Abstract
γ-Secretase, a multi-subunit transmembrane protease comprised of presenilin, nicastrin, presenilin enhancer 2, and anterior pharynx-defective 1, participates in the regulated intramembrane proteolysis of Type I membrane proteins including the amyloid precursor protein (APP). Although Aph-1 is thought to play a structural role in the assembly of γ-secretase complex and several transmembrane domains (TMDs) of Aph-1 have been shown to be critical for its function, the importance of the other domains of Aph-1 remains elusive. We screened a series of Aph-1 mutants and focused on 9 mutations distributed in 6 different TMDs of human APH-1aS, assessing their ability to complement mouse embryonic fibroblasts lacking Aph-1. We showed that mutations in TMD4 (G126) and TMD5 (H171) of Aph-1a prevented the formation of the Nct/Aph-1 subcomplex. Importantly, although mutations in TMD3 (Q83/E84/R85) and TMD6 (H197) of APH-1aS did not affect Nct/Aph-1 subcomplex formation, both mutations prevented further association/endoproteolysis of PS1. We propose a model that identifies critical TMDs of Aph-1 for associations with Nct and PS for the stepwise assembly of γ-secretase components.
Keywords: γ-Secretase, Aph-1, Nct, PS, mutagenesis, transmembrane domain
1. Introduction
γ-Secretase is an aspartyl-type protease membrane protein complex that catalyzes the regulated intramembrane proteolysis of numerous type I membrane proteins, including Notch and APP (Francis, et al., 2002). The requirement for γ-secretase processing of APP to generate amyloid-β, a neurotoxic peptide involved in the pathogenesis of Alzheimer's disease (AD) has lead to intense studies to understand the biology of γ-secretase and towards the development of rationally designed drugs targeting this enzyme for prevention or treatment of AD (Panza, et al., 2009).
The γ-secretase is comprised of PS (Takasugi, et al., 2003), the catalytic component of this enzyme complex (Haass and Steiner, 2002), and three other essential subunits (Edbauer, et al., 2003): Nct(Hu, et al., 2002), Pen-2 (Francis, et al., 2002), and Aph-1 (Francis, et al., 2002). Nct is thought to be the receptor that initially recognizes the processed substrates ;(Dries, et al., 2009;Shah, et al., 2005), although some studies challenged this view (Chavez-Gutierrez, et al., 2008). Pen-2 is believed to control the endoproteolysis of PS to form a stable heterodimer composed of N- and C-terminal fragments (PS-NTF and PS-CTF) (Luo, et al., 2003;Prokop, et al., 2004). Current studies support the view that the formation of mature, active γ-secretase requires the initial formation of Nct/Aph-1 sub-complex (LaVoie, et al., 2003), and the subsequent sequential assembly of PS and Pen-2, which assembles with the Nct/ Aph-1/PS ternary complex to initiate endoproteolysis of PS (Hu, et al., 2002).
In humans there are two APH-1 homologues, APH-1a and APH-1b, and APH-1a has two C-terminal splicing variants: APH-1aL (long variant) and APH-1aS (short variant). There is an additional homologue, Aph-1c in mice. Although the homologues are differentially expressed in various tissues (Serneels, et al., 2005), Aph-1aL, APH-1aS, and Aph-1b are functionally redundant in terms of their ability to form active γ-secretase complexes with the other three subunits (Shirotani, et al., 2004b). Aph-1 has 7 TMDs with its N-terminus in endoplasmic reticulum (ER)/extracellular space and its C-terminus in the cytosol (Fortna, et al., 2004). Direct interaction between Aph-1 and Nct for the initial subcomplex has been shown (Shirotani, et al., 2004a), and crosslinking experiments have also demonstrated close proximity between Aph-1 and Ps and between Aph-1 and Nct (Steiner, et al., 2008). The GXXXG motif (Kleiger, et al., 2002) in TMD4 of Aph-1 has been identified to be essential for the initial assembly and later maturation of γ-secretase (Lee, et al., 2004), recently H171 and H197 in TMD5 and TMD6 were also found to be critical for the function/maturation of γ-secretase (Pardossi-Piquard, et al., 2009).
To extend those findings, here we tested the functional role of TMDs of Aph-1 in the assembly of components of the γ-secretase complex using site-directed mutagenesis to generate 12 single, double, or triple mutations of the conserved amino acid residues within TMD1 to TMD6 of human APH-1aS (hAPH-1aS) and assessed their ability to complement Aph-1 null MEFs (Aph-1ab −/−; Aph-1c shRNA-suppressed). Our results are consistent with the previous findings that GXXXG, H171, and H197 are critical for structure/function of γ-secretase complex. In addition to an extra critical region identified, Q83/E84/Q87 in TMD3, we found those disrupting mutants differentially affected 2 steps in γ-secretase maturation: mutation in TMD4 or TMD5 of Aph-1 disrupted formation of the initial Aph-1/Nct sub-complex whereas those in TMD3 and TMD6 decreased association/endoproteolysis of PS after the formation of the Nct/Aph-1 subcomplex.
