Abstract
Ascorbic acid improves endothelial barrier function by decreasing the permeability of endothelial cells cultured on semi-porous membrane filters. This decrease was not due to enhanced collagen synthesis and was mimicked by the collagen synthesis inhibitor ethyl-3, 4-dihydroxybenzoic acid (EDHB). Since EDHB is known to chelate intracellular free iron, the effects of two membrane-permeant iron chelators were tested on endothelial permeability. Both 2,2′-dipyridyl and desferrioxamine decreased trans-endothelial permeability in a concentration-dependent manner. Increasing intracellular iron with a chelate of 8-hydroxyquinoline and ferric iron prevented effects of both EDHB and intracellular ascorbate. That EDHB and ascorbate did in fact chelate intracellular iron was supported by finding that they both decreased the cellular fluorescence quenching of the iron-sensitive dye Phen green SK. These results show that chelation of intracellular iron decreases endothelial barrier permeability and implicate this mechanism in the ability of EDHB and possibly intracellular ascorbate to tighten the endothelial barrier.
Keywords: paracellular transport, oxidative stress, iron chelation, endothelial permeability
1. Introduction
The movement of solutes, cells, and proteins out of the vascular bed is controlled by the permeability of the endothelial barrier lining the vessels. For molecules that do not directly move through cells, permeability is determined by the ability to move through various junctional structures connecting the cells or though gaps between the cells. Abnormal endothelial barrier function can lead to extravasation of blood components, resulting in tissue edema and dysfunction. Most organs and tissues take up vitamin C, or ascorbic acid, by transfer across the capillary bed. Recent studies from this laboratory showed that this transfer occurs by a paracellular route around the endothelial cells derived from veins and capillaries, rather than by a trans-cellular route across the cells [1]. Nonetheless, the intracellular content of the vitamin regulates the tightness of the endothelial barrier in human-derived endothelial cells from both large vessels and micro-capillaries. Increases in intracellular ascorbate rapidly decreased permeability of endothelial cells cultured on semi-porous supports to both the large molecule inulin and to ascorbate itself [1]. The effect of ascorbate on endothelial permeability was blocked by the microtubule agent colchicine, suggesting that it required rearrangement of the cell cytoskeleton and thus cell shape.
Another plausible mechanism by which ascorbate might affect endothelial barrier function is to facilitate collagen deposition. Utoguchi, et al. [2] showed that daily additions of physiologically relevant ascorbate concentrations (10–100 μM) for 5 days decreased transfer rates of high molecular weight fluorescein dextran across the endothelial barrier of bovine artery endothelial cells cultured on semi-permeable supports. This ascorbate-dependent decrease in barrier permeability was almost completely reversed in the presence of inhibitors of collagen synthesis, which led to the authors to conclude that collagen deposition was required for most of the increase in endothelial barrier function that they observed. The effects of ascorbate on endothelial barrier function observed in our study [1] occurred over minutes and not days after a single addition of the ascorbate precursor dehydroascorbate (DHA). Even after 18 h of culture, ascorbate had no effect on the permeability of the membrane and matrix laid down by the cells, suggesting that ascorbate-dependent changes in collagen deposition require longer times in culture to become significant. Nevertheless, it is possible that even the acute changes in endothelial barrier function we observed were somehow related to acute effects of ascorbate on collagen biosynthesis.
During testing of the role of ascorbate-dependent collagen hydroxylation in decreasing trans-endothelial permeability, we made the surprising observation that the collagen synthesis inhibitor ethyl-3,4-dihydroxybenzoate (EDHB) mimicked the effect of intracellular ascorbate to decrease paracellular permeability to ascorbate and inulin. Whereas the presence of ethyl group on EDHB allows its uptake into cells, the active form is likely to be 3,4-dihydroxybenzoate itself, which competitively inhibits ascorbate stimulation of the collagen prolyl hydroxylase by chelating but not reducing the active site ferric iron [3]. For this reason, and since EDHB has been shown to chelate and functionally deplete intracellular free iron [4], in this work we tested whether decreases in intracellular free iron due to chelation by dihydroxybenzoate and other known iron chelating agents might be involved in tightening of endothelial barrier function, especially as this might relate to the mechanism by which ascorbate improves endothelial barrier function.
2. Materials and methods
2.1 Materials
Sigma/Aldrich Chemical Co. (St. Louis, MO) supplied the reagent chemicals, including 3-aminopropionitrile (3-APN), ascorbate, cis-hydroxyproline, dehydroascorbic acid (DHA), desferrioxamine, 2,2′-dipyridyl, EDHB, 8-hydroxyquinoline, Phen green SK diacetate and N-2-hydroxyethylpiperazine N′-2-ethanesulfonic acid (Hepes). Dipyridyl and Phen green SK were initially dissolved in a small amount of dimethylsulfoxide, and then diluted with culture medium such that the final dimethylsulfoxide concentration was 0.8%. Perkin-Elmer Life and Analytical Sciences, Inc. (Boston, MA) supplied the [carboxyl-14C]inulin (molecular weight range 5000–5500, 2 mCi/g).
2.2 Cell Culture
EA.hy926 cells were originally provided by Dr. Dr. Cora Edgell (University of North Carolina, Chapel Hill, NC, USA). They were cultured in Dulbecco’s minimal essential medium and 10% (v/v) heat-inactivated fetal bovine serum, which contained 20 mM D -glucose and HAT media supplement (Sigma/Aldrich Chemical Co., St. Louis, MO). These cells are a hybridoma line derived from human umbilical vein endothelial cells that retain endothelial cell features of a cobblestone appearance with formation of capillary-like tubes in culture [5], express factor VIII antigen [6], oxidatively modify human low density lipoprotein [7], and have calcium-dependent endothelial nitric oxide synthase activity [7, 8]. Cells were cultured to confluence at 37 °C in humidified air containing 5% CO2.
