Abstract
Little is known regarding the involvement of the ventral pallidum (VP) in cocaine-seeking behavior, in contrast with considerable documentation of the involvement of its major afferent, the nucleus accumbens, over the past thirty years utilizing electrophysiology, lesion, inactivation, molecular, imaging, and other approaches. The VP is neuroanatomically positioned to integrate signals projected from the nucleus accumbens, basolateral amygdala, and ventral tegmental area. In turn, VP projects to thalamoprefrontal, subthalamic, and mesencephalic dopamine regions having widespread influence across mesolimbic, mesocortical, and nigrostriatal systems. Prior lesion studies have implicated VP in cocaine-seeking behavior, but the electrophysiological mechanisms underlying this behavior in the VP have not been investigated. In the present investigation, following two weeks of training over which animals increased drug intake, VP phasic activity comprised rapid-phasic increases or decreases in firing rate during the seconds prior to and/or following cocaine-reinforced responses, similar to those found in accumbens. As a population, the direction (increasing or decreasing) and magnitude of firing rate changes were normally distributed suggesting that ventral striatopallidal processing is heterogeneous. Since changes in firing rate around the self-administering lever press occurred in animals that escalated drug intake prior to neuronal recordings, a marker of “addiction-like behavior” in the rat, the present experiment provides novel support for a role of VP in drug-seeking behavior. This is especially important given that pallidothalamic and pallidomesencephalic VP projections are positioned to alter dopaminoceptive targets such as the medial prefrontal cortex, nucleus accumbens, and dorsal striatum, all of which have roles in cocaine self-administration.
Keywords: striatum, accumbens, striatopallidum, dopamine
Introduction
The mesolimbic dopamine system is critical for cocaine-seeking behavior (Roberts et al, 1977, 1980; Roberts and Koob, 1982). The primary target of mesolimbic dopaminergic neurons is the nucleus accumbens (NAcc) and lesions or GABAergic inactivation of this structure decrease cocaine-seeking (Roberts et al, 1977; McFarland and Kalivas, 2001, 2004). NAcc single unit recordings have revealed phasic changes (increases or decreases in firing rate) within seconds of cocaine-seeking responses (Carelli and Deadwyler, 1994; Ghitza et al, 2004). Because NAcc neurons exhibit rapid-phasic firing rate changes during movements prior to or following the completion of a cocaine-reinforced response but not during similar movements away from the lever within the same session (Chang et al, 1994), these phasic changes likely reflect appetitive neural processing related to drug-seeking behavior.
The primary target of NAcc efferents is the ventral pallidum (VP), which also integrates afferent signals from the basolateral amygdala, prefrontal cortex, and ventral tegmental area (VTA) (Zahm and Heimer, 1990; Fuller et al, 1987; Sesack et al, 1989; Klitenick et al, 1992). In turn, the VP predominantly projects to the mediodorsal (MD) thalamus, which influences the medial prefrontal cortex (Churchill et al, 1996; Zahm et al, 1996; O’Donnell et al, 1997). VP also projects to dopamine neurons of the VTA, thereby influencing mesolimbic and mesocortical structures (Kalivas et al, 1993; Groenewegen et al, 1993). VP additionally targets the subthalamic nucleus and substantia nigra, thereby affecting the nigrostriatal system (Haber et al, 1985; Groenewegen and Berendse 1990; Bell et al, 1995; Bevan et al, 1996).
Given its position within basal forebrain circuits implicated in motivated behavior, it is likely that the VP is involved in processing drug-seeking behavior. Indeed, lesions or GABAergic agonism of the VP decrease cocaine self-administration as well as the reinstatement of cocaine-seeking behavior by cocaine-priming or footshock (Hubner and Koob, 1990; Robledo and Koob, 1993; McFarland et al, 2001, 2004). However, results of microinjections studies are regarded by some as inconclusive, since saline or artificial cerebrospinal fluid microinjections into VP can disrupt motivated behavior (Chrobak and Napier, 2002). Therefore, using single unit recordings during cocaine self-administration behavior, we hypothesized that VP firing rate changes would be associated with instrumental responding, and present the first evidence in support of this hypothesis.
