Abstract
Afferent nerve fibers of the somatosensory system are a molecularly diverse cell population that detects a varied range of environmental stimuli, converting these external cues ultimately into a sensory percept. Afferents mediating detection of thermal stimuli express a repertoire of temperature sensitive ion channels of the TRP family which endow these nerves with the ability to respond to the breadth of temperatures in the environment. The cold and menthol receptor TRPM8 is responsible for detection of cold and, unlike other thermosensors, detects both innocuous and noxious temperatures. How this single molecule can perform such diverse functions is currently unknown, but expression analyses in adult tissues shows that TRPM8 neurons are a molecularly diverse population and it is likely that this diversity underlies differential functionality. To determine how this phenotype is established, we examined the developmental time course of TRPM8 expression using a mouse transgenic line in which GFP expression is driven by the TRPM8 transcriptional promoter (Trpm8GFP). We find that Trpm8GFP expression begins prior to embryonic day 15.5 (E15.5) after which expression reaches levels observed in adult neurons. By E18.5, central axons of Trpm8GFP neurons reach the spinal cord dorsal horn, but anatomical localization and in vivo measurements of neural activity suggest that fully functional cold circuits are not established until after the first postnatal week. Additionally, Trpm8GFP neurons undergo a transition in neurochemical phenotype, ultimately reaching adult expression of markers such TRPV1, CGRP, peripherin, and NF200 by postnatal day 14. Thus, based on immunochemical, anatomical and functional criteria, active cold neural circuits are fully established by the second week postnatal, thereby suggesting that important extrinsic or intrinsic mechanisms are active prior to this developmental stage.
List of Abbreviations: TRPM8, TRPV1, CGRP, TrkA, Trpm8GFP, P2X3
Sensory neurons of the dorsal root (DRG) and trigeminal (TG) ganglia convert environmental stimuli of a chemical, mechanical, or thermal nature into electrical activity, generating distinct percepts including touch, temperature and pain (Basbaum et al., 2009). Their remarkable ability to differentiate between the various modalities, as well as intensities, is fundamental for appropriate behavioral responses to environmental changes (McKemy, 2007, Basbaum et al., 2009). However, the detailed cellular and molecular mechanisms of each of these sensations are not completely understood (Julius and Basbaum, 2001). A wide repertoire of sensory transduction molecules that convert external environmental stimuli into neural activity have been recently identified, thereby allowing for the examination of the neurochemical, functional and anatomical properties of neurons to which a stimulus modality can be assigned (Basbaum et al., 2009). For example, ion channels of transient receptor potential (TRP) family are the primary detectors of thermal stimuli (Jordt et al., 2003), with the temperatures considered cool to cold mediated by TRPM8 (McKemy et al., 2002, Peier et al., 2002, Daniels and McKemy, 2007). Thus, an understanding of the properties of TRPM8-expressing neurons will provide insights into the means by which the peripheral nervous system detects cold temperatures.
Peripheral sensory neurons in general are specified early in development (Marmigere and Ernfors, 2007), with sensory sub-lineages identified even before neural crest precursors become committed to neuronal or glial fates (Zirlinger et al., 2002). However, functionally such distinctions require evidence for intact neural circuits in which neural activity or behaviors to environmental stimuli are produced. The central projections of DRG neurons terminate in a restricted region of the dorsal horn of the spinal cord. In growth of putative small-diameter afferents into the developing dorsal horn occurs in rodents beginning at E19, with nociceptive neurons predominantly terminating in lamina I and II (Mirnics and Koerber, 1995b, Snider, 1998, Hunt and Mantyh, 2001, Julius and Basbaum, 2001). While the establishment of functional spinal neural circuits developmentally is critical for afferent signaling, expression of sensory transduction molecules such as TRPM8 is also required for generation of functional neural responses. Therefore, we hypothesized that functional TRPM8-dependent cold circuits are established when channel expression is initiated and when TRPM8-afferent central projections are strictly localized at lamina I and IIo.
TRPM8 channels are expressed in functionally distinct subsets of neurons associated with unique neurochemical profiles (Takashima et al., 2007, Dhaka et al., 2008). Even more striking is that many of these cells are only visualized by TRPM8 expression and thus not easily defined molecularly (Takashima et al., 2007). In the adult, TRPM8 co-localizes with immunoreactivity to the intermediate filaments NF200 and peripherin, markers of Aδ- and C-fibers, respectively, which provide distinct perceptual cold sensations (Jyvasjarvi and Kniffki, 1987, Simone and Kajander, 1997, Takashima et al., 2007). Additionally, a cohort of TRPM8 neurons expresses nociceptor markers such as the painful heat-gated channel TRPV1 as well as calcitonin gene-related peptide (CGRP) (McNeill et al., 1988, Caterina et al., 1997). However, no co-localization between Trpm8GFP and IB4, a neuronal marker for non-peptidergic neurons, is observed which suggests that those presumptive non-peptidergic neurons (CGRP-negative) observed are not IB4+ neurons. Nevertheless, this strong heterogeneity may account for the broad range of functions attributed to TRPM8 (Welberg, 2008). However, what is not known is when this heterogeneity is established developmentally. To begin to answer this question we used immunochemistry and mouse genetics to examine the somal expression patterns of TRPM8 neurons in the developing mouse embryo, as well as determined when central projections of these afferents are likely to establish functional connections in the spinal cord dorsal horn. These data suggest that cold neurocircuits have the capacity to respond to cold temperatures very early in development, but do not form functional circuits until late in to the second week of postnatal development.
EXPERIMENTAL PROCEDURES
Immunohistochemistry
All animal work was performed in accordance with protocols approved by the Institutional Animal Care and Use Committees at the University of Southern California following the National Institutes of Health regulations. Embryos were collected from pregnant Trpm8GFP transgenic mice with the plug day designated as embryonic day 0.5 (E0.5). Mouse embryos from different embryonic stages were cut on a cryostat (Microm HM560). Sections (16 μm) were processed for immunochemistry as described (Zhao et al., 2009). Postnatal animals were transcardially perfused with 0.1M PBS followed with fixative (4% formaldehyde, 0.2% (v/v) saturated picric acid, 0.1M PBS, pH 7.3, at 4°C). DRGs from lumbar regions 4, 5 and 6 were collected and corresponding sections of spinal cord were carefully dissected and post-fixed overnight at 4°C in the same fixative solution. Samples were washed with 0.1M PBS for 30min. All tissues were cryo-protected in 30% sucrose in PBS at 4°C overnight, quickly frozen in OCT, sectioned with a cryostat at 12 μm (DRG) and 20 μm (spinal cord), and mounted on Superfrost Plus slides. Slides were stored at −80°C.
Frozen slides were dried at 4°C for 30min and then at 24°C for additional 30min and washed with deionized water for 30sec. Slide-mounted sections were washed three times with PBS, once with PBS plus 0.3% Triton X-100 (PBS-T) for 45min, and three times with PBS before a blocking step (1hr at room temperature with 20% normal goat serum in PBS). Primary antibodies were diluted in a PBS solution and incubated at 4°C overnight. Antibodies and dilution: 1:1000 chicken anti-GFP (GFP-1020; Aves Labs); 1:500 guinea pig anti-CGRP (T-5027; Peninsula); 1:500 rabbit anti-NF200 (N-4142; Sigma); 1:500 rabbit anti-peripherin (AB-1530; Millipore); 1:500 rabbit anti-TRPV1 (RA-14113; Neuromics); 1:500 rabbit anti-TrkA (a gift from L. Reichardt, UCSF), 1:500 mouse anti-TrkB (610102; BD Transduction Laboratories), 1:500 rabbit PV (AF-1404; R&D system), 1:1000 rabbit anti-c-fos (SC-253; Santa Cruz Biotechnology).
Sections were washed three times with PBS and incubated for 2hr at room temperature with secondary antibodies conjugated to Alexa-488 or Alexa-568 (Invitrogen) diluted 1:1000 in PBS. Slides were then washed three times with PBS, three times with deionized water, and mounted in Vectorshield mounting medium with DAPI (H-1200; Vector Laboratories, Inc). Digital images were acquired on an Olympus IX70 microscope. Quantification of overlap between eGFP expression and that of other neuronal markers was obtained per section and expressed as the percentage of GFP-positive cells that were immunoreactive for the respective markers with the s.e.m. between sections obtained from 5–12 animals.
For Fos antibody staining, sections were washed three times with PBS and incubated for 2hr at room temperature with (1:200) biotinylated goat anti-rabbit secondary antibody (PK-6101; Vector Laboratories, Inc) in PBS-T. Slides were washed three times with PBS and incubated for 1hr at room temperature with 1:350 Cy3-conjugated streptavidin (SA1010; Invitrogen) in PBS-T while protected from light. Sections were washed three times with PBS, three times with deionized water, and mounted in Vectorshield mounting medium with DAPI (H-1200; Vector Laboratories, Inc). Digital images were acquired as described.
