Abstract
Reduced intensity conditioning (RIC) regimens have been used extensively in adults with hematological malignancies. To address whether this is a feasible approach for children with acute lymphoblastic leukemia (ALL), we evaluated transplant outcomes in 38 recipients transplanted from 1995–2005 for whom this was their first transplant. The median age at transplant was 12 years and 47% had performance scores <90%. Disease status was first complete remission (CR) in 13%, ≥CR2 in 60% of patients and 22% had active disease at transplantation. Matched related donors were available for a third of patients and about half of whom received bone marrow (BM) and the others, peripheral blood progenitor cells (PBPC). Sixty percent of unrelated donor transplant recipients received PBPC. The day-100 probability of grade 2–4 acute GVHD was 37% and the 3-year probability of chronic GVHD, 26%. At 3-years, the probability of transplant related mortality was 40%, relapse, 37% and disease-free survival (DFS), 30%. These data indicate long-term DFS can be achieved using RIC regimens in children with ALL. Given the relatively small cohort, these findings must be validated in a larger population.
Keywords: pediatric, ALL, reduced intensity conditioning
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is one of the most commonly diagnosed pediatric malignancies. Using conventional chemotherapy, up to 80% of patients are expected to achieve long term hematological remission (1). However, the high prevalence of ALL in the pediatric population means that recurrent ALL remains a common indication for allogeneic hematopoietic cell transplantation (allo-HCT). Three-year disease free survival (DFS) for children transplanted in second complete remission (CR) using myeloablative conditioning and allo-HCT has been reported to be ~30–40%, with some studies reporting DFS as high as 70% (2). In common with most transplant studies, treatment failure is due to high rates of both transplant-related mortality (TRM) and disease recurrence.
Over the past 10 years considerable experience has been gained using reduced intensity conditioning (RIC) regimens for allo-HCT. While a number of different regimens have been tested, one unifying feature is that they have the potential for acceptable rates of donor engraftment and lower TRM relative to conventional or dose-intensive myeloablative conditioning. Because most children tolerate conventional dose-intensive conditioning and TRM increases with age, RIC has mainly been reserved for older patients or those with poor performance status.
One fundamental difference between RIC and myeloablative conditioning is the mechanism of disease control. With myeloablative conditioning, relapse protection is provided by the dose-intensive chemotherapy and/or total body irradiation and the allogeneic, graft-versus-leukemia (GVL) effect. In contrast, the lower dose of chemotherapy and/or irradiation associated with RIC may provide little up-front disease control and thus, the efficacy of RIC has been ascribed to the post-transplant GVL effect. While GVL reactions are difficult to document in real-time, both the kinetics of disease re-growth and responsiveness to GVL have had bearing on patient selection for RIC. Thus, RIC has been more commonly used in patients with chronic leukemia and indolent lymphoma (3). Enthusiasm for using RIC for ALL has been appropriately guarded, due to the poor responsiveness to post-HCT immune-based approaches in ALL, including rapid tapering of immune suppression and donor lymphocyte infusion following relapse (4, 5).
Under certain circumstances, RIC may be indicated for patients with ALL who require allo-HCT, but are ineligible for a dose-intensive conditioning. Such indications include: poor performance status, active infections, significant organ dysfunction or advanced age. Transplant outcome data after various RIC regimens for ALL are few and, with one exception are limited to reports in adults. To date, six reports have focused on the outcomes of HCT with RIC for ALL (6–11) while other reports have included patients with ALL and other leukemias (12–14). The report by Pulsipher and colleagues (14) is the only one limited to children and only 17 of 47 patients in that report had ALL. Thus, the effectiveness of RIC regimens for pediatric ALL has not been extensively reported. Here, we detail outcomes for children with ALL who received RIC allo-HCT as their first transplant and reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
METHODS
Data Collection
The CIBMTR is a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on consecutive allogeneic and autologous transplants to a Statistical Center at the Medical College of Wisconsin. Participating centers are required to report all consecutive transplants. Data collected include disease type, age, sex, pre-transplant disease stage and chemotherapy responsiveness, date of diagnosis, graft type, conditioning regimen, post-transplant disease progression and survival, development of a new malignancy, and cause of death. A subset of the reported transplants are selected for detailed reporting using a weighted randomization scheme and include detailed disease, and pre- and post-transplant clinical information. All subjects are followed longitudinally, with yearly follow-up. Computerized error checks, physicians’ review of submitted data and on-site audits of participating centers ensure data quality and compliance. As stated above, there has only been one other study to report outcomes of for RIC in pediatric ALL (PBMTC ONC313) (14). Based on a number of factors (participating centers, conditioning regimen, date of transplant), only one patient (UPN #9) has potentially participated in the above trial. This study was approved by the Institutional Review Board of the Medical College of Wisconsin.
