Abstract
Introduction
In thermally injured sheep, there is more than a 10-fold increase in bronchial blood flow following smoke inhalation. It was previously reported that sclerosis of the bronchial artery prior to smoke exposure would reduce the pathophysiology of the inhalation insult. We hypothesized that sclerosis of the bronchial artery after insult would attenuate smoke/burn-induced acute lung injury.
Methods
Through an incision at the 4th intercostal space, a catheter was placed via the esophageal artery into the bronchial artery such that the bronchial blood flow remained intact. Acute lung injury was induced by a 40% total body surface area, 3rd degree cutaneous burn and smoke inhalation. Adult female sheep (n = 18, 35.6 ± 1.0 kg) were divided into three groups following the injury: 1) Sclerosis group: 1 h post-injury, 4 mL 70% ethanol was injected into bronchial artery via bronchial catheter, n=6; 2) Control group: 1 h post-injury an equal dose of saline was injected into bronchia artery via bronchial catheter, n=6; 3) Sham group: no injury and no treatment, n=6. The experiment was conducted in awake condition for 24 h.
Results
Bronchial blood flow, measured by microspheres, was significantly reduced after ethanol injection in the sclerosis group. Pulmonary function, evaluated by measurement of blood gas analysis, pulmonary mechanics, and pulmonary transvascular fluid flux, was severely impaired in the control group. However, pulmonary function was significantly improved by bronchial artery sclerosis.
Conclusion
The results of our study clearly demonstrate a crucial role of enhanced bronchial circulation in thermal injury-related morbidity. Modulation of bronchial circulation using pharmacological agents may be an effective strategy in management of burn patients with concomitant smoke inhalation injury.
Keywords: Burn injury, Smoke inhalation injury, Bronchial artery, Acute respiratory distress syndrome, Sclerosis
1. Introduction
Smoke inhalation injury can result in severe respiratory distress, which is the leading cause of morbidity and mortality in burn victims [1]. After smoke inhalation, there is a rapid onset of hyperemia in the upper airway of humans and sheep [2]; hyperemia of the airway is the most effective way of diagnosing smoke inhalation [3]. Further, there is more than a 10-fold increase in bronchial blood flow immediately after inhalation injury [4]. Wagner et al. [5] previously found that an increase of bronchial blood flow directly contributes to lung lymph flow changes.
Likewise, smoke inhalation has also been shown to cause an increase in microvascular permeability to proteins in the pulmonary and bronchial circulations [6–8]. These physiological alterations in pulmonary microvasculature are delayed in onset, and the peak of increased microvascular permeability was observed approximately 24 h after injury [9]. In contrast, there is a marked increase in bronchial blood flow immediately after inhalation injury [4]. Because the increased bronchial blood flow enters into the pulmonary vasculature through various bronchopulmonary anastomoses [10,11], it has been suggested that the bronchial circulation plays a significant role in the spread of injury from the airway of the lung to the parenchyma [12–14]. Moreover, reduction of bronchial artery attenuated lung edema formation after inhalation in an anesthetized canine model [13] and in a conscious sheep model [12]. However, all these studies of attenuation of bronchial blood flow were performed before injury. To the best of our knowledge, it has not yet been shown that reduction of bronchial artery after injury attenuates lung dysfunction following burn and smoke inhalation injury. After inhalation injury, sclerosis of the bronchial circulation might be more effective since initial bronchial dilation may expose more of the vasculature to ablation. Recently, interventional therapy has made remarkable progress, and the infusion of pharmacological agents into the bronchial artery is achievable in humans [15,16]. Therefore, we hypothesized that sclerosis of bronchial artery after injury would attenuate smoke/burn-induced acute lung injury.
2. Materials and methods
2.1. Animal Model
Eighteen adult female sheep (30–40 kg) were cared for in the Investigative Intensive Care Unit at our institution, a facility accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The experimental procedures were approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston, TX). The National Institutes of Health and American Physiological Society guidelines for animal care were strictly followed.
All animals were endotracheally intubated and ventilated during the operative procedure while under isoflourane anesthesia. An arterial catheter (16 gauge, 24 in., Intracath, Becton Dickinson, Sandy, UT) was placed in the descending aorta via the femoral artery. A Swan-Ganz thermal dilution catheter (model 93A-131-7F, Edwards Critical-Care Division, Irvine, CA) was positioned in the right pulmonary artery via the right external jugular vein. A Silastic catheter was positioned in the left atrium through a left 4th thoracotomy. A 10-mm pneumatic occluding cuff (In Vivo Metrics, Healdsburg, CA) was implanted around the left pulmonary artery just distal to its origin in the right ventricle. After exposing the broncho-esophageal artery carefully, a 0.6 mm OD polyurethane catheter (Access Technologies, Sokie, IL) was inserted into the bronchial artery via the esophageal artery, and the tip of the catheter was placed in the bronchial artery. Only the esophageal artery (i.e., not the bronchial artery) was ligatured to secure the catheter. The bronchial artery catheter was continuously flushed using a heparin saline flush (3 units/mL, 2 mL/h) to prevent coagulation. Through a right 5th thoracotomy, an efferent lymph vessel from the caudal mediastinal lymph node was cannulated (Silastic catheter 0.025-in ID, 0.047-in OD; Dow Corning, Midland, MI) to evaluate changes in lung lymph flow according to a modification of the technique described by Staub et al. [17,18].
