Abstract
The mammalian main olfactory bulb (MOB) receives a dense noradrenergic innervation from the pontine nucleus locus coeruleus that is important for neonatal odor preference learning and odor processing in mature animals. Modulation of GABAergic granule cells (GCs) is thought to play a key role in the net functional impact of norepinephrine (NE) release in the MOB, yet there are few direct studies of the influence of NE on these cells. In the present study we investigated noradrenergic modulation of GC excitability using electrophysiological approaches in rat MOB slices. A moderate concentration of NE (10 µM) and the α1 receptor agonist phenylephrine (10 µM) depolarized and increased spontaneous or current injection-evoked spiking in GCs. By contrast, low NE concentrations (0.1–1.0 µM) or the α2 receptor agonist clonidine (10 µM) hyperpolarized and decreased the discharge of GCs. The effects of NE (10 µM) were blocked by antagonism of α1 and α2 receptors. Inhibitory effects of low NE concentrations were blocked or converted to excitatory responses by α2 receptor blockade, whereas excitatory effects of the moderate NE concentration were converted to inhibitory responses after α1 receptor blockade. NE (10 µM) and phenylephrine elicited inward currents that reversed near the potassium equilibrium potential. The effects of NE and phenylephrine were associated with increased membrane input resistance. Clonidine elicited an outward current associated with decreased membrane input resistance that reversed near the potassium equilibrium potential. These results indicate that α1 and α2 receptor activation exert opposing effects on GC excitability. Low concentrations of NE acting via α2 receptors suppress GC excitability, while higher concentrations of NE acting at α1 receptors increase GC excitability. These findings are consistent with recent findings that α1 and α2 receptor activation increase and decrease, respectively, GABAergic inhibition of mitral cells. The differential affinities of α1 and α2 noradrenergic receptor subtypes may allow for differential modulation of GABA release and olfactory processing as a function of the level of NE release, which in turn, is regulated by behavioral state.
Keywords: norepinephrine, main olfactory bulb, adrenergic receptor, granule cells, GABA
INTRODUCTION
The noradrenergic nucleus locus coeruleus (LC) provides the sole source of noradrenergic innervation of the main olfactory bulb (MOB) (Shipley et al. 1985). Noradrenergic fibers terminate in all but the most superficial MOB layers, densely targeting the internal plexiform and granule cell (GC) layers with a more moderate density in the mitral cell and external plexiform layers (McLean et al. 1989). The LC system plays well documented roles in modulation of behavioral state and sensory processing including olfactory function. Olfactory cues activate LC neurons and trigger norepinephrine (NE) release in the MOB (Ennis and Hayar, 2008). The widespread distribution of noradrenergic fibers in the MOB network provides a structural basis for potentially diverse physiological effects of NE release on olfactory processing. Consistent with this, noradrenergic input to the MOB is critical for the formation of conditioned odor preferences in neonates, as well as odor habituation and discrimination in mature animals (Sullivan et al. 2000; Harley et al. 2006; Doucette et al. 2007; Veyrac et al. 2007; Guerin et al., 2008; Mandairon et al. 2008).
The dense LC-NE innervation of the GC layer (GCL) suggests that modulation of GABAergic inhibition is a major component involved in noradrenergic modulation of olfactory processing in the MOB network. However, there are relatively few direct physiological studies on the impact of NE on the excitability of GABAergic GCs in the mammalian MOB. NE was reported to suppress GABAa receptor-mediated inhibition of mitral cells in the turtle MOB, an effect attributed to direct inhibition of GCs (Jahr and Nicoll 1982). NE, acting at α2 receptors, was similarly found to reduce GABAergic inhibition of cultured rat mitral cells due to presynaptic inhibition of transmitter release from mitral or GCs (Trombley 1992; Trombley and Shepherd 1992). However, α2 receptor activation did not directly influence GC cell excitability in the Xenopus laevis MOB (Czesnik et al. 2001). Smith et al. (2009) observed that NE or α1 receptor activation depolarized accessory olfactory bulb GCs. Recent studies in MOB slices demonstrated that GABAa receptor-mediated inhibition of mitral cells is bi-directionally regulated in a concentration-dependent manner by NE. Low NE concentrations in the sub-micromolar range, acting at α2 receptors, suppressed GABAergic inhibition while low micromolar concentrations increased GABAergic inhibition via α1 receptors (Nai et al., 2009). NE-induced, α1 receptor-mediated increase in GABAergic inhibition of mitral cells has also been observed in the accessory olfactory bulb (Araneda and Firestein, 2006). In both studies, the effects of NE were mediated on neuronal elements presynaptic to mitral cells, presumably by direct modulation of GC excitability.
Taken together, the preceding studies suggest that NE may modulate GABAergic inhibition of mitral cells via presynaptic actions on GCs involving both α1 and α2 receptors. The goal of the present study was to assess the direct postsynaptic effects of α1 and α2 receptor activation on the excitability of GCs using patch clamp electrophysiology in rat MOB slices. Our results indicate that α1 receptor activation increases, and α2 receptor activation decreases, spontaneous and evoked discharge in GCs in a manner consistent with recently observed effects of activation of these receptor subtypes on GABAa receptor-mediated inhibition of mitral cells (Nai et al., 2009).
MATERIALS AND METHODS
Slice preparation
Male and female 14–28- day-old Sprague Dawley rats were decapitated in accordance with Institutional Animal Care and Use Committee and National Institute of Health guidelines. Horizontal 400 µm-thick olfactory bulb slices were prepared as previously described (Dong et al. 2007). Briefly, the olfactory bulbs and a portion of the forebrain were dissected free from the surrounding skull, removed and immersed in oxygenated chilled sucrose-artificial cerebrospinal fluid (ACSF) composed of (in mM): 26 NaHCO3, 1 NaH2PO4, 3 KCl, 4 MgSO4, 0.1 CaCl2, 20 glucose and 234 sucrose; pH 7.3, 310 mOsm. Slices were cut using a Vibratome 3000 (Vibratome, St. Louis, MO) and transferred to an incubation chamber filled with normal ACSF saturated with 95% O2 and 5%CO2 and composed of (in mM): 124 NaCl, 26 NaHCO3, 1 NaH2PO4, 3 KCl, 2 MgSO4, 2 CaCl2, 10 glucose, 0.4 ascorbic acid, 2 sodium pyruvate (pH 7.3, 310 mOsm); osmolarity was 310 mOsm. NaH2PO4 was omitted in experiments where nickel and cadmium were added to the ACSF. Slices were held at 33°C for 15 min, and then at room temperature (22°C) until used. For recording, a single slice was placed in a recording chamber and continuously perfused with ACSF equilibrated with 95% O2 and 5%CO2.