Endpoints
Neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) of ≥0.5 × 109/L for three consecutive days and platelet recovery, ≥20 × 109/L for 7 days, unsupported. Diagnoses of grades 2, 3 and 4 acute graft-versus-host disease (GVHD) and chronic GVHD were based on published criteria (15, 16). TRM was defined as death in continuous CR and relapse, hematologic leukemia recurrence. Treatment failure (inverse of DFS) was defined as death from any cause or relapse.
Statistical Methods
The probability of neutrophil and platelet recovery, acute and chronic GVHD, TRM and relapse were calculated with the use of the cumulative-incidence-function method (17). For neutrophil and platelet recovery and GVHD, death without the event (hematopoietic recovery or GVHD) was the competing event. Data on patients without an event were censored at last contact. For TRM, relapse was the competing event and for relapse, TRM, the competing event. Univariate probabilities of DFS and overall survival were calculated using the Kaplan-Meier estimator (17). For DFS, death or relapse were events and patients alive and in remission were censored at last contact. For overall survival, death from any cause was an event and patients alive were censored at last contact. All p-values are two-sided and analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient Demographics
Table 1 shows the pre-transplant characteristics of the 38 patients aged ≤18 years with ALL who received RIC allo-HCT as their first transplant. Transplantations occurred between 1995 and 2005. Most patients (66%) were between the ages of 11 and 18 years. Approximately one half (47%) had performance scores <90. Most patients were in second or subsequent CR at HCT. For patients transplanted in CR2 or beyond CR2, the median duration of CR1 was 32 (range: 6–89) months. Approximately 20% of patients had active disease at time of HCT, (either primary induction failure [n=1] or in relapse [n=7]). The median follow-up for surviving patients was 48 (range 3–131) months.
Table 1.
Patient, disease and transplant characteristics
| Variables | Number |
|---|---|
| Number of patients | 38 |
| Age, years | |
| 1–10 | 13 |
| 11–18 | 25 |
| Recipient cytomegalovirus seropositivity | 20 |
| Performance score | |
| 90–100 | 18 |
| <90 | 19 |
| Unknown | 1 |
| Disease Status Prior to Transplant | |
| 1st complete remission | 5 |
| 2nd complete remission | 13 |
| 3rd complete remission | 5 |
| 4th complete remission | 5 |
| Relapse/primary induction failure | 8 |
| Unknown | 2 |
| Conditioning regimen | |
| Total body irradiation + other agents | 13 |
| Busulfan + other agents | 12 |
| Cyclophosphamide + other agents | 6 |
| Melphalan+ other agents | 7 |
| Type of donor | |
| HLA-identical sibling | 12 |
| Matched related | 1 |
| Unrelated donor | 25 |
| GVHD prophylaxis | |
| T-cell depletion | 2 |
| Calcineurin inhibitor ± other agents | 17 |
| Calcineurin inhibitor + methotrexate | 16 |
| Methotrexate + other agents | 1 |
| Unknown | 2 |
Stem cell Sources and Conditioning Regimens
There was an increase in the use of RIC during the study period; approximately one third of transplants in the current analysis were performed from 1995–2000 and two-thirds, from 2001–2005. Thirteen patients received allografts from matched related donors and the remaining patients received allografts from unrelated donors (Tables 2 and 3). Regimens were considered RIC if the cumulative dose of busulfan was <9 mg/kg, melphalan <150 mg/m2 or total body irradiation (TBI) ≤ 450 cGy, single fraction or 600–800 cGy, multiple fractions (18). Regimens that combined busulfan and melphalan were considered to be myeloablative. The decision to use RIC for patients was at the discretion of the transplant center and 10 of 38 patients were treated on institutional protocols. None of the patients were reported to have organ dysfunction (renal, cardiac or pulmonary) or a life threatening infection immediately prior to transplantation. GVHD prophylaxis varied though most patients received calcinuerin-inhibitor containing GVHD prophylaxis (Table 1).