Peripheral stimulation
Trpm8GFP transgenic mice at different ages P3, P10, P14, and P35 (P14 and P35 were first anesthetized with isoflurane) were treated with vehicle (70% ethanol) or menthol (1M) applied to the ipsilateral hind paw for 5sec repeated 10 times with 1min intervals. After 90min, mice were sacrificed and spinal cords dissected, fixed, sectioned, and stained with c-fos antibody as described above. Number of c-fos nuclei was obtained per field and expressed as the number of Fos-positive cells per optical field with the s.e.m. between fields from multiple sections obtained from 5–8 independent animals. All data sets were analyzed using two- or one-way ANOVA analysis followed by Tukey’s HSD post-hoc analysis.
RESULTS
Expression of TRPM8 in the developing mouse embryo
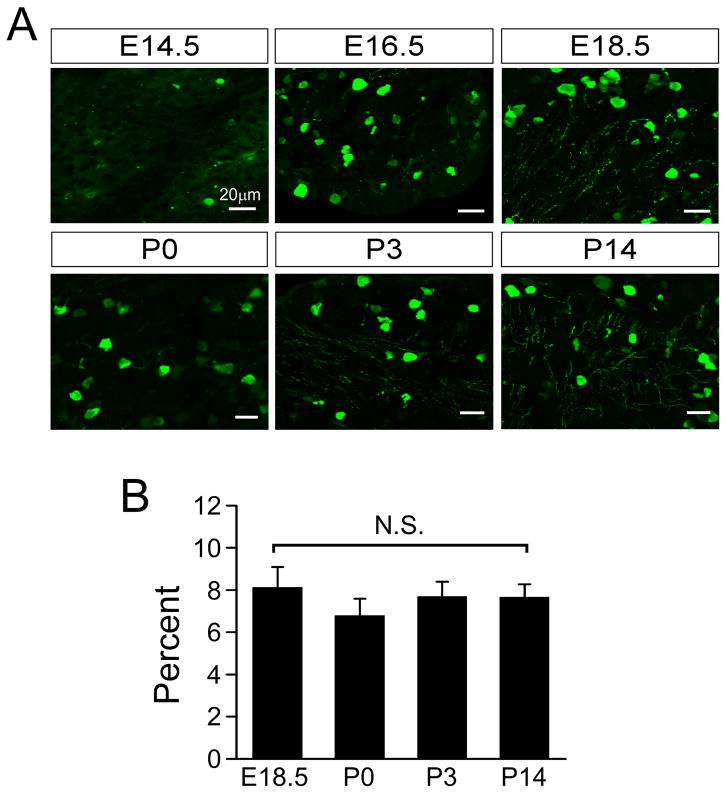
To examine the time course of TRPM8 expression developmentally we used a thoroughly characterized mouse transgenic line in which enhanced green fluorescent protein (eGFP) is expressed via the trpm8 transcriptional promoter (Trpm8GFP) (Takashima et al., 2007, Carr et al., 2009, Mandadi et al., 2009). Trpm8GFP is robustly expressed and recapitulates expression of TRPM8 channels in peripheral sensory neurons (Takashima et al., 2007), making it a suitable marker for TRPM8 expression and analysis of TRPM8-expressing neurons (Welberg, 2008). We first examined DRG neurons in the developing mouse embryo to establish the embryonic stage at which TRPM8 expression begins. At embryonic day 14.5 (E14.5), we observed very sparse eGFP expression in DRG neurons from Trpm8GFP mice, prior to which no transgene expression was detectable (Fig. 1A). However, by E16.5 eGFP labeling began to increase followed by more extensive labeling by E18.5 (Fig. 1A,B). At E18.5, 8.1±1.0% of DRG neurons (n=3249 cells) were labeled compared to 6.8±0.6% at P0 (n=2931 cells; Fig. 1B). After birth, Trpm8GFP expression was found in a subset of small- to medium-diameter neurons, similar to that we previously observed in adult tissues (Takashima et al., 2007). This time course is consistent with previous reports of expression of TRPM8 transcripts in mouse DRG (Hjerling-Leffler et al., 2007, Shibasaki et al., 2010). Of note, the extent of Trpm8GFP expression remained consistent postnatal with 7.7±0.7% (n=2974 cells) and 7.7±0.6% (n=2574 cells) of DRG neurons positive for Trpm8GFP at P3 and P14, respectively, even though there is significant neuronal death during this period (Fitzgerald, 2005). Thus, sensory afferents are molecularly prepared to detect cold temperatures at a developmental stage just preceding birth.
Fig. 1.
Developmental expression of TRPM8. (A) Trpm8GFP fluorescence in fixed thin sections from lumbar DRG taken at the stage indicated shows expression of TRPM8 in small diameter neurons. (B) Quantification of the percent of neurons within a ganglia that express Trpm8GFP. Data are mean ± s.e.m. (p>0.05) and bars equal 20 μm.
TRPM8 afferent axons innervate the dorsal horn of the spinal cord at approximately E16.5
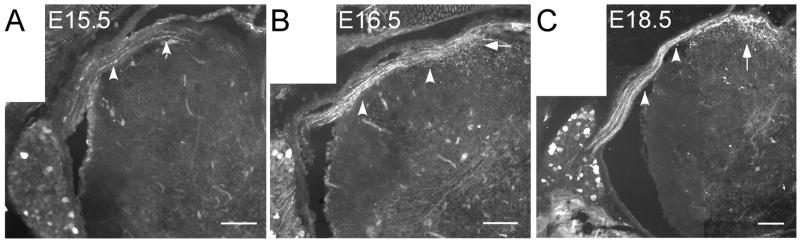
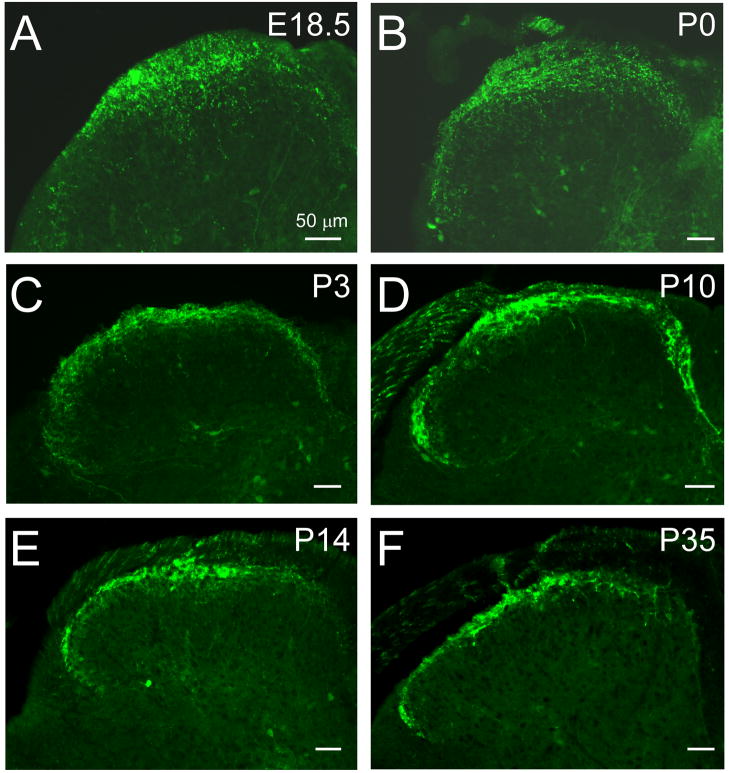
With expression beginning on or near E16.5, we next examined the central projections of Trpm8GFP afferent fibers to determine when these putative cold nerves are likely to establish functional connections in the spinal cord dorsal horn. In the rat, initial in growth of afferent fibers into the spinal gray matter occurs at approximately E14-E15, with a second wave of penetration of small diameter afferents at E19 (Mirnics and Koerber, 1995b). To determine when TRPM8 fibers establish their central projections into the mouse dorsal horn, we examined Trpm8GFP expression in embryonic whole mounts starting at day E15.5, a stage of development just prior to that of extensive eGFP expression (Fig. 1). A few eGFP-positive fibers were initially found in the dorsal roots by E15.5 (Fig. 2A) and then observed to reach the medial dorsal horn by E16.5 (Fig. 2B). By E18.5, Trpm8GFP fibers were still localized predominantly to the medial entry site, but had begun to project ventrally into the developing dorsal horn by this stage (Fig. 2C). However, the innervation observed at this time was restricted around the point of entry compared to that of adults (Fig. 2C and see Fig. 3A). Our finding that Trpm8GFP fibers enter the dorsal horn by E18.5 is consistent with previous dye labeling studies in which small-caliber afferent fibers, largely considered to be thermosensitive and nociceptive, were first observed to enter the developing dorsal horn at or near day E18-19 in the rat (Mirnics and Koerber, 1995b). By P0, eGFP-labeled fibers were observed lateral to the entry site, but still diffusely spread (Fig. 3B). However, by P3 eGFP positive fibers began to resolve their projection patterns towards one similar to adult tissues (Fig. 3C) and were fully refined by P10 (Fig. 3D), located in the most superficial laminar structures in the mouse spinal cord dorsal horn (Fig. 3E, F) (Takashima et al., 2007). Thus, Trpm8GFP fibers reach an adult topographical organization after the first week of postnatal development.