2. Experimental Procedures
Mutagenesis
Mutations were introduced into human APH-1aS cDNA and cloned into pcDNA3.1-V5/His (Invitrogen) using QuickChange site-directed mutagenesis as described by the manufacturer (Stratagene). Human APP695 expressing plasmid was transfected as the substrate to assess functional competence of γ-secretase; thus, the accumulation of its C-terminus (APP-CTF) would indicate the impaired γ-secretase function. LacZ expression vector served as the mock transfection control.
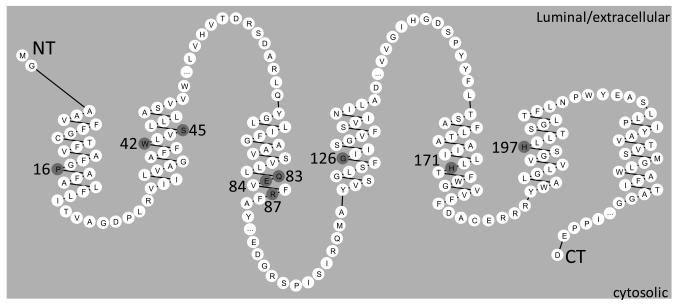
Nine amino acids were chosen for mutagenesis (Fig. 1A) as potential functionally critical residues using the following rationale: E84, R87, H171, or H197 are potentially charged; W42, S45, and Q83 are polar; P16 distorts the helix; G126 is known to disrupt assembly and activity of γ-secretase (Lee, et al., 2004). The targeted amino acids span TMD1-6 of the hAPH-1 peptide sequence. The targeted amino acids were replaced by residues with hydrophobic side chain that is compatible with preserved α-helix formation and intramembrane location.
Fig. 1.
Topology of Aph-1 depicted by Residue-based Diagram Editor (Campagne and Weinstein, 1999;Konvicka, et al., 2000;Skrabanek, et al., 2003). The residues in TMDs targeted for mutation highlighted in gray.
For most of the TMDs, there was only one site targeted for mutagenesis. However, TMD2 had 2 (W42V and S45A) and TMD3 had 3 (Q83L, E84V, R87L) sites targeted. Since multi-residue interaction could mediate TMD interactions, double or triple mutations were generated to enhance the sensitivity of complementation for TMDs with more than one targeted mutation. Thus, three hAPH-1aS compound-mutants were generated along with single-residue mutants: W42V/S45A (WS), Q83L/E84V (QE), Q83L/E84V/R87L (QER), P16L, W42V, S45A, Q83L, E84V, R87L, G126L, H171L, and H197L.
Cell lines
MEFs were isolated from Aph-1a/b −/− embryos (Ma, et al., 2005;Vetrivel, et al., 2008). To down regulate Aph-1c expression, short hairpin oligonucleotides corresponding to Aph-1c cDNA 905-924 (5′- T GAC CCC TGT ATC TTG GAA C TTCAAGAGA GTT CCA AGA TAC AGG GGT C TTTTTTC and 5′- TCGA GAA AAA AGA CCC CTG TAT CTT GGA ACT CTC TTG AAG TTC CAA GAT ACA GGG GTC A) were annealed and ligated into lentiviral vector pLentiLox3.7 between XhoI and HpaI sites. Aph-1a−/−;Aph-1b−/− cells were infected with lentivirus carrying the hairpin sequence. Single clones expressing EGFP were selected and screened for cell lines expressing reduced levels of Aph-1c. MEFs were cultured in DMEM high glucose supplemented with 10% fetal bovine serum in 5% CO2.
Transfection
To test function of γ-secretase, null MEFs at 40% confluence in a 6-well plate were transfected with 2 μ g of LacZ (mock control), Wt hAPH-1aS (positive control), or mutant hAPH-1aS vector together with 2 μ g of either APP695 (substrate +) or LacZ (substrate −) vector by Lipofectamine 2000 (Invitrogen). Cells and culture supernatants were harvested 24 hours later. For BN-PAGE and Co-IP, 4 μ g of of LacZ (mock), Wt (positive control), or mutant hAPH-1aS vector were transfected and harvested as stated. At least 3 independent transfections were done for density measurement and Aβ analysis.
Antibodies and blotting
For SDS gels, cell lysates were denatured at 55°C for 10 minutes, resolved in 4-20% Tris-glycine gels, and transferred to polyvinylidene difluoride (PVDF) membranes for probing. The antibodies used were as follows: anti-Nct (NCT-3925; 1:5000) (Li, et al., 2003); anti-PS1-CTF (1:2500) (Thinakaran, et al., 1996); anti-Pen-2 (Abcam 18189; 2 μg/ml); anti-V5 (Invitrogen R9600-25; 1:5000); anti-APP CTF(Sigma A8717, 1:5000); anti-Actin (Sigma A5541, 1:5000)
Enzyme-linked immunosorbent assay (ELISA)
25μL of each culture supernatant were analyzed for Aβ-40 or 42 using Aβ human ELISA kit (Invitrogen). The concentration of Aβ42 is below the detection limit (data not shown).