Table 2.
Characteristics of patients who died of leukemia relapse or transplant-related complication
| Patient | Age | Performance score | Disease status | Donor/Graft | Conditioning regimen | Acute GVHD | Chronic GVHD | Time to death | Primary cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | 80 | CR2 | MSD, BM | TBI (100 cGy) + Mel (136 mg/m2) | grade 4 | 3 months | GVHD | |
| 2 | 10 | 20 | relapse | MSD, BM | Bu (5mg/kg) + Cy | 1 month | organ failure | ||
| 3 | 2 | 90 | relapse | Unrelated, CB | Cy + etoposide + ATG | 1 month | infection | ||
| 4* | 16 | 70 | relapse | MSD, PBPC | TBI (450 cGy) + FLU + ATG | 1.5 month | relapse | ||
| 5* | 12 | 50 | CR2 | MSD, PBPC | Cy + FLU + ATG | 11 months | relapse | ||
| 6 | 3 | 40 | unknown | Unrelated, BM | Cy + FLU | 4 months | relapse | ||
| 7 | 7 | 90 | CR2 | Unrelated, PBPC | FLU + Mel (140 mg/m2) + ATG | grade 3 | 2 months | GVHD | |
| 8 | 12 | 90 | CR2 | Unrelated, PBPC | Bu (8 mg/kg)+ FLU + ATG | extensive | 7 months | relapse | |
| 9 | 8 | 80 | CR2 | Unrelated, CB | Bu (8 mg/kg) + FLU + ATG | 18 months | infection | ||
| 10 | 6 | 80 | relapse | Unrelated, PBPC | TBI (400 cGy) + Bu (6 mg/kg) + FLU | grade 4 | extensive | 7 months | GVHD |
| 11 | 11 | 80 | CR4 | Unrelated, PBPC | FLU + Mel (111 mg/m2) + ATG | 2 months | hemorrhage | ||
| 12 | 14 | 80 | Induction failure | MSD, PBPC | Bu (6 mg/kg) + etoposide + FLU + ATG | grade 4 | extensive | 4 months | GVHD |
| 13* | 16 | 100 | CR2 | MSD, BM | TBI (600 cGy, frac) + Cy | grade 2 | extensive | 11 months | GVHD |
| 14* | 12 | 90 | CR3 | Unrelated, PBSC | TBI (450 cGy)+ FLU + alemtuzumab | 7 months | relapse | ||
| 15 | 13 | 100 | CR3 | Unrelated, PBPC | FLU + Mel (120 mg/m2)+ thiotepa | 7 months | relapse | ||
| 16 | 13 | 90 | CR4 | Unrelated, PBPC | Bu (8 mg/kg) + FLU + ATG | grade 2 | extensive | 30 months | relapse |
| 17 | 13 | 80 | CR4 | Unrelated, BM | Bu (6 mg/kg) + FLU + ATG | grade 2 | extensive | 25 months | GVHD |
| 18 | 15 | 100 | CR2 | Unrelated, PBPC | Bu (6 mg/kg) + FLU + alemtuzumab | grade 3 | 2 months | GVHD | |
| 19* | 15 | 80 | CR1 | Unrelated, CB | TBI (600 cGy frac) + Cy + Mel (137 mg/m2) + ATG | 4 months | organ failure | ||
| 20 | 18 | 80 | CR4 | Unrelated, PBPC | FLU + Mel (100 mg/m2) + ATG | grade 2 | limited | 43 months | relapse |
| 21* | 17 | 70 | CR4 | Unrelated, PBPC | FLU + Mel (130 mg/m2) + alemtuzumab | grade 2 | 9 months | relapse | |
| 22 | 13 | 100 | CR2 | Unrelated, BM | TBI (600 cGy frac) + FLU + alemtuzumab | 2 months | relapse | ||
| 23 | 17 | 100 | CR2 | Unrelated, PBPC | Bu (7 mg/kg) + FLU | grade 3 | 3 months | GVHD |