After a 7–10 day recovery period, the sheep were deeply anesthetized with isoflourane and a third-degree flame burn of 20% was applied to each flank (total area 40%) using a Bunsen burner. Thereafter, inhalation injury was induced while sheep were in the prone position as described previously [7]. A modified bee smoker was filled with 50g burning cotton toweling and connected to the tracheostomy tube. The sheep were insufflated with a total of 48 breaths of cotton smoke. The sheep were removed from anesthesia after injury but were placed on ventilation (volume control) for 24 hours without any sedation. Adult female sheep (n = 18, 35.6 ± 1.0 kg) were divided into three groups: 1) Sclerosis group was injected with 4 mL 70% ethanol into the bronchial artery via bronchial artery cannulation at 1 h post-injury (n=6); 2) Control group was injected with an equal amount of saline (n=6) 1 h post-injury; 3) Sham group had no injury and no sclerosis administered, but same operation and same anesthesia were performed (n=6). The amount of injected ethanol is so small that pulmonary artery cannot be coagulated. Even if coagulated, the area of coagulated vessel may be small. We chose this agent and volume based on previously published experiments [19,20]. After burn and smoke inhalation injury, all sheep were placed on a ventilator (Servo Ventilator 300; Siemens-Elema AB, Solna, Sweden) with positive end-expiratory pressure set to 5 cm H2O and tidal volume maintained at 15 mL/kg. The latter tidal volume is equal to about 10 mL/kg in humans due to the large ventilatory dead space of sheep [21]. For the first 3 h post injury, all animals received an inspired oxygen concentration (FiO2) of 100% to expedite the removal of CO; thereafte2r, FiO was adjusted to maintain the arterial oxygen saturation >90%. All animals were given fluid resuscitation with Ringer’s lactate solution strictly according to the Parkland formula (4 mL/kg/% TBSA burned/24 h) [22]. The experiment was continued for 24 h in an awake condition.
2.2. Measurement of regional blood flow
To substantiate the changes in bronchial blood flow, measurements were taken using the fluorescent microsphere technique (Interactive Medical Technologies, West Los Angeles, CA) that we had previously developed [23]. At 0 h, Before Injection (BI), After Injection (AI), 6 h, and 24 h, ~12 × 106 fluorescent colored microspheres (15.0 ± 0.1 µm) were injected into the left atrium. Since some of the microspheres may pass through some of the larger vessels in the non-bronchial pulmonary circulation, the pneumatic occluder on the left pulmonary artery was inflated immediately after microsphere injection to prevent any pulmonary artifact to affect the bronchial blood flow measurement [23]. To calibrate microsphere numbers per blood flow, blood was withdrawn from the aorta with a Harvard pump (Harvard Apparatus model 55–1143, South Natick, MA) at a rate of 10 mL/min; the withdrawal was started before microsphere injection and continued for 2 min. Left proximal (6–8 mm bronchi near carina) and distal bronchi (2–4 mm bronchi in distal lower lobe) were obtained postmortem and used to quantify the bronchial blood flow to the airway.
2.3. Measurement of gas exchanges, lung lymph flow, pulmonary microvascular fluid flux, lung water content and activity of myeloperoxidase