Blue native polyacrylamide gel electrophoresis (BN-PAGE)
Proteins were extracted by native sample buffer (50 mM BisTris, 6N HCl, 50 mM NaCl, 10% w/v glycerol, 0.001% Ponceau S, 1X complete protease inhibitor, 0.5% w/v digitonin, PH 7.2), put on ice for 20 minutes, and subject to 11000XG centrifugation for 20 minutes at 4°C. The supernatant was collected and Coomassie G-250 was added to the final concentration of 0.125% w/v. The samples were run in 3-12% BisTris native PAGE gel (Invitrogen). Gels were immersed in 0.1% SDS for 10 minutes before transfer and the transferred PVDF membranes were post-fixed with 8% acetic acid before blocking.
Co-immunoprecipitation (Co-IP)
Cells were lysed and cleared as for BN-PAGE. 1/20 of the cleared total lysate was kept for loading as the total lysate. Metal-coupled paramagnetic beads (Invitrogen) were used to pull down hAPH-1 in supernatant for 10 minutes at 4°C. Beads were washed three times in native sample buffer without protease inhibitor (30X the volume of the initial bead solution) before elution by 200mM EDTA in 1X SDS sample buffer with reducing agent (Invitrogen) for SDS- PAGE.
Density measurement and statistical analysis
The average band intensities of each mutant or mock transfection relative to hAPH1aS transfection were normalized by actin in the SDS PAGE (Fig. 2D) or nonspecific band in Ps1-loop staining in the BN-PAGE (Fig. 3A, filled arrowhead) for statistical analysis. For SDS-PAGE, Nct glycosylation served as the index of γ-secretase complex maturation in Figure 2E. For BN-PAGE, the densities of upper and lower Nct bands were used as the indicator of γ-secretase holocomplex (Fig. 3E) and Nct/Aph-1 subcomplex (Fig. 3F), respectively. Statistical analyses were performed by one way analysis of variance (one way ANOVA) with Dunnett's multiple comparison test.
Figs. 2A-F.
Protein blot analysis of various hAPH-1aS mutations in complementing structure and function of γ-secretase. Immortalized Aph-1 deficient cells transiently transfected with expression plasmids encoding mutant hAPH-1aS and human APP695. Cell extracts were prepared and subjected to protein blot analysis using antisera specific for: A. Nct; glycosylated (arrowhead) and nonglycosylated (arrow) Nct; B. PS1-CTF (processed C-terminal fragment of PS1, arrowhead); C. V5-tagged hAPH-1aS; and D. Actin. E. Quantification of γ-secretase maturation by Nct glycosylation (panel A, arrowhead; mean±SEM). F. Assessment of APP processing as determined by secretion Aβ-40 (mean±SEM). Conditioned culture media were collected after transfection and analyzed by sandwich ELISA. * indicates p<0.05.
Figs. 3A-F.
BN-PAGE analysis of selected mutants on the formation of γ-secretase holocomplex and Nct/Aph-1 subcomplex. Membrane fractions of Aph-1 deficient cells transfected with mutant hAPH-1aS were prepared and subjected to blue native gel analysis using antiserum recognizing PS1-CTF (A), Pen-2 (B), Nct (C), and transfected hAph-1 (D). Note that PS and Pen-2 staining showed only holocomplex whereas Nct staining revealed both γ-secretase holocomplex and Nct/Aph-1 subcomplex. V5 staining showed the corresponding holocomplex and subcomplex, albeit with high background due to overexpression of hAPH-1. Hollow arrowhead, holocomplex; arrow, Nct/Aph-1 subcomplex; filled arrowhead, non-specific band as observed also in loading control; *, faint γ-secretase holocomplex by H171 complementation. E and F. Quantification (mean±SEM) of γ-secretase holocomplex (in panel E) and Nct/Aph-1 subcomplex (in panel F) using band intensity of the Nct blot (panel C). * indicates p<0.05.
3. Results
Identification of TMDs of Aph-1 critical for assembly of components of γ-secretase
To determine the functional role of TMDs of Aph-1 in assembly of components of γ-secretase complex, we mutagenized a series of conserved residues in the transmembrane helices (TMD1 to TMD6) of human APH-1aS tagged with V5 and 6His at the C-terminus and assessed their ability to functionally complement the phenotypes of Aph-1 null MEF. Since the vast majority of the Aph-1 peptide chain (and the majority of the entire γ-secretase complex) resides within the membrane, we reasoned that intramembrane residues within Aph-1 are likely to be crucial for its association with the other components of the complex. Further, we reasoned that the residues within the Aph-1 transmsmembrane domains that are both highly conserved and ‘unusual’ for a transmembrane α-helix (i.e. charged, hydrophilic, or helix-breaking residues) are the most likely to be functionally important. For these reasons, we chose residues P16 (TMD1), W42 and S45 (TMD2, either singly mutated or combined), Q83, E84, and R87 (TMD3, either singly mutated or combined), G126 (TMD4), H171 (TMD5), and H197 (TMD6) for mutagenesis. The rationale for the mutation of G126 was its participation in a conserved GxxxG intramembrane helical interaction motif, which has since been supported in studies by others (18).