Abbreviations: CR = complete remission; BM = bone marrow; PBPC = peripheral blood progenitor cells; MSD = matched sibling donor; TBI = total body irradiation; Frac = fractionated TBI dose; Bu = busulfan; Cy = cyclophosphamide; FLU = fludarabine; ATG = anti-thymocyte globulin; Mel = melphalan
Intervention post RIC HCT and outcome
Patient # 4: donor lymphocyte infusion (DLI) for leukemia relapse 1 month after RIC HCT and died 15 days after DLI
Patient # 5: DLI for mixed chimerism 5 months after RIC HCT and died 2 months after DLI
Patient # 13: DLI for leukemia relapse 6 months after RIC HCT and died 5 months after DLI
Patient # 14: DLI for leukemia relapse 5 months after RIC HCT and died 2 months after DLI
Patient # 19: Second allo-HCT (different donor) for mixed chimerism 3 months after RIC HCT and 1 month after second HCT. Conditioning regimen for second HCT is not known
Patient # 21: DLI for leukemia relapse 8 month after RIC HCT and died 1 month after DLI
Table 3.
Characteristics of patients who are alive
| Patient | Age | Performance score | Disease status at transplant | Donor/graft | Conditioning regimen | Acute GVHD | Chronic GVHD | Time to last contact |
|---|---|---|---|---|---|---|---|---|
| 24 | 9 | 100 | relapse | Matched relative, BM | TBI (800 cGy fractionated) + Bu + Cy | extensive | 131 months | |
| 25 | 12 | 100 | CR3 | MSD, BM | Bu (8 mg/kg) + Cy + etoposide | 100 months | ||
| 26 | 16 | 80 | CR1 | MSD, BM | Cy + etoposide | 67 months | ||
| 27 | 6 | 100 | relapse | MSD, BM | Cy + ARAC + Mel (140 mg/m2) | 57 months | ||
| 28 | 7 | 100 | CR3 | MSD, PBPC | Cy + ARAC + Mel (142 mg/m2) | extensive | 48 months | |
| 29 | 12 | 100 | CR3 | Unrelated, PBPC | Bu (7 mg/kg) + FLU + ATG | 16 months | ||
| 30* | 14 | 80 | CR1 | Unrelated, CB | TBI (200 cGy) + Cy + FLU + ATG | grade 3 | 25 months | |
| 31 | 6 | Not reported | CR2 | Unrelated, CB | TBI (200 cGy) + Cy + FLU + ATG | grade 2 | 8 months | |
| 32 | 9 | 100 | CR2 | Unrelated, CB | TBI (200 cGy) + Cy + FLU + ATG | grade 2 | limited | 4 months |
| 33 | 15 | 90 | CR1 | MSD, PBPC | Bu (4 mg/kg) + FLU | 3 months | ||
| 34 | 16 | 100 | CR2 | MSD, PBPC | FLU + Mel (140 mg/m2) | 3 months | ||
| 35 | 7 | 80 | CR2 | Unrelated, PBPC | Bu (5 mg/kg) + FLU + ATG | 36 months | ||
| 36* | 3 | 80 | relapse | Unrelated, BM | TBI (450 cGy) + Cy + alemtuzumab | 37 months | ||
| 37 | 13 | 70 | CR1 | Unrelated, PBPC | TBI (200 cGy) + FLU | grade 3 | extensive | 61 months |
| 38 | 13 | 100 | unknown | Unrelated, PBPC | FLU + Mel (137 mg/m2) | grade 3 | extensive | 24 months |