Arterial and mixed venous blood samples were taken at different time points for measurement of blood gases (IL GEM Premier 3000 Blood Gas Analyzer; GMI, Ramsey, MN). PaO2/FiO2 ratio was measured to help assess pulmonary gas exchange. Pulmonary shunt fraction (Qs/Qt) was calculated using standard equations. The pulmonary microvascular fluid flux was evaluated by measuring the lung lymph flow. Pulmonary vascular permeability index was calculated using the following formula: Permeability index = lung lymph flow × [lung lymph oncotic pressure (πi) / plasma oncotic pressure (πp)]. Peak and pause airway pressures were monitored on a ventilation machine (Servo Ventilator 300; Siemens-Elema AB, Solna, Sweden). Plasma and lymph oncotic pressures were measured at different time points using a colloid osmometer (4420 Colloid Osmometer, Wescor, Utah) that had been calibrated for sheep. Sheep were sacrificed under deep ketamine anesthesia 24 h after injury. The lower one-half of the right lung was used for the determination of bloodless wet-to-dry weight ratio [24]. The activity of myeloperoxidase (MPO), an indicator of neutrophil accumulation, was determined spectrophotometrically in whole lung homogenates. MPO concentrations were evaluated on homogenized right lung with a commercially available assay. Myeloperoxidase activity was reported as U/g dry tissue (CytoStore, Calgary, AB, Canada).
2.4. Statistical analysis
Summary statistics of data are expressed as means ± standard error of the mean. Statistical comparison was performed by ANOVA with post hoc Bonferroni test. Statistical significance was accepted at p < 0.05. All statistical calculations were performed with the Prism software (GraphPad Software Inc. San Diego, CA).
3. Results
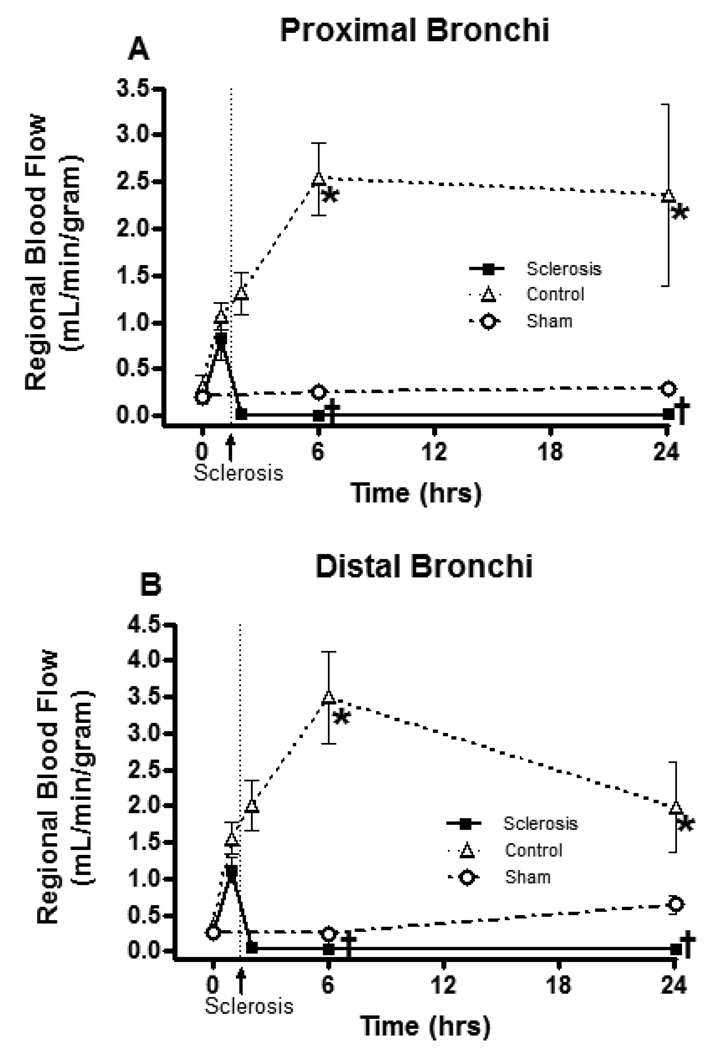
Figure 1 shows the regional blood flow changes measured with fluorescent colored microspheres. Burn and smoke inhalation injury significantly increased the airway blood flow in the control group: six hours after injury, blood flows were 2.54 ± 0.39 mL/g (Control: Burn and smoke inhalation injury) vs. 0.25 ± 0.05 mL/g (Sham: no injury) in proximal bronchi and 3.50 ± 0.63 mL/g (Control) vs. 0.25 ± 0.02 mL/g (Sham) in distal bronchi. Thus, the combination injury caused a 10- to 14-fold increase in blood flow. Ethanol injection in the bronchial artery significantly reduced the acute regional blood flow increase in proximal bronchi (Proximal Bronchi, Control: 2.54 ± 0.39 mL/g vs. Sclerosis: 0.01 ± 0.00 mL/g; Distal Bronchi, Control: 3.50 ± 0.63 mL/g vs. Sclerosis 0.02 ± 0.01 mL/g tissue at 6 h). These blood flow levels in the sclerosis group were lower than in sham group (Proximal Bronchi, Sham: 0.25 ± 0.05 vs. Sclerosis 0.01 ± 0.00; Distal Bronchi, Sham: 0.25 ± 0.02 vs. Sclerosis 0.02 ± 0.01 mL/g tissue at 6 h).
Fig 1.
Changes in airway blood flow after burn and smoke inhalation injury. Data are expressed as mean ± SEM. * Significant difference vs. sham group (p < 0.05); † Significant difference vs. control group (p < 0.05).
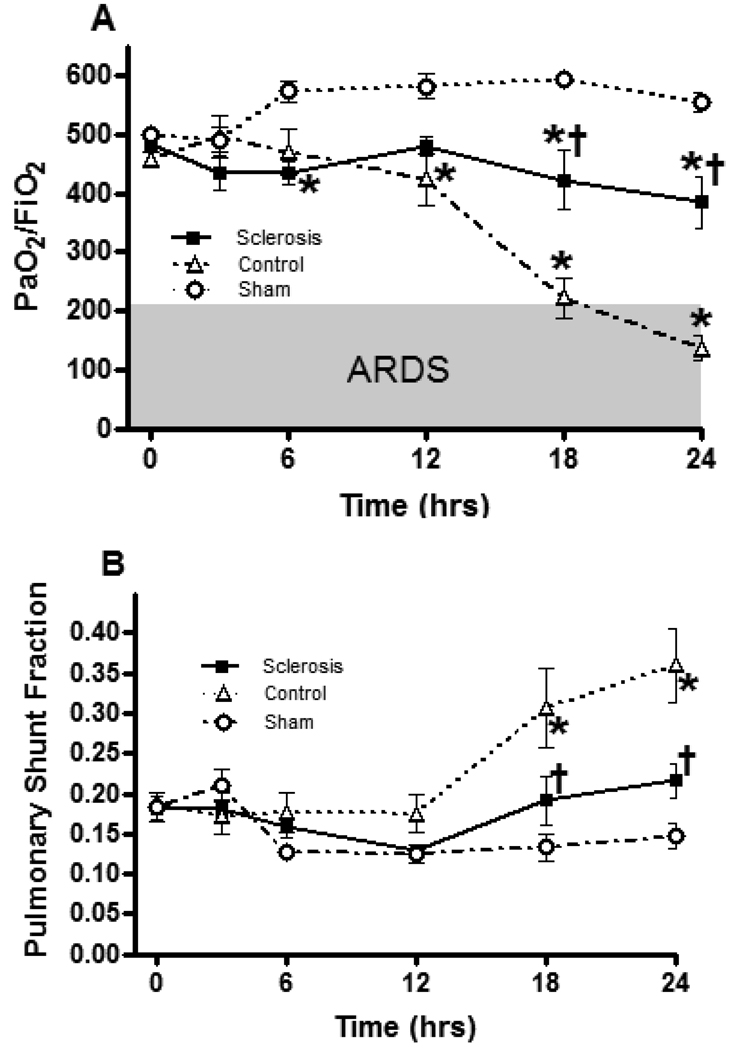
Figure 2 shows the effect of bronchial artery sclerosis on pulmonary gas exchange. Burn and smoke inhalation injury significantly deteriorated PaO2/ FiO2 ratio after 12 h and pulmonary shunt fraction after 18 h. Bronchial artery sclerosis significantly attenuated the pulmonary gas exchanges. Significant differences were observed at 18 and 24 h in PaO2/ FiO2 ratio and in pulmonary shunt fraction between the sclerosis group and control group.
Fig 2.
Effects of bronchial artery sclerosis on pulmonary gas exchanges. Data are expressed as mean ± SEM. * Significant difference vs. sham group (p < 0.05); † Significant difference vs. control group (p < 0.05).