Fig. 2.
TRPM8-positive central projections into the spinal dorsal horn. In fixed section of Trpm8GFP mice, GFP-positive fibers are observed in the dorsal roots (arrowheads) as early as E15.5 (A) and increase in density within 24 hrs (B). By E18.5, the central projections of Trpm8GFP fibers enter the dorsal horn but are restricted to the site of entry (C; arrow). Moreover, the numbers of GFP-positive soma are markedly increased between E15.5 and E18.5. Bars equal 50 μm.
Fig. 3.
Distribution of the central projections of TRPM8 fibers in the developing spinal dorsal horn. At E18.5 and P0, Trpm8GFP axons are densely innervating the dorsal horn, but largely restricted to the medial sites (A, B). By P3 and P10, Trpm8GFP axons have reached lateral regions of the dorsal horn (C, D) and by P14 (E) have resolved into an adult-like somatotopic organizational pattern (F). Bars equal 50 μm.
Development of TRPM8 spinal projections
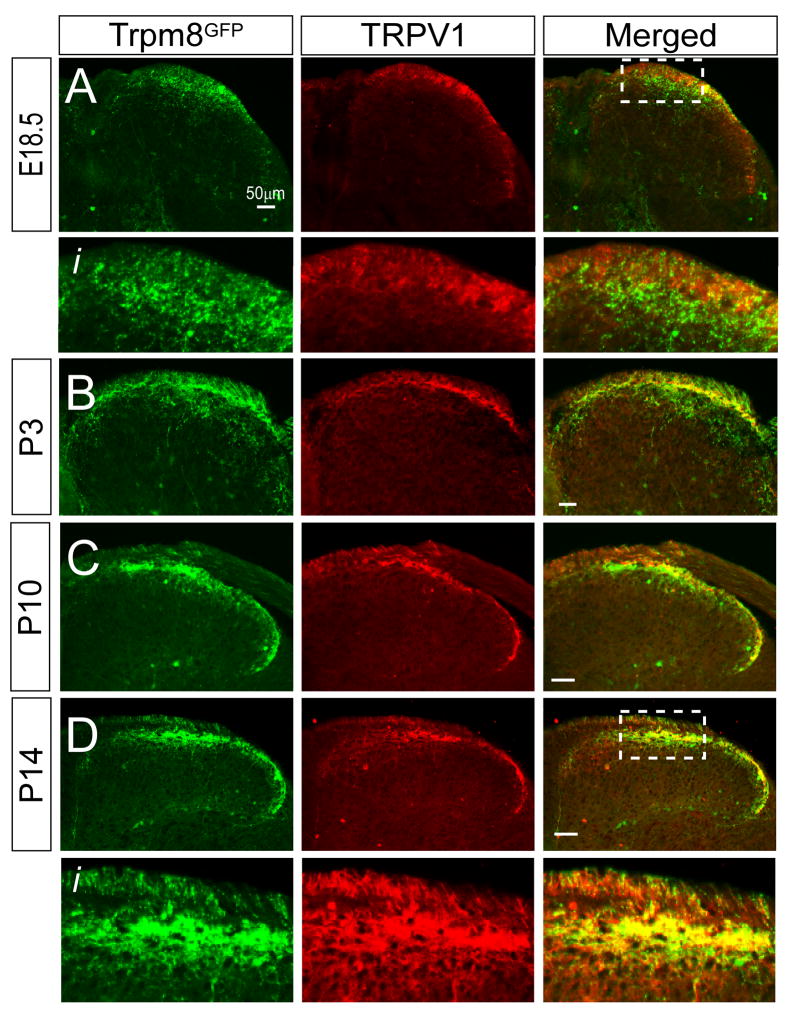
In rodents, the spinal cord dorsal horn has formed such that the general shape and structure is largely that of the adult by E17-E18 (Mirnics and Koerber, 1995b). We have previously reported that Trpm8GFP central afferent projections are restricted to lamina I and IIo in the adult (Takashima et al., 2007), consistent with thermosensitive and nociceptive neurons (Julius and Basbaum, 2001). However, in late embryonic and postnatal stages, Trpm8GFP fibers were located more diffusely and not in a topographical organization indicative of adult tissues (Fig. 3). To determine the extent of Trpm8GFP innervation in the dorsal horn relevant to other afferent subtypes, we compared the localization of eGFP-labeled fibers to that of the well characterized temperature-sensitive and nociceptive fiber type, those expressing the heat-gated channel TRPV1 (Caterina et al., 1997, Tominaga et al., 1998). In rats, TRPV1-positive afferents are known to terminate predominantly in lamina I with sparse projections in lamina II between E16.5 to postnatal day 2, but then extend ventrally into lamina II by P10 (Guo et al., 2001). We observed a similar pattern of TRPV1 fiber expression in Trpm8GFP mice in that the localization of TRPV1-immunoreactive (IR) afferents show a dorsal to ventral progression during the first week of postnatal development (Fig. 4). In contrast, we found eGFP-positive fibers extending well past TRPV1 terminals at E18.5 (Fig. 4A, Ai) and P3 (Fig. 4B). At E18.5, a visible distinction between Trpm8GFP and TRPV1-IR fibers was observed with little to no overlap in the projection patterns (Fig. 4Ai). However, by P10 this extended ventral innervation pattern of TRPM8 fibers had retracted dorsally (Fig. 4C) and formed into a pattern indicative of adult innervation and a strong overlap in Trpm8GFP and TRPV1-IR central projections (Fig. 4D, Di).
Fig. 4.
Somatotopic organization of TRPM8 and TRPV1 central projections in the developing dorsal horn. (A) At E18.5, both Trpm8GFP and TRPV1-IR fibers are observed in the dorsal horn, with Trpm8GFP axons projecting more ventral than those immunoreactive for TRPV1 (see boxed region Ai). By P3 (B), Trpm8GFP fibers are largely more ventral that TRPV1 axons but begin to retract and largely overlap with TRPV1 axons by P10 (C). By P14 (D), Trpm8GFP and TRPV1 axons are both localized to the same laminar region (Di) as is observed in the adult mouse. Bars equal 50 μm.
