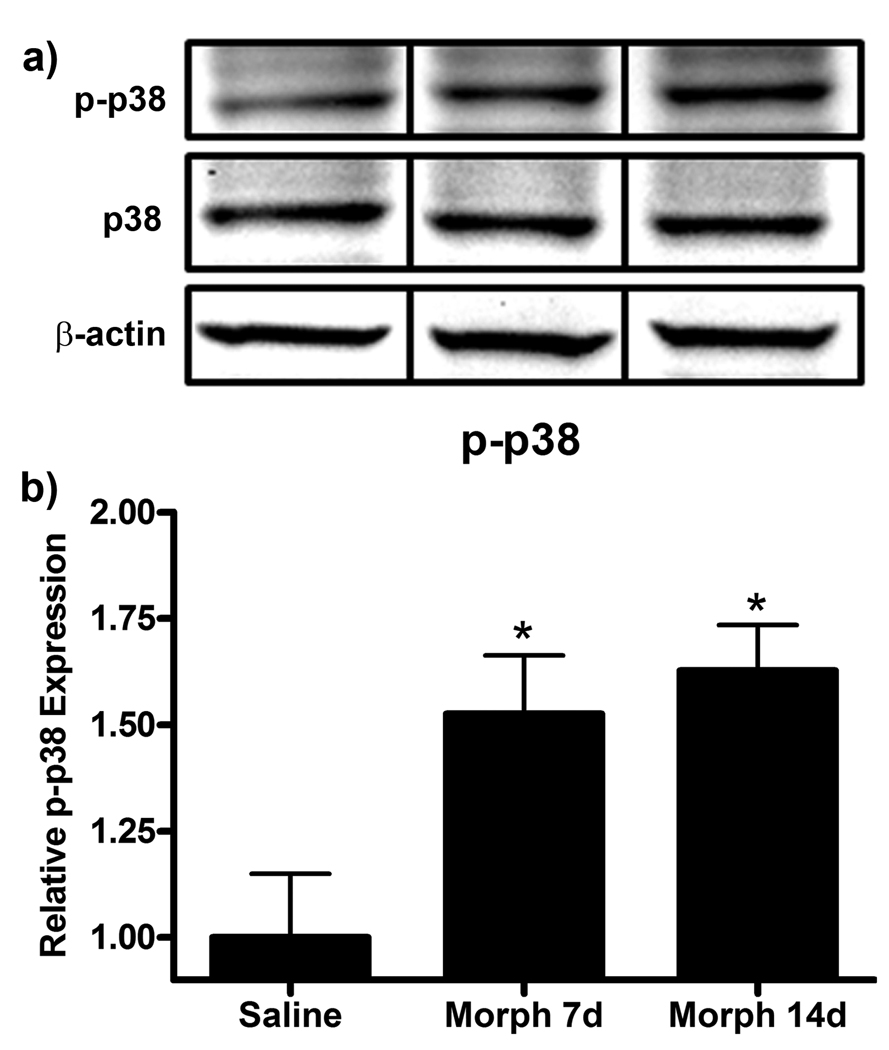
With no observable differences in Iba1 or GFAP expression to account for the behavioral effects observed between morphine and saline treated groups, we next assessed the phosphorylation state of several MAPKs via western blot on day 20. Phospho-p38 (p-p38) has been previously shown to be increased following paw incision injury in the ipsilateral dorsal horn of the lumbar spinal cord in association with increased behavioral hypersensitivity (Wen et al., 2009). Analysis of day 20 lumbar spinal cord protein showed that chronic morphine administration for 7 or 14 days increased p-p38 expression (Fig 6). p-p38 expression was increased from 1.00 ± 0.15 in saline to 1.53 ± 0.14 (p<0.05) in the morphine 7d group and 1.63 ± 0.11 (p<0.05).
Figure 6.
p-p38 expression is enhanced in the morphine 7d and 14d treatment groups following paw incision injury. (a) Representative western blot membranes of day 20 lumbar spinal cord protein from rats treated with saline or chronic subcutaneous morphine for 7 or 14 days probed for p-p38, p38 and β-actin protein expression. (b) Quantification of fold changes of p-p38 expression normalized to p38 expression and β-actin loading control ± SEM (n=6). *p<0.05 compared to saline.