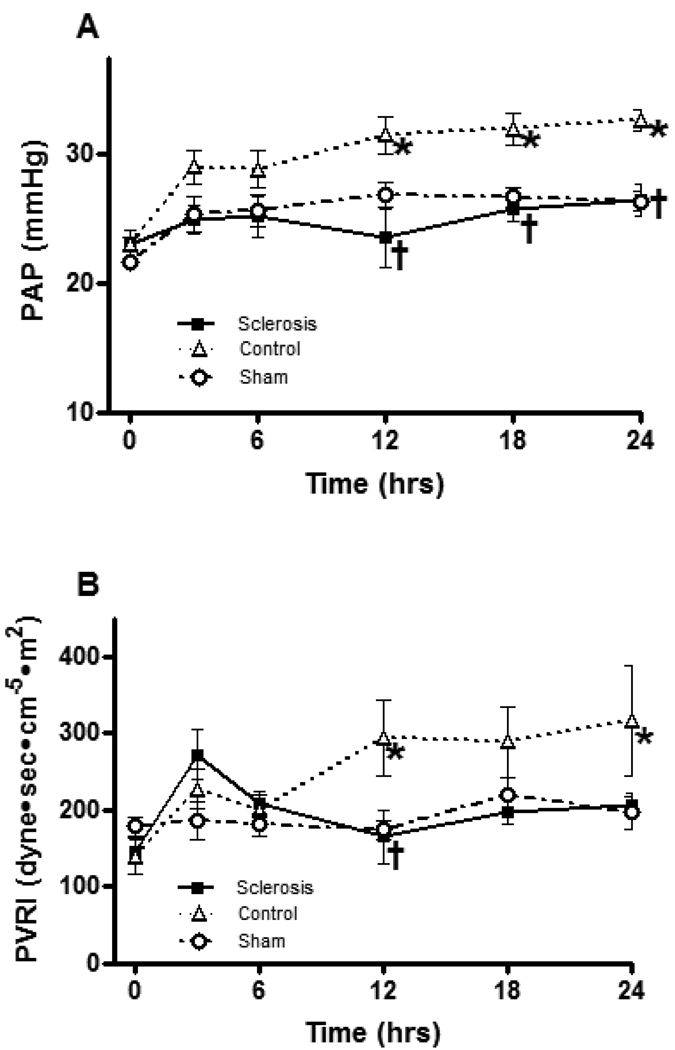
Burn and smoke inhalation injury significantly increased pulmonary artery pressure (PAP) and pulmonary artery vascular resistance index (PVRI). Bronchial artery sclerosis significantly attenuated the changes at 12, 18 and 24 h in PAP and 12 h in PVRI (Figure 3).
Fig 3.
Effects of bronchial artery sclerosis on pulmonary artery pressure (A) and pulmonary vascular resistance (B). Data are expressed as mean ± SEM. * Significant difference vs. sham group (p < 0.05); † Significant difference vs. control group (p < 0.05).
Figure 4 shows peak and pause airway pressure. Burn and smoke inhalation injury significantly increased the peak and pause airway pressure at 18 and 24 h. Bronchial artery sclerosis significantly suppressed this increase in the sclerosis group. Significant differences were observed at 18 and 24 h.
Fig 4.
Effects of bronchial artery sclerosis on peak (A) and pause (B) airway pressure. Data are expressed as mean ± SEM. * Significant difference vs. sham group (p < 0.05); † Significant difference vs. control group (p < 0.05).
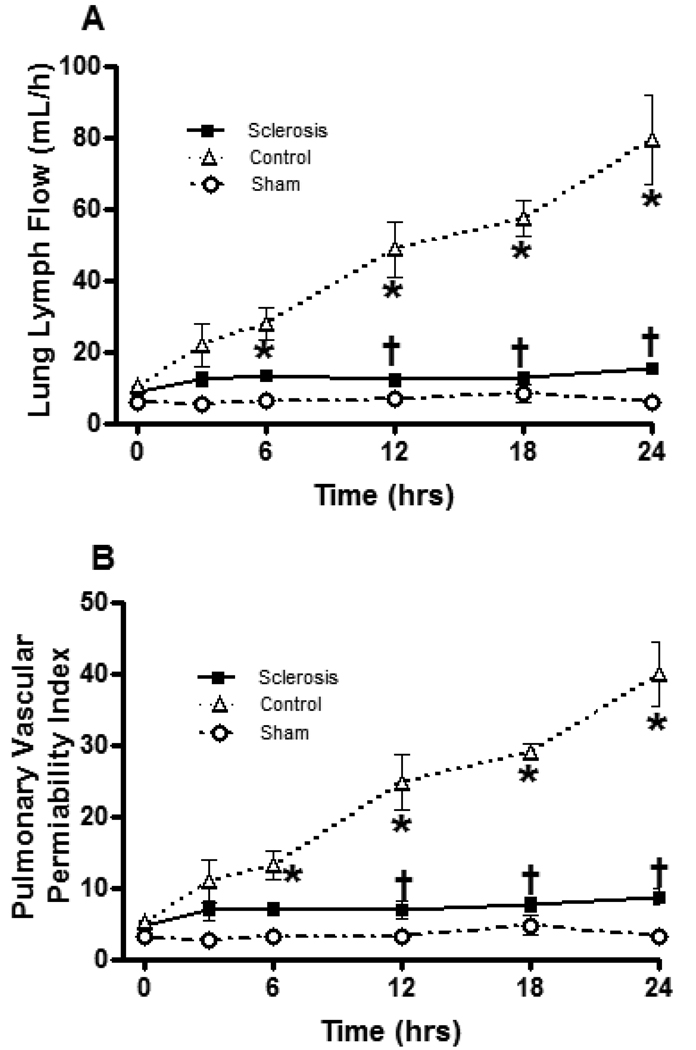
Lung lymph flow, a characteristic of pulmonary transvascular fluid flux, and pulmonary vascular permeability index were significantly increased in the control group after burn and smoke inhalation injury. The lymph flow began to increase at 6 h after insult, and a peak was observed at 24 h. However, bronchial artery sclerosis reversed this increase in pulmonary transvascular fluid flux and pulmonary vascular index. Significant differences were observed between the sclerosis group and control group at 12, 18 and 24 h (Figure 5).
Fig 5.
Effect of bronchial artery sclerosis on lung lymph flow (A) and lung pulmonary vascular permeability index (B). Data are expressed as mean ± SEM. * Significant difference vs. sham group (p < 0.05); † Significant difference vs. control group (p < 0.05).