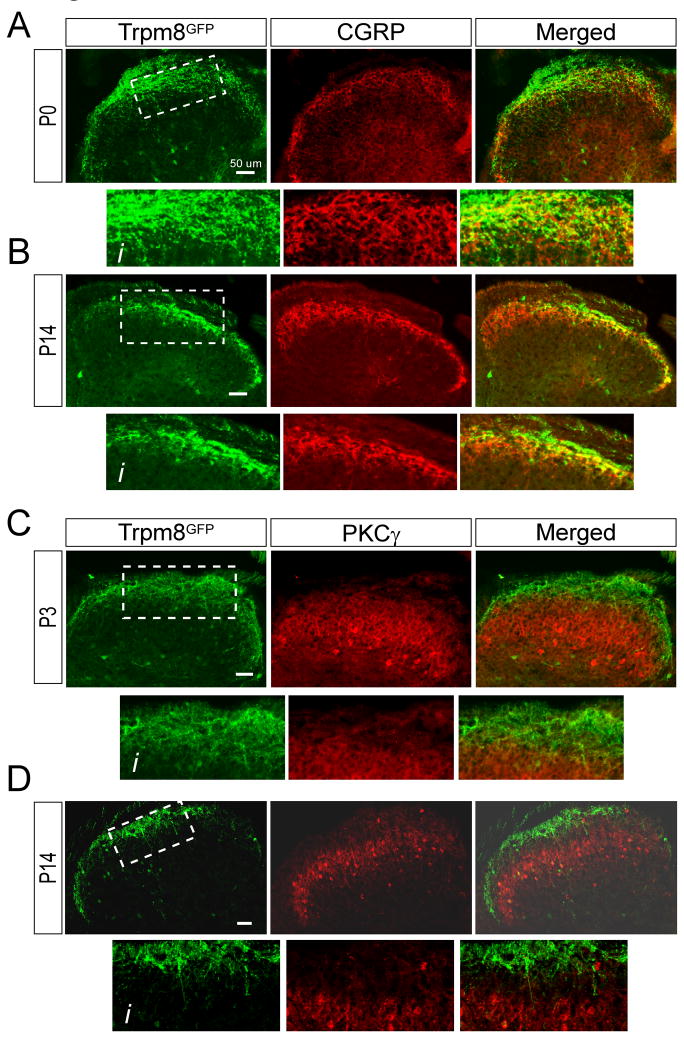
To further define the development of TRPM8 central projection we also compared Trpm8GFP labeling to two other lamina specific markers, CGRP and PKCγ, each of which labels distinct and non-overlapping regions of the dorsal horn (McNeill et al., 1988, Polgar et al., 1999, Hunt and Mantyh, 2001, Braz, 2009). CGRP characteristically labels lamina I and the outer layers of lamina II in the mouse spinal cord and largely overlaps with TRPM8 expressing afferents in the adult (McNeill et al., 1988, Takashima et al., 2007). We were unable to examine the locale of CGRP fibers embryonically as peptide expression begins at approximately E18 once afferent fibers reach their peripheral projections (Ai et al., 1998, Ai et al., 1999). By P0, CGRP+ neuronal projections were localized to the most superficial regions of the dorsal horn and Trpm8GFP axons largely overlapped with CGRP-positive fibers (Fig. 5A, Ai). However, by P14, TRPM8 projections were observed to take up a more refined localization suggesting that they had retracted and moved predominantly more dorsal than CGRP fibers (Fig. 5B, Bi), similar to that observed for TRPV1 afferents (Fig. 4). We next examined a more ventral lamina marker PKCγ which labels interneurons located to the inner regions of lamina II as well as lamina III and does not overlap with TRPM8 expressing fibers in the adult (Zylka et al., 2005, Takashima et al., 2007, Seal et al., 2009). At P14, axons labeled for TRPM8 are distinctly separated in the dorsal horn from spinal interneurons expressing PKCγ (Fig. 5D, Di). However, at P0, we found eGFP expressing axons to project close to spinal neurons expressing PKCγ (Fig. 5C, Ci), consistent with a more ventral projection of Trpm8GFP fibers in early development. Thus, TRPM8-positive afferents have a more diffuse projection pattern in the mouse spinal cord dorsal horn early in development compared to that found in adult animals.
Fig. 5.
Developmental refinement of TRPM8 central projections. (A) At P0, Trpm8GFP fibers are observed to overlap with CGRP-IR axons, but a number of GFP-positive fibers project more ventral than peptidergic fibers (Ai). By P14 (B, Bi), both fiber types have a more restricted somatotopic organization, but in contrast to P0, Trpm8GFP fibers are largely more dorsal than CGRP fibers. (C) PKCγ-IR interneurons are localized ventral to thermosensitive afferents in lamina III, but at P0, Trpm8GFP axons are in close proximity to PKCγ-IR (Ci). By P14 (D, Di) the labeling has separated with a distinct boundary (lamina II) between Trpm8GFP and PKCγ labeling. Bars equal 50 μm.
Establishment of functional TRPM8-dependent synaptic connections in the spinal cord developmentally
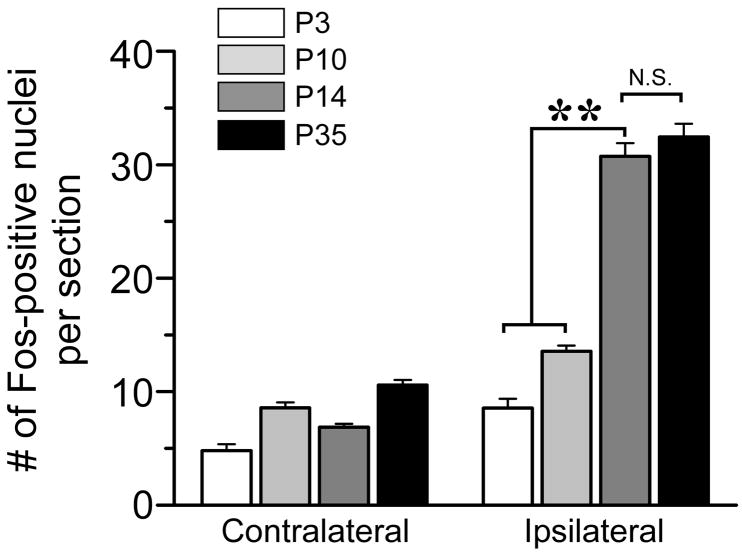
The somatotopic organization of Trpm8GFP fibers in the developing mouse spinal cord suggests that appropriate synaptic connections between these fibers and spinal resident second-order projection neurons and interneurons have yet to form, or are in the process of being established, during the first postnatal week. Precedence for temporal development of cold-activated circuits comes from recordings of menthol-evoked glutamatergic synaptic activity in spinal cord slices from postnatal rats (Baccei et al., 2003). Prior to P10, menthol does not alter the frequency of excitatory postsynaptic currents above baseline, but can evoke robust activity from this stage forward (Baccei et al., 2003). As this time course coincides with the progression of Trpm8GFP spinal projections to a more adult-like phenotype, we asked whether functional menthol-dependent circuits were present prior to P10 in vivo using protein expression of the immediate early gene c-fos (Fos) as a marker of neural activity (Coggeshall, 2005). Thus, we stimulated the hindpaw of anesthetized Trpm8GFP mice (at days P3, P10, P14 and P35) with menthol unilaterally (see Experimental Procedures) and examined the induction of Fos protein expression in the spinal cord. As shown in Fig. 6, peripheral menthol evokes robust Fos expression in the dorsal horn at day P14 (30.8±0.7 nuclei per section ipsilateral and 6.9±0.7 contralateral; p<0.01; n=6) to levels that are identical (p>0.05) to those observed in adult mice (>P35; 32.5±0.8 ipsilateral; n=5). However, at P3 and P10 little Fos expression was induced by peripheral menthol stimulation and at levels statistically lower than P14 (8.6±1.6 at P3, n=4; 13.6±0.9 at P10, n=4; p<0.01). Thus, as has been reported for menthol-evoked post-synaptic currents in rodent spinal cords (Baccei et al., 2003), little to no TRPM8 dependent activity occurs in the first few postnatal days. When considered in the context of the developmental delay in the formation of adult-like somatotopic organization of TRPM8 terminals in the spinal cord, these data suggest that functional cold neural circuits are not established until after the first postnatal week, and that there is likely either trophic or activity-dependent refinement of these projections to the spinal cord dorsal horn during this time.
Fig. 6.
Menthol-evoked neural activity over the first week of postnatal development. Quantitative data of the number of Fos positive nuclei in the lumbar spinal cord dorsal horn after hind paw stimulation with menthol at P3 (n = 11 sections), P10 (n = 32), P14 (n = 52), and P35 (n = 36). ** denote p<0.01
Temporal delay in development of the immunochemical phenotype of TRPM8 neurons
We have previously shown that Trpm8GFP neurons are immunoreactive to many classical somatosensory and nociceptive markers and exhibit a remarkable diversity in their neurochemical phenotypes (Takashima et al., 2007). These results, along with the striking complexity in the phenotype of mice lacking functional TRPM8 channels (Bautista et al., 2007, Colburn et al., 2007, Daniels and McKemy, 2007, Dhaka et al., 2007), suggests that the channel can serve a multitude of roles in sensory signaling (Welberg, 2008). Thus, we examined the developmental time course that establishes this diversity in functionality.