As expected, protein blot analysis showed that wild type (Wt) hAPH-1aS restored the level of PS1-CTF (Fig. 2B, lanes 2-3), maturation of Nct (Fig. 2A, lanes 2-3), and production of Aβ-40 (Fig. 2F) in Aph-1 null MEFs, indicating that the epitope-tagged hAPH-1aS could functionally complement the Aph-1 null phenotype.
Like wt APH-1aS, either single or compound mutations in TMD1 or TMD2 (P16L, W42V, S45A, and W42V/S45A) of APH-1aS could restore the maturation of γ-secretase in terms of PS1-CTF cleavage and Nct glycosylation (Figs. 2A-B, lanes 4-6, 15; Fig. 2E), thus indicating that these residues are not critical for physical interactions between APH-1 and the other components of the complex or for γ-secretase maturation.
Interestingly, while the single mutations in TMD3 (Q83L, E84V, R85L) were able to complement both structure and function of γ-secretase (Figs. 2A-B, lanes 10-12; Figs. 2E-F), however, the compound mutation in TMD3, Q83L;E84V, was defective to restore maturation of γ-secretase (Figs. 2A-B, lane 13; Fig. 2E, QE vs APH1aS, p<0.05), with only partial capability to rescue Aβ-40 production (Fig. 2F; QE vs APH1aS or QE vs Mock, both p<0.05). Importantly, the triple mutation, Q83L;E84V;R85L localized in TMD3, failed to structurally and functionally rescue the Aph-1 null phenotype (Figs. 2A-B, lane 14; Fig. 2E-F, QER vs APH1aS, P<0.05), indicating the critical role that these three residues play in regulating the assembly of components of γ-secretase complex. Similarly, failure to complement the Aph-1 null phenotype was observed for mutant H197L in TMD6 (Figs. 2A-B, lane 8; Fig. 2E-F, H197L vs APH1aS, p<0.05) and mutant G126L in TMD4 (Figs. 2A-B, lane 7; Fig. 2E-F, G126L vs APH1aS, p<0.05). Although, functionally, mutant H171L in TMD5 exhibited partial rescue (Fig. 2F, H171L vs APH1aS or Mock, both p<0.05), structurally as determined by the maturation of Nct glycosylation no significant difference were observed when compared with the mock transfection (Figs. 2A-B, lane 9, Fig. 2E, H171 vs Mock, p>0.05). Taken together, these results from our mutagenesis analysis of APH-1aS identified several TMDs in Aph-1 that are critical to facilitate the assembly of the components of the γ-secretase complex.
TMD4 and TMD5 of Aph-1 are critical determinants for the formation of Nct/Aph-1 subcomplex
Although we have identified several critical TMDs in Aph-1 for subunit assembly of γ-secretase, the precise role that these TMDs play is not known. To determine at which step in the subunit assembly of γ-secretase these Aph-1 mutants exert their effects, we assessed the impact of each of these mutants on their ability to form γ-secretase holo- or subcomplexes. As compared to controls (Figs. 3A-C, lanes 2), Blue Native-PAGE analysis revealed that mutants capable of rescuing the Aph-1 null phenotype (Q83L, E84V, R87L) also normalized the level of γ-secretase holocomplexes (Figs. 3A-C, lanes 3-5; Fig. 3E, Q83L, E84V, or R87L vs Mock, p<0.05). While mutant QE that partially rescued the Aph-1 null phenotype could restore the formation of γ-secretase holo-complexes (Figs. 3A 3-C, lane 6; Fig. 3E, QE vs Mock, p<0.05), mutants (QER, G126L, H171L, and H197L) that failed to complement the γ-secretase structurally as assessed by Nct glycosylation or PS1-CTF formation also showed low level of γ -secretase holo-complexes comparable to mock transfection. (Figs. 3A-C, lanes 7-10; Fig. 3E).