2.3 Assay of trans-endothelial ascorbate and inulin transfer
EA.hy926 cells were seeded in 6-well plates on polyethylene terephthalate cell culture inserts (0.4 micron pores at a density of 2 ± 0.2 × 106 pores per cm2, Falcon BD Biosciences, Franklin Lakes, NJ). Cells were cultured to confluence and thereafter for 6–7 days with 1.7 ml of medium in the upper well and 2.8 ml of medium in the lower well. Following treatments as noted, either ascorbate (0.3 mM) or [carboxyl-14C]inulin (10 μM) were added above the cells/filter and incubation was carried out at 37 °C for 60 min, except as noted. Medium above and below the cells/filter was sampled for assay of ascorbate or for liquid scintillation counting of radiolabeled inulin.
Assay of ascorbate and [carboxyl-14C]inulin permeability was determined as previously described [9] with minor modifications [1]. The permeability of both agents was calculated at the time points noted with correction for the rate of transfer across filters after removal of cells [2]. This corrects for any changes in permeability due to the matrix laid down by the cells during culture and the experiment. Transfer rates for both agents were previously shown to be linear for at least 60 min [1].
2.4 Assay of ascorbate and GSH
For assay of intracellular ascorbate, the cells on the filter were rinsed 3 times with Krebs-Ringer Hepes buffer (KRH) that consisted of 20 mM Hepes, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, and 1.4 mM CaCl2, pH 7.4. Following removal of the last rinse, the cell monolayer was treated with 0.1 ml of 25 % (w/v) metaphosphoric acid, detached from the filter with a rubber spatula, and then treated with 0.35 ml of 0.1M Na2HPO4 and 0.05 mM EDTA, pH 8.0. The lysate was removed and centrifuged at 3 °C for 1 min at 13,000 × g and the supernatant was taken for assay of ascorbate as described below and in some instances for GSH as described by Hissin and Hilf [10]. Intracellular concentrations of ascorbate and GSH were calculated based on the intracellular distribution space of 3-O-methylglucose in EA.hy926 cells, which was previously measured to be 3.6 ± 1.2 μl/mg protein [8]. For determination of ascorbate transfer across the cells and filter, ascorbate was measured in culture medium as follows. An aliquot of 0.1 ml of medium taken from below the cells was added to 0.1 ml of 25% metaphosphoric acid (w/v), mixed, neutralized with 0.35 ml of the above phosphate/EDTA buffer, and centrifuged to remove any precipitated solids before assay of ascorbate. Assay of ascorbic acid was performed in duplicate by high performance liquid chromatography as previously described [11].
2.5 Measurement of intracellular free iron
EA.926 cells were cultured for 6–7 days either on 6-well plates or on membrane filter supports, rinsed and incubated at 23 °C for 20 min with 12 μM Phen green SK diacetate, followed by addition of agents as noted. Cellular fluorescence was excited at 488 nm and emission was obtained at 505 nm with a long-pass filter using an inverted Leica DM IRB inverted microscope. Images were recorded digitally on a Nikon DXM 1200C CCD camera and image intensity was quantified over time using NIH ImageJ version 1.42q software and shown as a fraction of the initial value.
2.6 Data Analysis
Results are shown as mean + standard error. Statistical comparisons were made using SigmaStat 2.0 software (Jandel Scientific, San Rafael, CA). Differences between treatments were assessed by two-way analysis of variance with post-hoc testing using Tukey’s test.
3. Results
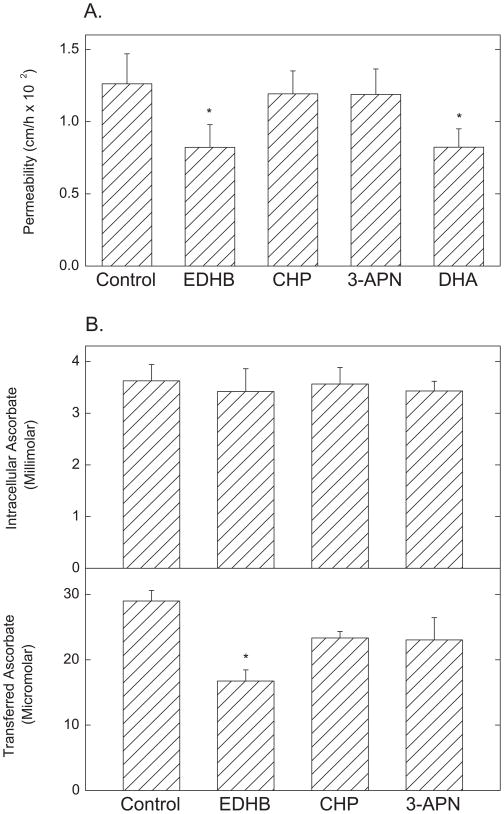
To test whether short-term treatment with collagen synthesis inhibitors affected transfer of [carboxyl-14C]inulin and ascorbate across the endothelial barrier, EA.hy926 cells were incubated for 30 min with concentrations of the inhibitors known to decrease collagen synthesis, followed by assay of [carboxyl-14C]inulin permeability for 1 h. Cells were also loaded with ascorbate by incubation with DHA, which is rapidly taken up and reduced to ascorbate within the cells, where it is effectively trapped. Under these conditions, DHA uptake and reduction did not generate a significant intracellular oxidative stress, since GSH levels were unaffected (results not shown). Using this method of loading cells with ascorbate, extracellular concentrations of the vitamin are <4 μM (results not shown). As indicated in Fig. 1A, EDHB and DHA loading both significantly decreased [carboxyl-14C]inulin transfer by about 30%, but cis-hydroxyproline and 3-aminoproprionitrile were without effect. The experiment was repeated by measuring transfer of 0.3 mM ascorbate over 60 min from above the cells to the compartment below the cells as another index of trans-endothelial permeability. During incubation with ascorbate, the cells accumulated intracellular concentrations of the vitamin to 3–4 mM; this was not affected by any of the collagen synthesis inhibitors (Fig. 1B, top panel). As shown in the bottom panel of Fig. 1B, only EDHB significantly decreased trans-endothelial transfer of ascorbate assessed as the final ascorbate concentration achieved in the medium below the cells after 60 min of transfer. The ascorbate transfer results confirm the inhibition of inulin transfer by EDHB and show that the effect of EDHB on ascorbate transfer is not due to changes in ascorbate uptake or accumulation.