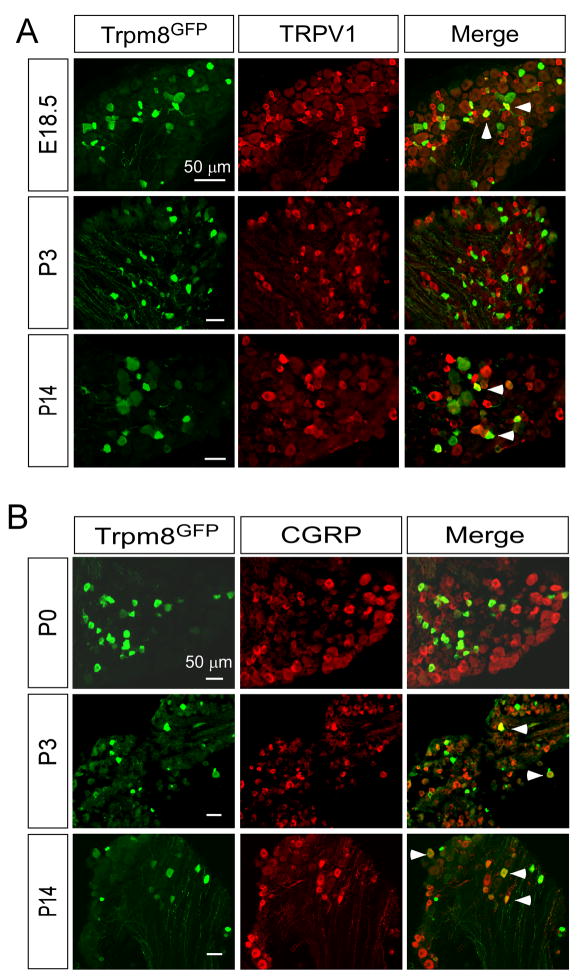
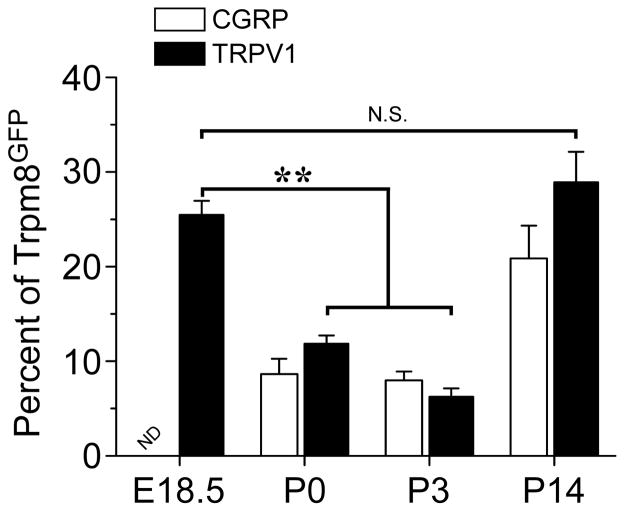
First, we looked for when eGFP-positive neurons became immunoreactive for TRPV1 and CGRP, markers of nociceptive neurons (Basbaum et al., 2009). Functional studies with cultured sensory neurons show that approximately 40–50% of menthol-sensitive neurons are also capsaicin-sensitive, suggesting overlap between TRPM8 and TRPV1 in adult mice (McKemy et al., 2002, Viana et al., 2002, Xing et al., 2006, Hjerling-Leffler et al., 2007, Shibasaki et al., 2010). Consistent with these functional data, in the adult mouse approximately 40% of Trpm8GFP neurons are immunoreactive for TRPV1 (Takashima et al., 2007). Functional assays (using Ca2+ microfluorimetry in acutely cultured DRG neurons) showed a greater than 90% overlap in the number of menthol-sensitive neurons with capsaicin-sensitive cells at E18.5, with this number decreasing to near 50% by P14 (Hjerling-Leffler et al., 2007). Thus these data predict a strong overlap in the expression of TRPM8 and TRPV1 early in development. For TRPV1 we found co-expression with Trpm8GFP as early as E18.5 and at levels not significantly different than found in P14 animals and adult mice (Fig. 7A and Fig. 8, 25.5±1.4% at E18.5, 28.9±3.2% at P14; p>0.05) (Takashima et al., 2007). Surprisingly, at postnatal days P0 and P3, a significant reduction in co-expression of TRPV1 and Trpm8GFP was observed with 11.9±0.9% and 6.2±0.9% (p<0.01) of TRPM8 neurons expressing TRPV1, respectively (Fig. 8). Of note, this fluctuation in TRPM8/TRPV1 co-expression coincides with the refining period of TRPM8 and TRPV1 central projections (Fig. 4 and Fig. 6).
Fig. 7.
Immunochemical expression of nociceptive markers in TRPM8 neurons. Fixed section of DRG from Trpm8GFP mice shows co-labeling of a fraction of TRPM8 neurons with TRPV1 (A) and CGRP (B). Arrows label cells co-expressing Trpm8GFP and the respective marker. Bars equal 50 μM.
Fig. 8.
Co-expression of TRPM8 with nociceptive markers. Quantification of the percent of Trpm8GFP neurons that co-label with TRPV1 or CGRP-IR. Among Trpm8GFP neurons co-staining for TRPV1 there were 25.5% at E18.5 (356/1398 in 14 sections), 11.9% at P0 (119/1000 in 10 sections), 6.2% at P3 (75/1210 in 12 sections), and 28.9% at P14 (550/1903 in 19 sections). For CGRP there were 8.6% at P0 (52/605 in 6 sections), 8.0% at P3 (168/2100 in 21 sections), and 20.9% at P14 (229/1096 in 11 sections). Data are means ± s.e.m. and ** denotes p<0.01.
For CGRP, as stated previously, expression begins near E18 in the rodent and thus we saw little overlap in CGRP immunoreactivity at E18.5 (not shown) but did observe a graded increase in CGRP expression in Trpm8GFP neurons at P0 (8.6±1.6%) and P3 (8.0±0.9%) with co-expression reaching levels observed in the adult by P14 (20.9±3.5%; Fig. 7B and Fig. 8). Since only a fraction of Trpm8GFP neurons labeled with CGRP, it suggests that many of these cells can be considered non-peptidergic and should label with the markers IB4 and P2X3 (Zylka et al., 2005). However, we and others have previously shown that adult TRPM8 neurons do not bind IB4 (Peier et al., 2002, Takashima et al., 2007, Dhaka et al., 2008), an expression phenotype we also observe in developing DRG neurons (data not shown). To further establish the neurochemical composition of Trpm8GFP neurons, we also examined co-expression with the ionotropic ATP receptor P2X3, which is known to not co-localize with CGRP and is almost exclusively localized in small-diameter non-peptidergic sensory neurons (Vulchanova et al., 1998). Similar to CGRP labeling, a small fraction of Trpm8GFP neurons were immunoreactive for P2X3 at P0 (2.5±0.3%) with expression increasing as the animal developed (10.8±1.4% at P14; Supplemental Fig. 1), further demonstrating that TRPM8 afferents express nociceptive markers, but that a significant number of these cells cannot be classified by traditional neuronal markers (Takashima et al., 2007).
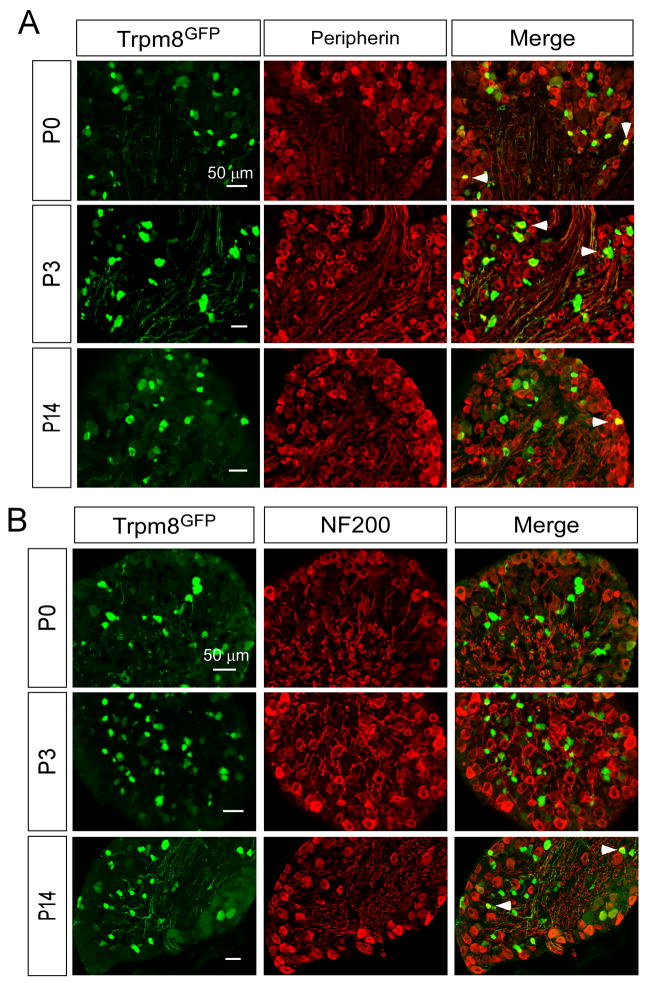
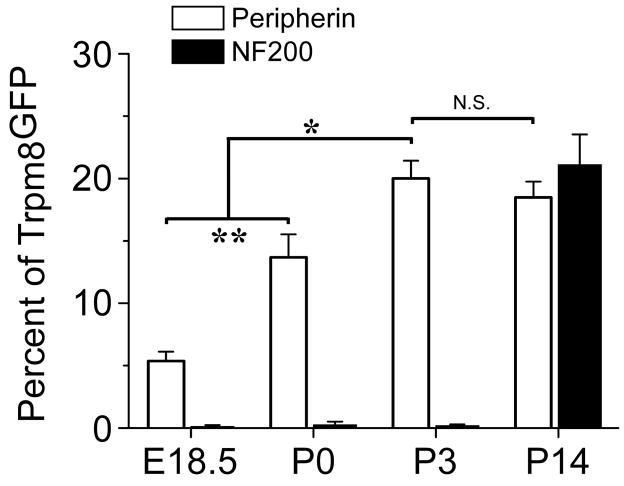
Aδ- and C-fibers are two functionally distinct fiber types that can be identified immunochemically by expression of the neurofilament markers NF200 and peripherin, respectively (Lawson and Waddell, 1991). In adult mice, Trpm8GFP-postive neurons were found to express both markers in adult DRG, albeit many of TRPM8 neurons were not immunoreactive for either marker and therefore were unable to be classified as C- or Aδ-fibers immunochemically (Takashima et al., 2007). Nonetheless, we examined when TRPM8 neurons begin to express these markers during development. As shown in Fig. 9A and Fig. 10, a subset of Trpm8GFP neurons were immunoreactive for peripherin at E18.5 (5.4±0.8%) and that the percentage of peripherin-positive neurons increased significantly after birth (13.7±1.9% at P0, p<0.01), reaching levels similar to that of adult mice by P3 (20.0±1.4% at P3; 18.5±1.3% at P14, p>0.05). In contrast, we observed little to no NF200 immunoreactivity in Trpm8GFP neurons during the first week postnatal (0.3±0.3% at P0 and 0.2±0.1% at P3), but then co-expression levels increased to adulthood by P14 (Fig. 9B and Fig. 10; 21.1±0.8%). These data show that a fraction of TRPM8 neurons can be immunochemically classified as C-fibers early in development, but fail to express Aδ-fiber markers until after the first week of development.