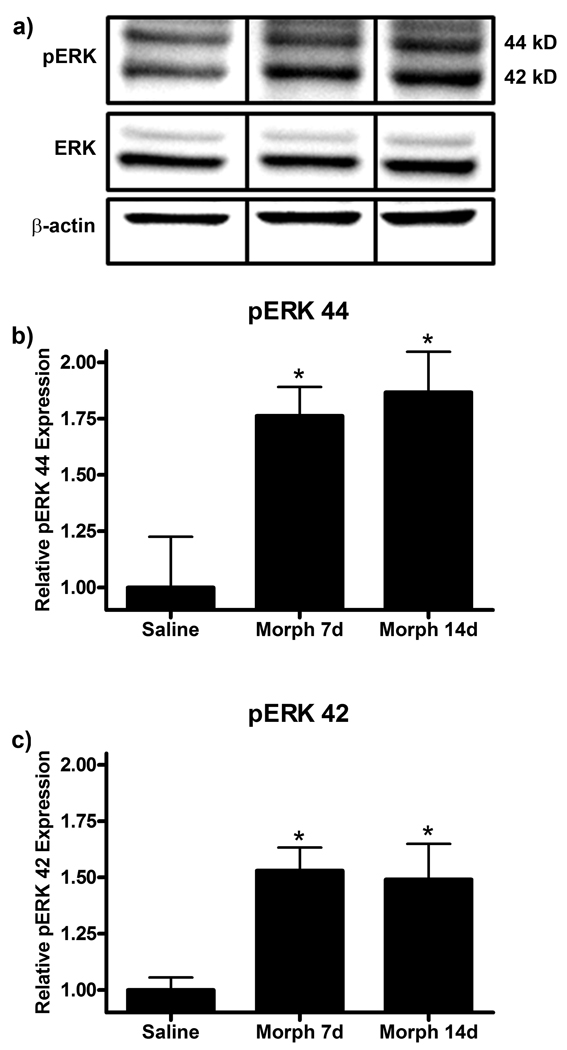
Phospho-ERK (pERK) has previously been shown to be increased in the lumbar spinal cord following spinal nerve injury (Zhuang et al., 2005). Western blot analysis of day 20 lumbar spinal cord protein revealed that chronic subcutaneous morphine administration enhanced both pERK 44 and 42 expression following paw incision surgery (Fig 7). pERK 44 expression was increased from 1.00 ± 0.25 in saline to 1.76 ± 0.14 (p<0.05) in the morphine 7d group and 1.87 ± 0.20 (p<0.05) in the morphine 14d group. pERK 42 expression was similarly increased from 1.00 ± 0.06 in saline to 1.53 ± 0.11 (p<0.05) in the morphine 7d group and 1.49 ± 0.17 (p<0.05) in the morphine 14d group.
Figure 7.
pERK expression is enhanced in the morphine 7d and 14d treatment group following paw incision injury. (a) Representative western blot membranes of day 20 lumbar spinal cord protein from rats treated with saline or chronic subcutaneous morphine for 7 or 14 days probed for pERK, ERK and β-actin protein expression. Quantification of fold changes of pERK 44 (b) and 42 (c) expression normalized to ERK expression and β-actin loading control ± SEM (n=6). *p<0.05 compared to saline.
c-Jun N-terminal kinase (JNK), the third major MAPK pathway, has also been shown to have a role in pain processing (Ji et al., 2009) and morphine tolerance (Chen and Sommer, 2009). Previously, we have shown that morphine treatment enhances the PI3K/ Akt intracellular signaling pathway in primary microglial cells (Horvath and DeLeo, 2009). No differences in JNK (54 or 46 kD isoforms) or Akt phosphorylation on day 20 were observed between the saline, morphine 7d and morphine 14d treatment groups following paw incision surgery via western blot analysis (Data not shown).