Materials and Methods
Subjects and surgery
Male Long–Evans rats (n=21, 300–350g; Charles River, USA) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). Prior to surgery, subjects received injections of atropine methyl nitrate (10 mg/kg, i.p.) and penicillin G (75,000 U/0.25 ml, i.m.) to reduce the risk of pulmonary edema and bacterial infection, respectively. Anesthesia was maintained with periodic i.p. injections of ketamine hydrochloride (60 mg/kg, i.p.). Following catheter implantation into the right jugular vein, a 2×8 or 5×3 array of Teflon-coated stainless steel microwires (California Fine Wire, Grover Beach, CA) was implanted into the right VP (2×8 array: 0.0–2.8mm AP; 1.6–2.2 ML; −7.7 DV; 5×3 array: −0.6–0.7mm AP; 1.2–2.7 ML; −7.7 DV; Paxinos and Watson, 1997) and secured with dental cement. The diameter of each uninsulated microwire tip was 50μm. For both arrays, anteroposterior distances between wires were 0.350 mm (wire center to center). Mediolateral distances were 0.5 mm in the 2×8 and 0.3 mm in the 5×3 array (wire center to center). An insulated 0.01 inch ground wire, stripped 5mm from the tip, was implanted 7mm ventral from skull in the left hemisphere. After surgery, rats were individually housed with access to food and water in the cocaine self-administration chambers to recover for at least a week. Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH, Publications 865–23) and were approved by the Institutional Animal Care and Use Committee, Rutgers University.
Cocaine self-administration
Prior to self-administration sessions, a nonretractable glass lever was mounted on a side wall of the chamber. Session onset was signaled by illumination of a stimulus light above the lever. Each lever press was immediately followed by an intravenous infusion of cocaine (0.24 mg/0.2 ml), a 7.5 sec tone (3.5 kHz, 70 dB) that corresponded with the operation of a syringe pump, and a 40 sec time-out during which the stimulus light was extinguished and lever presses had no programmed consequence. All self-administration sessions were six hours in duration. Rats were never drug primed.
Drug level calculation
Assuming first-order pharmacokinetics, calculated drug levels were determined over successive infusions by the equation:
where
Tn = the time since the previous cocaine infusion (min),
D = infusion dose (mg/kg),
Bn−1 = cocaine level at time of last infusion (mg/kg)
K = rate constant (0.693/t1/2) reflecting the metabolic half-life for cocaine (Nayak et al, 1976).
Electrophysiological procedures
Recordings occurred between days 15 and 20 of self-administration training. The rationale for recording at this time point corresponds with the development of escalated drug intake, posited to exemplify “addiction-like behavior” in rats (Ahmed and Koob, 1998; Deroche-Gamonet et al, 2004). Recording at this time point also corresponded to fully manifested drug-induced alterations in VP receptor expression (Hammer, 1989). In addition, well-trained animals quickly load to a relatively stable asymptotic drug level that exhibits little variability during the 6–10 hour recording session, mitigating pharmacological differences between lever presses. One recording session per microwire contributed to the dataset. Neural signals were led through a preamplifier that differentially amplified (10x) the signal on the recording electrode against another microwire that did not exhibit a single unit. The signal was then band-pass filtered (450 Hz to 10 kHz; roll off 1.5 dB/octave at 1 kHz and −6 dB/octave at 11 kHz) and amplified 700X. Using software and hardware of DataWave Technologies (Longmont, CO), electrical signals were sampled (50 kHz sampling frequency per wire) and stored for offline analysis. During each experiment, electrophysiological recordings began 30 minutes before the start of the self-administration session and continued for 1 hour after the session.
Isolation and separation of individual neural waveforms from background noise and waveforms of other neurons recorded from the same microwire were conducted post-hoc using Datawave spike sorting and separation software. First, neural discharges were sorted in terms of waveform parameters, including valley voltage, peak voltage, voltages at 4 user-defined time cursors, spike height, and peak time. A scatter plot of any 2 waveform parameters was displayed in a window, with 4 windows (eight parameters) displayed on one screen simultaneously. Each point plotted on the scatter plot corresponded to one recorded discharge. Each cluster of dots represented similar waveforms, which were separated from other clusters by enclosing it within a “cutting box”. All waveforms of the putative individual neuron during the entire session (6–10 hours) were then displayed in temporal order on a computer-simulated oscilloscope in order to assess the stability of neural waveforms within session. Waveforms whose parameters did not remain stable were discarded. Second, an interspike interval (ISI) histogram was constructed. If discharges occurred within the first 2 msec in the ISI, corresponding to a neuron’s natural refractory period, the recording was not considered that of a single neuron and was discarded. When more than a single sample of neural waveforms appeared to have been recorded from a given wire, cross-correlation histograms were used to confirm that the sample corresponded to distinct neurons. If discharges occurred within the first 2 msec in the cross-correlation and both neurons contained 0 discharges within their individual ISI’s, both neurons were considered independent single units. Neurons exhibiting signal-to-noise ratios less than 2:1 were discarded.