Fig. 9.
Immunochemical expression of Aδ - and C-fiber markers in TRPM8 neurons. Fixed section of DRG from Trpm8GFP mice shows co-labeling of a fraction of TRPM8 neurons with peripherin (A) and NF200 (B). Arrows label cells co-expressing Trpm8GFP and the respective marker. Bars equal 50 μM.
Fig. 10.
Co-expression of TRPM8 with Aδ- and C-fiber markers. Quantification of the percent of Trpm8GFP neurons that co-label with peripherin or NF200-IR. Among Trpm8GFP neurons co-staining for peripherin there were 5.4% at E18.5 (64/1185 in 12 sections), 13.7% at P0 (96/701 in 7 sections), 20.0% at P3 (660/3300 in 33 sections), and 18.5% at P14 (351/1897 in 19 sections). For NF200 there were 0.1% at E18.5 (2/2000 in 14 sections), 0.3% at P0 (3/1000 in 10 sections), 0.2% at P3 (10/5000 in 57 sections), and 21.1% at P14 (211/1000 in 10 sections). Data are means ± s.e.m. and ** denotes p<0.01.
TRPM8 neurons exhibit limited expression of neurotrophic receptors
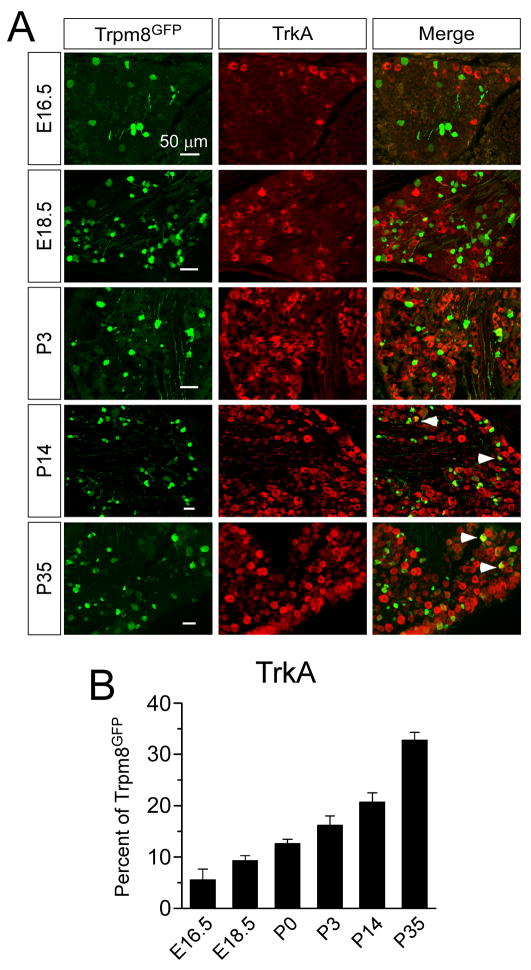
Neurotrophic growth factor receptors and their corresponding ligands are the earliest recognized markers of sensory neuron subtypes and known to control cell development and death, target innervation, and the establishment of functional contacts to spinal cord neurons (Smeyne et al., 1994, Silos-Santiago et al., 1995, Marmigere and Ernfors, 2007). In addition, these receptors are critical for the establishment of neuronal phenotypes in that they regulate expression of both ion channels and peptides. Small diameter thermosensory and nociceptive neurons largely express the nerve growth factor (NGF) receptor TrkA, while large diameter low-threshold mechanosensitive and proprioceptive neurons express the brain derived neurotrophic factor (BDNF) and neurotrophin 4 (NT-4) receptor TrkB, and the NT-3 receptor TrkC, respectively. Several elegant studies have shown that the majority of DRG sensory neurons are dependent on NGF for their survival early in development (Marmigere and Ernfors, 2007). Thus, we examined the expression of specific receptor types in developing TRPM8 neurons to determine if the diversity in the expression pattern of these neurons correlates with receptor subtype. Surprisingly, only a small percentage of Trpm8GFP neurons are immunoreactive for TrkA embryonically (5.6±2.1% at E16.5 and 9.3±1.0% at E18.5; Fig. 11), but the co-expression levels gradually increased postnatal, reaching a peak of 32.8±1.5% at P35 (Fig. 11B). These data suggest that TRPM8 neurons are likely to be dependent on trophic factors other than NGF. However, Trpm8GFP neurons are not immunoreactive for the brain-derived neurotrophic factor (BDNF) receptor TrkB (data not shown), and only a very small percentage were immunoreactive for parvalbumin, a surrogate marker of TrkC-expressing neurons (Supplemental Fig. 2).
Fig. 11.
Immunochemical expression of TrkA in TRPM8 neurons. (A) Fixed section of DRG from Trpm8GFP mice shows co-labeling of a fraction of TRPM8 neurons with TrkA. Arrows label cells co-expressing Trpm8GFP and the respective marker. Bars equal 50 μM. (B) Quantification of the percent of Trpm8GFP neurons that co-label with TrkA. Among Trpm8GFP neurons 5.6% at E16.5 (67/1196 in 12 sections), 9.3% at E18.5 (102/1097 in 11 sections), 12.6% at P0 (51/405 in 4 sections), 16.2% at P3 (179/1105 in 11 sections), 20.8% at P14 (270/1298 in 13 sections) and 32.8% at P35 (1575/4802 in 48 sections) co-stained with TrkA-IR. Data are means ± s.e.m.
We also examined co-expression of Trpm8GFP with the glial-derived neurotrophic factor (GDNF) receptor Ret, of which a subset of labeled neurons are small-diameter non-peptidergic neurons that initially co-express TrkA, but begin to co-express Ret at E16 (Molliver et al., 1997, Chen et al., 2006, Luo et al., 2007). These neurons undergo a postnatal switch in trophic factor dependence and eliminate TrkA expression, which is complete by P14 (Molliver et al., 1997). Thus, we examined if a subset of TRPM8 neurons go through this differentiation by determining co-localization between Trpm8GFP and Ret. However, we found little to no co-localization between Trpm8GFP neurons and Ret (Supplemental Fig. 3), suggesting these neurons are not Ret-dependent, non-peptidergic neurons, consistent with observations in mice lacking Ret expression in sensory afferents in which TRPM8 expression was not affected (Luo et al., 2007).
DISCUSSION
Developmental profile of TRPM8 expression in DRG
A requirement for active sensory transduction is the expression and correct localization of transduction molecules in the appropriate cell type and cellular domain. In the case of temperature-sensing afferents, select TRP ion channel expression is fundamental for the establishment of thermal sensitivity. In the mouse, neural crest cells coalesce and ultimately differentiate into small diameter neurons at or near E11.5 (Lawson and Biscoe, 1979), a subpopulation that correlates to nociceptive and thermosensitive neurons (Julius and Basbaum, 2001). Remarkably, one-day later TRPV1 transcripts can be detected (Hjerling-Leffler et al., 2007, Shibasaki et al., 2010), which is then followed shortly thereafter by protein expression (Funakoshi et al., 2006), demonstrating that a subpopulation of DRG neurons establish their functional identity very early in development. At the cellular level, TRPV1 channels are functional at this stage (Hjerling-Leffler et al., 2007, Shibasaki et al., 2010), and capsaicin can evoke spinal glutamate release as early as P0 (Baccei et al., 2003), suggesting the presence of functional neural circuits.