Figure 1. Effects of collagen synthesis inhibitors on trans-endothelial movement of inulin and ascorbate EA.hy926 cells.
Cells cultured on porous membrane filters for 6 days were incubated at 37 °C for 30 min without additions (Control), or with EDHB (0.3 mM), cis-hydroxyproline (CHP, 1.5 mM), 3-aminopropionitrile (3-APN, 0.6 mM) or DHA (0.3 mM), all added to media above the filter and cells. Panel A: After the preliminary incubation, [carboxy-14C]inulin (10μM) was added above the filter and cells and the incubation was continued for 60 min, followed by determination of inulin permeability across the cell layer as described under Materials and Methods. Results are shown for 6 assays, with p < 0.05 for bars not sharing the same letter. Panel B: After incubation with the agents, ascorbate was added to a concentration of 0.3 mM above the cells, and the incubation was continued for 60 min. Aliquots of the medium below the cells and filter were removed for assay of ascorbate (lower panel) and the cells were rinsed 3 times in KRH before extraction for assay of intracellular ascorbate as described under Materials and Methods (upper panel). Results are shown from 5 experiments, with an “*” indicating p < 0.05 compared to control.
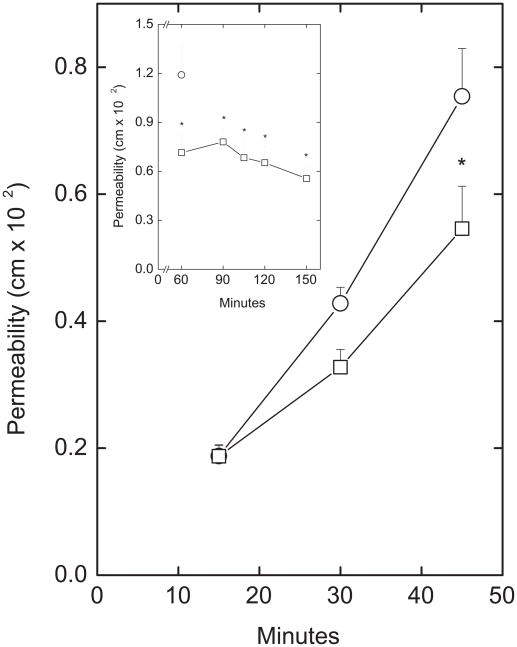
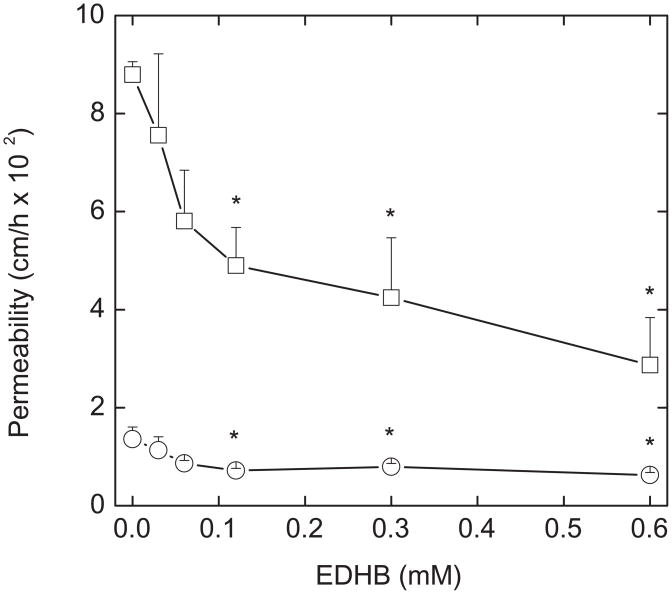
The time and concentration dependence of inhibition of paracellular [carboxyl-14C]inulin transfer by EDHB are shown in Figs. 2 and 3, respectively. Compared to a control well that did not receive EDHB, inhibition of [carboxyl-14C]inulin transfer by 0.3 mM EDHB was significant by 45 min (Fig. 2) and almost complete by after 60 min (Fig. 2, inset). The concentration dependence of EDHB to inhibit paracellular transfer was measured as permeability of both [carboxyl-14C]inulin (Fig. 3, circles) and ascorbate (Fig. 3, squares). The results showed that permeability of the endothelial barrier at baseline to ascorbate was about 6 times greater than for [carboxyl-14C]inulin. Nonetheless, decreases in permeability for both [carboxyl-14C]inulin and ascorbate showed a similar dependence on the EDHB concentration and were significant at EDHB concentrations of 0.12 mM and higher. EDHB at 0.25 mM did not affect intracellular GSH (results not shown), showing that like DHA, it did not generate a significant oxidative stress. Nor did it affect the permeability of the filter and any matrix that had been laid down by the cells in this experiment (results not shown).