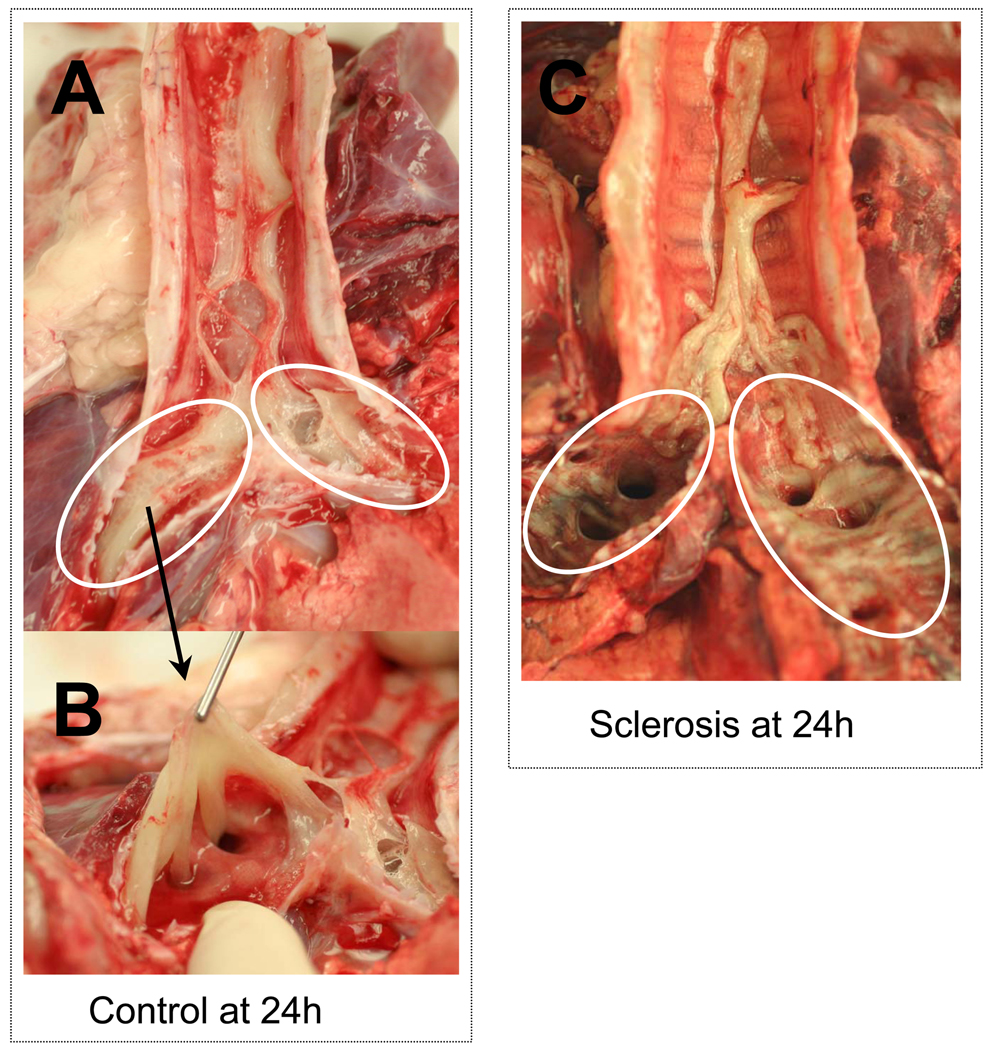
Figure 6 shows cast formation in airways. Burn and smoke inhalation injury caused heavy cast formation in trachea and bronchi, and it proved very difficult to remove the cast from bronchi in control animals (Figure 6A, B). Bronchial artery sclerosis suppressed this cast formation in bronchi of the sclerosis group (Figure 6C).
Fig 6.
Photographs showing representative cast formation in trachea and bronchi in control animals at 24 h (A), cast formation in bronchia in control animals (B) and cast formation in trachea and bronchi in sclerosis animals at 24 h (C). Burn and smoke inhalation injury causes cast formation in airway (A, B). However, bronchial artery reduced the cast formation in bronchi (white circle) but not in trachea (C).
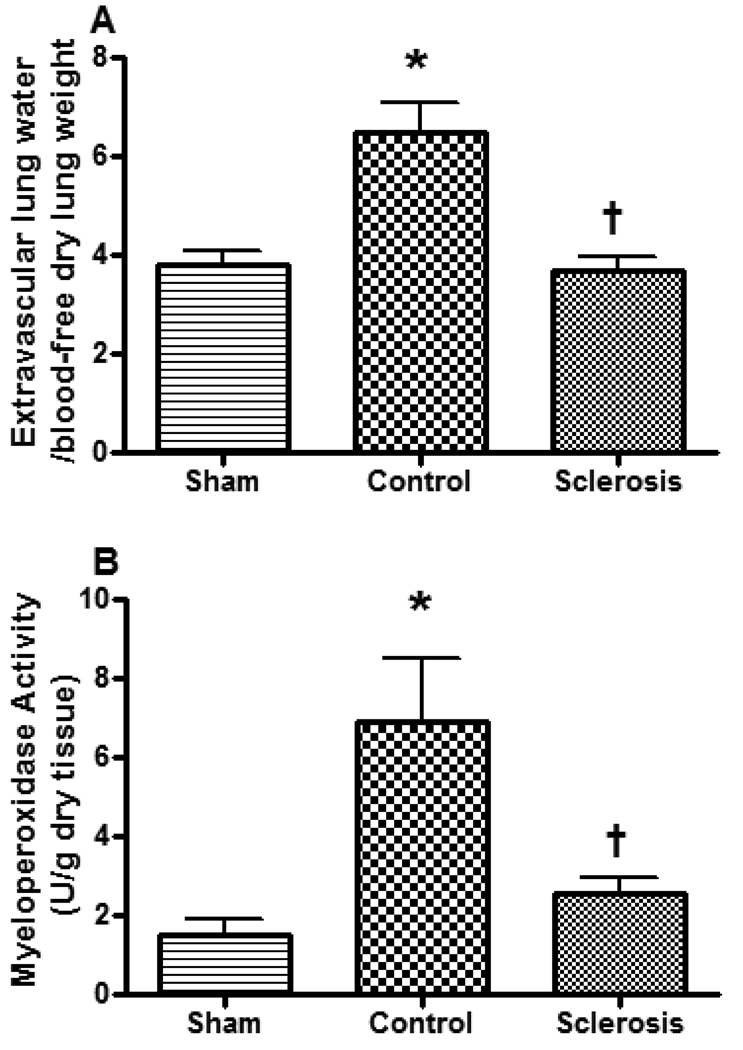
Lung bloodless wet to dry weight ratio, a measure of lung water content, and myeloperoxidase activity were significantly increased in the control group. However, the lung wet to dry ratio was much lower in the sclerosis group (Figure 7A). Bronchial artery sclerosis also significantly reduced the myeloperoxidase activity (Figure 7B).