Electrophysiology
Whole-cell recordings were performed at 30°C. Drugs were applied by bath perfusion (1.5–2 ml/min) for 4 min. The recording pipette contained (in mM): 120 potassium gluconate, 6 KCl, 2 NaCl, 2 MgCl2, 10 phosphocreatine ditris salt, 3 MgATP, 0.3 Na2GTP, 0.2 EGTA, 10 HEPES, and (pH 7.3, 290 mOsm). The intracellular solution also contained 0.4% biocytin and 0.02% Lucifer Yellow. Neurons were visualized using an upright microscope (BX51WI; Olympus Optical, Tokyo, Japan) equipped with epifluorescence and near-infrared differential interference contrast optics.
Whole-cell current and voltage clamp techniques were used to record membrane potential and currents. Analog signals were low-pass filtered at 2 kHz (Axopatch 200B) and digitized at 5 kHz using a Digidata-1322A interface and Clampex 9.0 software (Molecular Devices, Sunnyvale, CA). In current clamp membrane input resistance was determined by voltage changes elicited by negative current pulses (−2 to −100 pA, 500 msec). I–V relations of NE and agonist evoked currents were studied with a voltage ramp protocol (−120 mV to −10 mV, 70 mV/s, 1.4 sec duration) from a holding potential (HP) of −80 mV; the current attributable to NE or agonists was determined by subtracting the curves recorded in their presence and absence. The membrane potential and firing frequency were analyzed with ClampFit 9.0 (Molecular Devices) and Mini Analysis program (Synaptosoft, Decatur, GA). OriginPro 8 (OriginLab Corporation, Northampton, MA) was used for further data analysis.
GCs were identified by soma location, and distinct morphological (small soma size, single apical dendrite ramifying in the external plexiform layer) and electrophysiological (high input impedance, relatively hyperpolarized resting membrane potential, low rate of spontaneous spiking) properties. The majority of cells recorded in the present study were superficial GCs located in the mitral cell and internal plexiform layers. Recordings were also obtained from a smaller subset of deep GCs within the GCL proper.
Data analysis
Data, expressed as mean ± SEM, were statistically analyzed using one-way repeated measurement Anova followed by posthoc comparisons (Newman-Keuls tests), or with Students t-tests (SigmaStat, Aspire Software International, Ashburn, VA). Percentage data from different groups were analyzed with the Mann-Whitney U test.
Immunohistochemistry and microscopy
Slices containing the recorded cells were treated using methods described previously (Dong and Buonomano, 2005). Briefly, the slices were fixed with 4% paraformaldehyde overnight and then rinsed thoroughly with 0.1 M phosphate buffered saline (PBS, pH 7.4) at room temperature. Slices were incubated with 0.6 % Triton X-100 (0.1M PBS) at room temperature for 1 hr, then in 0.1 M PBS with 1:200 Avidin-Oregon Green 488 conjugate at 4 °C overnight. After rinsing in 0.1 M PBS for three times, 10 min each, the slices were mounted with the Vectashield mounting medium with DAPI (H-1200, Burlingame, CA). Images of labeled cells were captured using an upright Olympus BX50 microscope equipped with a BioRad MRC 1024 Confocal system with krypton-argon laser (Hemel Hempstead, UK).
Drugs and solutions
Drugs were applied by switching the bath perfusion solution with a three-way valve system. Biocytin and Avidin-Oregon Green 488 were purchased from Molecular Probe (Carlsbad, CA). Clonidine (Clon), prazosin (Praz), idazoxan (Idaz), LY367385, tetrodotoxin (TTX), 2-amino-5-phosphonopentanoic acid (APV), and 6-cyano-2,3-dihydroxy-7-nitro-quinoxaline (CNQX) were obtained from Tocris Bioscience (Ellisville, MO). NE, phenylephrine (PE), gabazine and other chemicals were purchased from Sigma (St. Louis, MO).
RESULTS
Recordings were obtained from 109 superficial and 17 deep GCs. The mean resting membrane potential (−64.5 ± 1.1 mV vs. −64.9 ± 2.0 mV) and input impedance (1.2 ± 0.1 GΩ vs. 1.7 ± 0.3 GΩ) did not differ between the two GCs subtypes (p>0.05, t-tests). Unless specified otherwise, the data reported were obtained from superficial GCs. An example of a biocytin-filled superficial GC is shown in Figure 1A.
Figure 1.
Effects of NE and α1 and α2 receptor agonists on GC membrane potential and discharge. A: A superficial GC filled with biocytin. GL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer; GCL, GC layer; scale bar: 20 µm. B–D: Current clamp recordings in normal ACSF showing that NE (10 µM, B) or PE (10 µM, C) depolarized and increased the firing rate of GCs, while Clon (10 µM, D) hyperpolarized and decreased spontaneous discharge. E: Group data showing the effects of NE, PE and Clon on firing frequency (black bars) and membrane potential (unfilled bars); hatched bars show peak changes (see text). Data are expressed as increase or decrease in firing frequency or membrane potential from control (0). F–H: Bath application of CNQX (10 µM), APV (50 µM), and gabazine (10 µM) reduced baseline activity and spontaneous discharge in GCs. Under these conditions, 10 µM NE, PE, and Clon produced changes in GC membrane potential similar to those observed in normal ACSF. I: Group data for experiments shown in (F–H). J–M: In the presence CNQX-APV-gabazine, GCs were depolarized by steady positive current injection to elicit spiking activity. A low concentration of NE (0.1 µM) hyperpolarized and decreased the firing rate of GCs (J). 10 µM NE (K) and PE (L) depolarized and increased the firing rate of GCs, while Clon hyperpolarized and decreased the firing frequency (M). N: Group data for experiments shown in (J–M). Note that application of NE in the presence of Idaz (10 µM) and Praz (1 µM) (NE+Praz+Ida) did not significantly alter membrane potential or firing rate. *p<0.05 compared to control, paired t-tests. The normalized firing frequency was calculated as: (firing frequency at the end of drug application – basal frequency)/basal frequency.