p-p38 and pERK colocalization
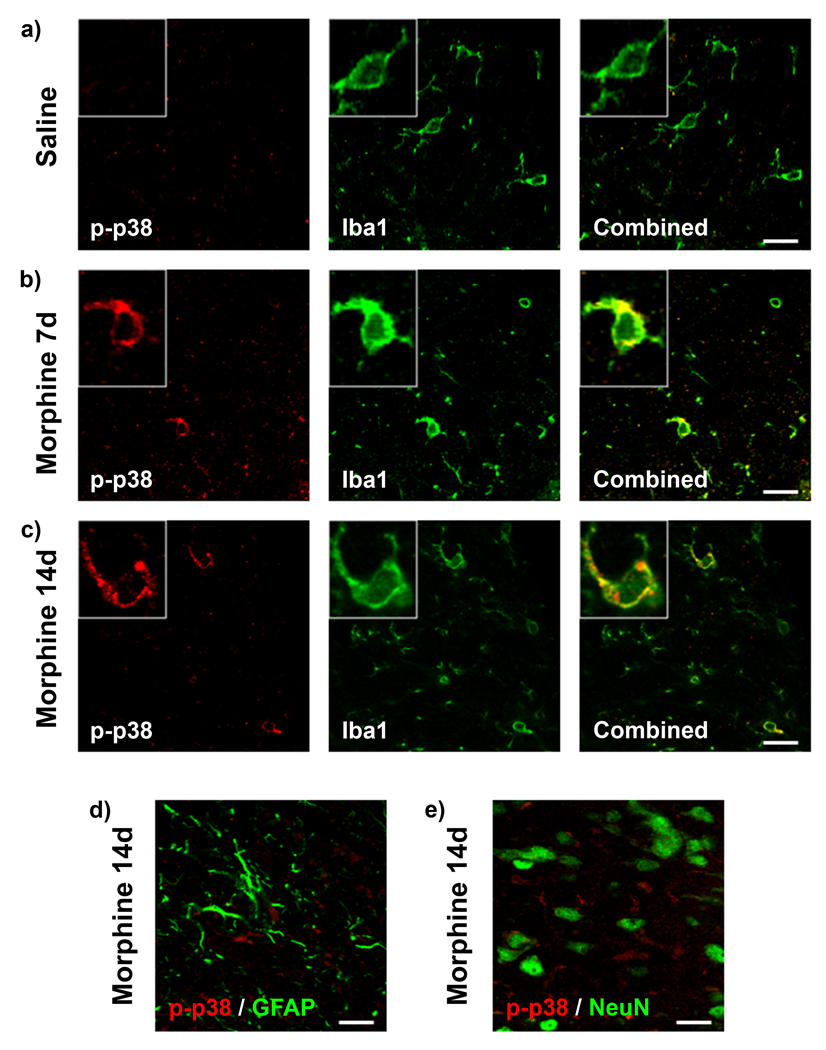
MAPKs are known to have roles in both pain processing and morphine tolerance (Chen and Sommer, 2009, Ji et al., 2009). Following nerve injury, paw incision surgery or the induction of morphine tolerance, p38 and ERK phosphorylation can be observed to be differentially expressed in microglia, astrocytes and neurons. Therefore, to assess which cell types in the dorsal horn of the spinal cord express p-p38 and pERK on day 20, a series of colocalization studies were completed.
p-p38 immunofluorescence was assessed for colocalization with the microglial marker Iba1, the astrocytic marker GFAP and the neuronal marker NeuN (Fig 8). In accordance with the western blot results, there was significantly higher immunofluorescence for p-p38 in the morphine 7d and 14d treatment groups compared to saline (Fig 8 a–c). p-p38 immunofluorescence was only observed to colocalize with the microglial marker Iba1 and was never found to colocalize with GFAP or NeuN (Fig 8 d, e).
Figure 8.
Microglial p-p38 expression is enhanced in the morphine 7d and 14d treatment groups. Confocal images of p-p38 (red) and Iba1 (green) immunofluorescence colocalization in saline (a), morphine 7d (b) and morphine 14d (c) treatment groups on day 20 in the superficial dorsal horn of the spinal cord ipsilateral to paw incision injury. Higher magnification images of single cells are found in boxes. (d) Confocal images of p-p38 (red) and GFAP (green) immunofluorescence showing no colocalization in the morphine 14d treatment group on day 20. (e) Confocal images of p-p38 (red) and NeuN (green) immunofluorescence showing no colocalization in the morphine 14d treatment group on day 20. Scale bar equals 20 µm, magnified box width equals 20 µm.