Fig 7.
Effect of bronchial artery sclerosis on the bloodless lung wet to dry (A) and myeloperoxidase activity (B). Data are expressed as mean ± SEM. * Significant difference vs. sham group (p < 0.05); † Significant difference vs. control group (p < 0.05).
4. Discussion
Recently, interventional therapy has made remarkable progress in several diseases such as cardiovascular disease, cancer therapy and venous malformation therapy [25–27]. In inflammatory disease, continuous regional arterial infusion (CRAI) therapy of protease inhibitors and antibiotics on acute necrotizing pancreatitis has been reported to reduce mortality rates [28–30]. In regards to the bronchial artery, bronchial artery infusion (BAI) and bronchial artery embolism (BAE) are performed for the treatment of lung cancer and hemoptysis therapy, respectively [15,31]. Thus, interventional therapy has been widely used in various patient illnesses. As we showed previously, ablation of bronchial artery before insult reduced blood flow increases and airway hyperemia, and prevented pulmonary pathophysiological changes [13,20]. However, there is no report of sclerosis of the bronchial artery after insult, which would connect to interventional therapy of acute lung injury induced by burn and smoke inhalation injury. We therefore devised the novel bronchial artery cannulation technique, which preserved bronchial circulation and kept the animals alive for the long term. Using the cannulation technique, we were able to ablate the bronchial circulation after burn and smoke inhalation injury.
The bronchial artery sclerosis prevented the fall in PaO2/FiO2 ratio and increased pulmonary shunt fraction noted after burn and smoke inhalation injury in the intact control animals. The mechanism by which the bronchial circulation contributes to the pathogenesis in lung parenchyma has not been clearly defined. The bronchial circulation provides 97% of the blood supply to the intrapulmonary airway, but only 13% of the bronchial circulation drains into bronchial vein that flows into the right side of the heart [32,33]. Most of intrapulmonary airway blood flow drains into the pulmonary microcirculation at the pre-capillary level [10,34]. Thus, with smoke inhalation injury, inflammation occurs in the airways, which could release mediators or cells into the bronchial circulation that would then be delivered to the whole pulmonary parenchyma where they could elicit lung damage. Airway injury may result in the formation of mediators that enter into the bronchial circulation and are thus delivered to the pulmonary microvasculature [35]. We did not measure any inflammation mediators, but there are several reports that some inflammation mediators increase after smoke inhalation injury [14,36,37].
Activated neutrophils in the bronchial circulation also flow out into the pulmonary microcirculation [38]. In the present study, the myeloperoxidase activity increased after burn and smoke injury, and this activity was significantly reduced by bronchial artery sclerosis. Treatment of the cells with an antibody to L-selectin prevents the changes in transvascular fluid flux and other aspects of parenchymal damage [39]. Baile et al. have previously reported that 85% of the bronchial blood venous return was into the pulmonary circuit, and that 50% of the neutrophils injected into the bronchial artery became lodged in the pulmonary microvasculature [34]. In addition, activated neutrophils may likewise enter the pulmonary parenchymal areas and lodge in the pulmonary vasculature. Certainly the lack of neutrophils in the lungs of the sclerosed sheep as indexed by MPO would support this hypothesis.
Burn and smoke inhalation injury induced the rapid increase of bronchial circulation, which increased the pulmonary fluid flux, pulmonary vascular permeability, airway exudation and pulmonary edema, in turn causing airway cast formation and increased airway pressure. Likewise, the increase of bronchial circulation could spread inflammatory mediators and cells into the whole pulmonary parenchyma and accelerate lung damage. The combination of these components could cause burn and smoke inhalation-induced acute lung injury and pulmonary failure. Therefore, modulation of bronchial circulation using pharmacological agents may be an effective strategy in the management of burn patients with concomitant smoke inhalation injury. One limitation of this experiment is that sheep were insufflated with a total of 48 breaths of cotton smoke, which causes severe inhalation injury but does not necessarily correspond to the less severe smoke inhalation injury seen in patients who survive long enough to be admitted to a burn ICU. Another limitation of this experiment was that we used 70% ethanol to ablate bronchial blood flow and terminated the experiment at 24 h. No adverse effects of sclerosis were observed within 24 h, but the sclerosis of bronchial artery may have some adverse effects beyond this time point. In clinical situation, continuous regional arterial infusion therapy may be preferred to sclerosis, which coagulates all bronchial arteries. Also, the anatomy of the bronchial artery in humans is one hindrance to intravenous therapy. Sheep have a single common bronchial artery [40], but humans have several branches of the bronchial artery [41–44]. Nonetheless, interventional therapy has progressed rapidly and multiple cannulation techniques have been developed. In the future, we will be able to infuse pharmacological agents into human bronchial arteries. Consequently, it will be possible to suppress the bronchial artery flow in situations of pulmonary hyperemia in humans via the interventional technique. Thus, interventional therapy to modulate the bronchial circulation using pharmacological agents may be an effective strategy in the management of ARDS induced by burn and smoke inhalation injury.
Acknowledgments
We thank Jeffrey D. Meserve for his editorial assistance and Nettie Biondo and John R. Salsbury for their technical assistance.