Effects of NE and Receptor Agonists on GC Membrane Potential and Discharge
A previous study from our laboratory reported that α1 and α2 receptor activation modulates GABAergic input to mitral cells via presynaptic actions on inhibitory interneurons (Nai et al., 2009). Therefore, the present experiments focused on the effects of NE and α1/α2 agonists on GC excitability. Concentrations of NE and agonists were based on those that produced significant effects on GABAergic IPSCs in mitral cells (Nai et al., 2009). We first investigated the actions of NE, the α1 receptor agonist phenylephrine (PE), and the α2 receptor agonist clonidine (Clon), each at 10 µM on GC membrane potential and spontaneous discharge using whole-cell current clamp recordings. The effects of these ligands on membrane potential and spiking frequency were measured at the end of the drug application. In normal ACSF, application of NE or PE (Fig.1B,C,E) elicited slow and relatively small amplitude depolarization of GCs, 2.2 ± 0.2 mV for NE (n=16, p<0.05, paired t-test) and 3.0 ± 0.3 mV for PE (n=17; p<0.05, paired t-test). The depolarizations were associated with an increase in the spontaneous discharge rate from 0.6 ± 0.2 to 1.5 ± 0.5 Hz for NE (n=9, p<0.05, paired t-test), and from 0.7 ± 0.3 to 1.9 ± 0.7 Hz for PE (n=10, p<0.05, paired t-test) (Fig.1B,C,E). In contrast, Clon hyperpolarized the membrane potential by −1.9 ± 0.1 mV (Fig.1D,E, n=15; p<0.05, paired t-test), and reduced the firing rate (Fig.1D–E, from 1.1 ± 0.4 to 0.7 ± 0.3 Hz; n=12, p<0.05, paired t-test). The effects of NE and noradrenergic receptor agonists lasted 5–15 min and were partially or fully reversible by 30 min after washout (Supplemental Figure 1).
In many cells the peak agonist effects, especially NE and PE occurred after the end of the drug application, and in some cases (Fig. 1C) the large depolarization resulted in cessation of firing (i.e., depolarization block). Therefore, we further analyzed the peak effects of NE and PE on cells that displayed maximal membrane potential changes within five min after the drug delivery. NE depolarized the cells by 6.2 ± 2.2 mV (n=11, p<0.05, paired t-test). PE depolarized the membrane potentials by 8.3 ± 2.5 mV (n=12, p<0.05, paired t-test). The peak hyperpolarization by Clon was −2.3 ± 0.1 mV (n=13, p<0.05, paired t-test)
In order to assess if NE and agonist effects are directly mediated, similar experiments were conducted in the presence of antagonists of ionotropic glutamate and GABA receptors: APV (50 µM), CNQX (10 µM) and gabazine (10 µM). As previously reported, these antagonists reduced spontaneous synaptic activity and action potential generation in GCs (Heinbockel et al., 2007). Under these conditions, depolarizations were induced by NE (2.7 ± 0.3 mV, n=10; p<0.05) and PE (2.5 ± 0.3 mV, n=6; p<0.05) comparable to those observed in normal ACSF (Fig. 1F,G,I). Clon hyperpolarized the membrane potential (Fig. 1H,I, −2.2 ± 0.4 mV, n=14). For cells showing the maximal membrane potential changes within five min following drug application, the peak depolarization induced by NE and PE were 6.9 ± 1.6 mV (n=8) and 9.6 ± 4.3 mV (n=6), respectively (p<0.05, paired t-test for both agonists); the peak hyperpolarization by Clon was −2.6 ± 0.2 mV (n=11, p<0.05, paired t-test). When steady depolarizing current injection (2–20 pA) was used to restore spontaneous spiking in GCs in the presence of APV-CNQX-gabazine (Fig. 1K,L,N), NE (10 µM) and PE (10 µM) depolarized, 2.8 ± 0.4 mV, n=10 for NE; 2.8 ± 0.5 mV, n=9 for PE) and increased the firing rate (1.3 ± 0.7 to 3.4 ± 1.4 Hz, n=10, p<0.05 for NE; 0.7 ± 0.2 to 2.1 ± 0.4 Hz, n=9, p<0.05 for PE). The depolarization and increase in firing elicited by 10 µM NE were completely blocked (Fig. 1N, Fig. 3B,C) when applied in the presence of in the presence of the α1 and α2 receptor antagonists prazosin (Praz, 1 µM) and idazoxan (Idaz, 10 µM), respectively (firing rate = 13.7 ± 2.9% increase, n=3, p>0.05, paired t-test; depolarization = 0.28 ± 0.1 mV [0.6 ± 0.2% increase], n=4, p>0.05, paired t-test). A low concentration of NE (0.1 µM) and Clon (10 µM) hyperpolarized GCs (Fig. 1J,M,N, −2.0 ± 0.3 mV, n=10, p<0.05 for Clon), and suppressed their firing rate (Fig. 1J,M,N, 1.3 ± 0.3 to 0.3 ± 0.1 Hz; n=10, p<0.05 for Clon). The peak depolarization by NE and PE in cells reaching their maximal response within five min after drug application were 8.0 ± 2.3 mV, (n=9) and 12.7 ± 4.1 mV (n=8), respectively (p<0.05, paired t-test for both agonists). The peak hyperpolarization induced by Clon was −2.6 ± 0.6 mV (n=10, p<0.05, paired t-test).
Figure 3.
GC excitability and GABAergic inhibition of mitral cells is bi-directionally regulated by NE in a concentration-dependent manner. A: Group data from GC current clamp recordings in the presence of CNQX-APV-gabazine show that 0.1–1 µM NE hyperpolarized and reduced the spike discharge rate compared to the pre-NE baseline. By contrast, 10 µM NE depolarized and increased the discharge rate of GCs. GC membrane potential is expressed as absolute change from baseline, while discharge is normalized to a baseline value of 1.0. Data from 0.1–1 µM and 10 µM NE were obtained from separate groups of GCs; n=6–7 GCs for each concentration, *p<0.05, Anova followed by Newman-Keuls tests (0–1 µM data) or paired t-test (10 µM data). The level of GABAergic inhibition of mitral cells, expressed as the normalized frequency of mIPSCs, was bi-directionally modified by NE with a decrease elicited by NE concentrations less than 1.0 µM NE and an increase with 10 µM NE; #p<0.05, n=6–12 mitral cells per concentration. Mitral cell data were adapted from Fig. 6B in Nai et al. (2009) and mIPSCs were recorded in the presence of CNQX-APV-gabazine-TTX (1 µM). The frequency of GC spiking and mitral cell IPSCs was calculated as: frequency at the end of drug application/basal frequency. B: Open circles show group data illustrating concentration-dependent effects of NE on GC firing rate (in APV-CNQX gabazine); replotted from (A). Closed circles show the effects when 0.3 and 1 µM NE were applied in the presence of Idaz (10 µM), and when 10 µM NE was applied in the presence of Praz (1 µM). Open diamond symbol shows that NE was without affect when applied in the presence of Idaz and Praz combined. C: The experimental conditions and cells are identical to (B), except that change in membrane potential is illustrated. *p<0.05 (B–C), Anova followed by Newman-Keuls tests.