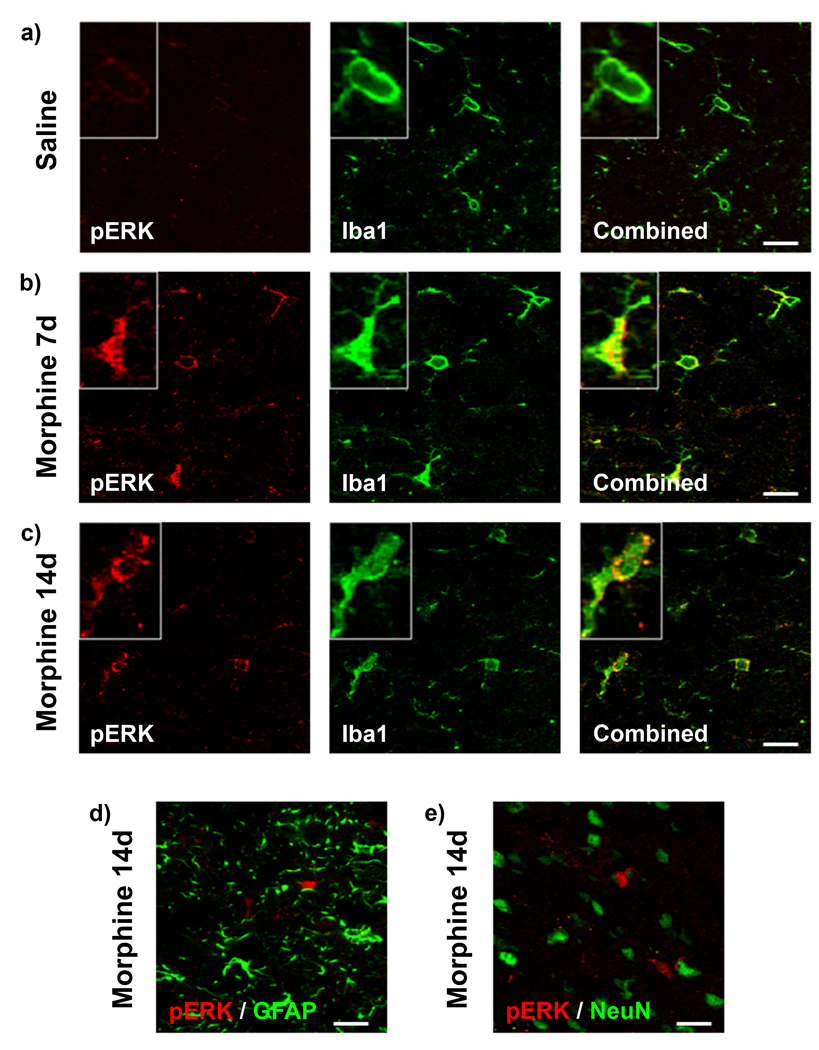
Immunofluorescence for pERK was similarly assessed for colocalization with Iba1, GFAP and NeuN expression (Fig 9). pERK immunofluorescence was observed to be much greater in the morphine 7d and 14d groups compared to saline treatment (Fig 9 a–c). pERK immunofluorescence colocalization was only observed with microglial Iba1 and never with GFAP or NeuN in all of the treatment groups (Fig 9 d, e).
Figure 9.
Microglial pERK expression is enhanced in the morphine 7d and 14d treatment groups. Confocal images of pERK (red) and Iba1 (green) immunofluorescence colocalization in saline (a), morphine 7d (b) and morphine 14d (c) treatment groups on day 20 in the superficial dorsal horn of the spinal cord ipsilateral to paw incision injury. Higher magnification images of single cells are found in boxes. (d) Confocal images of pERK (red) and GFAP (green) immunofluorescence showing no colocalization in the morphine 14d treatment group on day 20. (e) Confocal images of pERK (red) and NeuN (green) immunofluorescence showing no colocalization in the morphine 14d treatment group on day 20. Scale bar equals 20 µm, magnified box width equals 20 µm.
Discussion
In this study, we demonstrate that: 1) Prior morphine tolerance attenuates the resolution of postoperative allodynia; 2) Prior morphine tolerance enhances spinal microglial p38 and pERK phosphorylation concomitant with behavioral hypersensitivity; 3) MAPK activation is independent of changes in microglial Iba1 or astrocytic GFAP expression. These results suggest that morphine tolerance primes microglia, enhancing their subsequent cellular responses to CNS insults, possibly mediated by MAPKs. MAPK activation in primed microglia could maintain behavioral hypersensitivity and thus delay the resolution of postoperative allodynia.
