Abstract
Protein C is a vitamin K-dependent anticoagulant serine protease zymogen in plasma which upon activation by the thrombin-thrombomodulin complex down-regulates the coagulation cascade by degrading cofactors Va and VIIIa by limited proteolysis. In addition to its anticoagulant function, activated protein C (APC) also binds to endothelial protein C receptor (EPCR) in lipid-rafts/caveolar compartments to activate protease- activated receptor 1 (PAR-1) thereby eliciting antiinflammatory and cytoprotective signaling responses in endothelial cells. These properties have led to FDA approval of recombinant APC as a therapeutic drug for severe sepsis. The mechanism by which APC selects its substrates in the anticoagulant and antiinflammatory pathways is not well understood. Recent structural and mutagenesis data have indicated that basic residues of three exposed surface loops known as 39-loop (Lys-37, Lys-38, and Lys-39), 60-loop (Lys-62, Lys-63, and Arg-67), and 70-80-loop (Arg-74, Arg-75, and Lys-78) (chymotrypsin numbering) constitute an anion binding exosite in APC that interacts with the procoagulant cofactors Va and VIIIa in the anticoagulant pathway. Furthermore, two negatively charged residues on the opposite side of the active-site of APC on a helical structure have been demonstrated to determine the specificity of the PAR-1 recognition in the cytoprotective pathway. This article will review the mechanism by which APC exerts its proteolytic function in two physiologically inter-related pathways and how the structure-function insights into determinants of the specificity of APC interaction with its substrates in two pathways can be utilized to tinker with the structure of the molecule to obtain APC derivatives with potentially improved therapeutic profiles.
Keywords: APC, EPCR, PAR-1, Thrombomodulin, Anticoagulant, Antiinflammatory, Specificity
1. Introduction
Protein C is a single chain vitamin K-dependent plasma serine protease zymogen that upon activation by the thrombin-thrombomodulin (TM) complex down-regulates the clotting cascade by a feedback loop inhibition mechanism [1–3]. Activated protein C (APC) circulates in plasma as a light and heavy chain molecule held together by a single disulfide bond [2]. The N-terminal light chain of APC contains the non-catalytic γ-carboxyglutamic (Gla) domain followed by two epidermal growth factor (EGF)-like domains [2]. The catalytic domain of APC, with a trypsin-like primary specificity pocket, is located on the C-terminal heavy chain of the molecule [2,4]. The Gla domain, with nine vitamin K-dependent γ-carboxylated Glu residues, mediates the Ca2+-dependent interaction of APC with protein S on negatively charged membrane surfaces [2,5]. Protein S is a vitamin K-dependent plasma cofactor which promotes the anticoagulant function of APC in the proteolytic degradation of the procoagulant cofactors factors Va (FVa) and VIIIa (FVIIIa) [3,5]. The APC cleavage of these procoagulant cofactors shuts down thrombin generation through both intrinsic and extrinsic pathways [1–3]. Insight into the importance of the APC anticoagulant pathway in the regulation of blood coagulation can be gleaned from the observation that a heterozygous protein C deficiency is associated with a high risk of venous thrombosis and its homozygous deficiency causes purpura fulminans, which is fatal unless treated by protein C replacement therapy [6]. A complete protein C deficiency in mice results in lethal perinatal consumptive coagulopathy, as demonstrated by the targeted gene disruption [7].
In addition to its anticoagulant function, APC also exhibits potent cytoprotective, antiinflammatory and profibrinolytic properties [8–11]. The protective cellular activities of APC require the Gla domain-dependent interaction of the protease with endothelial protein C receptor (EPCR) on the surface of vascular endothelial cells [10,12,13]. The importance of the EPCR-dependent APC regulation of the inflammatory pathways has been demonstrated in animal models of septic shock where blocking either the thrombin-TM activation of protein C or blocking the interaction of APC with EPCR by specific monoclonal antibodies converts a sub-lethal dose of E. coli to a lethal phenotype with the characteristic multiple organ failure observed in severe sepsis [8,14]. The protective anticoagulant and antiinflammatory activities of APC have led to FDA approval of recombinant APC as a therapeutic drug for treating severe sepsis [15]. The mechanism by which APC functions in the antiinflammatory pathway is not fully understood. It has been hypothesized that the interaction of APC with EPCR renders the protease capable of cleaving the exodomain of protease activated receptor 1 (PAR-1), thereby eliciting cytoprotective and antiinflammatory signaling responses in vascular endothelial cells [9,10,16]. Nevertheless, since thrombin is the only known physiological activator of protein C which can activate PAR-1 with 3–4 orders of magnitude higher catalytic efficiency to elicit proinflammatory responses [17,18], it is not yet clear how APC initiates protective responses in endothelial cells through the cleavage of the same receptor. This article will provide an overview of the mechanism through which thrombin activates protein C and how APC recognizes its different substrates and cofactors in the anticoagulant and protective cellular signaling pathways. Insights into the molecular determinants of the APC specificity in these two pathways can lead to rational design of second generation APC therapeutics with improved safety profiles.
2. Protein C Activation
Protein C circulates in plasma as a single chain zymogen [2]. Thrombin removes a 12-residue peptide from the activation peptide of protein C to convert the zymogen to a two-chain active protease held together by a disulphide bond [1,2]. The physiological activation of protein C by thrombin occurs on vascular endothelial cell surfaces and requires the cofactor function of the integral membrane glycoprotein, TM, and the divalent cation, Ca2+ [1,19]. TM accelerates the rate of protein C activation by thrombin in the presence of Ca2+ ~1000-fold [1,19,20]. The activation of protein C by the thrombin-TM complex is further improved ~10-fold if the zymogen is bound via its Gla domain to EPCR on the surface of vascular endothelial cells [21]. The mechanisms by which the three cofactors (TM, EPCR, and Ca2+) improve the rate of protein C activation by thrombin by approximately 4 orders of magnitude have been extensively studied. The extracellular domain of TM contains six epidermal growth factor-like domains [1]. It has been shown that the isolated 4–6 domains of TM (TM4-6) can bind to a basic exosite on thrombin (exosite-1), thereby enabling the protease to recognize and rapidly activate protein C [1,20,22]. In addition to promotion of the activation of protein C, TM4-6 also inhibits the activity of thrombin toward the procoagulant substrates, fibrinogen, PAR-1, and cofactors V and VIII [1,23,24]. The interaction of these procoagulant substrates with exosite-1 is a prerequisite for their recognition and subsequent activation by thrombin [1,23,24]. The high-affinity interaction of TM4-6 with exosite-1 competitively inhibits the binding of procoagulant substrates to this site, thus the cofactor essentially converts thrombin from a procoagulant to a potent anticoagulant protease upon binding [1].
The exact molecular mechanism by which TM4-6 improves the catalytic efficiency of thrombin toward protein C is not fully understood and has been the subject of investigation by several laboratories for many years [1,25–28]. An attractive hypothesis that has emerged more than two decades ago is that TM may function by inducing allosteric changes in the active-site pocket of thrombin to alleviate its potential inhibitory interactions with protein C in the presence of Ca2+ [1,29]. Interestingly, unlike its stimulatory role in the presence of TM, physiological levels of Ca2+ potently inhibits the activation of protein C by thrombin when TM is absent [1,25]. Since thrombin has no known Ca2+-binding site, it has been hypothesized that the TM-altered conformer of thrombin optimally recognizes the Ca2+-altered conformer of protein C [1,29,30].
We have localized the Ca2+-binding site critical for protein C activation by the thrombin-TM complex to the 70-80-loop (chymotrypsinogen numbering system [31]) of the zymogen [32], the same loop that also binds Ca2+ in trypsin [33]. The presence of two conserved acidic residues (Glu-70 and Glu-80) is the signature for Ca2+ binding to this loop, and the carboxylic oxygen atoms of both residues contribute to the ligation of Ca2+ in this loop [33]. In thrombin, residue 70 is a Lys, thus the loop is stabilized by a salt-bridge between Lys-70 and Glu-80, providing a structural basis for thrombin functioning independent of Ca2+ [31]. Although the x-ray crystal structure of protein C has not been resolved, the same residues are also thought to be involved in ligating Ca2+ in the 70-80-loop of the zymogen. In support of this hypothesis, we have shown that the substitution of Glu-80 of protein C with a Lys results in a functionally active mutant which is activated normally by the thrombin-TM complex independent of Ca2+, presumably by the Glu-70 and Lys-80 establishing a salt-bridge in the mutant zymogen [32]. The mutant APC also exhibits normal amidolytic activity independent of Ca2+ [32].