Figure 2. Time course of inhibition of trans-endothelial inulin movement by EDHB.
EA.hy926 cells cultured on filters for 6 days were treated above the filter without (circles) or with (squares) 0.3 mM EDHB and 10 μM [carboxy-14C]inulin for the times indicated before determination of inulin permeability as described under Materials and Methods. Results are from 5 experiments, with “*” indicating p < 0.05 compared to the corresponding well not treated with EDHB. Inset: Cells were treated with 0.3 mM EDHB (squares) for the times noted which included 60 min of incubation with 10 μM [carboxy-14C]inulin before determination of inulin permeability coefficients. The single circle represents the 60 min inulin permeability coefficient in cells not exposed to EDHB. Results are shown from 4 experiments, with an “*” indicating p < 0.05 compared to the 60 min control.
Figure 3. Concentration dependence of inhibition of trans-endothelial movement by EDHB.
Cells cultured for 6 days on membrane filters were treated for 15 min with the indicated concentration of EDHB added above the cells and filter, followed by addition of either 10 μM [carboxy-14C]inulin (circles) or 0.3 mM ascorbate. After another 60 min of incubation, permeability coefficients were measured for both inulin and ascorbate. Results are shown for 6 experiments with inulin and 3 with ascorbate. An “*” indicates p < 0.05 compared to the sample not treated with EDHB.
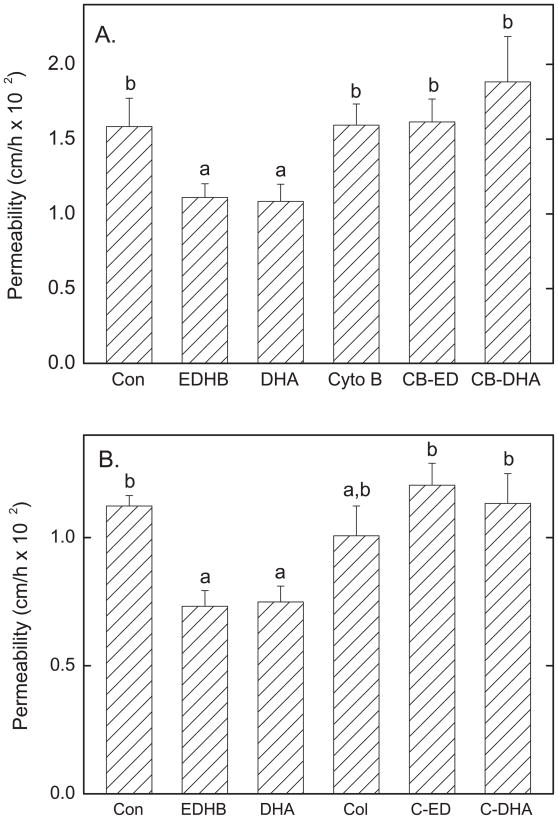
To determine whether the inhibitory effects of EDHB on endothelial permeability were due to changes in the cytoskeleton that might change cell shape or contacts, effects of a microfilament inhibitor (cytochalasin B) and a microtubule inhibitor (colchicine) were studied. Cytochalasin B (15 μM) had no effect on basal permeability, but prevented the decrease in permeability induced by both EDHB and DHA (Fig. 4A). Similarly, colchicine (10 μM) had no effect on basal rates of inulin transfer, but also prevented the decreases induced by both EDHB and DHA (Fig. 4B). These results suggest that the cytoskeleton is involved in tightening the endothelial barrier in response to EDHB and DHA.
Figure 4. Cytoskeletal inhibitors prevent DHA and EDHB inhibition of inulin transfer.
Cells cultured on filters for 6 days were incubated for 30 min with additions as noted above the cells and filter followed by assay of [carboxy-14C]inulin (10 μM) transfer over 60 min. Panel A shows results of 7 experiments for cytochalasin B (Cyto B, 15 μM) and Panel B shows results of 5 experiments for colchicine (Col, 10 μM). DHA and EDHB were added at concentrations of 0.3 mM where noted with cytochalasin B (CB-ED, CB-DHA) or colchicine (C-ED, C-DHA). Bars not sharing the same letters above them indicate results that are statistically different.