We report that prior exposure to morphine has a detrimental effect on the resolution of behavioral hypersensitivity following paw incision surgery. Post-surgical pain is a well-described phenomenon and opioids, including morphine, have been shown to reduce both mechanical hyperalgesia and allodynia when administered postoperatively in animal models (Whiteside et al., 2004). Investigation into the effects of pre-operative morphine administration on postoperative allodynia is in its infancy. It has been shown that generation of opioid-induced hyperalgesia prior to skin incision enhances nociceptive sensitivity (Li et al., 2001, Liang et al., 2008). In these studies, rats were administered increasing doses of morphine until opioid-induced hyperalgesia was achieved, which was characterized by lowered nociceptive thresholds. Following surgery, the rats were found to have exaggerated behavioral hypersensitivity. It is not surprising that the rats experienced enhanced allodynia after surgery, given their baseline sensitivities were decreased. In our experiments, the chronic subcutaneous morphine dosing paradigm we employed did not alter baseline nociception once antinociceptive tolerance was established by day 4. Thus, it is even more intriguing that such a marked effect of prior morphine exposure following paw incision surgery was observed.
Of particular importance is that the behavioral hypersensitivity elicited by chronic morphine administration prior to and concomitant with paw incision surgery lasted days and weeks after the cessation of morphine release from the implanted osmotic minipumps. Rats in the morphine 7d group received morphine for seven days preceding paw incision surgery. However, increased behavioral hypersensitivity was observed for up to thirteen days after cessation of morphine in thermal testing. Rats in the morphine 14d group received morphine for seven days preceding and seven days following paw incision surgery. Behavioral hypersensitivity was observed six days after cessation of morphine in both thermal and mechanical testing. In both behavioral paradigms, rats in the morphine 14d group failed to resolve their hypersensitivity and return to baseline levels of nociception by the last day of testing. This suggests that the morphine primed- and paw incision-induced molecular changes in microglia are long lived, as evidenced by the enhanced phosphorylation of p38 and ERK at day 20 in both the morphine 7d and 14 d groups. This attenuation of resolution of postoperative allodynia might also mark a shift from acute to persistent postoperative pain. No effect of prior morphine exposure was observed following L5 nerve transection surgery. This could be due to the robust nature of the injury and subsequent persistent allodynia or a result of the behavioral tests completed that have limited resolution of very low thermal withdrawal latencies and mechanical withdrawal thresholds.
Microglial CD11b/ Iba1 and astrocytic GFAP protein expression are commonly used as protein markers of glial cells, however, their use as markers of cellular activation has been increasingly questioned. We have recently reported that Iba1 and GFAP protein expression is enhanced through 7 days of continuous morphine treatment, which paralleled antinociceptive tolerance behaviors (Horvath et al., 2010). Here we show that days after cessation of morphine treatment, Iba1 and GFAP protein expression is not different from controls, while behavioral hypersensitivity persists. Evidence is building that there is a disconnect between CD11b/ Iba1 and GFAP expression, the function of glial cells and overall nociceptive behavior. Our laboratory has previously shown that local anesthetic administration prior to spinal nerve cryolysis reduced CD11b expression and microglial hypertrophy without affecting allodynia (Colburn et al., 1997). Similarly, it has been shown that minocycline reduced CD11b expression in both acute and chronic pain models, but only reduced allodynia in the chronic pain model (Ito et al., 2009). A recent study also showed that the amount of GFAP expressed in cortical and hippocampal astrocytes was not correlated with the membrane potential and function of these cells (Mishima and Hirase, 2010). Finally, minocycline has been shown to reduce tactile allodynia following formalin injection, without reducing CD11b in microglia (Li et al., 2010). Li et al. did, however, find that minocycline reduced microglial p38 phosphorylation in association with reduced tactile allodynia. These studies suggest that CD11b/ Iba1 and GFAP, while morphological markers of glial cells, are not adequate markers of changes in functional states.