This work was supported by National Institute for General Medical Sciences Grant GM66312, GM060688 and Grants 8954, 8450 and 8460 from the Shriners of North America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barrow RE, Spies M, Barrow LN, Herndon DN. Influence of demographics and inhalation injury on burn mortality in children. Burns. 2004;30:72–77. doi: 10.1016/j.burns.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Linares HA, Herndon DN, Traber DL. Sequence of morphologic events in experimental smoke inhalation. J Burn Care Rehabil. 1989;10:27–37. doi: 10.1097/00004630-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Pruitt BA, Jr, Cioffi WG, Shimazu T, Ikeuchi H, Mason AD., Jr Evaluation and management of patients with inhalation injury. The Journal of trauma. 1990;30:S63–S68. doi: 10.1097/00005373-199012001-00015. [DOI] [PubMed] [Google Scholar]
- 4.Abdi S, Herndon D, McGuire J, Traber L, Traber DL. Time course of alterations in lung lymph and bronchial blood flows after inhalation injury. J Burn Care Rehabil. 1990;11:510–515. doi: 10.1097/00004630-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Wagner EM, Blosser S, Mitzner W. Bronchial vascular contribution to lung lymph flow. J Appl Physiol. 1998;85:2190–2195. doi: 10.1152/jappl.1998.85.6.2190. [DOI] [PubMed] [Google Scholar]
- 6.Herndon DN, Traber LD, Linares H, Flynn JD, Niehaus G, Kramer G, et al. Etiology of the pulmonary pathophysiology associated with inhalation injury. Resuscitation. 1986;14:43–59. doi: 10.1016/0300-9572(86)90006-7. [DOI] [PubMed] [Google Scholar]
- 7.Kimura R, Traber LD, Herndon DN, Linares HA, Lubbesmeyer HJ, Traber DL. Increasing duration of smoke exposure induces more severe lung injury in sheep. J Appl Physiol. 1988;64:1107–1113. doi: 10.1152/jappl.1988.64.3.1107. [DOI] [PubMed] [Google Scholar]
- 8.Traber DL, Schlag G, Redl H, Traber LD. Pulmonary edema and compliance changes following smoke inhalation. J Burn Care Rehabil. 1985;6:490–494. doi: 10.1097/00004630-198511000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Isago T, Noshima S, Traber LD, Herndon DN, Traber DL. Analysis of pulmonary microvascular permeability after smoke inhalation. J Appl Physiol. 1991;71:1403–1408. doi: 10.1152/jappl.1991.71.4.1403. [DOI] [PubMed] [Google Scholar]
- 10.Charan NB, Turk GM, Dhand R. Gross and subgross anatomy of bronchial circulation in sheep. J Appl Physiol. 1984;57:658–664. doi: 10.1152/jappl.1984.57.3.658. [DOI] [PubMed] [Google Scholar]
- 11.Bernard S, Luchtel DL, Polissar N, Hlastala MP, Lakshminarayan S. Structure and size of bronchopulmonary anastomoses in sheep lung. The anatomical record. 2005;286:804–813. doi: 10.1002/ar.a.20216. [DOI] [PubMed] [Google Scholar]
- 12.Abdi S, Herndon DN, Traber LD, Ashley KD, Stothert JC, Jr, Maguire J, et al. Lung edema formation following inhalation injury: role of the bronchial blood flow. J Appl Physiol. 1991;71:727–734. doi: 10.1152/jappl.1991.71.2.727. [DOI] [PubMed] [Google Scholar]
- 13.Hales CA, Barkin P, Jung W, Quinn D, Lamborghini D, Burke J. Bronchial artery ligation modifies pulmonary edema after exposure to smoke with acrolein. J Appl Physiol. 1989;67:1001–1006. doi: 10.1152/jappl.1989.67.3.1001. [DOI] [PubMed] [Google Scholar]
- 14.Efimova O, Volokhov A, Hales CA. Injection of prostaglandin F2alpha into the bronchial artery in sheep increases the pulmonary vascular permeability to protein. Pulmonary pharmacology & therapeutics. 2007;20:167–171. doi: 10.1016/j.pupt.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 15.So T, Osaki T, Nakata S, Hanagiri T, Sugio K, Yasumoto K. Carinal resection after induction bronchial arterial infusion for locally advanced non-small cell lung cancer. Jpn J Thorac Cardiovasc Surg. 2004;52:143–147. doi: 10.1007/s11748-004-0131-y. [DOI] [PubMed] [Google Scholar]
- 16.Koshiishi H, Okamura T, Takahashi M, Ohtsubo Y, Azuma Y, Tamamoto F, et al. [Evaluation of bronchial arterial infusion (BAI) for lung cancer with brain metastasis] Gan To Kagaku Ryoho. 2006;33:1860–1862. [PubMed] [Google Scholar]
- 17.Staub N, Bland R, Brigham K, Demling R, Erdmann A, Woolverton W. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975;19:315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- 18.Traber D, Adams T, Henriksen NJ, Traber D, Thomson P. Reproducibility of cardiopulmonary effects of different endotoxins in the same sheep. J Appl Physiol. 1983;54:1167–1171. doi: 10.1152/jappl.1983.54.4.1167. [DOI] [PubMed] [Google Scholar]
- 19.Baile EM, Minshall D, Harrison PB, Dodek PM, Pare PD. Systemic blood flow to the lung after bronchial artery occlusion in anesthetized sheep. J Appl Physiol. 1992;72:1701–1707. doi: 10.1152/jappl.1992.72.5.1701. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai H, Johnigan R, Kikuchi Y, Harada M, Traber LD, Traber DL. Effect of reduced bronchial circulation on lung fluid flux after smoke inhalation in sheep. J Appl Physiol. 1998;84:980–986. doi: 10.1152/jappl.1998.84.3.980. [DOI] [PubMed] [Google Scholar]