To ascertain the reasons for the inability of these mutants to complement the assembly and maturation of γ-secretase holo-complexes, we first examined their impact on formation of Nct/Aph-1 sub-complexes, a critical step of subunit assembly for the maturation of γ-secretase. Nct staining was used to mark the formation of γ-secretase holocomplex and Nct/Aph-1 subcomplex. By staining for APH1aS, an upper band of γ-secretase holocomplex (hollow arrowhead, Fig. 3D) and a lower band of Nct/Aph-1 subcomplex (arrow, Fig. 3D) were identified in the blot corresponding to Nct staining, thus verifying the identity of holocomplex and Nct/Aph-1 subcomplex (Fig. 3C). Blue-native PAGE analysis showed that the mutants (QER and H197L) that failed to form γ-secretase holocomplexes (Fig. 3C, lanes 7 and 10; Fig. 3E), were capable of forming the Nct/Aph-1 subcomplexes to a similar extent as that observed for wild-type hAPH-1aS (Fig. 3C lanes 7 and 10; Fig. 3F, QER or H197 vs Mock, p<0.05), indicating that mutant QER and H197L retained their ability to efficiently bind Nct. However, mutants G126L and H171L failed to complement the formation of Nct/Aph-1 subcomplexes (Fig. 3C, lanes 8 and 9; Fig. 3F, G126L or H171L vs APH1aS, p<0.05), indicating that TMD4 and TMD5 of Aph-1 are critical for facilitating the interaction with Nct for the formation of Nct/Aph-1 subcomplex.
TMD3 and TMD6 of Aph-1 are important determinants for subunit assembly of Aph-1/Nct subcomplex with PS/Pen2
Although our demonstration that mutations in TMD4 and TMD5 prevent formation of Nct/Aph-1 subcomplexes can explain the failure for maturation of γ-secretase holocomplexes, this did not appear to be applicable for the mutations in TMD3 or TMD6. Since mutants QER in TMD3 and H197L in TMD6 are competent for formation of Nct/Aph-1 subcomplexes, we speculated that these mutants may affect the next step of the subunit assembly process, namely the interaction with and processing of PS. To test this notion, we employed a coimmunoprecipitation approach using the four mutants (QER in TMD3, G126L in TMD4, H171L in TMD5, and H197L in TMD6) that exhibited significant impairment in complex assembly as shown by our BN-PAGE analysis. Antisera recognizing Wt APH-1aS was able to pull down both glycosylated (Figs. 4B-C, arrowhead, lane 1b) and nonglycosylated (Figs. 4B-C, arrow, lane 1b) Nct, PS1-CTF (Fig. 4A, lane 1b, band CTF), indicating that Wt hAPH-1 restored the maturation and assembly of γ-secretase holocomplexes in Aph-1 null cells. Importantly, antisera recognizing APH-1aS mutants in TMD3 (QER) or TMD6 (H197L), can efficiently pull-down immature, but not mature Nct (Figs. 4B-C, lanes 2b and 5b), however, they failed to pull-down PS1-CTF (Fig. 4A, lanes 2b and 5b, band CTF), indicating that these mutants interfere with subsequent steps of maturation of Nct and of complex assembly with PS/Pen2. Since mutations in TMD4 (G126L) and TMD5 (H171L) of Aph-1 severely disrupted its ability to assemble with Nct, we observed much less efficient pull-downs of Nct using with these mutants (Figs. 4B-C, lanes 3b and 4b). A small amount of Nct was, neveretheless, co-precipitated with these two mutants. In the case of the G126L mutant this low level of co-precipitated Nct was purely immature (unglycosylated), while in the case of H171L the co-precipitated Nct has higher relatively maturation ratio, albeit reduced in overall amount (compare ratios of mature and immature Nct in Figs. 4B-C lanes 1b and 4b), indicating that although the H171L mutation severely impairs the initial association between Aph-1 and Nct, it does not affect subsequent maturation of the low level of Aph-1/Nct sub-complex that is able to form. This also explains the partially retained ability of this mutant to complement production of Aβ-40 (Fig. 2F) and the maturation of γ-secretase (Figs. 2A-B, 3A-C). Our results indicate that while mutants H197L in TMD6 and QER in TMD3 were competent in binding to Nct, the Nct/Aph-1 subcomplex failed to proceed further to assembly with PS and its subsequent endoproteolysis, suggesting that TMD3 and TMD6 of Aph-1 play crucial roles in association of the Aph-1/Nct subcomplex with PS/Pen2.
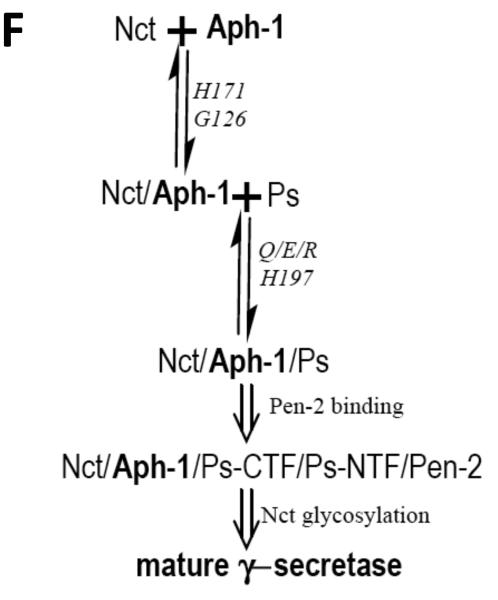
Fig. 4A-G.