Activation α1 and α2 Receptors Exert Opposing Effects on GC Excitability
The preceding findings indicate that activation of α1 and α2 receptors exert direct and opposing effects on the excitability of GCs. We wondered if individual GCs express both α1 and α2 receptors. In all 9 cells tested in normal ACSF, PE (10 µM) depolarized and increased the firing rate while Clon hyperpolarized and reduced the firing rate (Fig. 2A,B). Similar results were observed for 5 cells tested in ACSF containing APV-CNQX-gabazine (Fig. 2C); cells were depolarized by steady positive current injection to elicit spiking activity as in Figure 1J–M. These results suggest that most GCs express both α1 and α2 receptors, but do not exclude the possibility that some GCs may express one of the two noradrenergic receptor subtypes.
Figure 2.
GCs express α1 and α2 receptors. A1–2: Traces showing the opposing effects of PE (10 µM) and Clon (10 µM) on the same GC. B1: In 9 GCs tested in normal ACSF, PE increased, but Clon decreased, spontaneous spiking. B2: In the same cells as in (B1), PE and Clon induced depolarization and hyperpolarization, respectively. C1 and C2: In the presence of APV-CNQX-gabazine, 5 GCs tested responded to PE and Clon in a manner similar to that observed in normal ACSF; cells were depolarized by steady positive current injection to elicit spiking activity as in Figure 1J–M. *mean values differ significantly than basal values, p<0.05, paired t-tests.
A recent study reported that low concentrations of NE (0.01–1.0 µM) suppressed, while intermediate concentrations (3–10 µM) increased, GABAa receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs) in mitral cells (Nai et al., 2009). The findings above indicate that an intermediate concentration of NE (10 µM) increases GC excitability. To determine if lower concentrations of NE suppress excitability, we measured changes in membrane potential and firing rate produced by 0.1, 0.3 and 1.0 NE µM in the presence of APV-CNQX-gabazine (Fig. 3A). Compared to basal membrane potential and firing rate (1.2 ± 0.4 Hz), 0.1–1.0 µM NE significantly hyperpolarized and reduced the discharge rate of GCs: 0.1 µM (−1.5 ± 0.1 mV, 0.5 Hz ± 0.3 Hz), 0.3 µM (1.7 ± 0.3 mV, 0.5 ± 0.3 Hz), and 1.0 µM 1.4 ± 0.3 mV, 0.7 ± 0.4 Hz); n=6–7 cells per concentration, p<0.05; see also Fig, 1J Combined with the 10 µM NE results, the dose-response profile for NEs actions on GC is similar to the change in the frequency of sIPSCs in mitral cells across the same NE concentration range (Nai et al., 2009), as shown in Figure 3A. Based on previous studies (Nai et al., 2009) and the present results, we hypothesize that low concentrations (≤ 1µM) of NE inhibit GC excitability via α2 receptors, while intermediate concentrations (3–30 µM) excite GCs via α1 receptors. To test this hypothesis, we applied low concentrations of NE in the presence of the α2 receptor antagonist Idaz, and an intermediate concentration in the presence of the α1 receptor antagonist Praz. In the presence of the Idaz (10 µM), the hyperpolarization and decreased firing elicited by 0.1 NE were blocked, while 0.3 µM NE produced a depolarization and increased firing rate (Fig. 3B,C). By contrast, in the presence of Praz (1 µM), 10 µM NE hyperpolarized and inhibited the firing rate of GCs. These findings further suggest that individual GC express both α1 and α2 receptors, and that inhibitory effects of low NE concentrations are mediated by α2 receptors and the excitatory effects of intermediate concentrations are mediated by α1 receptors.
Effects of NE and Receptor Agonists on GC Responses to Current Injection
To further investigate the direct effects of activation of α1 and α2 receptors, we measured spiking and membrane hyperpolarization elicited by injection of positive or negative current pulses (2 to 100 pA, 0.5 sec), respectively, into GCs in the presence of APV-CNQX-gabazine (Fig. 4A,B,C). NE (10 µM) or PE (10 µM) increased spiking responses to positive current pulses, measured as the number of spikes/pulse; NE: 2.5 ± 0.2 to 6.6 ± 0.6 spike/pulse (n=8, p<0.05, paired t-test), PE: 3.1 ± 0.4 to 5.1 ± 0.5 spike/pulse (n=9; p<0.05, paired t-test). The increases in evoked spiking were not due to depolarization as the membrane potential was manually reset to the baseline level during agonist application. NE and PE also reduced the latency of the first evoked spike (by 73.6 ± 18.6 ms and 42.2 ± 13.8 ms respectively; p<0.05, paired t-tests). NE and PE increased the amplitude of hyperpolarizing responses to negative current pulses (Fig 4. A,B), translating to an increase in membrane resistance of 176.1 ± 59.2 MΩ (Fig. 4E, n=8; p<0.05, paired t-test) for NE and 193.8 ± 76.8 MΩ for PE (Fig. 4E; n=9; p<0.05, paired t-test).