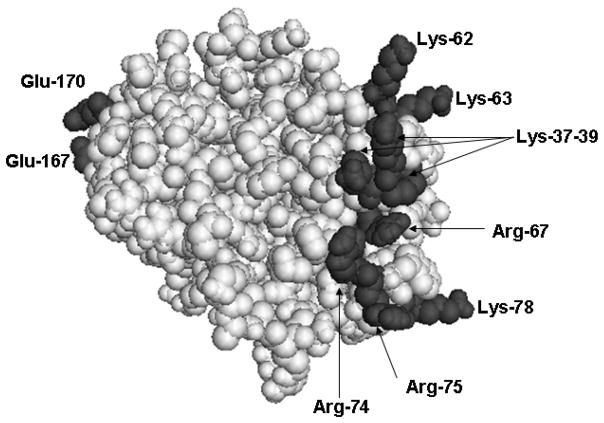
The stabilization of the 70-80-loop of protein C by the Ca2+ ion is essential for the TM-dependent recognition and activation of the zymogen by thrombin [28,34]. Structural and mutagenesis data have indicated that basic residues of this loop (Arg-74, Arg-75, and Lys-78) together with basic residues of two nearby surface loops known as 39-loop (Lys-37-39) and 60-loop (Lys-62, Lys-63, and Arg-67) constitute an anion binding exosite which interacts with the acidic residues of the fourth EGF-like domain of TM in the protein C activation complex in the presence of Ca2+ [22,34]. The three dimensional positions of these residues in the x-ray crystal structure of the catalytic domain of APC are presented in Fig. 1. There are several lines of evidence supporting the hypothesis that the conformation of the 70-80-loop is allosterically linked to the thrombin recognition site on the activation peptide of protein C [32,35,36]. Thus it has been hypothesized that the binding of Ca2+ to 70-80-loop induces a conformational change in the activation peptide of protein C, thereby improving its complimentarity with the TM-altered conformation of the active-site pocket of thrombin [1,29,30]. The inhibitory effect of Ca2+ in the absence of TM further supports the hypothesis that the metal ion-induced conformer of the zymogen is not optimal for docking into the active-site pocket of free thrombin [1,25,29].
Fig. 1.
Crystal structure of the catalytic domain of APC. The side chains of basic residues of three loops (39, 60, and 70–80) and acidic residues of 162-helix (Glu-167 and Glu-170) are shown. The coordinates (Protein Data Bank code 1AUT) were used to prepare the figure [4].
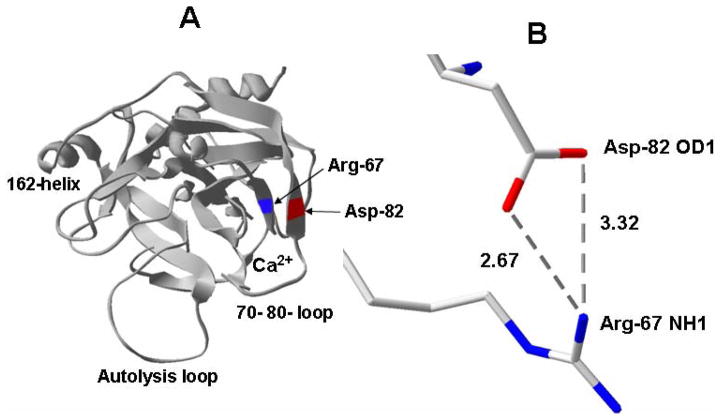
Recently, based on structural data, we used a mutagenesis approach to understand the mechanism by which Ca2+ regulates the activation of protein C by thrombin in the absence and presence of TM. Initially, we conducted an Ala-scanning of the basic residues of all three loops of protein C (39, 60 and 70–80) constituting the anion-binding exosite of the zymogen [28,34]. The kinetic characterization of these mutants in activation studies by thrombin suggested that the basic residues of the protein C exosite are required for the TM-dependent recognition of the zymogen by thrombin, however, these residues played a negative role in the recognition of protein C by thrombin when TM was absent [28,34]. Interestingly, the activation of the Arg-67 to Ala mutant of protein C by thrombin was improved 20-fold independent of both TM and Ca2+ [28]. The examination of the three dimensional position of the 70-80-loop in the x-ray crystal structure of APC suggested that this loop is located between two anti-parallel β strands comprised of residues 64–69 and 81–91 (Fig. 2). It was noticed that the NH1 guanidyl group of Arg-67 on the former strand is located within 2.67 Å from the OD2 carboxylic oxygen of Asp-82 on the latter strand (Fig. 2). We hypothesized that the formation of a salt-bridge between these two residues may modulate the unique Ca2+-dependence of protein C activation by thrombin in the absence and presence of TM [28]. To test this hypothesis, we substituted both Arg-67 and Asp-82 of the substrate with Cys residues, thereby engineering a new disulfide bond between the two residues [28]. Interestingly, we discovered that the activation of the Cys-67/Cys-82 mutant of protein C by thrombin was improved 60–80-fold independent of both TM and Ca2+ [28]. In an earlier study, we had demonstrated that the substitution of Arg-35 of thrombin with a Glu (R35E) leads to a 20–25-fold improvement in the rate of wild-type protein C activation by the mutant thrombin also independent of TM and Ca2+ [30]. We discovered that the activation of the Cys-67/Cys-82 mutant of protein C by R35E thrombin is enhanced by three orders of magnitude independent of both TM and Ca2+ [28]. Thus, the accelerating effect of the two mutations, one in the zymogen and the other in the enzyme, was additive [28]. This effect is essentially identical to the accelerating effect of TM and Ca2+ in the wild-type system [1,28]. Thus, it appears that the primary function of TM in protein C activation by thrombin involves the alleviation of the inhibitory interactions of Arg-67 of protein C and Arg-35 of thrombin in the presence of Ca2+. Structural data suggest that the guanidyl group of Arg-35 is pointing toward the active-site pocket of thrombin [31], possibly impeding access of the active-site pocket by the Ca2+-stabilized conformer of protein C in the absence of TM.
Fig. 2.
Crystal structure of the catalytic domain of APC. (A) The three dimensional positions of Arg-67 and Asp-82 on two anti-parallel β structures of APC are shown. (B) Intra-atomic distances between NH1 guanidyl of Arg-67 and carboxylic oxygens of Asp-82.
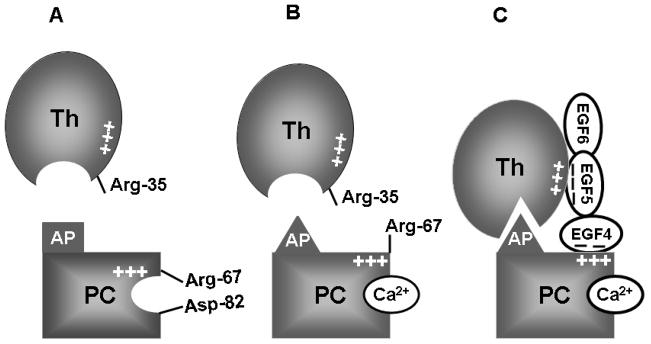
Based on these results, we proposed a model (Fig. 3) for protein C activation by thrombin and TM which supports and extends the previous model presented by Esmon et al [1,28]. This model predicts that in the absence of Ca2+, the guanidyl group of Arg-67 of protein C is in a salt-bridge/hydrogen bond contact with Asp-82, a residue immediately outside of the 70-80-loop of protein C [28]. The binding of Ca2+ to the 70-80-loop of protein C is associated with a conformational change, leading to disruption of the electrostatic interaction between Arg-67 and Asp-82 of the protein. The side chain of Arg-67 in protein C, which remains exposed in the presence of Ca2+, makes an inhibitory for interaction with thrombin, nevertheless, this residue is also required for the ability of the zymogen to interact with an acidic site on the fourth EGF-like domain of TM in the activation complex [22,28,34]. In the Ca2+-stabilized conformer of protein C, the side chain of Arg-67 is free and not engaged in a salt-bridge interaction with Asp-82, thereby impeding the docking of protein C into the active-site groove of thrombin possibly due to its repulsive interaction with Arg-35 of thrombin [28].
Fig. 3.
Hypothetical model of protein C activation by the thrombin-TM complex. (A) Arg-67 and Asp-82 are involved in electrostatic interactions in the zymogen protein C in the absence of Ca2+. The activation peptide (AP) of protein C is not complimentary to fit optimally into the active-site pocket of thrombin in the absence of Ca2+. (B) The binding of Ca2+ to the 70-80-loop of protein C disrupts the salt-bridge/hydrogen bond between Arg-67 and Asp-82, thereby relocating Arg-67 to an inhibitory position for interaction with thrombin in the absence of TM. The metal ion also induces conformational change in the activation peptide of protein C, which is still not complimentary for the active-site pocket of free thrombin. (C) The acidic EGF5 domain of TM binds to exosite-1 of thrombin and the acidic EGF4 domain binds to the basic exosite of the Ca2+-stabilized protein C (including Arg-67), thereby altering the conformation of the active-site and/or the extended binding pocket residues of thrombin (including Arg-35), thus facilitating the docking of the activation peptide of protein C in the catalytic groove of thrombin (see the text for further details).