Construction of perievent time histograms (PETHs)
The initial 10 rapidly-spaced “loading” self-infusions were excluded from analysis in order to remove pharmacological differences across lever presses. All other reinforced lever presses were analyzed, which averaged 41.27 ± 0.39 reinforced presses per session.
Rapid-phasic changes in firing that occurred within seconds of the reinforced lever press were determined by constructing rasters and PETHs that displayed neuronal discharges within ±12 s of each lever press. Offset of the cocaine-reinforced lever press was used as the node around which PETHs were constructed. Using these histograms, the magnitude of changes in firing was standardized and calculated for all neurons. Neurons that exhibited rapid changes in firing related to the instrumental response did not exhibit these changes in firing prior to −3 s before the lever press. Therefore the period between −9 to −3 s prior to each lever press served as the baseline period. For changes in firing that commenced within the 3 s prior to the lever press, a ratio, B/(A + B), was calculated for every neuron as a measure of change in firing relative to baseline. The ratio equally weights increases and decreases in firing rate. ‘A’ was equal to the mean firing rate of the neuron during the baseline time window (−9 to −3 s) before each lever press. ‘B’ was equal to the firing rate of the neuron during the firing window. Analysis of the firing window began at −3 s. The firing window was determined as follows (as described in Ghitza et al, 2003; 2004). 1) Onset of the firing window was defined as the first of four consecutive 100-ms bins in which the neuron exhibited at least a 20% change from baseline firing rate. These criteria were used to rule out any spurious fluctuations in activity and yet to be sensitive enough to detect even relatively small changes. 2) Offset of the firing window was defined as either the first of four consecutive 100-ms bins after the onset of the firing window when the neuron no longer exhibited at least a 20% change from baseline or as the time of the lever press (defined as time 0), whichever occurred first.
For changes in firing that commenced following the lever press, a ratio, B/(A + B), was calculated for every neuron in the following manner. ‘A’ was equal to the baseline firing rate (−9 to −3 s before each lever press). ‘B’ was equal to the mean firing rate of the neuron during the firing window. The firing window was determined as follows. 1) Onset of the firing window was defined as the first of four consecutive 100-ms bins in the 6 s following the lever press in which the neuron exhibited at least a 20% change from baseline. 2) Offset of the firing window was defined as the first of four consecutive 100-ms bins after the onset of the firing window when the neuron no longer exhibited at least a 20% change in firing relative to baseline. While pre-press firing was separated from post-press firing for purposes of interpreting their possible correlations with behavior, individual neurons can exhibit changes in firing both before and after the lever press (Ghitza et al, 2004). Some neurons failed to exhibit a change of 20%, but to include them in the analysis, along with all other neurons, a standard firing window was assigned to them. This was defined as the average firing window exhibited by neurons that showed at least a 20% change. The average pre-press firing window started at −1.9 sec and ended at −0.2 sec. The average post-press firing window started at 0.4 sec and ended at 3.5 sec.
Histological procedures
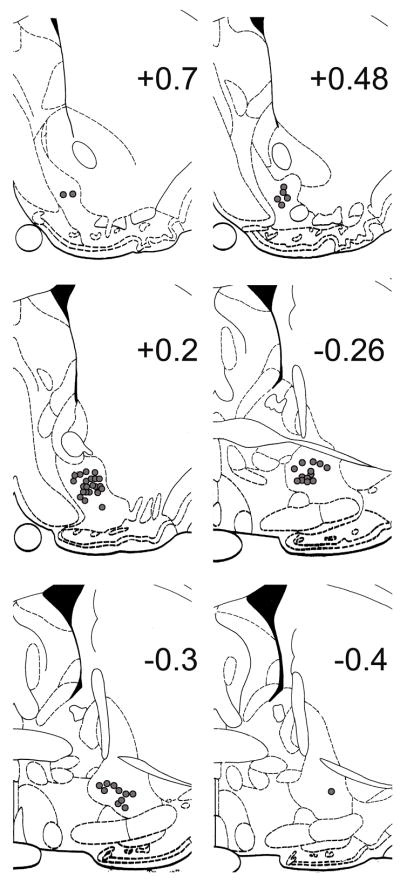
The histological procedures used to verify the location of each recorded neuron were described in previous reports (Fabbricatore et al, 2009; Ghitza et al, 2003; 2004). The location of all wire tips were marked by anodal current (50 mA, 4 seconds) leaving an iron deposit that was subsequently visualized with a 5% potassium ferrocyanide and 10% HCl solution. The sections (50μm) were counterstained with a 0.2% solution of Neutral Red and coverslipped. If all implanted microwire tracks were identified from their entry into cortex to their tips (blue spots by potassium ferrocyanide staining of iron deposits), microwire tip positions were subsequently histologically localized. If any of the implanted microwires could not be identified, neural data from the animal were discarded. Two investigators blind to recorded neural activity reconstructed all microwire three-dimensional positions (inter-rater reliability: 96.23%) according to the atlas of Paxinos and Watson (1997). Placement reliability was defined as microwires localized 1) onto the identical anteroposterior plate; 2) within 300μm of placements (hypotenuse of mediolateral and dorsoventral); and 3) within the same brain region. Neurons localized outside the subcommissural VP or within ±150μm of any noncommissural VP border were discarded.