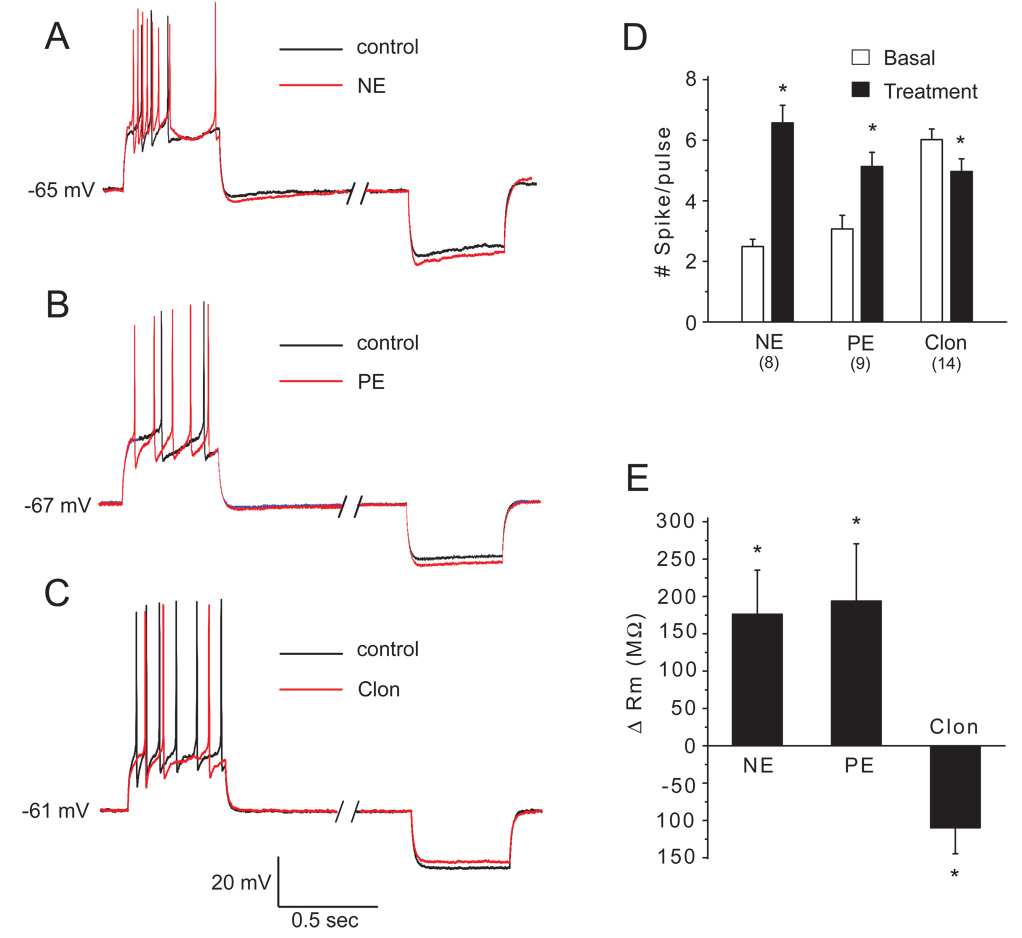
Figure 4.
Superimposed current clamp traces showing membrane potential responses to +20 pA and −50 pA current steps before and during application of NE and receptor agonists (each at 10 µM) in the presence of APV-CNQX-gabazine. A–B: NE and PE increased evoked discharge to positive current injection and increased hyperpolarizing responses to negative current injection. C: Clonidine decreased evoked discharge and decreased hyperpolarization to negative current injection. D–E: Group data summary of the effects of NE and receptor agonists on the number of spikes/pulse (D) and changes in membrane resistance (E). *p<0.05; n=8–14 cells per group, paired t-tests.
Clon (10 µM) reduced the number of evoked spikes (6.0 ± 0.3 to 4.9 ± 0.4 spikes/pulse; n=14, p<0.05, paired t-test) and increased the latency of the first spike by 30.9 ± 9.4 ms (p<0.05, paired t-test). Clon decreased responses to negative current pulses, corresponding to a decrease in membrane resistance of 109.8 ± 34.8 MΩ; (Fig. 4 C,D,E; n=14, p<0.05, paired t-test). In a population of deep GCs tested, PE (10 µM, n=6) and Clon (n=5), respectively, increased and decreased evoked spiking (n=5, p<0.05 for both, paired t-tests). The magnitude of effects of PE and Clon on evoked spiking did not differ between superficial and deep GCs (p>0.05, Mann-Whitney U tests, data not shown).
The Group I metabotropic glutamate receptor, mGluR1, was recently reported to mediate the excitatory effects of NE and α1 receptor agonists on GCs in the mouse accessory olfactory bulb (Smith et al., 2009). We therefore tested if the selective mGluR1 antagonist LY367385 altered the response of rat GCs to PE. In the presence APV-CNQX-gabazine and LY367385 (100 µM), PE (10 µM) increased the number of spikes induced by depolarizing pulses (6.2 ± 0.7 to 9.2 ± 0.9 spikes/pulse, n=4, p<0.05); this increase did not differ from that elicited by PE in the absence of LY367385 (p>0.05, Mann-Whitney U test, data not shown). Similarly, the increase in membrane resistance elicited by PE did not differ in the presence or absence of LY367385 (p>0.05, Mann-Whitney U test, data not shown).
Effects of PE and Clon on Membrane Currents
Voltage clamp recordings were used to investigate the current-voltage (I–V) relationship of responses elicited by NE and agonists. NE-induced changes of cellular excitability are commonly mediated by modulation of potassium conductances (see Discussion). Therefore the following experiments were conducted in the presence of APV-CNQX-gabazine, TTX (1 µM), nickel (100 µM), and cadmium (100 µM). NE (10 µM) and PE (10 µM) produced an inward current that was largest at −10 mV and declined as the membrane potential was shifted to more negative potentials (Fig. 5A, B). The slope of the I–V curves were reduced by NE or PE, indicative of an increase in input resistance due to closure of channels that were open at the range of holding potentials tested (−120 to −10 mV). Potassium channels were assumed to provide the major contribution to the membrane conductance under these recording conditions, suggesting that NE and PE lead to a decreased potassium conductance. Consistent with this, the NE and PE currents reversed in polarity at −95.8 ± 4.3 mV (range, −85 to −106 mV; n=5) and −94.9 ± 4.1 mV (range, −85 to −105 mV; n=5), respectively, near the calculated equilibrium potential of potassium channels (−97.6 mV). Clon induced an outward current that was maximal at positive membrane potentials and progressively declined at more negative potentials, reversing in polarity at −93.1 ± 2.4 mV (range, −86 to −110 mV; n=10, Fig. 5C). Clon increased the slope of the I–V curve, indicative of a decrease in membrane resistance. At −60 mV, NE and PE induced inward currents of −3.5 ± 1.5 pA and −4.2 ± 0.6 pA, respectively, while Clon induced an outward current of 3.9 ± 1.3 pA (Fig. 5D).
Figure 5.
Effects of NE and α1 and α2 receptor agonists on GC membrane currents. Membrane currents produced by slow voltage ramps (−120 to −10 mV) were recorded in the presence of TTX (1 µM), CNQX(10 µM), APV(50 µM), gabazine (10 µM), NiCl2 (100 µM) and CdCl2 (100 µM). Figures show I–V plots before (basal) and during NE or agonist application, as well as difference curves obtained by subtraction of the basal and NE/agonist plots; insets show enlarged difference curve. A–B: NE (A, 10 µM, n=5) and PE (B, 10 µM, n=5) induced an inward current that progressively increases at membrane potentials from −120 to −10 mV. The NE and PE current reversed in polarity at −95.8 ± 4.3 mV and −94.9 ± 4.1 mV, respectively. C: Clonidine (10 µM, n=10) induced an outward current with a reversal potential of −93.1 ± 2.4 mV). D: Group data showing the amplitude of the NE and agonist currents at −60 mV. *p<0.05, paired t-tests.