The activation peptide of protein C, at its primed site, contains several basic residues which are also shown to play an inhibitory role in zymogen recognition by thrombin [37]. It is possible that the repulsive interaction of these residues with Arg-35 also contributes to the inability of thrombin to accommodate the activation peptide of protein C in its active-site pocket in the presence of Ca2+. It appears that the allosteric modulation of the extended active-site pocket residues of thrombin (including the side chain of Arg-35) by TM overcomes these inhibitory interactions [28,38,39,40]. Thus, Arg-35 of thrombin and Arg-67 of protein C cooperatively drive the unique TM and Ca2+ dependence of protein C activation by thrombin [28]. It is worth noting that two Asp residues, present at the P3 and P3′ sites of the protein C activation peptide, have been shown to play an inhibitory role in interaction of protein C with thrombin, presumably due to their repulsive interactions with Glu-192 of thrombin [41–43]. It is likely that TM also plays a role in alleviating these inhibitory interactions [1,43].
3. Anticoagulant Function of APC
APC down-regulates the clotting cascade by proteolytically degrading the procoagulant cofactors FVa and FVIIIa which are essential cofactors for factors Xa and IXa in the extrinsic and intrinsic pathways of thrombin generation, respectively [1–3,44]. Both cofactors are homologous glycoproteins, synthesized as single chain precursors with domain structures A1-A2-B-A3-C1-C2 [45–47]. During activation by the physiological activator thrombin, the B domain from both cofactors is released and the resulting A1-A2 heavy chain subunits remain non-covalently associated with the A3-C1-C2 light chain in a divalent cation-dependent mechanism [48–50]. While A1-A2 subunits in FVa remain contiguous, the cleavage at Arg-372 of FVIII by thrombin converts the heavy chain of the cofactor into two separate subunits [51]. The A1 subunit of FVIIIa retains a stable metal-ion dependent linkage with the A3-C1-C2 subunit, whereas the A2 subunit remains weakly associated with the dimer through electrostatic interactions [52]. The C2 subunit in both cofactors binds to negatively charged membranes [53–56].
The mechanism by which APC recognizes the two cofactors in the anticoagulant pathway has been extensively investigated by several laboratories in recent years [57–61]. It has been demonstrated that the same basic residues of three surface loops (39-, 60- and 70-80-loops) (Fig. 1) that are critical for the recognition of the zymogen protein C by the thrombin-TM complex are also involved in determining the recognition specificity of APC with FVa [61–63] and FVIIIa [64] in the anticoagulant pathway. In addition to basic residues of these loops, the autolysis loop (148-loop) of APC is also highly basic, with 5 Lys/Arg between residues 143–154. Mutagenesis studies have indicated that these residues may also be critical for the APC recognition of FVa in anticoagulant pathway [60,65–67].
The inactivation of FVa by APC requires two cleavages at Arg-306 and Arg-506 sites, located on the A1 and A2 domains of the cofactor, respectively [58,59]. It has been demonstrated that APC cleaves these two sites in a sequential kinetic order, with an initial cleavage occurring rapidly at the Arg-506 site followed by a slower cleavage occurring at the Arg-306 site [58]. While the cleavage of the Arg-506 site is relatively fast and independent of a cofactor, the cleavage of the Arg-306 site is accelerated 20-fold by protein S, a vitamin K-dependent cofactor for APC in plasma [57]. Mutagenesis data have indicated that the interaction of the basic exosite of APC with a complimentary site, flanking the Arg-506 site of FVa, is responsible for the ability of APC to preferentially cleave this bond with a higher catalytic specificity [62,63]. The site on APC that determines the cleavage specificity of the Arg-306 peptide bond has not been identified. Since the cleavage of the Arg-306 site by APC is membrane-dependent, it has been hypothesized that protein S binding to APC leads to the relocation of the active-site topography of the membrane-bound APC, thereby allowing the protease to recognize and cleave the Arg-306 scissile bond on the membrane surface [68,69]. The affinity of protein S for membrane is very high, thus protein S also improves the affinity of APC for the negatively charged membrane surfaces [70].
In the case of FVIIIa, APC cleaves the cofactor at the homologous Arg-336 and Arg-562 sites, located on the A1 and A2 subunits, respectively [51,52]. Unlike FVa, however, in which a single cleavage at Arg-506 site only partially inactivates the cofactor [57,59], the APC cleavage of either site in FVIIIa leads to a near-complete inactivation of the cofactor [71]. In vitro data has indicated that protein S and FV function synergetically as cofactors to accelerate the rate of FVIIIa degradation by APC [72]. Both the A1 and A2 subunits of FVIIIa contain acidic C-terminal sequences that might potentially provide contact sites for the basic exosite of APC [48,64]. It is however worth noting that FVIIIa has a low concentration and a short half-life in plasma due to the spontaneous A2 subunit dissociation which leads to a complete loss of cofactor activity [48,73]. Thus a physiological role for APC in the regulation of the procoagulant activity of FVIIIa has not been fully established. By contrast, the critical role of APC in the regulation of the clotting cascade through the proteolytic degradation of FVa has been well-established [26,58]. A natural common variant of FVa in which the protease recognition site at residue Arg-506 is mutated to Gln is resistant to efficient inactivation by APC [74,75]. This mutation, which is named FVa Leiden [75], occurs with a high frequency of ~5–10% in the general population and is associated with a high incidence of venous thrombosis [58,74].
In addition to the heavy chain, an interactive site for APC on the light chain of both FVa and FVIIIa has been reported [76,77]. This site has been mapped to the C-terminal end of the A3 domain in both cofactors [76,77]. Peptide sequences derived from the C-terminal end of the A3 domain of both FV and FVIII inhibit the APC inactivation of the cofactors [76]. The association of FVa and FVIIIa with their target proteases factor Xa and factor IXa in the prothrombinase and Tenase complexes, respectively, prevents the APC recognition and inactivation of cofactors [78–80]. This is possible by both factors Xa and IXa having overlapping binding sites with APC on the cofactors, thereby protecting the susceptible cleavage sites from recognition by APC upon binding to cofactors on negatively charged membrane surfaces [78–80].
4. Intracellular Signaling Function of APC
In addition to its anticoagulant activity, APC also exhibits potent cytoprotective and antiinflammatory properties. This has been demonstrated both in in vitro endothelial cell culture systems and in vivo animal models of inflammation [8–10,81,82]. The mechanism of the protective cellular signaling activity of APC is poorly understood. It has been hypothesized that when the Gla domain of APC binds to EPCR it acquires a different specificity, thus activating PAR-1 and thereby initiating protective signaling events in endothelial cells [9,10]. The specific Gla domain residues that differentially mediate the interaction of APC with either protein S in the anticoagulant pathway or EPCR in the cytoprotective pathway has been mapped by a mutagenesis approach [83]. It appears that several residues at the N-terminal Gla domain play critical roles in determining the specificity of APC binding to EPCR and several others in the C-terminal end of the Gla domain are involved in specific interactions with protein S at the membrane surface [83].
In a recent study, we demonstrated that Glu-167 and Glu-170, two residues unique for the 162-helix of APC on the catalytic domain (Fig. 1), which are not conserved on the homologous helix of other vitamin K-dependent coagulation proteases, constitute a specific PAR-1-binding exosite on APC that facilitates the recognition and cleavage of the receptor by the protease on the endothelial cell surface [84]. We showed that the specific interaction of APC with an unknown site of the PAR-1 extracellular domain through this exosite is essential for the EPCR- and PAR-1-dependent cytoprotective signaling function of APC [84]. Both APC mutants exhibited normal anticoagulant activity in both FVa-inactivation and plasma-based clotting assays, suggesting that neither one of the acidic residues of 162-helix are involved in interaction of APC with FVa and FVIIIa [84]. Previous results have demonstrated that the substitutions of the basic residues of either 39-loop (Lys-37, Lys-38 and Lys-39) or the Ca2+-binding 70-80-loop (Arg-74 and Arg-75) (Fig. 1) specifically abrogate the anticoagulant activity, but not the protective cell signaling activity of APC [85]. Thus, the specificity of the APC for interaction with its natural substrates in the alternative anticoagulant and cytoprotective pathways are determined by two distinct exosites with different polarity. The acidic residues of the 162-helix, on the left side of the active-site toward the back of the molecule, determine the specificity of the protease interaction with PAR-1, and the basic residues of 39- and 70-80-loops on the right side of the active-site, determine the specificity of the protease interaction with the procoagulant cofactors (Fig. 1).