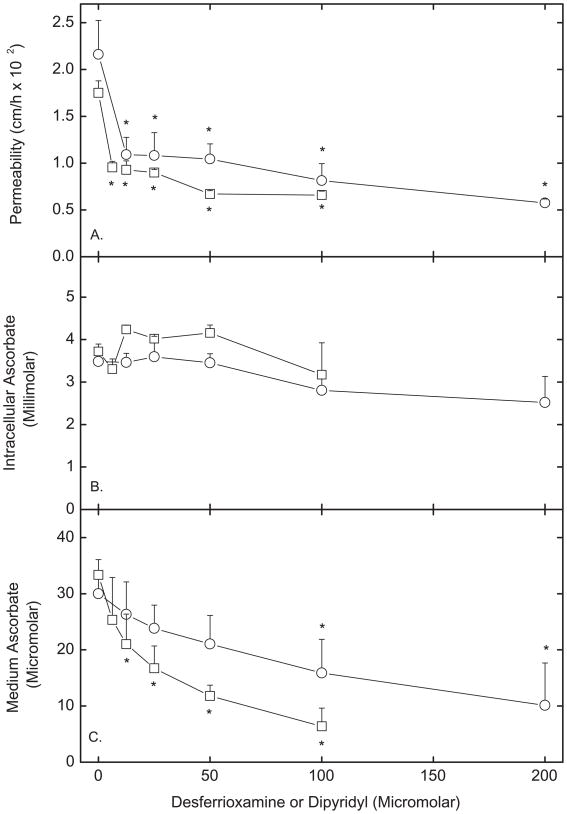
To examine whether changes in intracellular iron might mediate the inhibitory effects of EDHB and intracellular ascorbate on endothelial permeability, the effects of two cell-penetrant iron chelators were studied. As shown in Fig. 5A, both desferrioxamine (circles), which chelates ferric iron, and dipyridyl (squares), which chelates ferrous iron, inhibited permeability to [carboxyl-14C]inulin in a concentration-dependent manner. Similar results were also observed for transfer of ascorbate (Fig. 5C). Neither agent significantly affected accumulation of intracellular ascorbate during the 60 min transfer assay (Fig 5B). The decrease in [carboxyl-14C]inulin transfer induced by the iron chelators was prevented by cytochalasin B and colchicine (results not shown), suggesting that as for DHA and EDHB, the effects were related to changes in the cytoskeleton.
Figure 5. Inhibition of endothelial barrier permeability by cell-penetrant iron chelating agents.
Cells cultured on filters for 6 d were incubated at 37 °C in culture medium that contained the indicated concentrations of desferrioxamine (circles) or dipyridyl (squares) above the cells and filters. After 120 min for desferrioxamine and 60 min for dipyridyl, 60 min transfer assays of 10 μM [carboxy-14C]inulin (Panel A) or 300 μM ascorbate (Panel C) were carried out. Intracellular ascorbate was also measured in the ascorbate transfer assay (Panel B). Results are shown from at least 3 experiments for each agent, with an “*” indicating p < 0.05 compared to untreated control.
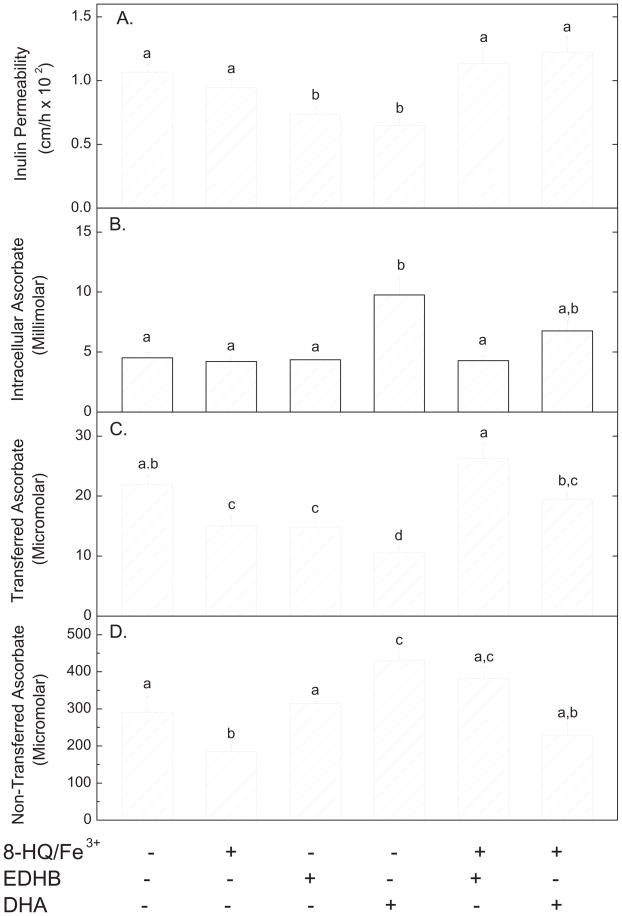
To test whether increases in intracellular free iron affect endothelial paracellular permeability, cells were treated with 8-hydroxyquinoline/Fe3+ for 1 h and the permeability was compared to EDHB and DHA alone and plus 8-hydroxyquinoline/Fe3+. As shown in Fig. 6, 8-hydroxyquinoline/Fe3+ did not affect transfer of radiolabeled inulin (Panel A, compare 1st two bars), but did decrease transfer of ascorbate (Panel C, compare 1st two bars). The decrease with ascorbate was likely due to oxidation of ascorbate added above the cells during the transfer assay, since 8-hydroxyquinoline/Fe3+ significantly decreased the final ascorbate concentration above the cells at the end of the transfer assay (Fig. 6, Panel D, compare 1st two bars). Thus, there was less ascorbate to be transferred. One might also expect intracellular ascorbate to be decreased after the transfer assay, but this was not observed (Fig. 6, Panel B). This can be explained by saturation of ascorbate uptake on the SVCT2 transporter (apparent Km = 84 μM, [12]) present in these cells [13], since even after 8-hydroxyquinoline/Fe3+ treatment, the ascorbate concentration above the cells was 185 μM. The main finding in Fig. 6 is that 8-hydroxyquinoline/Fe3+ completely prevented the inhibition by EDHB and DHA loading of transfer of both [carboxyl-14C]inulin (Fig. 6A) and ascorbate (Fig. 6C). As expected, intracellular ascorbate was increased by DHA loading beyond that due to ascorbate itself in the ascorbate transfer experiment (Fig. 6, Panel B, 4th bar). These results show that 8-hydroxyquinoline/Fe3+ prevented the tightening of the endothelial permeability barrier induced by both EDHB and DHA.