Discussion
The results of our study indicate that NE directly and bi-directionally modifies the excitability of MOB GCs via activation of α1 and α2 receptors. Specifically, low concentrations of NE or the α2 receptor agonist Clon suppressed, while a higher concentration of NE or the α1 receptor agonist PE enhanced, GC excitability. Taken together with previous studies (Nai et al., 2009), these results indicate that low levels of NE in the MOB attenuate GC-mediated GABAergic inhibition of mitral cells, whereas higher concentrations lead to increased inhibition of mitral cells.
NE and the α1 receptor agonist PE, at concentrations recently shown to increase GABAergic inhibition of mitral cells (Nai et al., 2009), depolarized and increased the spontaneous or evoked spiking in GC. By contrast, relatively low concentrations of NE, or the α2 receptor agonist Clon, hyperpolarized and suppressed spike generation in GCs. In all cases, these effects persisted in the presence of APV-CNQX-gabazine, indicating that they result from direct postsynaptic actions of NE or agonists on GCs. Corresponding voltage clamp recordings demonstrated that PE and Clon induced inward and outward currents, respectively, that reversed in polarity near the reversal potential for potassium ions. Combined with the resistance changes produced by the agonists, these overall findings suggest that α1 and α2 receptor activation decreases and increases potassium conductances in GCs, respectively. The modest amplitude of the agonist-evoked changes in membrane potential, as well as modulation of potassium channels, is consistent with previous α1 and α2 receptor-mediated actions in the substantial gelatinosa (North and Yoshimura, 1984), laterodorsal tegmental nucleus (Williams and Reiner, 1993) cerebral cortex (Wang and McCormick, 1993; Pralong and Magistretti, 1995; Kawaguchi and Shindou, 1998), supraoptic nucleus (Ogata and Massuo, 1986; Yamashita et al., 1987), hippocampus (Bergles et al., 1996), dorsal raphe (Pan et al., 1994) and periaqueductal gray (Vaughn et al., 1996). Similar to our study, α1 receptor activation elicited depolarizations, increased membrane resistance and reduced potassium currents, while α2 activation produced hyperpolarization, decreased membrane resistance and increased potassium currents in sympathetic preganglionic, dorsal vagal, periaqueductal gray neurons (Fukada et al., 1987; Inokuchi et al., 1992; Vaughn et al., 1996).
The potassium conductance modified by α1 and α2 agonists was active over the range of membrane potentials tested, suggesting modulation of a resting or leak potassium. Although the I–V relationship of leak potassium currents is typically linear, some leak channel subtypes (i.e., KCNK) exhibit outward rectification at membrane potentials positive to the potassium equilibrium potential (Talley et al., 2000; Goldstein et al., 2001), as observed in the present study. NE, acting via α1, receptors has been reported to inhibit rectifying and non-rectifying leak potassium currents (Inokuchi et al., 1992; Wang and McCormick, 1993; Pan et al., 1994; Vaughn et al., 1996; Hayar et al., 2001). Outwardly rectifying leak potassium channel subunits are highly expressed by neurons in the GCL (Talley et al., 2001). In view of the finding that the current modified by Clon exhibited similar properties, it is possible that α1 and α2 receptor activation bi-directionally modulate GC excitability by opposing actions on a same leak potassium current in these cells. Additional studies are needed to conclusively identify the voltage-dependency of potassium conductances, as well as other ion channels, modified by NE in GCs.
NE was recently reported to depolarize accessory olfactory bulb GCs via activation of α1 receptors (Smith et al., 2009). The properties of the α1 receptor-mediated excitation of accessory olfactory bulb GCs differs markedly from those observed in the present study. The depolarization was mediated by a non-selective cation current with a reversal potential near −20 mV, and was blocked by extracellular cadmium. The effects of α1 receptor agonists were also blocked by the mGluR1 antagonist LY367385, suggesting that the excitatory action of NE on accessory olfactory bulb GCs is due to potentiation of basal activity of mGluRs. By contrast, the PE-evoked depolarization in the present study persisted in extracellular cadmium and was unaffected by LY367385. Thus, while α1 receptor activation increases GC excitability and GABAergic inhibition of mitral cells in both the main and accessory olfactory bulbs, the signaling pathways coupled to the receptors differ markedly in the two olfactory structures.
Our physiological findings are consistent with the expression of both α1 and α2 receptors in the GCL, including GCs (Young and Kuhar 1979, 1980; Jones et al. 1985; McCune et al. 1993; Pieribone et al. 1994; Talley et al. 1996; Day et al. 1997; Domyancic and Morilak 1997; Winzer-Serhan et al. 1997a,b). Responses of single GCs to both PE and Clon suggest that some GCs express both α1 and α2 receptor subtypes as observed for brainstem neurons (Grudt et al., 1995; Fukada et al., 1987; Vaughn et al., 1996). The effects of NE were blocked by Praz and Idaz, indicating that α1 and α2 receptors are the major GC noradrenergic receptors engaged by NE. Noradrenergic receptor subtypes have differential affinities for NE and LC neuronal discharge, and consequently extracellular NE levels, vary as a function of attentional state or level of vigilance (Berridge and Waterhouse 2003). Therefore, the net impact of the LC system on GABAergic inhibition in the MOB will be modulated as a function of the level of NE release and the specific NE receptors that are engaged by the prevailing extracellular concentration of NE. α2 receptors have a higher affinity for NE than α1 receptors (Ramos and Arnsten, 2007). Consistent with this, the suppression of GC excitability by low NE concentrations (0.1 – 1.0 µM) was eliminated or converted to an excitatory response by α2 receptor blockade. By contrast, the excitation observed at a higher NE concentration (10 µM) was converted to inhibition by α1 receptor blockade.
These concentration-dependent effects of NE on GC excitability correspond well to the dose-response profile for NE modulation of GABAergic inhibition of mitral cells (Fig. 3A; Nai et al., 2009). The ability of higher NE concentrations to override the initial low concentration inhibition of GCs may be due to a higher density of α1 than α2 receptors. Alternatively, α1 receptors may be preferentially distributed to GC dendrites where they may be more influential in controlling GABA release than somatic α2 receptors. The subcellular distribution of distribution of noradrenergic receptors on GCs is unknown. It is noteworthy, however, that the highest density of α1 receptors in the brain is in the external plexiform layer where GC distal dendrites ramify (Young and Kuhar, 1980).