Abbreviations: CR = complete remission; BM = bone marrow; PBPC = peripheral blood progenitor cells; MSD = matched sibling donor; TBI = total body irradiation; Bu = busulfan; Cy = cyclophosphamide; Mel = melphalan; FLU = fludarabine; ATG = anti-thymocyte globulin
Intervention post RIC HCT and outcome
Patient # 30: Second HCT (same donor, TBI 1200 cGY + Cy) for leukemia relapse 21 months after RIC HCT, leukemia relapse and third HCT 36 months after second HCT. Patient is alive, 4 months from the third HCT
Patient # 36: DLI for cytogenetic relapse 4 months after RIC HCT, and alive, 33 months after DLI
Transplant Associated Outcomes
All patients developed severe neutropenia (ANC ≥ 0.5 × 109/L) after transplant conditioning and thirty-five of 38 patients achieved neutrophil recovery. The day-28 probability of neutrophil recovery was 82% (95% CI 67 – 92); 31 of 38 patients. The remaining 4 patients remained neutropenic for a longer period. The day-100 probability of platelet recovery was 79% (95% CI 65 – 90). Twenty-four patients with ANC ≥ 0.5 × 109/L had chimerism assay performed. Donor engraftment (>90% donor derived cells in the peripheral blood or marrow) was observed in 21 patients (88%) <3 months after transplant. Some patients (n=9) had serial chimerism assays performed as late as 2 years. The frequency and timing of chimerism assay was the discretion of the transplant center. One patient received donor leukocyte infusion (DLI) and another patient, second transplant for treatment of mixed chimerism without evidence of relapse. For the patient who received DLI, this occurred 4 months after the first transplant and this patient died 7 months later of recurrent leukemia and the second transplant recipient underwent this procedure 4 months after the first transplant and died one month later from organ failure (Table 2).
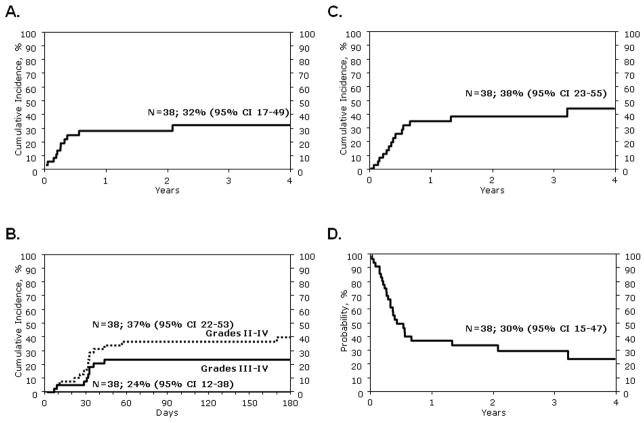
In univariate analysis, the probability of TRM at 100 days and 3 years was 19% (95% CI 8–33%) and 32% (95% CI 17–49%), respectively (Figure 1A). Sixteen patients developed grade 2 and 9 patients, grade 3–4 acute GVHD (Tables 2, 3). The day-100 probability of grade 2–4 acute GVHD was 37% (95% CI 22–53%) and grade 3–4 acute GVHD, 24% (95% CI 12–38%) (Figure 1B). The probability of grade 2–4 or 3–4 acute GVHD did not change significantly at day-180. Chronic GVHD occurred in 12 of 38 patients (Tables 2, 3). The 3-year probability of chronic GVHD was 26% (95% CI 12–42%), with the severity reported as limited in 2 patients and extensive in 10 patients.