Co-IP analysis using WT or mutant 6his-tagged hAPH-1aS. Aph-1 deficient cells transfected with complex assembly defective Aph-1aS mutants were prepared as for analysis by BN-PAGE. Lysates, subjected to immunoprecipation and SDS- PAGE, were immunoblotted for: A. PS1-CTF (arrow); B and C. Nct; Shorter (B) and longer (C) exposure of Nct blot. Arrowhead, glycosylated Nct; arrow, nonglycosylated Nct; D. hAPH1aS (arrow); and E. Actin. T, total lysate; P, pulled-down fraction. F. Aph-1 mutations differentially disrupt steps of γ-secretase maturation. G. Proposed model of domain-specific functions of Aph-1 in interaction with Nct and Ps/Pen-2.
4. Discussion
Correct assembly of Aph-1 with Nct, PS and Pen2 is essential for γ-secretase activity. Initial biochemical analysis suggested that Aph-1 interacts with both PS1-CTF and Nct (Fraering, et al., 2004;Steiner, et al., 2008). However, except for the GXXXG motif in TMD4 (Lee, et al., 2004) and histidine residues in TMDs 5 and 6 (Pardossi-Piquard, et al., 2009), the other critical regions or residues responsible for the subunit assembly of Aph-1 into mature γ-secretase complex are not known. Through our Aph-1 mutagenesis studies, we have now identified six residues localized within TMDs 3 to 6 of Aph-1 that are critical for the assembly of γ-secretase complex.
Previous work has shown the critical role of G122 and G126 in the TMD 4 of Aph-1 for γ-secretase maturation (Lee, et al., 2004). The GXXXG motif has been implicated in the TMD interactions of membrane proteins (Lemmon, et al., 1994) (Senes, et al., 2000). We confirmed that G126L mutation abrogates the formation of Nct/Aph-1 subcomplex, the first step required for maturation of γ-secretase (Hu and Fortini, 2003). The absence of a corresponding GXXXG motif in Nct suggests that Nct interacts with Aph-1 by mechanisms other than through this classical GXXXG homodimer formation (Lee, et al., 2004). It is also possible that the GXXXG motif is essential for Aph-1 intramolecular interaction to facilitate recognition of a different Aph-1 TMD by Nct. Although the active γ-secretase complex is thought most likely to contain only one subunit each of the four essential components (Sato, et al., 2007), it is possible that Aph-1 could associate with Nct through an accessory factor by dimerization through the GXXXG motif or possibly by first homodimerizing with a second APH-1 molecule through GxxxG interactions. This transient assembly could provide contact surface for Nct or could trigger a conformational change in Aph-1, allowing it to associate with Nct. The observation that the G126L APH-1 mutant is unable to associate with Nct and forms this initial sub-complex is consistent with this potential model.
Consistent with findings by Pardossi-Piquard and co-workers, we demonstrated that H171 and H197 have essential, but differential roles in γ-secretase assembly. H171 in TMD5 of Aph-1 is necessary for assembly with Nct indicates that intact GXXXG in TMD4 of Aph-1 is necessary but not sufficient to form a stable Nct/Aph-1 subcomplex. Alternatively, it is plausible that the H171 of Aph-1 directly interacts with Nct to form the Nct/Aph-1subcomplex and that the mutation in TMD5 of Aph-1 greatly reduces the affinity for Nct. Supporting this view is the observation that TMD5-7 of Aph-1 is sufficient to pull down Nct (Fortna, et al., 2004). Thus, our data identify two determinants (G126 and H171) in TMD4 and TMD5 of Aph-1 required for the initial subunit assembly of Nct and Aph-1 to form the Nct/Aph-1 subcomplex (see our model in Fig. 4G).
Interestingly, our analysis of mutants QER and H197 in TMD3 and TMD6, respectively, suggests that these TMDs are critical for binding/endoproteolysis of PS (Fig. 4). Furthermore, single mutants of Q/E/R could still complement assembly/function of γ-secretase while QE and QER, respectively, showed partial to complete loss in complementation ability demonstrates these 3 residues act synergistically for interaction with Nct, probably by forming a hydrophilic binding surface in the membrane. The requirement for a combination of mutations also explains the absence of disrupting effects in identical positions (Pardossi-Piquard et al., 2009). The retention of ability to bind Nct also argues against complete disruption of the protein by the QER triple mutation.
In addition, although QER and H197 mutants predominantly reduce interaction between Nct/Aph-1 subcomplex and PS, a mild decrease in Aph-1 interaction with Nct observed by Co-IP (Figs. 4B-C) would suggest that those residues in TMD3 and TMD6 may also have some involvement in the initial Nct/Aph-1 interaction, albeit clearly less critical than the role of G126 and H171 in this initial interaction. We cannot discount the possibility, however, that the somewhat decreased interaction of these mutants with Nct could be due to steric hindrance by the bulkiness of the leucine residues.