As indicated above, recombinant APC has been approved by the FDA in the US for treating severely septic patients, reducing the overall mortality rate by nearly 20% [15]. However, there are at least two relatively major problems with the APC-therapy that need to be addressed. The first problem is that the use of APC is associated with an increased risk (~ 2%) of bleeding in certain patients [10,15]. This problem is greater than it appears to be since it also limits the threshold of APC dosage in treatments, thus potentially masking the beneficial effect of APC in a higher number of patients. The second major problem is the high cost of the APC-therapy, with a 96 hour continuous infusion of recombinant APC [drotrecogin alfa (activated)™ Eli Lilly] costing ~$10,000, thus limiting its wide utilization due to socioeconomic reasons [86]. Recent results, using a mouse non-anticoagulant APC derivative in which all three basic residues of the 39-loop and two basic residues of the 70-80-loop have been replaced with Ala residues (APC-5A), have demonstrated that the cellular signaling activity of APC is primarily responsible for its beneficial protective property in animal models of severe sepsis [87,88]. If similar results hold true for human APC, such mutants could eliminate the increased bleeding risk of the APC-therapy in severe sepsis.
Recently, we showed that the 70-80-loop stabilized Cys-67/Cys-82 mutant of human APC, described above (Fig. 2), has a dramatically reduced anticoagulant, but near normal protective intracellular signaling activity in endothelial cells [89]. Moreover, our preliminary in vivo data suggests that the non-anticoagulant APC Cys-67/Cys-82 improves the neurological outcome of experimental stroke in mice [90] and exhibits cardio-protective activities in a mouse model of ischemia/reperfusion injury [91]. In the latter model, the 162-helix mutant of APC which possesses normal anticoagulant activity but lacks a PAR-1-dependent cytoprotective activity, did not exhibit a cardio-protective activity [91]. In a recent study, another APC mutant with no cytoprotective activity but improved anticoagulant and antithrombotic activity exhibited diminished protective activity in an endotoxin-induced murine sepsis model [92]. These mutants provide excellent tools to initiate further in vivo studies to understand the extent to which the anticoagulant activity versus direct cellular activity of APC contributes to the beneficial effect of APC in severe sepsis. If it turns out that the direct cellular effect of APC is responsible for its protective effect in severe sepsis, it may be possible to use the structure-function knowledge described above to develop new APC variants with no anticoagulant but improved specific activity toward PAR-1, thereby reducing the bleeding side effect and the high cost of the APC-therapy in the septic setting.
4. Mechanism of the Protective Intracellular Signaling Function of APC
The mechanism by which the APC activation of PAR-1 elicits protective intracellular responses is not fully understood. The complexity of this question is underscored by the observation that thrombin activates PAR-1 with at least three orders of magnitude higher catalytic efficiency to initiates disruptive, hyperpermeability and proinflammatory responses in endothelial cells [17,18]. The PAR-1-dependent proinflammatory properties of thrombin have been reported to be mediated through the protease activating the nuclear factor (NF)-κ B pathway in endothelial cells [93–95]. It has been demonstrated that the APC-EPCR complex can inhibit the thrombin- or proinflammatory cytokines-mediated activation of NF-κB and down-regulate the expression of proinflammatory cytokines by activation of PAR-1 in endothelial cells [93–95]. The mechanism by which the activation of PAR-1 by the two proteases initiates two opposite responses in in vitro cellular models is not known. Recently, we investigated whether the level of receptor activation by thrombin and APC determines the type of response in endothelial cells. Thus, we engineered a chimeric meizothrombin (thrombin intermediate which has both Gla and Kringle-1 and -2 domains of prothrombin) in which the Gla domain of the thrombin intermediate was substituted with the corresponding domain of APC (Fig. 4) [96]. This meizothrombin derivative retained its thrombin-like high specific activity toward PAR-1 and interacted with EPCR with normal affinity [96]. Interestingly, we discovered that PAR-1 cleavage by this meizothrombin derivative elicits a protective signaling response in endothelial cells, suggesting that the binding of Gla domain of APC to EPCR rather than the protease type cleaving the receptor determines the type of PAR-1 response in endothelial cells [96]. To investigate the mechanism of this effect, we studied the effect of PAR-1 cleavage by thrombin in endothelial cells which were pretreated with the catalytically inactive Ser-195 to Ala substitution mutant of protein C. Both APC and the zymogen protein C interact with EPCR with identical affinities. The results revealed that when EPCR is occupied by its ligand protein C, the cleavage of PAR-1 by thrombin elicits only protective signaling responses in endothelial cells [96].
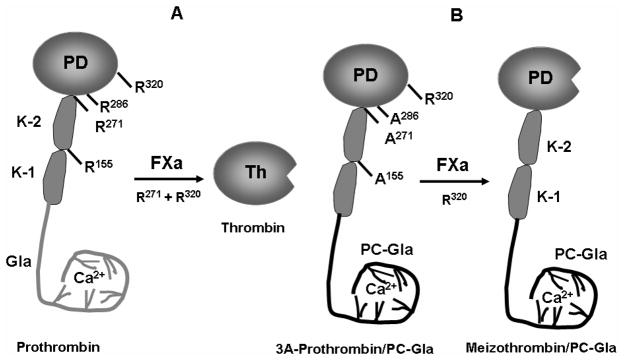
Fig. 4.
Cartoons of wild-type and mutant prothrombin activation by factor Xa. (A) The proteolytic cleavage of prothrombin at Arg-320 by factor Xa yields an activation intermediate that is an active product called meizothrombin. A second cleavage at Arg-271 by factor Xa is required to separate the catalytic domain of prothrombin from the non-catalytic domain (Gla, Kringle-1 and Kringle-2 domains) to yield thrombin. Further cleavages can occur at Arg-155 and Arg-286 in a feed-back reaction by both thrombin and meizothrombin. (B) The substitution of the Gla domain of prothrombin with the corresponding domain of protein C and its Arg-155, Arg-271 and Arg-286 residues with 3 Ala’s yields a mutant of prothrombin (3A-prothrombin/PC-Gla) which can be activated by factor Xa through the cleavage of Arg-320 to yield meizothrombin/PC-Gla which cannot be further processed by either factor Xa or the resulting mutant meizothrombin.
To provide further support for our hypothesis, we pre-incubated human umbilical vein endothelial cells or human pulmonary artery endothelial cells with a physiologically relevant concentration of the zymogen protein C (wild-type or the S195A mutant) and then used the thrombin receptor agonist peptides (TRAP) SFLLRN or TFLLRN to activate PAR-1. Both PAR-1 agonist peptides elicited a barrier disruptive response in endothelial cells that could be effectively reversed to a protective response if endothelial cells were pretreated with the zymogen protein C prior to their stimulation with TRAP [96,97]. Further studies demonstrated that when EPCR was occupied by protein C, similar to APC, the thrombin activation of PAR-1 inhibits the activation of RhoA and enhances the activation of Rac1 pathways in the TNF-α-stimulated endothelial cells [95]. Furthermore, thrombin inhibited NF-κB pathway by a PAR-1-dependent mechanism if cells were pretreated with the zymogen protein C [95]. Based on these results, we concluded that the cleavage of PAR-1 by thrombin on intact endothelium expressing EPCR would initiate potent protective intracellular responses in the presence of physiological concentrations of the zymogen protein C [95–97]. Thus, the in vitro data proposing a PAR-1-dependent proinflammatory effect for thrombin in cellular models may have no physiological relevance.
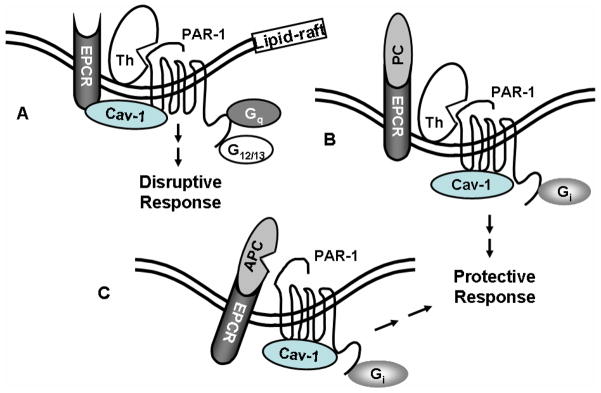
How the occupancy of EPCR by protein C changes the PAR-1-dependent signaling specificity of thrombin is not known. PAR-1 can signal through interaction with different members of the G protein subfamilies including Gi, Gq and G12/13 [98]. It has been hypothesized that thrombin disrupts endothelial barrier function through the activation of PAR-1 that is coupled to Gq and/or G12/13, but APC signals through the cleavage of PAR-1 coupled to Gi to reverse the hyperpermeability induced by proinflammatory cytokines [96,98]. However, in a series of studies, we discovered that the ligand occupancy of EPCR recruits PAR-1 to a protective pathway by coupling PAR-1 to Gi in endothelial cells independent of the protease cleaving the receptor [95–97]. Our studies revealed that both PAR-1 and EPCR associate with caveolin-1 in the cholesterol rich lipid-rafts and that the occupancy of EPCR by either APC or protein C leads to its dissociation from caveolin-1, a process which appears to change the specificity of PAR-1 signaling from a disruptive to a protective response in cultured endothelial cells (Fig. 5). Our further studies revealed that when EPCR is occupied, the disruptive hyperpermeability activity of thrombin is mediated through the activation of PAR-4 [96]. The activation of PAR-4 requires a higher concentration of thrombin since, unlike PAR-1, PAR-4 lacks a hirudin-like sequence to bind exosite-1 of thrombin [18].