The MAPK family of kinases is comprised of p38, ERK and JNK. These intracellular signaling pathways respond to an array of stimuli including nociceptive activity, nerve injury, cytokines, chemokines and growth factors. Their downstream effectors include the transcription factors CREB, ATF-2 and c-Jun, which mediate the transcription of many proinflammatory and pronociceptive factors (ex. IL-1β, IL-6, TNFα, COX, iNOS) and the expression of membrane receptors (ex. CD11b, TLR-4, P2X4, CB2) (Ji et al., 2009). The role of MAPKs in the integration of external stimuli and cellular responses suggest that they may be better indicators of glial function.
Recently, the roles of MAPKs, specifically p38 and ERK, have been studied in models of morphine tolerance and surgical incision injury. Chronic morphine administration has been shown to enhance microglial p38 (Cui et al., 2006) and astrocytic ERK (Wang et al., 2009) activation in association with the development of antinociceptive tolerance. Inhibition of microglial p38 activation, through the use of the glial modulator minocycline, has also been shown to attenuate morphine tolerance (Cui et al., 2008). Similarly, p38 activation in spinal microglia has been shown following paw incision injury (Wen et al., 2009). Administration of a p38 inhibitor reduced p38 activation, however, there have been conflicting reports of the effects of p38 inhibition on postoperative allodynia (Ito et al., 2009, Wen et al., 2009). Taken together, these results show that the activation of glial MAPKs are critical to the development of both morphine tolerance and postoperative allodynia. Herein, we show that microglial p38 and ERK phosphorylation is enhanced postoperatively up to two weeks after cessation of chronic morphine administration. This implicates MAPKs as possible key molecular changes that occur in primed microglia. Future studies using selective p38 and ERK inhibitors must be completed to determine the mechanism of this activity and provide a timeline of MAPK activation before and after morphine and paw incision stimuli.
It is also important to note that p38 and ERK expression and phosphorylation are both temporally regulated and cell type specific depending upon the stimuli. It has been shown that within minutes of an insult to a peripheral nerve, ERK phosphorylation is increased in dorsal spinal cord neurons (Ji et al., 1999). Peripheral nerve injury has also been shown to increase ERK phosphorylation in microglia in the acute phase and astrocytes in the chronic phase following nerve injury (Ji et al., 2009). Peripheral nerve ligation and transection and paw incision injury have been shown to enhance p38 and ERK phosphorylation in microglia (Jin et al., 2003, Tsuda et al., 2004, Wen et al., 2009). Recently, our laboratory has also shown that antagonism of cannabinoid type 1 and type 2 receptor signaling enhanced p38 phosphorylation in astrocytes and perivascular microglia following paw incision surgery (manuscript in preparation).The effect of morphine on p-p38 and pERK expression is similarly cell type specific depending upon the route and duration of administration. Daily intrathecal injection of morphine, which has been associated with the mini-withdrawal phenomenon, has been shown to enhance p38 phosphorylation in microglia and ERK phosphorylation in astrocytes (Cui et al., 2006, Wang et al., 2009). Morphine administration from implanted subcutaneous pellets, which can lead to variable drug release, has been show to decrease pERK expression and have no effect on p-p38 expression in the presence of peripheral inflammation (Almela et al., 2009).
For our studies we employed a well-defined paw incision surgery model of acute postoperative pain (Brennan et al., 1996, Romero-Sandoval et al., 2008) and subcutaneous minipump morphine administration model to induce antinociceptive tolerance (Horvath et al., 2010). While both paw incision-induced allodynia and subcutaneous morphine-induced tolerance are peripheral insults, we have previously shown that they are mediated by central nervous system effects in the spinal cord (Romero-Sandoval and Eisenach, 2007, Romero-Sandoval et al., 2008, Horvath et al., 2010). The combination of morphine priming and paw incision surgery produced marked p38 and ERK phosphorylation in microglia two weeks after surgery and the cessation of morphine administration in association with impaired resolution of postoperative allodynia. This does not mean, however, that p38 and ERK phosphorylation is necessarily restricted to microglia at time points not assessed in this study. It is possible that following surgery or morphine treatment, pERK can be expressed in neurons and astrocytes at other time points not tested in the current study.