Figure 1.
Figure 1A. Probability of transplant-related mortality
Figure 1B. Probabilities of grade 2–4 and 3–4 acute graft-versus-host disease
Figure 1C. Probability of leukemia relapse
Figure 1D. Probability of disease-free survival
Leukemia relapse occurred in 14 patients post-HCT; the 3-year probability of relapse was 38% (95% CI 23–55%) (Figure 1C). One patient received a second HCT from a different unrelated donor and 5 patients, DLI. Twenty-three patients are dead, 14 from recurrent disease and 9 from a treatment-related complication (Table 2). Fifteen patients are alive at last follow-up (Table 3). The 3-year probabilities of DFS and OS were 30% (95% CI 15–47%) (Figure 1D) and 36% (95% CI 20–53%), respectively.
Transplant outcomes according to performance score at transplantation were evaluated as none of these patients were reported to have organ dysfunction or a life threatening infection just prior to initiation of transplant conditioning. We did not observe statistically significant differences in TRM (39% vs. 22%, p=0.27) and DFS (28% vs. 31%, p=0.84) in patients with performance scores <90 and 90–100, respectively. The relatively small numbers of patients may explain our inability to detect a significant difference despite an absolute difference of 17% for TRM. We also examined for an effect of TBI-containing regimens on DFS and found none (30% with TBI containing vs. 27% with non-TBI regimens, p=0.85).
DISCUSSION
In this report we describe the transplant outcomes for children aged ≤18 years with ALL who received RIC before their first allo-HSCT. We found that RIC regimens were associated with myelosuppression in all patients along with high rates of TRM and acute and chronic GVHD. Similarly, leukemia recurrence was also high, resulting in modest DFS rates at 3 years. Several patients reported herein had performance scores of 70 or 80 (Karnofsky or Lansky scale) and/or were in second or subsequent CR at HCT and were thus at significant risk for transplant-related complications and/or leukemia relapse.
An important limitation of this registry study is the lack of information regarding the rationale for the selection of RIC. We speculate patients who received RIC were either treated on an institutional protocol (26% of study population) or judged to be at high risk for transplant-related mortality by the treating physician based on intensity of therapies received prior to HCT even though none of the patients were reported to have renal, cardiac or pulmonary function dysfunction, poor performance score and/or more advanced disease status (beyond second CR) in some patients. There may also be several unmeasured factors that may have contributed to the selection of RIC for allo-HCT in these children. In this context, these data lend support to the notion that RIC regimens can expand HCT options for children and adolescents otherwise unsuitable for dose-intensive myeloablative conditioning who would succumb to their disease with chemotherapeutic regimens alone.
One of the perceived benefits to RIC has been the lower TRM relative to traditional dose-intensive conditioning. We observed TRM rates (19% at day-100 and 32% at 3-years) consistent with other reports describing outcomes for RIC with adult ALL, where TRM ranged from 17–40% (6–11). Moreover, in these reports, there appears to be a trend for higher TRM when patients with advanced disease or chemotherapy refractory leukemia are included. The high rate of TRM in our study sharply contrasts with the only other large multicenter pediatric RIC study were TRM was much lower (11%) (14). This difference might be explained by the use of a uniform conditioning regimen in the report by Pulsipher and colleagues (14), together with the fact that transplants were carried out in a more contemporary era than this analysis which spanned over a decade. Interestingly, Pulsipher and colleagues report relapse rates of 43% at 2-years which are higher than that observed in the current analysis. This may in part be attributed to the inclusion patients who had failed prior transplantation and at higher risk for recurrent leukemia compared to the population in this analysis. TRM is the competing event for relapse and TRM is expected to be low when recurrence rate is high.