Taken together with previous findings (Nai et al., 2009), our results suggest that modest increases in LC discharge leading to relatively small increases in NE in the bulb would decrease GABAergic inhibition of mitral cells. Functionally, mitral cell disinhibition would be expected to increase sensitivity to odorants (i.e., lower detection threshold) and/or impair discrimination among odorants (Yokoi et al., 1995). More vigorous levels of LC discharge and higher levels of NE release would lead to preferential activation of α1 receptors and increased inhibition. This would increase odorant detection threshold, but increase odorant discrimination (Linster and Hasselmo, 1997). Partially consistent with these predictions, α1 receptor blockade in the MOB impaired spontaneous odor discrimination in adult rats (Mandairon et al. 2008). Blockade of α2 receptors produced modest but non-significant reductions in odor discrimination, a result that may be complicated by presynaptic enhancement of NE release in vivo by α2 antagonists.
Previous studies have demonstrated non-linear relationships between LC neuronal firing rates (and presumably NE release) and vigilance in visual discrimination tasks (Aston-Jones et al 1994). Similar non-linear relationships have been observed between postsynaptic NE levels and thalamic and cortical neuronal excitability (Kawaguchi and Shindou 1998; Waterhouse et al. 1998; Berridge and Waterhouse 2003; Devilbiss and Waterhouse 2000, 2004; Devilbiss et al. 2006). Our findings suggest that there may be a non-linear relationship between the level of NE and the excitability of GCs. Functionally, the differing affinity of noradrenergic receptor subtypes appears to allow for dynamic modulation of GABAergic inhibition in MOB as function of the extracellular NE concentration, which in turn, is regulated by behavioral state.
Supplementary Material
Supplemental Figure 1. Long lasting but reversible effects of noradrenergic receptor agonists on GC membrane potential. Application of PE (10 µM, n=8 GCs) or Clon (10 µM, n=6 GC cells) for four min (denoted at horizontal lines beginning at time 0) in the presence of APV-CNQX-gabazine produced depolarizing or hyperpolarizing effects, respectively, that peaked at 10−15 min. Membrane potentials significantly declined from peak value at 20 min and returned to pre-agonist basal values by 30 min. *p<0.05; NS, non-significant (p>0.05), Anova followed by Newman-Keuls tests.
Acknowledgement
This study was supported by NIH grants DC008702 (CRCNS Award Program) and DC03195, and the contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We thank Ms. Ying Jin for excellent assistance with confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Araneda RC, Firestein S. Adrenergic enhancement of inhibitory transmission in the accessory olfactory bulb. J Neurosci. 2006;26(12):3292–3298. doi: 10.1523/JNEUROSCI.4768-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DW, Doze VA, Madison DV, Smith SJ. Excitatory actions of norepinephrine of multiple classes of hippocampal CA1 interneurons. J Neurosci. 1996;16:572–585. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge C, Waterhouse B. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Czesnik D, Nezlin L, Rabba J, Muller B, Schild D. Noradrenergic modulation of calcium currents and synaptic transmission in the olfactory bulb of Xenopus laevis. Eur J Neursci. 2001;13:1093–1100. doi: 10.1046/j.0953-816x.2001.01479.x. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Page ME, Waterhouse BD. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci. 2006;26:9860–9872. doi: 10.1523/JNEUROSCI.1776-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action in sensory cortical neuronal responses to excitatory stimuli. Synapse. 2000;37:273–282. doi: 10.1002/1098-2396(20000915)37:4<273::AID-SYN4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci. 2004;24:10773–10785. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyancic AV, Morilak DA. Distribution of alpha1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J Comp Neurol. 1997;386:358–378. doi: 10.1002/(sici)1096-9861(19970929)386:3<358::aid-cne3>3.0.co;2-0. 1997. [DOI] [PubMed] [Google Scholar]
- Dong HW, Buonomano DV. A technique for repeated recordings in cortical organotypic slices. J Neurosci Methods. 2005;146:69–75. doi: 10.1016/j.jneumeth.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Dong HW, Hayar A, Ennis M. Activation of group I metabotropic glutamate receptors on main olfactory bulb granule cells and periglomerular cells enhances synaptic inhibition of mitral cells. J Neurosci. 2007;27:5654–5663. doi: 10.1523/JNEUROSCI.5495-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn Mem. 2007;14:539–547. doi: 10.1101/lm.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Hayar A. Physiology of the Olfactory Bulb, The Senses: A Comprehensive Reference, Basbaum A, Kaneko A, Shepherd G, Eds.-In Chief. In: Firestein S, Beauchamp G, editors. Olfaction and Taste. Vol. 4. San Diego: Academic Press; 2008. pp. 641–686. [Google Scholar]
- Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol (Lond) 1987;393:213–231. doi: 10.1113/jphysiol.1987.sp016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Williams JT, Travagli RA. Inhibition by 5-hydroxytryptamine and noradrenaline in substantia gelatinosa of guinea-pig spinal trigeminal nucleus. J Physiol. 1995;485:113–120. doi: 10.1113/jphysiol.1995.sp020716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin D, Peace ST, Didier A, Linster C, Cleland TA. Noradrenergic neuromodulation in the olfactory bulb modulates odor habituation and spontaneous discrimination. Behav Neurosci. 2008;122:816–826. doi: 10.1037/a0012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C, Darby-King A, McCann J, McLean J. Beta1-adrenoceptor or alpha1-adrenoceptor activation initiates early odor preference learning in rat pups: support for the mitral cell/cAMP model of odor preference learning. Learn Mem. 2006;13:8–13. doi: 10.1101/lm.62006. [DOI] [PubMed] [Google Scholar]
- Hayar A, Heyward PM, Heinbockel T, Shipley MT, Ennis M. Direct excitation of mitral cells via activation of alpha1-noradrenergic receptors in rat olfactory bulb slices. J Neurophysiol. 2001;86:2173–2182. doi: 10.1152/jn.2001.86.5.2173. [DOI] [PubMed] [Google Scholar]
- Heinbockel T, Laaris N, Ennis M. Metabotropic glutamate receptors in the main olfactory bulb drive granule cell-mediated inhibition. J Neurophysiol. 2007;97:858–870. doi: 10.1152/jn.00884.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi H, Yoshimura M, Polosa C, Nishi S. Adrenergic receptors (α1 and α2) modulate different potassium conductances in sympathetic preganglionic neurons. Can J Physiol Pharmacol. 1992;70:S92–S97. doi: 10.1139/y92-249. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. Noradrenergic modulation of dendrodendritic inhibition in the olfactory bulb. Nature. 1982;297:227–229. doi: 10.1038/297227a0. [DOI] [PubMed] [Google Scholar]