Figure 6. Effects of 8-hydroxyquinoline/Fe3+ on endothelial barrier permeability.
Cells that had been cultured on filters for 6 d were incubated where indicated in culture medium at 37 °C for 1 h with a mixture of 8-hydroxyquinoline (30 μM) and ferric chloride (15 μM), followed by addition of 0.3 mM DHA or 0.3 mM EDHB, all added above the cells and filter. After 30 min of further incubation, the transfer assays for [carboxyl-14C]inulin (10 μM) and ascorbate (0.3 mM) were carried out. Panel A shows [carboxyl-14C]inulin permeability and Panels B – D show ascorbate results. Panel B shows intracellular ascorbate, Panel C shows ascorbate transferred across the filters, and Panel D shows ascorbate remaining above the filters. Results are shown from 3–6 experiments for each assay, with bars not sharing the same symbol indicating p < 0.05.
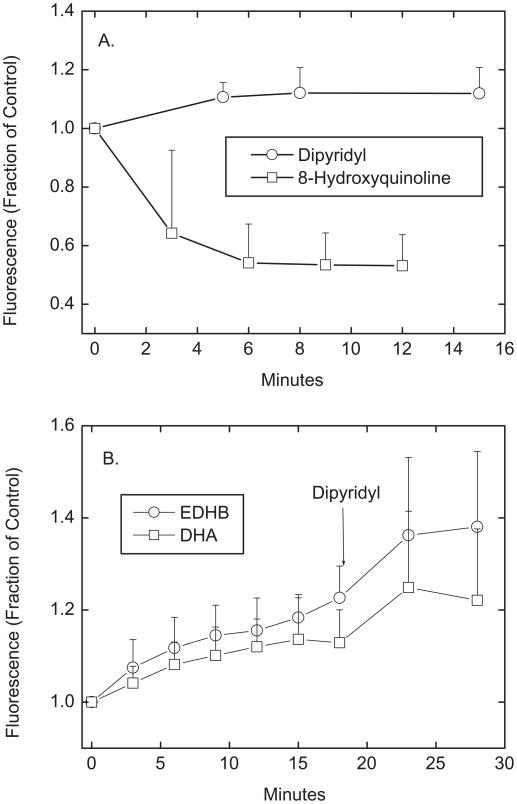
If either EDHB or ascorbate interacts with intracellular iron, then they should compete with a fluorescent iron-binding compound. Phen green SK is known to enter cells and chelate intracellular iron [14], primarily in its ferrous form [15]. This in turn decreases the fluorescence of Phen green SK. Cells loaded with Phen green SK for 20 min in KRH in the absence of D-glucose had stable levels of cellular fluorescence for at least 30 min thereafter (results not shown). Treatment of the cells with the cell-penetrant ferric iron chelator dipyridyl significantly increased Phen green SK fluorescence by 12% within about 5 min (Fig. 7A, circles), whereas 8-hydroxyquinoline/Fe3+ caused a marked 50% decrease in Phen green SK fluorescence (Fig. 7A, squares). This shows that the Phen green SK assay effectively measures chelatable intracellular iron in EA.hy926 cells. When Phen green SK-loaded cells were treated with either EDHB or DHA at concentrations known to affect paracellular transfer, there was a gradual increase in cellular fluorescence that stabilized after about 10 min (Fig. 7B). The fluorescence increase was slightly greater with EDHB than with DHA loading. Subsequent addition of dipyridyl caused a further increase in fluorescence following both EDHB and DHA treatments. These results indicate that both EDHB and possibly intracellular ascorbate compete with Phen green SK for intracellular iron, but that their chelation is only partial.
Figure 7. Fluorescence changes of Phen green SK.
Ea.hy926 cells that had been cultured on filters for 6 days in culture were rinsed in 3 times in KRH above and below the cells, and then incubated at 37 °C in KRH with 12 μM Phen green SK diacetate added above the cells and filters. After 20 min, the cells were transferred to the microscope stage and treated above the cells and filters for the times noted at room temperature with the following agents: Panel A: 50 μM dipyridyl or a mixture of 30 μM 8-hydroxyquinoline and 15 μM ferric chloride (N = 3 experiments); Panel B: 0.18 mM EDHB or 0.18 mM DHA, both followed by 50 μM dipyridyl, where indicated (N = 6 experiments). Results are shown as the fluorescence signal relative to a control well in the same experiment that received no additions. All values after the 3 min time point were different from the time zero point for each agent at p < 0.05.
4. Discussion
This study was undertaken to determine why EDHB improved endothelial barrier function and whether this might have relevance to the ability of intracellular ascorbate to do the same. The results show that both agents chelate intracellular iron to a modest extent and that stronger chelators of iron also tighten the endothelial permeability barrier. The latter conclusion is based on the finding that both dipyridyl and desferrioxamine, known cell-penetrant chelators of ferrous and ferric iron, respectively, decreased transfer of both radiolabeled inulin and ascorbate across the EA.hy926 cells cultured on semi-porous membrane filters. That dipyridyl actually did chelate intracellular iron was evident by its ability to increase fluorescence of Phen green SK, indicating that less ferrous iron was available to quench the fluorescence of Phen green SK. On the other hand, an increase in intracellular iron due to treatment with 8-hydroxyquinoline/Fe3+ did not affect endothelial barrier permeability, suggesting that it was iron removal that was important.