Although RIC regimens are commonly used for adults with chronic leukemia and low to intermediate grade lymphoma (3), a major concern with using RIC regimens for pediatric ALL is the faster growth kinetic of ALL that might result in higher relapse rates. This concern is, perhaps, magnified by the fact that RIC relies mainly on immunological mechanisms for eradication of disease (i.e., GVL) and yet, post-HCT adoptive immunotherapies designed to exploit GVL, like infusions of donor lymphocytes or NK cells have been disappointing for treating relapsed ALL (4, 19). However, in the current study, the relapse rate at 3-years was 38% which is comparable to that after dose-intensive myeloablative conditioning regimens in children with ALL (2) and to that reported after RIC allo-HCT in adults with ALL (6–8, 10, 11). Most patients in the current analysis were in second or subsequent CR or had active disease at HCT, and despite the relatively high relapse rate, one third of the patients are alive and disease-free which supports the notion that sustained remission in ALL can also be achieved in children after RIC and allo-HCT. Given the relatively small numbers of patients in the current analysis these findings must be validated in a larger series. Further, the relatively small numbers of patients prevented us from examining for an effect of acute or chronic GVHD on relapse in the current analysis. Nevertheless, a potent effect of acute GVHD in reducing relapse in children with ALL receiving unrelated donor transplants after dose-intensive myeloablative conditioning has been reported, suggesting a role for immune based mediation of relapse risk (20). Similarly, lower doses of immune suppression (cyclosporine A) after transplantation have been associated with relapse protection in ALL (21, 22). In some studies adoptive transfer of donor lymphocytes can reverse rising host chimerism associated with minimal residual disease (23). As well, an association between chronic GVHD and sustained remission following RIC and allo-HCT has been reported for ALL (11). Taken together, these reports support the assertion that ALL is sensitive to a GVL effect (24–26).
Despite the small numbers of patients and their heterogeneity with respect to performance score, disease status at HCT and donor source, this is the first report on use of RIC regimens for pediatric ALL. All children received RIC and allo-HCT as their first transplant. The observed 3-year DFS rates are comparable to those after dose-intensive myeloablative conditioning regimens and allo-HCT. While our observations must be interpreted with caution, the modest success reported herein offers a potentially life-saving treatment option to children who may otherwise not be eligible for allo-HCT. Only a clinical trial that uses a uniform RIC regimen, GVHD prophylaxis and donor-graft source can further establish the role of RIC allo-HCT for pediatric ALL.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Eisai, Inc.; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gamida Cell, Ltd.; GE Healthcare; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Saladax Biomedical, Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; Teva Pharmaceutical Industries;; THERAKOS, Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 2.Hahn T, Wall D, Camitta B, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in children: an evidence-based review. Biol Blood Marrow Transplant. 2005;11:823–861. doi: 10.1016/j.bbmt.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Alousi A, de Lima M. Reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Clin Adv Hematol Oncol. 2007;5:560–570. [PubMed] [Google Scholar]
- 4.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 5.Elmaagacli AH, Beelen DW, Trenn G, Schmidt O, Nahler M, Schaefer UW. Induction of a graft-versus-leukemia reaction by cyclosporin A withdrawal as immunotherapy for leukemia relapsing after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;23:771–777. doi: 10.1038/sj.bmt.1701672. [DOI] [PubMed] [Google Scholar]