- Jones LS, Gauger LL, Davis JN. Anatomy of brain alpha1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J Comp Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J Neurosci. 1998;18:6963–6976. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Hasselmo M. Modulation of inhibition in a model of olfactory bulb reduces overlap in the neural representation of olfactory stimuli. Behav Brain Res. 1997;84:117–127. doi: 10.1016/s0166-4328(97)83331-1. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Peace S, Karnow A, Kim J, Ennis M, Linster C. Noradrenergic modulation in the olfactory bulb influences spontaneous and reward-motivated discrimination, but not the formation of habituation memory. Eur J Neurosci. 2008;27(5):1210–1219. doi: 10.1111/j.1460-9568.2008.06101.x. [DOI] [PubMed] [Google Scholar]
- McCune SK, Voigt MM, Hill JM. Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neurosci. 1993;57:143–151. doi: 10.1016/0306-4522(93)90116-w. [DOI] [PubMed] [Google Scholar]
- McLean JH, Shipley MT, Nickell WT, Aston-Jones G, Reyher CKH. Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol. 1989;285:339–349. doi: 10.1002/cne.902850305. [DOI] [PubMed] [Google Scholar]
- Nai Q, Dong HW, Hayar A, Linster C, Ennis M. Noradrenergic regulation of GABAergic inhibition of main olfactory bulb mitral cells varies as a function of concentration and receptor subtype. J Neurophysiol. 2009;101(5):2472–2484. doi: 10.1152/jn.91187.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Yoshimura M. The actions of noradrenaline on neurones of the rat sunstantia gelatinosa in vitro. J Physiol. 1984;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Matsuo T. The effects of catecholamines on electrical activity of neurons the guinea pig supraoptic nucleus in vitro. Brain Res. 1986;385:122–135. doi: 10.1016/0006-8993(86)91553-2. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Grudt TJ, Williams JT. Alpha 1-adrenoceptors in rat dorsal raphe neurons: regulation of two potassium conductances. J Physiol. 1994;478:437–447. doi: 10.1113/jphysiol.1994.sp020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of α1 adrenoreceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong E, Magistretti PJ. Noradrenaline increase K-conductance and reduces glutamatergic transmission in the mouse entorhinal cortex by activation of α2-adrenoreceptors. Eur J Neurosc. 1995;7:2370–2378. doi: 10.1111/j.1460-9568.1995.tb01034.x. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Halloran FJ, de la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res. 1985;329:294–299. doi: 10.1016/0006-8993(85)90537-2. [DOI] [PubMed] [Google Scholar]
- Smith RS, Weitz CJ, Araneda RC. Excitatory actions of noradrenaline and metabotropic glutamate receptor activation in granule cells of the accessory olfactory bulb. J Neurophysiol. 2009;102(2):1103–1114. doi: 10.1152/jn.91093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Stackenwalt G, Nasr F, Lemon C, Wilson D. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci. 2000;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley E, Lei Q, Sirois J, Bayliss D. TASK-1, a two–pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of α2a-adrenergic receptor like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ. Norepinephrine inhibits calcium currents and EPSPs via a G-protein-coupled mechanism in olfactory bulb neurons. J Neurosci. 1992;12:3992–3998. doi: 10.1523/JNEUROSCI.12-10-03992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ, Shepherd GM. Noradrenergic inhibition of synaptic transmission between mitral and granule cells in mammalian olfactory bulb cultures. J Neurosci. 1992;12:3985–3991. doi: 10.1523/JNEUROSCI.12-10-03985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Bandler R, Christie MJ. Differential responses of lateral and ventrolateral rat periaqueductal grey neurones to noradrenaline in vitro. J Physiol. 1996;490:373–381. doi: 10.1113/jphysiol.1996.sp021151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrac A, Nguyen V, Marien M, Didier A, Jourdan F. Noradrenergic control of odor recognition in a nonassociative olfactory learning task in the mouse. Learn Mem. 2007;17:847–854. doi: 10.1101/lm.708807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J Neurosci. 1993;13:2199–2216. doi: 10.1523/JNEUROSCI.13-05-02199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse BD, Devilbiss D, Fleischer D, Sessler FM, Simpson KL. New perspectives on the functional organization and postsynaptic influences of the locus ceruleus efferent projection system. Adv Pharmacol. 1998;42:749–754. doi: 10.1016/s1054-3589(08)60856-x. [DOI] [PubMed] [Google Scholar]
- Williams JA, Reiner PB. Noradrenaline hyperpolarizes identified rat mesopontine cholinergic neurons in vitro. J Neurosci. 1993;13:3878–3883. doi: 10.1523/JNEUROSCI.13-09-03878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoreceptors during rat brain development-I. α2A messenger RNA expression. Neurosci. 1997a;76:241–260. doi: 10.1016/s0306-4522(96)00368-5. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoreceptors during rat brain development-II. α2C messenger RNA expression and [3H]rauwolscine binding. Neurosci. 1997b;76:261–272. doi: 10.1016/s0306-4522(96)00369-7. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Inenaga K, Kannan H. Depolarizing effect of noradrenaline in neurons the rat supraoptic nucleus in vitro. Brain Res. 1987;405:348–352. doi: 10.1016/0006-8993(87)90304-0. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, Kuhar MJ. Noradrenergic a1 and a2 receptors: autoradiographic visualization. Eur J Pharmacol. 1979;59:317–319. doi: 10.1016/0014-2999(79)90299-1. [DOI] [PubMed] [Google Scholar]
- Young WS, Kuhar MJ. Noradrenergic alpha1 and alpha2 receptors: light microscopic autoradiographic localization. Proc Natl Acad Sci USA. 1980;77:1696–1700. doi: 10.1073/pnas.77.3.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Long lasting but reversible effects of noradrenergic receptor agonists on GC membrane potential. Application of PE (10 µM, n=8 GCs) or Clon (10 µM, n=6 GC cells) for four min (denoted at horizontal lines beginning at time 0) in the presence of APV-CNQX-gabazine produced depolarizing or hyperpolarizing effects, respectively, that peaked at 10−15 min. Membrane potentials significantly declined from peak value at 20 min and returned to pre-agonist basal values by 30 min. *p<0.05; NS, non-significant (p>0.05), Anova followed by Newman-Keuls tests.