The cellular and molecular mechanisms driving morphine tolerance, glial priming and the transition from acute to persistent pain are areas of intense interest. Herein, we show that morphine antinociceptive tolerance prior to paw incision surgery attenuated the resolution of postoperative allodynia in association with p38 and ERK phosphorylation in spinal microglial cells. Chronic morphine administration is known to enhance microglial MAPK signaling, which can lead to the enhanced expression of membrane surface receptors for ATP (ex. P2X4) and cytokines (ex. CX3CR1) and production and release of proinflammatory and pronociceptive factors (Ji et al., 2009). The summation of these events could cause neuronal sensitization and antinociceptive tolerance. Prior chronic morphine exposure could prime microglia, causing exacerbated MAPK signaling pathway activation following subsequent paw incision injury. This would cause more robust microglial responses in rats with a history of morphine tolerance versus naïve states, which is manifest by further neuronal sensitization, behavioral hypersensitivity and inhibition of the resolution of postoperative allodynia. Our results indicate that microglial MAPKs play an integral role in the mechanisms by which morphine attenuates the resolution of postoperative pain and suggest that patients abusing opioids or on chronic opioid therapy may be more susceptible to develop chronic pain syndromes following acute injury.
Acknowledgements
The authors would like to thank the National Institute on Drug Abuse F30DA024933 (RJH) and the National Institute on Drug Abuse DA11276 and DA025987 (JAD).
Abbreviations
- CNS
central nervous system
- ERK
extracellular receptor kinase
- GFAP
glial fibrillary acidic protein
- Iba1
ionized calcium adaptor-binding protein 1
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen activated protein kinase
- NeuN
neuronal nuclei
- PBS
phosphate buffered saline
- PI3K
phosphoinositide 3-kinase
- TBS
tris buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest for the studies presented herein.
References
- Almela P, Garcia-Nogales P, Romero A, Milanes MV, Laorden ML, Puig MM. Effects of chronic inflammation and morphine tolerance on the expression of phospho-ERK ½ and phospho-P38 in the injured tissue. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:315–323. doi: 10.1007/s00210-008-0356-x. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol. 2009;40:101–107. doi: 10.1007/s12035-009-8074-z. [DOI] [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–123. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29:998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath RJ, Romero-Sandoval EA, DeLeo JA. Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and μ opioid receptor protein expression while enhancing perivascular microglial ED2. Pain. 2010 doi: 10.1016/j.pain.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Obata H, Saito S. Spinal microglial expression and mechanical hypersensitivity in a postoperative pain model: comparison with a neuropathic pain model. Anesthesiology. 2009;111:640–648. doi: 10.1097/ALN.0b013e3181b05f42. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Reichling DB, Levine JD. Shared mechanisms for opioid tolerance and a transition to chronic pain. J Neurosci. 2010;30:4660–4666. doi: 10.1523/JNEUROSCI.5530-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- Li K, Fu KY, Light AR, Mao J. Systemic minocycline differentially influences changes in spinal microglial markers following formalin-induced nociception. J Neuroimmunol. 2010;221:25–31. doi: 10.1016/j.jneuroim.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Angst MS, Clark JD. Opioid-induced hyperalgesia and incisional pain. Anesth Analg. 2001;93:204–209. doi: 10.1097/00000539-200107000-00040. [DOI] [PubMed] [Google Scholar]
- Liang D, Shi X, Qiao Y, Angst MS, Yeomans DC, Clark JD. Chronic morphine administration enhances nociceptive sensitivity and local cytokine production after incision. Mol Pain. 2008;4:7. doi: 10.1186/1744-8069-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima T, Hirase H. In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous. J Neurosci. 2010;30:3093–3100. doi: 10.1523/JNEUROSCI.5065-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104:655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29:327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106:787–794. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, LaCroix-Fralish ML, Nutile-McMenemy N, DeLeo JA. Transcriptional and translational regulation of glial activation by morphine in a rodent model of neuropathic pain. J Pharmacol Exp Ther. 2005;313:1239–1247. doi: 10.1124/jpet.104.082420. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23:2576–2586. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ, Wang CC. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009;110:155–165. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Harrison J, Boulet J, Mark L, Pearson M, Gottshall S, Walker K. Pharmacological characterisation of a rat model of incisional pain. Br J Pharmacol. 2004;141:85–91. doi: 10.1038/sj.bjp.0705568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]