In contrast, we find that TRPM8 expression (Trpm8GFP) begins several days after TRPV1 (E15.5-16.5), a developmental time point that coincides with previous analyses of TRPM8 transcript expression (Hjerling-Leffler et al., 2007, Shibasaki et al., 2010). Likewise, menthol-evoked responses are observed at the cellular level near this time, evidence to the functional expression of TRPM8 channels. Of note, Hjerling-Leffler and colleagues also report that cold temperatures (down to 5°C) evoke activity (measured by increased intracellular calcium) in a larger percentage of lumbar DRG neurons (45%) than menthol (Hjerling-Leffler et al., 2007). Moreover, cellular cold-sensitivity starts as early as E12.5-14.5, a full 2–3 days prior to the onset of menthol-sensitivity and TRPM8 expression. Thus, these expression and functional differences suggest that cold-evoked activity in embryonic neurons may use mechanisms distinct from TRPM8. TRPA1 has also been reported to act as a cold sensor in DRG neurons (Story et al., 2003), but transcript expression and TRPA1-specific agonist evoked responses were not observed until after birth (Hjerling-Leffler et al., 2007), indicating these early embryonic cold responses were not TRPA1-mediated. Moreover, the transduction mechanism that provides for the increased calcium response to cold temperatures in this study is not clear, nor is it evident that such activity is biologically relevant in the development of cold inputs.
TRPM8 dependent cold circuits are established postnatal
Cellular expression and evidence for channel activation in vitro does not necessarily correlate to functional neural circuits in the developing nervous system. Therefore, we examined when TRPM8 afferents likely make synaptic connections with their secondary partner neurons in the spinal cord during development. Our study reveals that Trpm8GFP afferents innervate the dorsal horn of the spinal cord starting at approximately E18.5, but likely are not part of a fully functional neural circuit due to an immature somatotopic organization in the spinal cord dorsal horn. This suggests that fine-tuning of synaptic connections is still possibly occurring at this time. To test this, we investigated when the functional synaptic connections are made during development. Previous studies show the establishment of functional cold circuits in rats by P10 by measuring mEPSC frequency in response to menthol by neurons in the dorsal horn of the spinal cord (Baccei et al., 2003). We hypothesized that TRPM8 afferents establish functional synaptic connections when the termination pattern is restricted within lamina I and IIo. If this is true we should be able to observe markers of synaptic activity, namely Fos upon menthol stimulation after the refinement period rather than before. Our results show that at all postnatal developmental stages tested in this study except for P3, c-fos expression in the dorsal horn of the spinal cord was significantly higher in menthol-treated animals ipsilateral side to the simulation site than in either the contralateral dorsal horn or in vehicle-treated animals. Moreover, the relative number of Fos-positive nuclei observed in P14 was similar to number at P35. Therefore, we conclude that functional central cold circuits are established by P14 a stage much later than when the peripheral projections of these fibers are likely to reach their peripheral targets. Previous studies show that fibers originating from lumbar DRG reach the hind paw by E14.5 to E15, and the epidermis of the most distal toes by E16 to E16.5 (Mirnics and Koerber, 1995a) approximately the same time Trpm8GFP expression begins in DRG neurons. This indicates that peripheral targeting occurs before central targeting and suggests that peripheral innervation could be more important for specification of TRPM8 neurons.
Growth factor receptor expression phenotype of TRPM8 neurons during development
Neurotrophic growth factor receptors and their corresponding soluble ligands are the earliest recognized markers of sensory neuron subtypes and are critical for the establishment of neuronal phenotypes (Smeyne et al., 1994, Silos-Santiago et al., 1995, Marmigere and Ernfors, 2007). Small diameter thermosensory and nociceptive neurons largely express the nerve growth factor (NGF) receptor TrkA, and several elegant studies have shown that the majority of DRG sensory neurons are dependent on NGF for their survival early in development (Marmigere and Ernfors, 2007). Surprisingly, only a small percentage of Trpm8GFP neurons express TrkA in early development (E16.5 and E18.5) with expression gradually increases throughout development. These data were somewhat of surprise for two reasons. First, TrkA expression is known to decrease during development, whereas here we find that TRPM8 neuronal expression of TrkA increases. Secondly, in adult mice Trpm8GFP neurons do not label with IB4, suggesting that these neurons do not fit the classification of “non-peptidergic” by current neurochemical standards (Takashima et al., 2007). Therefore, we expected a higher percentage of TRPM8 neurons to be TrkA dependent. However, this result is consistent with a previous report where NGF−/−;Bax−/− mice showed reduced expression of TRPM8 in P0 by in situ hybridization, but TRPM8 expression is not completely eliminated (Luo et al., 2007). Thus, the data suggest that not all Trpm8GFP neurons are dependent on NGF for survival. Moreover, we did not observe any Trpm8GFP neurons immunoreactive for Ret at P0 and P3 and little to no co-localization at P14. This suggest that Trpm8GFP neurons are not part of the TrkA sub-population which switch to Ret dependence after birth, supporting data showing that TRPM8 expression is unaffected in Retf/f;Wnt1-Cre mice (Luo et al., 2007). This further supports the hypothesis that Ret functions downstream of Runx1, due to the fact that expression of TRPM8 is eliminated in Runx1−/− DRGs (Chen et al., 2006). Additionally, we see little to no co-localization between Trpm8GFP neurons and either TrkB or TrkC at any given developmental stage. This suggests that TRPM8-expressing neurons are neither mechanoreceptive nor proprioceptive neurons. Moreover, it also suggests that TRPM8-expressing neurons do not switch their neurotrophic factors dependency throughout the development.
Neurochemical phenotype of TRPM8 neurons during development
We have previously shown that TRPM8 neurons express a wide cohort of neurochemical markers in adult mice, demonstrating that these cells are varied molecularly (Zirlinger et al., 2002, Takashima et al., 2007). This phenotype also suggests that these neurons are also functionally diverse consistent with TRPM8-null phenotypes (Bautista et al., 2007, Colburn et al., 2007, Dhaka et al., 2007). We asked if this diversity is present during development to understand the developmental basis for the broad range of cold sensations mediated by TRPM8. Thermosensitive and nociceptive DRG neurons can be grossly categorized into several distinct subsets based on neuronal marker expression. For instance, first and second pain sensations in teeth are attributed to activation of Aδ- and C-fibers, respectively (Jyvasjarvi and Kniffki, 1987, Mengel et al., 1993). These subtypes can be largely identified by the expression of NF200 and peripherin for Aδ- and C-fibers, respectively, and we have shown previously that approximately one-quarter of TRPM8 neurons co-express each of these markers in the adult (Takashima et al., 2007). Our current results show Trpm8GFP neurons do not co-localize with NF200+ until after day P3 (between P3 and P14). This increase in co-localization with NF200 coincides with a reported postnatal increase in the number of NF200+ rat neurons which occurs between days P0 and P3 (Beland and Fitzgerald, 2001). Conversely, Trpm8GFP expression in C-fibers identified by peripherin immunoreactivity is observed as early as E18.5 and increases to adult levels by P3. However, as in the adult, a large percentage of Trpm8GFP neurons do not label with either marker and thus cannot be distinguished as either fiber neurochemcial phenotype.
In addition to markers of fiber-type, we also determined the expression patterns of Trpm8GFP DRG neurons in comparison to nociceptor markers, such as TRPV1 and CGRP. In adult mice, we have shown that approximately one-quarter of Trpm8GFP DRG neurons express either TRPV1 or CGRP (Takashima et al., 2007). These results are in agreement with functional studies in cultured sensory neurons from both rat and mouse in which there is overlap in menthol and capsaicin sensitivity (Guo et al., 2001, McKemy et al., 2002, Viana et al., 2002, Xing et al., 2006, Hjerling-Leffler et al., 2007). Indeed, as many as 80% of acutely cultured P0 DRG neurons that are menthol-sensitive are reported to also be capsaicin sensitive (Hjerling-Leffler et al., 2007). This overlap in agonist sensitivity drops to nearly 50% by P7 after which these values stay constant. Thus, these data suggest that TRPM8 and TRPV1 neurons arise from an embryonically related lineage (Hjerling-Leffler et al., 2007). However, our data show a dramatic decrease in co-localization between TRPM8 and TRPV1 after birth. Interestingly, co-expression of Trpm8GFP and TRPV1 was biphasic in nature in that by day P14, co-expression returned to adult levels.