Fig. 5.
Cartoons of PAR-1 activation by APC and thrombin when EPCR is either free or occupied by its ligand protein C. (A) EPCR is associated with caveolin-1 (Cav-1) in the lipid-rafts of endothelial cells when the receptor is not occupied by the Gla domain of protein C/APC. Thrombin cleavage of PAR-1 elicits disruptive signaling responses through coupling the receptor to G12/13 and Gq proteins. (B) The occupancy of EPCR by protein C (PC) results in the dissociation of EPCR from caveolin-1, thereby switching the specificity of PAR-1 signaling by coupling it to the Gi protein. Thus, thrombin cleavage of PAR-1 initiates protective response when EPCR is occupied. (C) The same as (B) except that the EPCR and PAR-1 dependent protective signaling response is elicited by APC.
Given our in vitro cellular data that both thrombin and APC exert their PAR-1-dependent protective effects by an EPCR-dependent mechanism (Fig. 5), the appropriate question that needs to be addressed is: since in animal models of inflammation thrombin is expected to be generated and thrombin is the only known physiological activator of protein C, how then does APC exert its protective effect in vivo through the activation of PAR-1 in the presence of thrombin, which has three orders of magnitude higher catalytic efficiency toward PAR-1? Clearly, numerous animal models of inflammation have established an EPCR and PAR-1-dependent protective effect for APC and neither the active-site inhibited protease nor the S195A mutant of protein C exhibit protective activity in these models [10]. One possibility is that thrombin partitioned to the vasculature may not be capable of activating the microvascular endothelial cell surface PAR-1 due to its high-affinity interaction with TM which occupies exosite-1 of thrombin. In support of this hypothesis, we recently demonstrated that all three receptors, TM, EPCR and PAR-1 are colocalized within lipid-rafts of endothelial cells [99]. We also discovered that the interaction of exosite-1 of thrombin with the acidic hirudin-like sequence present on the exodomain of PAR-1 is required for recognition and cleavage of the receptor by thrombin [100]. Noting that the affinity of thrombin for endothelial cell TM (KD < 1 nM) [101] is much higher than that of PAR-1 (in μM range for the exodomain of the receptor) [102] and the effective concentration of TM in the microcirculation can be as high as 500 nM [103,104], it is possible that thrombin partitioned onto the microvascular endothelial cell surface would all bind TM, with the complex being capable of only activating the EPCR-bound protein C but not PAR-1. Thus, in the microcirculation, the activation of the endothelial cell PAR-1 may proceed solely via the APC pathway. Noting the membrane lipid-raft localization of all three receptors, this pathway would be sufficiently robust since the activation of protein C by the thrombin-TM complex would be mechanistically linked to the activation of PAR-1 by APC, as has been previously proposed [105].
Another possibility is that the PAR-1-dependent endothelial cell signaling does not contribute significantly to the antiinflammatory role of APC, but rather that leukocytes are major targets for the protective effect of APC in severe sepsis. In this venue, it has recently been demonstrated that APC has an RGD sequence that can bind to leukocyte integrins, thereby inhibiting the migration of neutrophils into tissues [106]. Results of several other recent studies have indicated that the EPCR-dependent barrier protective activity of APC is mediated through a crosstalk with other G- and non-G-protein coupled receptors [94,107,108]. Thus, the activation of PAR-1 by APC and thrombin can differentially couple PAR-1 to different trans-activators in various cells. In support of this hypothesis, it has been shown that PAR-1 crosstalk with sphingosine 1-phosphate receptor 1 (S1P1) elicits a protective response [107], however, a crosstalk between PAR-1 and S1P3 evokes proinflammatory responses [109]. Another recent study showed that the protective effect of PAR-1 requires transactivation of the PAR-2 signaling pathway [110]. Two other studies demonstrated that the APC-mediated signaling via both apolipoprotein E receptor 2 (ApoER2) and angiopoietin (Ang)/Tie2 pathways also contribute to cytoprotective and antiinflammatory properties of APC [111,112]. Finally, to put the complexity of this question into perspective, a very interesting recent study showed that extracellular histones are major mediators of endothelial dysfunction, organ failure and death during severe sepsis and that the protective effect of APC in an LPS-induced murine sepsis model is primarily mediated through the direct APC degradation of histones independent of both EPCR and PAR-1 [113]. Thus, much further research work is required to understand the exact mechanism by which APC modulates the inflammatory pathways in the severe sepsis syndrome.
5. Regulation of the Proteolytic Activity of APC in Plasma
The proteolytic activity of APC in plasma is primarily regulated by the serpins, protein C inhibitor (PCI), plasminogen activator inhibitor 1 (PAI-1) and α1-proteinase inhibitor (α1-PI) [114–116]. The general non-serpin inhibitor α2-macroglobulin also contributes to regulation of APC activity in plasma [117]. However, APC reacts very slowly with all of these inhibitors, thus it has a long circulating half-life of approximately 20–25 min in plasma [115,117]. Another mechanism, which may be involved in the regulation of APC in plasma, is the EPCR-mediated endocytosis that can facilitate the transcytosis of the protease and its clearance from circulation [118,119]. Among the three serpins, the reactivity of APC with α1-PI is the slowest (~10 M−1s−1). However, owing to its high concentration (~40 μM), α1-PI is believed to contribute to the regulation of APC activity in plasma [115]. The reactivity of APC with the other two serpins is also relatively slow (102–103 M−1s−1). However, the two cofactors heparin and vitronectin markedly accelerate the reactivity of APC with PCI and PAI-1, respectively [114,120].
Therapeutic high molecular weight heparins can accelerate the inhibition of APC by PCI by three orders of magnitude in the presence of physiological levels of Ca2+ [121], possibly suggesting a negative outcome for heparin-therapy in severe sepsis. The heparin-mediated acceleration of the PCI inhibition of APC requires the interaction of heparin with the same basic exosite that is also critical for protein C activation by the thrombin-TM complex and the anticoagulant activity of the protease [121]. PCI may also play an important role in the regulation of protein C activation by thrombin since it rapidly inhibits thrombin when the protease forms a complex with TM [122]. It has been demonstrated that TM provides a binding site for PCI in the thrombin-TM complex, thereby facilitating the optimal recognition of the serpin by the protein C activation complex [123,124]. How vitronectin accelerates the PAI-1 inhibition of APC has not been investigated. Vitronectin is abundant plasma and platelet glycoprotein and its ability to promote the interaction of APC with PAI-1 may lead to the depletion of the plasma pool of PAI-1, thereby increasing the effective concentration of plasminogen activators in plasma and possibly accounting for the reported profibrinolytic property of APC [11,116,120]. Insight into mechanisms by which serpins neutralize the proteolytic activity of APC in plasma can lead to the design of novel protease variants with reduced serpin reactivity, thus providing another approach for potentially improving the therapeutic profile of APC by protein engineering. Indeed, such knowledge has been utilized to engineer APC derivatives which exhibit resistance to inactivation by PCI and α1-PI without alteration in their anticoagulant activity [125].
Acknowledgments
The research discussed herein was supported by grants awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (Grants No. HL 68571 and HL 62565).
I would like to thank Audrey Rezaie for proofreading the manuscript.