Although most intracellular iron is bound to ferritin, heme, or various non-heme iron-containing enzymes, it is well established that there is a small pool of intracellular free or labile iron [16]. This labile iron pool likely functions to provide iron for essential cellular functions such as active site components of enzymes, as well as to regulate synthesis of iron-responsive proteins, including ferritin and heme oxygenase-1 [16]. It also may participate in redox chemistry to generate reactive oxygen species, which may either serve a cell signaling role, or if present in excess, it may cause oxidative damage to the cell [17]. Ascorbate or EDHB might affect this labile intracellular iron by at least two mechanisms, based on known actions of each agent. First, they could change the redox state of intracellular iron. Given the reducing environment of the cytosol [14], most labile iron in the cell is expected to already be in the reduced or ferrous state. Ascorbate readily donates an electron to convert ferric to ferrous iron. Indeed, this effect to maintain iron in the active site of various dioxygenase enzymes may be one of its major cellular functions [18]. On the other hand, 3,4-dihydroxybenzoate chelates rather than reduces ferric iron [4]. Thus, it competes with both ascorbate and 2-oxoglutarate to inhibit the collagen prolyl hydroxylases [3]. It appears to do the same for the HIF-1α prolyl hydroxylases in cells [19], although it was noted to be a poor inhibitor of HIF-1α prolyl hydroxylases in extracts of insect cells engineered to express the human enzymes [20]. Whereas ascorbate might have at least part of its effects on endothelial permeability due to maintaining free or active site iron in the reduced state, this mechanism does not mediate the similar effect of EDHB.
Another possible mechanism is that the two agents could chelate free intracellular iron. This would decrease its availability for cellular processes, but protect the cell against iron-dependent oxidative stress. Ascorbate is well known to form a low affinity chelate with ferrous iron that stabilizes it and renders it more available for intestinal absorption [21]. As noted above, 3,4-dihydroxybenzoate chelates ferric iron, albeit with an affinity constant for iron only about 1/40th that of desferrioxamine [4]. Nonetheless, treatment of murine fibroblasts for 12 h with EDHB concentrations as low as 200 μM caused cellular iron deficiency, manifest as activation iron-responsive protein 1 with associated up-regulation of transferrin receptor expression and decreased cellular ferritin [4]. In agreement with these results, we found a modest but consistent effect of both ascorbate and EDHB to increase the fluorescence of intracellular Phen green SK (i.e., by chelating iron that would otherwise quench Phen green SK fluorescence). We consider this chelation modest, since Phen green SK fluorescence in ascorbate- or 3,4-dihydroxybenzoate-loaded cells could be further increased by treating them with dipyridyl, which strongly chelates ferrous iron.
That a decrease in intracellular free iron might change endothelial permeability is supported by the present finding that two cell-permeable iron chelators, dipyridyl and desferrioxamine, decreased endothelial cell permeability. Dipyridyl is a bidentate ligand that strongly chelates ferrous iron, while desferrioxamine is a hexadentate ligand that chelates ferric iron. The effects of these agents suggest that it is total iron that is important. In contrast to our results, a previous report noted that desferrioxamine did not affect the permeability barrier of endothelial cells cultured on filter supports [22]. The reason for this difference may relate to slow cell penetration by desferrioxamine. The previous work followed a time course of 100 min, whereas we found it necessary to incubate the cells for a total of 3 h with the agent to observe an effect on basal rates of radiolabeled inulin or ascorbate transfer.
Whereas chelating intracellular free iron tightened the endothelial barrier, simply increasing intracellular iron with a chelate of ferric iron and the ionophore 8-hydroxyquinoline did not affect paracellular endothelial permeability. Nonetheless, increased intracellular iron due to 8-hydroxyquinoline/Fe3+ completely prevented effects of both EDHB and ascorbate to decrease endothelial permeability. This result also supports the hypothesis that tightening the endothelial barrier by the two agents involves chelation of intracellular iron. It could be that even ambient free iron levels in cultured endothelial cells are sufficient to decrease endothelial barrier function, a process that is reversed by chelating some of the labile iron. Endothelial cells cultured in oxygenated medium are known to be under oxidative stress, a process that is reversed by ascorbate [23]. It is possible that some of this oxidative stress is caused by higher intracellular free iron than optimal for this level of oxygen and down-stream by-products of oxygen. Indeed, treatment of endothelial cells with the oxidant H2O2 is well known to increase endothelial barrier permeability [22]. This may occur by alterations in cytoskeletal protein arrangement [24], possibly by activation of myosin light chain kinase [25]. In this mechanism, reactive oxygen species generated by redox cycling of intra- or even extracellular iron will increase basal endothelial permeability, which is then decreased by chelation of intracellular ferric or ferrous iron by 3,4-dihydroxybenzoate or ascorbate, respectively. Ascorbate, and to a lesser extent 3,4-dihydroxybenzoate [26], might also decrease endothelial permeability by directly scavenging cellular reactive oxygen species.
In conclusion, the results of the present study implicate intracellular free iron in regulation of endothelial barrier function. Chelation of intracellular free iron by both 3,4-dihydroxybenzoate and ascorbate may account for at least part of their affects to decrease endothelial barrier permeability through changes in the cytoskeleton, although the mechanism remains to be elucidated at the molecular level.
Acknowledgments
This work was supported by the NIH grants DK050435 and DK020593 (Vanderbilt Diabetes Research and Training Center.