- 6.Arnold R, Massenkeil G, Bornhauser M, et al. Nonmyeloablative stem cell transplantation in adults with high-risk ALL may be effective in early but not in advanced disease. Leukemia. 2002;16:2423–2428. doi: 10.1038/sj.leu.2402712. [DOI] [PubMed] [Google Scholar]
- 7.Martino R, Giralt S, Caballero MD, et al. Allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning in acute lymphoblastic leukemia: a feasibility study. Haematologica. 2003;88:555–560. [PubMed] [Google Scholar]
- 8.Hamaki T, Kami M, Kanda Y, et al. Reduced-intensity stem-cell transplantation for adult acute lymphoblastic leukemia: a retrospective study of 33 patients. Bone Marrow Transplant. 2005;35:549–556. doi: 10.1038/sj.bmt.1704776. [DOI] [PubMed] [Google Scholar]
- 9.Cho BS, Lee S, Kim YJ, et al. Reduced-intensity conditioning allogeneic stem cell transplantation is a potential therapeutic approach for adults with high-risk acute lymphoblastic leukemia in remission: results of a prospective phase 2 study. Leukemia. 2009;23:1763–1770. doi: 10.1038/leu.2009.102. [DOI] [PubMed] [Google Scholar]
- 10.Bachanova V, Verneris MR, DeFor T, Brunstein CG, Weisdorf DJ. Prolonged survival in adults with acute lymphoblastic leukemia after reduced-intensity conditioning with cord blood or sibling donor transplantation. Blood. 2009;113:2902–2905. doi: 10.1182/blood-2008-10-184093. [DOI] [PubMed] [Google Scholar]
- 11.Mohty M, Rocha V, Chevallier P, Harousseau JL, Nagler A. Reduced-intensity conditioning for allogeneic stem cell transplantation: 10 years later. Curr Opin Oncol. 2009;21 (Suppl 1):S1. doi: 10.1097/01.cco.0000357466.38219.0f. [DOI] [PubMed] [Google Scholar]
- 12.Michallet M, Bilger K, Garban F, et al. Allogeneic hematopoietic stem-cell transplantation after nonmyeloablative preparative regimens: impact of pretransplantation and posttransplantation factors on outcome. J Clin Oncol. 2001;19:3340–3349. doi: 10.1200/JCO.2001.19.14.3340. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Arguelles GJ, Gomez-Almaguer D, Ruiz-Arguelles A, Gonzalez-Llano O, Cantu OG, Jaime-Perez JC. Results of an outpatient-based stem cell allotransplant program using nonmyeloablative conditioning regimens. Am J Hematol. 2001;66:241–244. doi: 10.1002/ajh.1051. [DOI] [PubMed] [Google Scholar]
- 14.Pulsipher MA, Boucher KM, Wall D, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114:1429–1436. doi: 10.1182/blood-2009-01-196303. [DOI] [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 16.Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75:2459–2464. [PubMed] [Google Scholar]
- 17.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 20.Davies SM, Wang D, Wang T, et al. Recent decrease in acute graft-versus-host disease in children with leukemia receiving unrelated donor bone marrow transplants. Biol Blood Marrow Transplant. 2009;15:360–366. doi: 10.1016/j.bbmt.2008.12.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacigalupo A, Van Lint MT, Occhini D, et al. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood. 1991;77:1423–1428. [PubMed] [Google Scholar]
- 22.Locatelli F, Zecca M, Rondelli R, et al. Graft versus host disease prophylaxis with low-dose cyclosporine-A reduces the risk of relapse in children with acute leukemia given HLA-identical sibling bone marrow transplantation: results of a randomized trial. Blood. 2000;95:1572–1579. [PubMed] [Google Scholar]
- 23.Bader P, Klingebiel T, Schaudt A, et al. Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children. Leukemia. 1999;13:2079–2086. doi: 10.1038/sj.leu.2401581. [DOI] [PubMed] [Google Scholar]
- 24.Gassas A, Sung L, Saunders EF, Doyle J. Graft-versus-leukemia effect in hematopoietic stem cell transplantation for pediatric acute lymphoblastic leukemia: significantly lower relapse rate in unrelated transplantations. Bone Marrow Transplant. 2007;40:951–955. doi: 10.1038/sj.bmt.1705853. [DOI] [PubMed] [Google Scholar]
- 25.Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol. 2008;142:877–888. doi: 10.1111/j.1365-2141.2008.07260.x. [DOI] [PubMed] [Google Scholar]
- 26.Passweg JR, Tiberghien P, Cahn JY, et al. Graft-versus-leukemia effects in T lineage and B lineage acute lymphoblastic leukemia. Bone Marrow Transplant. 1998;21:153–158. doi: 10.1038/sj.bmt.1701064. [DOI] [PubMed] [Google Scholar]