References
- 1.Esmon CT. Molecular events that control the protein C anticoagulant pathway. Thromb Haemost. 1993;70:1–5. [PubMed] [Google Scholar]
- 2.Stenflo J. Structure-function relationships of epidermal growth factor modules in vitamin K-dependent clotting factors. Blood. 1991;78:1637–1651. [PubMed] [Google Scholar]
- 3.Walker FJ, Fay PJ. Regulation of blood coagulation by the protein C system. FASEB J. 1992;6:2561–2567. doi: 10.1096/fasebj.6.8.1317308. [DOI] [PubMed] [Google Scholar]
- 4.Mather T, Oganessyan V, Hof P, Huber R, Foundling S, Esmon C, Bode W. The 2.8 Å crystal structure of Gla-domainless activated protein C. EMBO J. 1996;15:6822–6831. [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlbäck B. Protein S and C4b-binding protein: Components involved in the regulation of the protein C anticoagulant system. Thromb Haemostas. 1991;66:49–61. [PubMed] [Google Scholar]
- 6.Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981;68:1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jalbert LR, Rosen ED, Moons L, Chan JC, Carmeliet P, Collen D, Castellino JF. Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest. 1998;102:1481–1488. doi: 10.1172/JCI3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor FB, Jr, Chang A, Esmon CT, D’Angelo A, Vigano-D’Angelo S, Blick KE. Protein C prevents the coagulopathic and lethal effects of E coli infusion in the baboon. J Clin Invest. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 10.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C patheway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 11.Comp PC, Esmon CT. Generation of fibrinolytic activity by infusion of activated protein C into dogs. J Clin Invest. 1981;68:1221–1228. doi: 10.1172/JCI110368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukudome K, Esmon CT. Identification, cloning and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269:26486–26491. [PubMed] [Google Scholar]
- 13.Oganesyan V, Oganesyan N, Terzyan S, Qu D, Dauter Z, Esmon NL, Esmon CT. The crystal structure of the endothelial protein C receptor and a bound phospholipid. J Biol Chem. 2002;277:24851–24854. doi: 10.1074/jbc.C200163200. [DOI] [PubMed] [Google Scholar]
- 14.Taylor FB, Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang AC, Laszik Z, Kosanke S, Peer G, Esmon CT. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- 15.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJJ. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Eng J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 16.Ruf W, Dorfleutner A, Riewald M. Specificity of coagulation factor signaling. J Thromb Haemost. 2003;1:1495–1503. doi: 10.1046/j.1538-7836.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 17.Ludeman MJ, Kataoka H, Srinivasan Y, Esmon NL, Esmon CT, Coughlin SR. PAR1 cleavage and signaling in response to activated protein C and thrombin. J Biol Chem. 2005;280:13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 18.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 19.Castellino FJ. Human protein C and activated protein C: Components of the human anticoagulation system. Trends Cardiovasc Med. 1995;5:55–62. doi: 10.1016/1050-1738(94)00031-X. [DOI] [PubMed] [Google Scholar]
- 20.Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005;106:2605–2612. doi: 10.1182/blood-2005-04-1710. [DOI] [PubMed] [Google Scholar]
- 21.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci (USA) 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuentes-Prior P, Iwanaga Y, Huber R, Pagila R, Rumennik G, Seto M, Morser J, Light DR, Bode W. Structural basis for the anticoagulant activity of the thrombin-thrombomodulin complex. Nature. 2000;404:518–525. doi: 10.1038/35006683. [DOI] [PubMed] [Google Scholar]
- 23.Hofsteenge J, Stone SR. The effect of thrombomodulin on the cleavage of fibrinogen and fibrinogen fragments by thrombin. Eur J Biochem. 1987;168:49–56. doi: 10.1111/j.1432-1033.1987.tb13385.x. [DOI] [PubMed] [Google Scholar]
- 24.Esmon CT, Lollar P. Involvement of thrombin anion-binding exosites 1 and 2 in the activation of factor V and factor VIII. J Biol Chem. 1996;271:13882–13887. doi: 10.1074/jbc.271.23.13882. [DOI] [PubMed] [Google Scholar]
- 25.Esmon NL, DeBault LE, Esmon CT. Proteolytic formation and properties of gamma-carboxyglutamic acid-domainless protein C. J Biol Chem. 1983;258:5548–5553. [PubMed] [Google Scholar]
- 26.Dahlbäck B, Villoutreix BO. The anticoagulant protein C pathway. FEBS Letters. 2005;579:3310–3316. doi: 10.1016/j.febslet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Vindigni A, White CE, Komives EA, Di Cera E. Energetics of thrombin-thrombomodulin interaction. Biochemistry. 1997;36:6674–6681. doi: 10.1021/bi962766a. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Manithody C, Rezaie AR. Activation of protein C by the thrombin-thrombomodulin complex: cooperative roles of Arg-35 of thrombin and Arg-67 of protein C. Proc Natl Acad Sci (USA) 2006;103:879–884. doi: 10.1073/pnas.0507700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esmon CT, Esmon NL. Protein C activation. Semin Hemost Thromb. 1984;10:122–130. doi: 10.1055/s-2007-1004414. [DOI] [PubMed] [Google Scholar]
- 30.Rezaie AR, Yang L. Thrombomodulin allosterically modulates the activity of the anticoagulant thrombin. Proc Natl Acad Sci (USA) 2003;100:12051–12056. doi: 10.1073/pnas.2135346100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bode W, Mayr I, Baumann U, Huber R, Stone SR, Hofsteenge J. The refined 1.9 Å crystal structure of human α-thrombin: interaction with D-Phe-Pro-Arg chlorometheylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989;8:3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezaie AR, Mather T, Sussman F, Esmon CT. Mutation of Glu 80 [to] Lys results in a protein C mutant that no longer requires Ca2+ for rapid activation by the thrombin-thrombomodulin complex. J Biol Chem. 1994;269:3151–3154. [PubMed] [Google Scholar]
- 33.Bode W, Schwager P. The refined crystal structure of bovine beta-trypsin at 1.8 Å resolution. II. Crystallographic refinement, calcium binding site, benzamidine binding site and active site at pH 7.0. J Mol Biol. 1975;98:693–717. doi: 10.1016/s0022-2836(75)80005-2. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Rezaie AR. The fourth epidermal growth factor-like domain of thrombomodulin interacts with the basic exosite of protein C. J Biol Chem. 2003;278:10484–10490. doi: 10.1074/jbc.M211797200. [DOI] [PubMed] [Google Scholar]
- 35.Rezaie AR, Esmon CT. Tryptophans 231 and 234 in protein C report the Ca(2+)-dependent conformational change required for activation by the thrombin-thrombomodulin complex. Biochemistry. 1995;34:12221–12226. doi: 10.1021/bi00038a016. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Prasad S, Di Cera E, Rezaie AR. The conformation of the activation peptide of protein C is influenced by Ca2+ and Na+ binding. J Biol Chem. 2004;279:38519–38524. doi: 10.1074/jbc.M407304200. [DOI] [PubMed] [Google Scholar]
- 37.Grinnell BW, Gerlitz B, Berg DT. Identification of a region in protein C involved in thrombomodulin-stimulated activation by thrombin: potential repulsion at anion-binding site I in thrombin. Biochem J. 1994;303:929–933. doi: 10.1042/bj3030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L, Manithody C, Walston TD, Cooper ST, Rezaie AR. Thrombomodulin enhances the reactivity of thrombin with protein C inhibitor by providing both a binding site for the serpin and allosterically modulating the activity of thrombin. J Biol Chem. 2003;278:37465–37470. doi: 10.1074/jbc.M307243200. [DOI] [PubMed] [Google Scholar]
- 39.Koeppe JR, Beach MA, Baerga-Ortiz A, Kerns SJ, Komives EA. Mutations in the fourth EGF-like domain affect thrombomodulin-induced changes in the active site of thrombin. Biochemistry. 2008;47:10933–10039. doi: 10.1021/bi8008278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandhi PS, Chen Z, Mathews FS, Di Cera E. Structural identification of the pathway of long-range communication in an allosteric enzyme. Proc Natl Acad Sci (USA) 2008;105:1832–1837. doi: 10.1073/pnas.0710894105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrlich HJ, Grinnell BW, Jaskunas SR, Esmon CT, Yan SB, Bang NU. Recombinant human protein C derivatives: altered response to calcium resulting in enhanced activation by thrombin. EMBO J. 1990;9:2367–2373. doi: 10.1002/j.1460-2075.1990.tb07411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rezaie AR, Esmon CT. The function of calcium in protein C activation by thrombin and the thrombin-thrombomodulin complex can be distinguished by mutational analysis of protein C derivatives. J Biol Chem. 1992;267:26104–26109. [PubMed] [Google Scholar]