Taken together, our results support a model in which H171 in TMD5 and G126 in TMD4 of Aph-1facilitates the formation of the Nct/Aph-1 subcomplex as the first step of γ-secretase assembly, whereas H197 in TMD6 and Q83/E84/R87 in TMD3 and of Aph-1 are required for the subsequent steps of γ-secretase maturation including the association of PS with the Nct/Aph-1 subcomplex and endoproteolysis of PS. Future studies are required to clarify the exact mechanism whereby the Nct/Aph-1 subcomplex interacts with PS. Based on our findings, this association may occur at least partially through intramembrane interactions between Aph/Nct and PS, likely mediated by residues Q83/E84/R87 in TMD3 and/or H197 in TMD6 of Aph-1; extramembranous interactions (e.g. mediated by residues C248/E333/G339 of Nct ectodomain (Shirotani, et al., 2004a)) may also contribute to this association (Fig. 4G).
5. Acknowledgement
We thank R. Doms (University of Pennsylvania) for support of generation of Aph-1 mutants and G. Thinakaran (University of Chicago) for Pen-2 antiserum. This work was supported by National Institutes of Health NINDS Grants R01 NS045150 and P01 NS047308 (to P.C.W.).
Abbreviations
- PS
presenilin
- Nct
nicastrin
- Pen-2
presenilin enhancer 2
- Aph-1
anterior pharynx-defective 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Disclosure Statement
All authors disclose no conflict of interest of any kind in this paper.
7. Reference
- Campagne F, Weinstein H. Schematic representation of residue-based protein context-dependent data: an application to transmembrane proteins. J Mol Graph Model. 1999;17(3-4):207–13. doi: 10.1016/s1093-3263(99)00032-7. [DOI] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Tolia A, Maes E, Li T, Wong PC, de Strooper B. Glu(332) in the Nicastrin ectodomain is essential for gamma-secretase complex maturation but not for its activity. J Biol Chem. 2008;283(29):20096–105. doi: 10.1074/jbc.M803040200. [DOI] [PubMed] [Google Scholar]
- Dries DR, Shah S, Han YH, Yu C, Yu S, Shearman MS, Yu G. GLU333 of nicastrin directly participates in gamma-secretase activity. J Biol Chem. 2009 doi: 10.1074/jbc.M109.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5(5):486–8. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Fortna RR, Crystal AS, Morais VA, Pijak DS, Lee VM, Doms RW. Membrane topology and nicastrin-enhanced endoproteolysis of APH-1, a component of the gamma-secretase complex. J Biol Chem. 2004;279(5):3685–93. doi: 10.1074/jbc.M310505200. [DOI] [PubMed] [Google Scholar]
- Fraering PC, LaVoie MJ, Ye W, Ostaszewski BL, Kimberly WT, Selkoe DJ, Wolfe MS. Detergent-dependent dissociation of active gamma-secretase reveals an interaction between Pen-2 and PS1-NTF and offers a model for subunit organization within the complex. Biochemistry. 2004;43(2):323–33. doi: 10.1021/bi035748j. [DOI] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3(1):85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Haass C, Steiner H. Alzheimer disease gamma-secretase: a complex story of GxGD-type presenilin proteases. Trends Cell Biol. 2002;12(12):556–62. doi: 10.1016/s0962-8924(02)02394-2. [DOI] [PubMed] [Google Scholar]
- Hu Y, Fortini ME. Different cofactor activities in gamma-secretase assembly: evidence for a nicastrin-Aph-1 subcomplex. J Cell Biol. 2003;161(4):685–90. doi: 10.1083/jcb.200304014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ye Y, Fortini ME. Nicastrin is required for gamma-secretase cleavage of the Drosophila Notch receptor. Dev Cell. 2002;2(1):69–78. doi: 10.1016/s1534-5807(01)00105-8. [DOI] [PubMed] [Google Scholar]
- Kleiger G, Grothe R, Mallick P, Eisenberg D. GXXXG and AXXXA: common alpha-helical interaction motifs in proteins, particularly in extremophiles. Biochemistry. 2002;41(19):5990–7. doi: 10.1021/bi0200763. [DOI] [PubMed] [Google Scholar]
- Konvicka K, Campagne F, Weinstein H. Interactive construction of residue-based diagrams of proteins: the RbDe web service. Protein Eng. 2000;13(6):395–6. doi: 10.1093/protein/13.6.395. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Fraering PC, Ostaszewski BL, Ye W, Kimberly WT, Wolfe MS, Selkoe DJ. Assembly of the gamma-secretase complex involves early formation of an intermediate subcomplex of Aph-1 and nicastrin. J Biol Chem. 2003;278(39):37213–22. doi: 10.1074/jbc.M303941200. [DOI] [PubMed] [Google Scholar]