Unlike the other markers examined, CGRP expression does not begin until late in embryonic development (approximately at E18) and coincides with peripheral DRG axons reaching their target tissues, such as the skin (Ai et al., 1999). Thus, as predicted we observed little to no co-expression of Trpm8GFP and CGRP at E18.5, but did find that these two markers are co-expressed postnatally, with expression reaching adult levels by P14 (Takashima et al., 2007). These data suggest that the phenotype of TRPM8 neurons during the first week of postnatal development is largely non-nociceptive, but that by P14 a fraction of these neurons have developed qualities characteristic of nociceptors, which are maintained into adulthood. However, although our data suggests that early postnatal TRPM8 neurons are presumptive non-nociceptive neurons, the IB4, cRet, and P2X3 data suggest that they are not “classical” non-peptidergic neurons. It is interesting to note that a significant proportion of Trpm8GFP neurons cannot be classified into a particular subset in that they do not express markers such as TRPV1, CGRP, NF200, or peripherin, suggesting that this small population of afferents (~2% of all afferent neurons within a respective ganglia) can only be distinguished by TRPM8 expression.
In conclusion, our data from TRPM8 axon projection patterns and functional synaptic connection determined by Fos expression show that TRPM8-dependent functional cold circuits are not established until at least the second week of postnatal development. Moreover, during postnatal development the neurochemical phenotype of TRPM8-expressing neurons changes dramatically until the same time period when functional cold circuits are established, approximately at P14. This suggests that neurochemical phenotype correlates with the establishment of cold-sensing circuits. Thus, our results from the time course of terminal formation, the functional synaptic connections, and the developmental neurochemical phenotype suggest that maturation of cold circuitry is completed at P14 in mice. This process seems to be completed soon after connections are formed between A-fiber dorsal root afferents, interneurons, and motor neurons (Narayanan et al., 1971, Dahlhaus et al., 2005). All of the data combined suggests that maturation of cold circuits could be activity-dependent. Additionally, our data suggest that those neurons which express TRPM8 in DRG are committed to their fate before they form contacts with their future central targets. Here, we provided an insight that brings us one step closer to determining how different types of sensory neurons could be established during development.
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health Grant NS054069 (D.D.M.). We thank members of the McKemy lab for their helpful insights and discussions during this study and also thank Q. Wu for help with embryonic whole mounts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai X, Cappuzzello J, Hall AK. Activin and bone morphogenetic proteins induce calcitonin gene-related peptide in embryonic sensory neurons in vitro. Mol Cell Neurosci. 1999;14:506–518. doi: 10.1006/mcne.1999.0798. [DOI] [PubMed] [Google Scholar]
- Ai X, MacPhedran SE, Hall AK. Depolarization stimulates initial calcitonin gene-related peptide expression by embryonic sensory neurons in vitro. J Neurosci. 1998;18:9294–9302. doi: 10.1523/JNEUROSCI.18-22-09294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccei ML, Bardoni R, Fitzgerald M. Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. J Physiol. 2003;549:231–242. doi: 10.1113/jphysiol.2003.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Beland B, Fitzgerald M. Influence of peripheral inflammation on the postnatal maturation of primary sensory neuron phenotype in rats. J Pain. 2001;2:36–45. doi: 10.1054/jpai.2001.17697. [DOI] [PubMed] [Google Scholar]
- Braz JM, Basbaum AI. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience. 2009;163:1220–1232. doi: 10.1016/j.neuroscience.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RW, Pianova S, McKemy DD, Brock JA. Action potential initiation in the peripheral terminals of cold-sensitive neurones innervating the guinea-pig cornea. J Physiol. 2009;587:1249–1264. doi: 10.1113/jphysiol.2008.167023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. Fos, nociception and the dorsal horn. Progress in Neurobiology. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Dahlhaus A, Ruscheweyh R, Sandkuhler J. Synaptic input of rat spinal lamina I projection and unidentified neurones in vitro. J Physiol. 2005;566:355–368. doi: 10.1113/jphysiol.2005.088567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RL, McKemy DD. Mice left out in the cold: commentary on the phenotype of TRPM8-nulls. Mol Pain. 2007;3:23. doi: 10.1186/1744-8069-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nature Reviews Neuroscience. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Funakoshi K, Nakano M, Atobe Y, Goris RC, Kadota T, Yazama F. Differential development of TRPV1-expressing sensory nerves in peripheral organs. Cell Tissue Res. 2006;323:27–41. doi: 10.1007/s00441-005-0013-3. [DOI] [PubMed] [Google Scholar]
- Guo A, Simone DA, Stone LS, Fairbanks CA, Wang J, Elde R. Developmental shift of vanilloid receptor 1 (VR1) terminals into deeper regions of the superficial dorsal horn: correlation with a shift from TrkA to Ret expression by dorsal root ganglion neurons. Eur J Neurosci. 2001;14:293–304. doi: 10.1046/j.0953-816x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Jyvasjarvi E, Kniffki KD. Cold stimulation of teeth: a comparison between the responses of cat intradental A delta and C fibres and human sensation. J Physiol. 1987;391:193–207. doi: 10.1113/jphysiol.1987.sp016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Biscoe TJ. Development of mouse dorsal root ganglia: an autoradiographic and quantitative study. J Neurocytol. 1979;8:265–274. doi: 10.1007/BF01236122. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A Hierarchical NGF Signaling Cascade Controls Ret-Dependent and Ret-Independent Events during Development of Nonpeptidergic DRG Neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Nakanishi ST, Takashima Y, Dhaka A, Patapoutian A, McKemy DD, Whelan PJ. Locomotor networks are targets of modulation by sensory transient receptor potential vanilloid 1 and transient receptor potential melastatin 8 channels. Neuroscience. 2009;162:1377–1397. doi: 10.1016/j.neuroscience.2009.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- McKemy DD. Temperature sensing across species. Pflugers Arch. 2007;454:777–791. doi: 10.1007/s00424-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- McNeill DL, Coggeshall RE, Carlton SM. A light and electron microscopic study of calcitonin gene-related peptide in the spinal cord of the rat. Exp Neurol. 1988;99:699–708. doi: 10.1016/0014-4886(88)90186-0. [DOI] [PubMed] [Google Scholar]
- Mengel MK, Stiefenhofer AE, Jyvasjarvi E, Kniffki KD. Pain sensation during cold stimulation of the teeth: differential reflection of A delta and C fibre activity? Pain. 1993;55:159–169. doi: 10.1016/0304-3959(93)90145-F. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Koerber HR. Prenatal development of rat primary afferent fibers: I. Peripheral projections. J Comp Neurol. 1995a;355:589–600. doi: 10.1002/cne.903550408. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Koerber HR. Prenatal development of rat primary afferent fibers: II. Central projections. J Comp Neurol. 1995b;355:601–614. doi: 10.1002/cne.903550409. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Narayanan CH, Fox MW, Hamburger V. Prenatal development of spontaneous and evoked activity in the rat (Rattus norvegicus albinus) Behaviour. 1971;40:100–134. doi: 10.1163/156853971x00357. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M. TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J Neurosci. 2010;30:4601–4612. doi: 10.1523/JNEUROSCI.5830-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silos-Santiago I, Molliver DC, Ozaki S, Smeyne RJ, Fagan AM, Barbacid M, Snider WD. Non-TrkA-expressing small DRG neurons are lost in TrkA deficient mice. J Neurosci. 1995;15:5929–5942. doi: 10.1523/JNEUROSCI.15-09-05929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Kajander KC. Responses of cutaneous A-fiber nociceptors to noxious cold. J Neurophysiol. 1997;77:2049–2060. doi: 10.1152/jn.1997.77.4.2049. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Snider WD. How do you feel? Neurotrophins and mechanotransduction. Nat Neurosci. 1998;1:5–6. doi: 10.1038/199. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Viana F, de la Pena E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Welberg L. Sensory perception: One TRPM8 fits all. Nature Reviews Neuroscience. 2008;9:79–79. [Google Scholar]
- Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol. 2006;95:1221–1230. doi: 10.1152/jn.01035.2005. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Wang Z, Gu Y, Feil R, Hofmann F, Ma L. Regulate axon branching by the cyclic GMP pathway via inhibition of glycogen synthase kinase 3 in dorsal root ganglion sensory neurons. J Neurosci. 2009;29:1350–1360. doi: 10.1523/JNEUROSCI.3770-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirlinger M, Lo L, McMahon J, McMahon AP, Anderson DJ. Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc Natl Acad Sci U S A. 2002;99:8084–8089. doi: 10.1073/pnas.122231199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.