- 43.Le Bonniec BF, Esmon CT. Glu-192→Gln substitution in thrombin mimics the catalytic switch induced by thrombomodulin. Proc Natl Acad Sci (USA) 1991;88:7371–7375. doi: 10.1073/pnas.88.16.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- 45.Guinto ER, Esmon CT, Mann KG, MacGillivray RTA. The complete cDNA sequence of bovine coagulation factor V. J Biol Chem. 1992;267:2971–2978. [PubMed] [Google Scholar]
- 46.Vehar GA, Keyt B, Eaton D, Rodriguez H, O’Brien DP, Rotblat F, Oppermann H, Keck R, Wood WI, Harkins RN, et al. Structure of human factor VIII. Nature. 1984;312:337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- 47.Kane WH, Davie EW. Blood coagulation factors V and VIII: Structural and functional similarities and their relationship to hemorrhagic and thrombotic disorders. Blood. 1988;71:539–555. [PubMed] [Google Scholar]
- 48.Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998;92:3983–3996. [PubMed] [Google Scholar]
- 49.Esmon CT. The subunit structure of thrombin-activated factor V: isolation of activated factor V, separation of subunits and reconstitution of biological activity. J Biol Chem. 1979;254:964–973. [PubMed] [Google Scholar]
- 50.Lollar P, Fay PJ, Fass DN. Factor VIII and factor VIIIa. Methods Enzymol. 1993;222:128–143. doi: 10.1016/0076-6879(93)22010-d. [DOI] [PubMed] [Google Scholar]
- 51.Eaton D, Rodriguez H, Vehar GA. Proteolytic processing of human factor VIII: correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986;25:505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- 52.Fay PJ, Smudzin TM. Characterization of the interaction between the A2 subunit and A1/A3-C1-C2 dimer in Human Factor VIIIa. J Biol Chem. 1992;267:13246–13250. [PubMed] [Google Scholar]
- 53.Nicolaes GA, Villoutreix BO, Dahlbäck B. Mutations in a potential phospholipid binding loop in the C2 domain of factor V affecting the assembly of the prothrombinase complex. Blood Coagul Fibrinol. 2000;11:89–100. [PubMed] [Google Scholar]
- 54.Peng W, Quinn-Allen MA, Kim SW, Alexander KA, Kane WH. Trp2063 and Trp2064 in the factor Va C2 domain are required for high-affinity binding to phospholipid membranes but not for assembly of the prothrombinase complex. Biochemistry. 2004;43:4385–4393. doi: 10.1021/bi035763o. [DOI] [PubMed] [Google Scholar]
- 55.Gilbert GE, Kaufman RJ, Arena AA, Miao H, Pipe SW. Four hydrophobic amino acids of the factor VIII C2 domain are constituents of both the membrane-binding and von Willebrand factor-binding motifs. J Biol Chem. 2002;277:6374–6381. doi: 10.1074/jbc.M104732200. [DOI] [PubMed] [Google Scholar]
- 56.Majumder R, Quinn-Allen MA, Kane WH, Lentz BR. A phosphatidylserine binding site in factor Va C1 domain regulates both assembly and activity of the prothrombinase complex. Blood. 2008;112:2795–2802. doi: 10.1182/blood-2008-02-138941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosing J, Hoekema L, Nicolaes GA, Thomassen MC, Hemker HC, Varadi K, Schwarz HP, Tans G. Effects of protein S and factor Xa on peptide bond cleavages during inactivation of factor Va and factor VaR506Q by activated protein C. J Biol Chem. 1995;270:27852–27858. doi: 10.1074/jbc.270.46.27852. [DOI] [PubMed] [Google Scholar]
- 58.Nicolaes GA, Dahlbäck B. Factor V and thrombotic diseases: description of a Janus-faced protein. Arterioscler Thromb Vasc Biol. 2002;22:530–538. doi: 10.1161/01.atv.0000012665.51263.b7. [DOI] [PubMed] [Google Scholar]
- 59.Kalafatis M, Rand MD, Mann KG. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J Biol Chem. 1994;269:31869–31880. [PubMed] [Google Scholar]
- 60.Gale AJ, Heeb MJ, Griffin JH. The autolysis loop of activated protein C interacts with factor Va and differentiates between the Arg506 and Arg306 cleavage sites. Blood. 2000;96:585–593. [PubMed] [Google Scholar]
- 61.Yang L, Manithody C, Rezaie AR. Contribution of basic residues of the 70-80-loop to heparin binding and anticoagulant function of activated protein C. Biochemistry. 2002;41:6149–6157. doi: 10.1021/bi015899r. [DOI] [PubMed] [Google Scholar]
- 62.Gale AJ, Tsavaler A, Griffin JH. Molecular characterization of an extended binding site for coagulation factor Va in the positive exosite of activated protein C. J Biol Chem. 2002;277:28836–28840. doi: 10.1074/jbc.M204363200. [DOI] [PubMed] [Google Scholar]
- 63.Friedrich U, Nicolaes GA, Villoutreix BO, Dahlbäck B. Secondary substrate-binding exosite in the serine protease domain of activated protein C is important for cleavage at Arg-506 but not at Arg-306 in factor Va. J Biol Chem. 2001;276(23):105–23108. doi: 10.1074/jbc.M103138200. [DOI] [PubMed] [Google Scholar]
- 64.Manithody C, Fay PJ, Rezaie AR. Exosite-dependent regulation of factor VIIIa by activated protein C. Blood. 2003;101:4802–4807. doi: 10.1182/blood-2003-01-0126. [DOI] [PubMed] [Google Scholar]
- 65.Shen L, Villoutreix BO, Dahlbäck B. Interspecies loop grafting in the protease domain of human protein C yielding enhanced catalytic and anticoagulant activity. Thromb Haemostas. 1999;82:1078–1087. [PubMed] [Google Scholar]
- 66.Yang L, Manithody C, Rezaie AR. The functional significance of the autolysis loop in protein C and activated protein C. Thromb Haemostas. 2005;94:60–68. doi: 10.1267/THRO05010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qureshi SH, Manithody C, Bae JS, Yang L, Rezaie AR. Autolysis loop restricts the specificity of activated protein C: analysis by FRET and functional assays. Biophys Chem. 2008;134:239–245. doi: 10.1016/j.bpc.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yegneswaran S, Wood GM, Esmon CT, Johnson AE. Protein S alters the active site location of activated protein C above the membrane surface. J Biol Chem. 1997;272:25013–25021. doi: 10.1074/jbc.272.40.25013. [DOI] [PubMed] [Google Scholar]
- 69.Yegneswaran S, Smirnov MD, Safa O, Esmon NL, Esmon CT, Johnson AE. Relocating the active site of activated protein C eliminates the need for its protein S cofactor. J Biol Chem. 1999;274:5462–5468. doi: 10.1074/jbc.274.9.5462. [DOI] [PubMed] [Google Scholar]
- 70.Dahlbäck B. Protein S and C4b-binding protein: Components involved in the regulation of the protein C anticoagulant system. Thromb Haemostas. 1991;66:49–61. [PubMed] [Google Scholar]
- 71.Regan LM, Lamphear BJ, Huggins CF, Walker FJ, Fay PJ. Factor IXa protects factor VIIIa from activated protein C. J Biol Chem. 1994;269:9445–9452. [PubMed] [Google Scholar]
- 72.Shen L, Dahlbäck B. Factor V and protein S as synergistic cofactors to activated protein C in degradation of factor VIIIa. J Biol Chem. 1994;269:18735–18738. [PubMed] [Google Scholar]
- 73.Parker ET, Doering CB, Lollar P. A1 subunit-mediated regulation of thrombin-activated factor VIII A2 subunit dissociation. J Biol Chem. 2006;281:13922–13930. doi: 10.1074/jbc.M513124200. [DOI] [PubMed] [Google Scholar]
- 74.Dahlbäck B. Resistance to activated protein C, the Arg506 to Gln mutation in the factor V gene, and venous thrombosis. Thromb Haemost. 1995;73:739–742. [PubMed] [Google Scholar]
- 75.Bertina RM, Koeleman BPC, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, van der Velden PA, Reitsma PH. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 76.Walker FJ, Scandella D, Fay PJ. Identification of the binding site for activated protein C on the light chain of factors V and VIII. J Biol Chem. 1990;265:1484–1489. [PubMed] [Google Scholar]
- 77.Stoilova-McPhie S, Villoutreix BO, Mertens K, Kemball-Cook G, Holzenburg A. 3-dimensional structure of membrane-bound coagulation factor VIII: modeling of the factor VIII heterodimer within a 3-dimensional density map derived by electron crystallography. Blood. 2002;99:1215–1223. doi: 10.1182/blood.v99.4.1215. [DOI] [PubMed] [Google Scholar]
- 78.O’Brien LM, Mastri M, Fay PJ. Regulation of factor VIIIa by human activated protein C and protein S: inactivation of cofactor in the intrinsic factor Xase. Blood. 2000;95:1714–1720. [PubMed] [Google Scholar]
- 79.Lapan KA, Fay PJ. Localization of a factor X interactive site in the A1 subunit of factor VIIIa. J Biol Chem. 1997;272:2082–2088. doi: 10.1074/jbc.272.4.2082. [DOI] [PubMed] [Google Scholar]
- 80.Gale AJ, Yegneswaran S, Xu X, Pellequer JL, Griffin JH. Characterization of a factor Xa binding site on factor Va near the Arg-506 activated protein C cleavage site. J Biol Chem. 2007;282:21848–21855. doi: 10.1074/jbc.M702192200. [DOI] [PubMed] [Google Scholar]
- 81.Iwaki T, Cruz DT, Martin A, Castellino FJ. A cardioprotective role for the endothelial protein C receptor in lipopolysaccharide-induced endotoxemia in the mouse. Blood. 2005;105:2364–2371. doi: 10.1182/blood-2004-06-2456. [DOI] [PubMed] [Google Scholar]