- 21.Vidal Melo MF, Harris RS, Layfield D, Musch G, Venegas JG. Changes in regional ventilation after autologous blood clot pulmonary embolism. Anesthesiology. 2002;97:671–681. doi: 10.1097/00000542-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Baxter CR. Problems and complications of burn shock resuscitation. The Surgical clinics of North America. 1978;58:1313–1322. doi: 10.1016/s0039-6109(16)41693-2. [DOI] [PubMed] [Google Scholar]
- 23.Wu CH, Lindsey DC, Traber DL, Cross CE, Herndon DN, Kramer GC. Measurement of bronchial blood flow with radioactive microspheres in awake sheep. J Appl Physiol. 1988;65:1131–1139. doi: 10.1152/jappl.1988.65.3.1131. [DOI] [PubMed] [Google Scholar]
- 24.Pearce ML, Yamashita J, Beazell J. Measurement of Pulmonary Edema. Circulation research. 1965;16:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- 25.Sharkawi T, Cornhill F, Lafont A, Sabaria P, Vert M. Intravascular bioresorbable polymeric stents: a potential alternative to current drug eluting metal stents. J Pharm Sci. 2007;96:2829–2837. doi: 10.1002/jps.20957. [DOI] [PubMed] [Google Scholar]
- 26.Simons ME. Peripheral vascular malformations: diagnosis and percutaneous management. Can Assoc Radiol J. 2001;52:242–251. [PubMed] [Google Scholar]
- 27.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 28.Takeda K, Matsuno S, Sunamura M, Kakugawa Y. Continuous regional arterial infusion of protease inhibitor and antibiotics in acute necrotizing pancreatitis. American journal of surgery. 1996;171:394–398. doi: 10.1016/S0002-9610(97)89617-1. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Matsuno S, Ogawa M, Watanabe S, Atomi Y. Continuous regional arterial infusion (CRAI) therapy reduces the mortality rate of acute necrotizing pancreatitis: results of a cooperative survey in Japan. Journal of hepato-biliary-pancreatic surgery. 2001;8:216–220. doi: 10.1007/s005340170019. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi J, Kawarada Y, Isaji S, Yokoi H, Higashiguchi T. Therapeutic effects of continuous intraarterial antibiotic infusion in preventing pancreatic infection in experimental acute necrotizing pancreatitis. Pancreas. 1996;13:184–192. doi: 10.1097/00006676-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Osaki T, Hanagiri T, Nakanishi R, Yoshino I, Taga S, Yasumoto K. Bronchial arterial infusion is an effective therapeutic modality for centrally located early-stage lung cancer: results of a pilot study. Chest. 1999;115:1424–1428. doi: 10.1378/chest.115.5.1424. [DOI] [PubMed] [Google Scholar]
- 32.Bernard SL, Glenny RW, Polissar NL, Luchtel DL, Lakshminarayan S. Distribution of pulmonary and bronchial blood supply to airways measured by fluorescent microspheres. J Appl Physiol. 1996;80:430–436. doi: 10.1152/jappl.1996.80.2.430. [DOI] [PubMed] [Google Scholar]
- 33.Charan NB, Thompson WH, Carvalho P. Functional anatomy of bronchial veins. Pulmonary pharmacology & therapeutics. 2007;20:100–103. doi: 10.1016/j.pupt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Baile EM, Pare PD, Ernest D, Dodek PM. Distribution of blood flow and neutrophil kinetics in bronchial vasculature of sheep. J Appl Physiol. 1997;82:1466–1471. doi: 10.1152/jappl.1997.82.5.1466. [DOI] [PubMed] [Google Scholar]
- 35.Lin YS, Ho CY, Tang GJ, Kou YR. Alleviation of wood smoke-induced lung injury by tachykinin receptor antagonist and hydroxyl radical scavenger in guinea pigs. Eur J Pharmacol. 2001;425:141–148. doi: 10.1016/s0014-2999(01)01184-0. [DOI] [PubMed] [Google Scholar]
- 36.Noonan TC, Selig WM, Burhop KE, Burgess CA, Malik AB. Pulmonary microvascular response to LTB4: effects of perfusate composition. J Appl Physiol. 1988;64:1989–1996. doi: 10.1152/jappl.1988.64.5.1989. [DOI] [PubMed] [Google Scholar]
- 37.Noonan TC, Selig WM, Kern DF, Malik SB. Mechanism of peptidoleukotriene-induced increases in pulmonary transvascular fluid filtration. J Appl Physiol. 1986;61:1928–1934. doi: 10.1152/jappl.1986.61.5.1928. [DOI] [PubMed] [Google Scholar]
- 38.Traber D, Herndon D, Enkhbaatar P, Maybauer MO, Maybauer DM. The pathophysiology of inhalation injury. In: Herndon D, editor. Total Burn Care. Philadelphia: Saunder Elsevier; 2007. pp. 248–261. [Google Scholar]
- 39.Sakurai H, Schmalstieg FC, Traber LD, Hawkins HK, Traber DL. Role of L-selectin in physiological manifestations after burn and smoke inhalation injury in sheep. J Appl Physiol. 1999;86:1151–1159. doi: 10.1152/jappl.1999.86.4.1151. [DOI] [PubMed] [Google Scholar]
- 40.Magno MG, Fishman AP. Origin, distribution, and blood flow of bronchial circulation in anesthetized sheep. J Appl Physiol. 1982;53:272–279. doi: 10.1152/jappl.1982.53.1.272. [DOI] [PubMed] [Google Scholar]
- 41.Cauldwell E, Siekert R, Lininger R, Anson B. The bronchialarteries. An anatomical study of 150 human cadavers. Surgery Gynecol Obstet. 1948;86:395–412. [PubMed] [Google Scholar]
- 42.Suzuki K, Kamata N, Tanaka J, Nomura S, Asumi M, Kutsukake Y, et al. [Clinical investigation of the branching formation of the bronchial arteries in the Japanese] Nippon Igaku Hoshasen Gakkai Zasshi. 1989;49:979–985. [PubMed] [Google Scholar]
- 43.Botenga AS. The significance of broncho-pulmonary anastomoses in pulmonary anomalies: a selective angiographic study. Radiol Clin Biol. 1969;38:309–328. [PubMed] [Google Scholar]
- 44.Kasai T, Chiba S. Macroscopic anatomy of the bronchial arteries. Anat Anz. 1979;145:166–181. [PubMed] [Google Scholar]