Abbreviations used
- DHA
dehydroascorbic acid
- EDHB
ethyl-3.4-dihydroxybenzoic acid
- Hepes
N-2-hydroxyethylpiperazine-NN-2-ethanesulfonic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.May JM, Qu ZC, Qiao H. Transfer of ascorbic acid across the vascular endothelium: mechanism and self-regulation. Am J Physiol Cell Physiol. 2009;297:C169–C178. doi: 10.1152/ajpcell.00674.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utoguchi N, Ikeda K, Saeki K, Oka N, Mizuguchi H, Kubo K, Nakagawa S, Mayumi T. Ascorbic acid stimulates barrier function of cultured endothelial cell monolayer. J Cell Physiol. 1995;163:393–399. doi: 10.1002/jcp.1041630219. [DOI] [PubMed] [Google Scholar]
- 3.Majamaa K, Gunzler V, Hanauske-Abel HM, Myllylä R, Kivirikko KI. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986;261:7819–7823. [PubMed] [Google Scholar]
- 4.Wang J, Buss JL, Chen G, Ponka P, Pantopoulos K. The prolyl 4-hydroxylase inhibitor ethyl-3,4-dihydroxybenzoate generates effective iron deficiency in cultured cells. FEBS Lett. 2002;529:309–312. doi: 10.1016/s0014-5793(02)03389-6. [DOI] [PubMed] [Google Scholar]
- 5.Bauer J, Margolis M, Schreiner C, Edgell CJ, Azizkhan J, Lazarowski E, Juliano RL. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J Cell Physiol. 1992;153:437–449. doi: 10.1002/jcp.1041530302. [DOI] [PubMed] [Google Scholar]
- 6.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pech-Amsellem MA, Myara I, Pico I, Maziere C, Maziere JC, Moatti N. Oxidative modifications of low-density lipoproteins (LDL) by the human endothelial cell line EA.hy 926. Experientia. 1996;52:234–238. doi: 10.1007/BF01920713. [DOI] [PubMed] [Google Scholar]
- 8.Jones W, Li X, Perriott LM, Whitesell RR, May JM. Uptake, recycling, and antioxidant functions of α-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 9.Siflinger-Birnboim A, del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol. 1987;132:111–117. doi: 10.1002/jcp.1041320115. [DOI] [PubMed] [Google Scholar]
- 10.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 11.May JM, Qu ZC, Mendiratta S. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 12.May JM, Qu ZC. Transport and intracellular accumulation of vitamin C in endothelial cells: relevance to collagen synthesis. Arch Biochem Biophys. 2005;434:178–186. doi: 10.1016/j.abb.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Qiao H, Li L, Qu ZC, May JM. Cobalt-induced oxidant stress in cultured endothelial cells: Prevention by ascorbate in relation to HIF-1alpha. Biofactors. 2009;35:306–313. doi: 10.1002/biof.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds IJ. Fluorescence detection of redox-sensitive metals in neuronal culture: focus on iron and zinc. Ann N Y Acad Sci. 2004;1012:27–36. doi: 10.1196/annals.1306.003. [DOI] [PubMed] [Google Scholar]
- 15.Lehnen-Beyel I, Groot HD, Rauen U. Enhancement of iron toxicity in L929 cells by D-glucose: accelerated(re-)reduction. Biochem J. 2002;368:517–526. doi: 10.1042/BJ20020639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakhlon O, Cabantchik ZI. The labile iron pool: Characterization, measurement, and participation in cellular processes. Free Radic Biol Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 17.Stäubli A, Boelsterli UA. The labile iron pool in hepatocytes: prooxidant-induced increase in free iron precedes oxidative cell injury. Am J Physiol Gastrointest Liver Physiol. 1998;274:G1031–G1037. doi: 10.1152/ajpgi.1998.274.6.G1031. [DOI] [PubMed] [Google Scholar]
- 18.Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta Gen Subj. 2002;1569:1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 19.Wright G, Higgin JJ, Raines RT, Steenbergen C, Murphy E. Activation of the prolyl hydroxylase oxygen-sensor results in induction of GLUT1, heme oxygenase-1, and nitric-oxide synthase proteins and confers protection from metabolic inhibition to cardiomyocytes. J Biol Chem. 2003;278:20235–20239. doi: 10.1074/jbc.M301391200. [DOI] [PubMed] [Google Scholar]
- 20.Hirsilä M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 21.Hallberg L, Brune M, Rossander L. The role of vitamin C in iron absorption. Int J Vitam Nutr Res Suppl. 1989;30:103–108. [PubMed] [Google Scholar]
- 22.Okayama N, Kevil CG, Correia L, Jourd’heuil D, Itoh M, Grisham MB, Alexander JS. Nitric oxide enhances hydrogen peroxide-mediated endothelial permeability in vitro. Am J Physiol. 1997;273:C1581–C1587. doi: 10.1152/ajpcell.1997.273.5.C1581. [DOI] [PubMed] [Google Scholar]
- 23.Smith AR, Visioli F, Hagen TM. Vitamin C matters: increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. FASEB J. 2002;16:1102–1104. doi: 10.1096/fj.01-0825fje. [DOI] [PubMed] [Google Scholar]
- 24.Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res. 2004;68:231–238. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Davis HW. Hydrogen peroxide-induced cytoskeletal rearrangement in cultured pulmonary endothelial cells. J Cell Physiol. 1998;174:370–379. doi: 10.1002/(SICI)1097-4652(199803)174:3<370::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Ueda J, Saito N, Shimazu Y, Ozawa T. A comparison of scavenging abilities of antioxidants against hydroxyl radicals. Arch Biochem Biophys. 1996;333:377–384. doi: 10.1006/abbi.1996.0404. [DOI] [PubMed] [Google Scholar]