- 82.Liu D, Cheng T, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 83.Preston RJS, Ajzner E, Razzari C, Karageorgi S, Dua S, Dahlbäck B, Lane DA. Multifunctional specificity of the protein C/activated protein C Gla domain. J Biol Chem. 2006;281:28850–28857. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 84.Yang L, Bae JS, Manithody C, Rezaie AR. Identification of a specific exosite on activated protein C for interaction with protease-activated receptor 1. J Biol Chem. 2007;282:25493–25500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 85.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–1744. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 86.Brar SS, Manns BJ. Activated protein C: cost effective or costly? Crit Care. 2007;11:164. doi: 10.1186/cc6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant-activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niessen F, Furlan-Freguia C, Fernandez JA, Mosnier LO, Castellino FJ, Weiler H, Rosen H, Griffin JH, Ruf W. Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced vascular leakage and lethality. Blood. 2009;113:2859–2866. doi: 10.1182/blood-2008-12-192385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Bae JS, Yang L, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium binding loop of activated protein C eliminates its anticoagulant but not protective signaling properties. J Biol Chem. 2007;282:9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 90.Gruber A, Hurst S, McCarty OJT, Hurns PD, Rezaie AR. Non-anticoagulant activated protein C analog improves the neurological outcome of experimental stroke in mice. International Stroke Conference; Feb. 20–22, 2008; New Orleans, LA. [Google Scholar]
- 91.Wang J, Yang L, Rezaie AR, Li J. Activated protein C protects heart against ischemia reperfusion injury via activating AMPK signaling pathway and decreasing inflammatory response. Circulation. 2009;120(S1489):217. [Google Scholar]
- 92.Mosnier LO, Zampolli A, Kerschen EJ, Schuepbach RA, Banerjee Y, Fernández JA, Yang XV, Riewald M, Weiler H, Ruggeri ZM, Griffin JH. Hyperantithrombotic, noncytoprotective Glu149Ala-activated protein C mutant. Blood. 2009;113:5970–5978. doi: 10.1182/blood-2008-10-183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-κB. Crit Care Med. 2002;30:S288–S293. doi: 10.1097/00003246-200205001-00019. [DOI] [PubMed] [Google Scholar]
- 94.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 95.Bae JS, Rezaie AR. Thrombin inhibits nuclear factor kappaB and RhoA pathways in cytokine-stimulated vascular endothelial cells when EPCR is occupied by protein C. Thromb Haemost. 2009;101:513–520. [PMC free article] [PubMed] [Google Scholar]
- 96.Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the PAR-1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bae JS, Rezaie AR. Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand. Thromb Haemost. 2008;100:101–109. doi: 10.1160/TH08-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McLaughlin JN, Shen L, Holinstat M, Brooks JD, DiBenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 99.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci (USA) 2007;104:2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bae JS, Yang L, Rezaie AR. lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost. 2008;6:954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 101.Ye J, Rezaie AR, Esmon CT. Glycosaminoglycan contributions to both protein C activation and thrombin inhibition involve a common arginine-rich site in thrombin that includes residues arginine 93, 97, and 101. J Biol Chem. 1994;269:17965–17970. [PubMed] [Google Scholar]
- 102.Nieman MT, Schmaier AH. Interaction of thrombin with PAR1 and PAR4 at the thrombin cleavage site. Biochemistry. 2007;46:8603–8610. doi: 10.1021/bi700597p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Busch C, Cancilla P, DeBault L, Goldsmith J, Owen W. Use of endothelium cultured on microcarriers as a model for the microcirculation. Lab Invest. 1982;47:498–504. [PubMed] [Google Scholar]
- 104.Esmon CT. Cell mediated events that control blood coagulation and vascular injury. Ann Rev Cell Biol. 1993;9:1–26. doi: 10.1146/annurev.cb.09.110193.000245. [DOI] [PubMed] [Google Scholar]
- 105.Feistritzer C, Schuepbach RA, Mosnier LO, Bush LA, Di Cera E, Griffin JH, Riewald M. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006;281:20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 106.Elphick GF, Sarangi PP, Hyun YM, Hollenbaugh JA, Ayala A, Biffl WL, Chung HL, Rezaie AR, McGrath JL, Topham DJ, Reichner JS, Kim M. Recombinant human activated protein C inhibits integrin-mediated neutrophil migration. Blood. 2009;113:4078–4085. doi: 10.1182/blood-2008-09-180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 108.Minhas N, Xue M, Fukudome K, Jackson CJ. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 2009 Oct 26; doi: 10.1096/fj.09-134445. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 109.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 110.Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8:1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang XV, Banerjee Y, Fernandez JA, Deguchi H, Xu X, Mosnier LO, Urbanus RT, de Groot PG, White-Adams TC, McCarty OJT, Griffin JH. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci (USA) 2009;106:274–279. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Minhas N, Xue M, Fukudome K, Jackson CJ. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 2009 Oct 26; doi: 10.1096/fj.09-134445. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 113.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pratt CW, Church FC. General features of the heparin-binding serpins antithrombin, heparin cofactor II and protein C inhibitor. Blood Coag Fibrinol. 1993;4:479–490. doi: 10.1097/00001721-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 115.Heeb MJ, Griffin JH. Physiologic inhibition of human activated protein C by α1-antitrypsin. J Biol Chem. 1988;263:11613–11616. [PubMed] [Google Scholar]
- 116.Sakata Y, Loskutoff DJ, Gladson CL, Hekman CM, Griffin JH. Mechanism of protein C-dependent clot lysis: Role of plasminogen activator inhibitor. Blood. 1986;68:1218–1223. [PubMed] [Google Scholar]
- 117.Heeb MJ, Gruber A, Griffin JH. Identification of divalent metal ion-dependent inhibition of activated protein C by alpha 2-macroglobulin and alpha 2-antiplasmin in blood and comparisons to inhibition of factor Xa, thrombin, and plasmin. J Biol Chem. 1991;266:17606–17612. [PubMed] [Google Scholar]
- 118.Nayak RC, Sen P, Ghosh S, Gopalakrishnan R, Esmon CT, Pendurthi UR, Rao LV. Endothelial cell protein C receptor cellular localization and trafficking: potential functional implications. Blood. 2009;114:1974–1986. doi: 10.1182/blood-2009-03-208900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iakhiaev AV, Rezaie AR, Idell S. Thrombomodulin-mediated catabolism of protein C by pleural mesothelial and vascular endothelial cells. Thromb Haemost. 2007;98:627–634. [PubMed] [Google Scholar]
- 120.Rezaie AR. Vitronectin functions as a cofactor for rapid inhibition of activated protein C by plasminogen activator inhibitor-1. Implications for the mechanism of profibrinolytic action of activated protein C. J Biol Chem. 2001;276:15567–15570. doi: 10.1074/jbc.C100123200. [DOI] [PubMed] [Google Scholar]
- 121.Rezaie AR. Exosite-dependent regulation of the protein C anticoagulant pathway. Trends Cardiovasc Med. 2003;13:8–15. doi: 10.1016/s1050-1738(02)00191-3. [DOI] [PubMed] [Google Scholar]
- 122.Rezaie AR, Cooper ST, Church FC, Esmon CT. Protein C inhibitor is a potent inhibitor of the thrombin-thrombomodulin complex. J Biol Chem. 1995;270:25336–25339. doi: 10.1074/jbc.270.43.25336. [DOI] [PubMed] [Google Scholar]
- 123.Yang L, Manithody C, Walston TD, Cooper ST, Rezaie AR. Thrombomodulin enhances the reactivity of thrombin with protein C inhibitor by providing both a binding site for the serpin and allosterically modulating the activity of thrombin. J Biol Chem. 2003;278:37465–37470. doi: 10.1074/jbc.M307243200. [DOI] [PubMed] [Google Scholar]
- 124.Li W, Adams TE, Nangalia J, Esmon CT, Huntington JA. Molecular basis of thrombin recognition by protein C inhibitor revealed by the 1.6-Å structure of the heparin-bridged complex. Proc Natl Acad Sci (USA) 2008;105:4661–4666. doi: 10.1073/pnas.0711055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berg DT, Gerlitz B, Shang J, Smith T, Santa P, Richardson MA, Kurz KD, Grinnell BW, Mace K, Jones BE. Engineering the proteolytic specificity of activated protein C improves its pharmacological properties. Proc Natl Acad Sci (USA) 2003;100:4423–4428. doi: 10.1073/pnas.0736918100. [DOI] [PMC free article] [PubMed] [Google Scholar]