- Lee SF, Shah S, Yu C, Wigley WC, Li H, Lim M, Pedersen K, Han W, Thomas P, Lundkvist J, Hao YH, Yu G. A conserved GXXXG motif in APH-1 is critical for assembly and activity of the gamma-secretase complex. J Biol Chem. 2004;279(6):4144–52. doi: 10.1074/jbc.M309745200. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Treutlein HR, Adams PD, Brunger AT, Engelman DM. A dimerization motif for transmembrane alpha-helices. Nat Struct Biol. 1994;1(3):157–63. doi: 10.1038/nsb0394-157. [DOI] [PubMed] [Google Scholar]
- Li T, Ma G, Cai H, Price DL, Wong PC. Nicastrin is required for assembly of presenilin/gamma-secretase complexes to mediate Notch signaling and for processing and trafficking of beta-amyloid precursor protein in mammals. J Neurosci. 2003;23(8):3272–7. doi: 10.1523/JNEUROSCI.23-08-03272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo WJ, Wang H, Li H, Kim BS, Shah S, Lee HJ, Thinakaran G, Kim TW, Yu G, Xu H. PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. J Biol Chem. 2003;278(10):7850–4. doi: 10.1074/jbc.C200648200. [DOI] [PubMed] [Google Scholar]
- Ma G, Li T, Price DL, Wong PC. APH-1a is the principal mammalian APH-1 isoform present in gamma-secretase complexes during embryonic development. J Neurosci. 2005;25(1):192–8. doi: 10.1523/JNEUROSCI.3814-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Solfrizzi V, Frisardi V, Capurso C, D'Introno A, Colacicco AM, Vendemiale G, Capurso A, Imbimbo BP. Disease-modifying approach to the treatment of Alzheimer's disease: from alpha-secretase activators to gamma-secretase inhibitors and modulators. Drugs Aging. 2009;26(7):537–55. doi: 10.2165/11315770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Pardossi-Piquard R, Yang SP, Kanemoto S, Gu Y, Chen F, Bohm C, Sevalle J, Li T, Wong PC, Checler F, Schmitt-Ulms G, George-Hyslop P, Fraser PE. APH1 polar transmembrane residues regulate the assembly and activity of presenilin complexes. J Biol Chem. 2009;284(24):16298–307. doi: 10.1074/jbc.M109.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop S, Shirotani K, Edbauer D, Haass C, Steiner H. Requirement of PEN-2 for stabilization of the presenilin N-/C-terminal fragment heterodimer within the gamma-secretase complex. J Biol Chem. 2004;279(22):23255–61. doi: 10.1074/jbc.M401789200. [DOI] [PubMed] [Google Scholar]
- Sato T, Diehl TS, Narayanan S, Funamoto S, Ihara Y, De Strooper B, Steiner H, Haass C, Wolfe MS. Active gamma-secretase complexes contain only one of each component. J Biol Chem. 2007;282(47):33985–93. doi: 10.1074/jbc.M705248200. [DOI] [PubMed] [Google Scholar]
- Senes A, Gerstein M, Engelman DM. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J Mol Biol. 2000;296(3):921–36. doi: 10.1006/jmbi.1999.3488. [DOI] [PubMed] [Google Scholar]
- Serneels L, Dejaegere T, Craessaerts K, Horre K, Jorissen E, Tousseyn T, Hebert S, Coolen M, Martens G, Zwijsen A, Annaert W, Hartmann D, De Strooper B. Differential contribution of the three Aph1 genes to gamma-secretase activity in vivo. Proc Natl Acad Sci U S A. 2005;102(5):1719–24. doi: 10.1073/pnas.0408901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122(3):435–47. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Shirotani K, Edbauer D, Kostka M, Steiner H, Haass C. Immature nicastrin stabilizes APH-1 independent of PEN-2 and presenilin: identification of nicastrin mutants that selectively interact with APH-1. J Neurochem. 2004a;89(6):1520–7. doi: 10.1111/j.1471-4159.2004.02447.x. [DOI] [PubMed] [Google Scholar]
- Shirotani K, Edbauer D, Prokop S, Haass C, Steiner H. Identification of distinct gamma-secretase complexes with different APH-1 variants. J Biol Chem. 2004b;279(40):41340–5. doi: 10.1074/jbc.M405768200. [DOI] [PubMed] [Google Scholar]
- Skrabanek L, Campagne F, Weinstein H. Building protein diagrams on the web with the residue-based diagram editor RbDe. Nucleic Acids Res. 2003;31(13):3856–8. doi: 10.1093/nar/gkg552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Winkler E, Haass C. Chemical crosslinking provides a model of the gamma -secretase complex subunit architecture and evidence for close proximity of the C-terminal fragment of presenilin with APH-1. J Biol Chem. 2008 doi: 10.1074/jbc.M709067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422(6930):438–41. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17(1):181–90. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Zhang X, Meckler X, Cheng H, Lee S, Gong P, Lopes KO, Chen Y, Iwata N, Yin KJ, Lee JM, Parent AT, Saido TC, Li YM, Sisodia SS, Thinakaran G. Evidence that CD147 modulation of beta-amyloid (Abeta) levels is mediated by extracellular degradation of secreted Abeta. J Biol Chem. 2008;283(28):19489–98. doi: 10.1074/jbc.M801037200. [DOI] [PMC free article] [PubMed] [Google Scholar]