Results
Behavior
Consistent with the “long-access” model of cocaine self-administration (Ahmed and Koob, 1998), animals significantly increased the number of self-administered infusions over two weeks of training (F(20, 273) = 6.927, p < 10−14). The cumulative drug intake (mg/kg) significantly increased over daily self-administrations (F(20, 273) = 7.203, p < 10−15) from 23.49 ± 3.18 mg/kg on day 1 to 37.40 ± 1.29 mg/kg on day 14.
During neural recording sessions, self-administration behavior was characterized by an initial period of rapid lever pressing followed by self-infusions at regular intervals such that calculated blood level remained within stable limits, assuming constant pharmacokinetics. Due to differences in body weight, drug dose varied across animals ranging between 0.668 to 0.827 mg/kg/inf. The average infusion size was 0.743 ± 0.007 mg/kg/inf and animals maintained asymptotic drug levels of 4.036 ± 0.081 mg/kg.
Neural
Fifty-four single units were recorded from 53 microwires out of 82 total microwires localized in the subcommissural VP (Fig 1; Paxinos and Watson, 1997). VP neurons exhibited predominantly biphasic waveforms (96.30%) with a paucity of triphasic waveforms (3.70%), similar to a previous report (McDaid et al, 2005). Baseline firing rates of VP neurons, defined as −9 to −3 sec prior to reinforced lever presses during self-administration, averaged 2.97 ± 1.15 impulses/sec, ranging between 0.04 to 44.20 impulses/sec, similar to a prior awake behaving VP recording that ranged from less than 0.5 impulses/sec to greater than forty impulses/sec (Tindell et al, 2004).
Figure 1.
Histological localization of potassium ferrocyanide lesioned microwire tips in serial coronal sections (Paxinos and Watson, 1997). Number on each plate indicates anteroposterior distance (mm) from bregma. Each filled circle represents a single microwire within the VP that recorded neural activity.
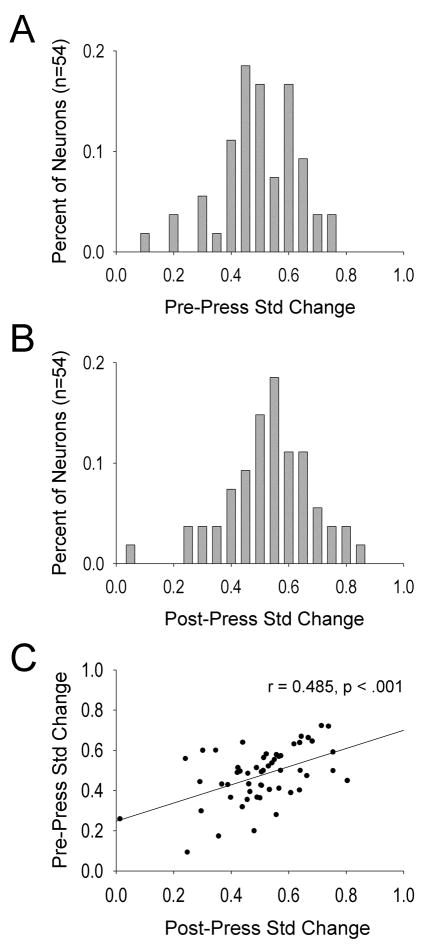
VP neurons exhibited relatively normal distributions in their standardized pre- and post-press firing rate changes as illustrated in Figures 2A and 2B, respectively. As a population, pre- and post-press changes in firing rate were directionally sensitive. That is, the standardized change in pre-press firing rates significantly correlated with standardized changes in post-press firing rates, r = 0.485, p < .001 (Fig. 2C). Although many neurons displayed relatively small firing change magnitudes, the neurons with the greatest behavioral relevance may be those exhibiting large changes in firing before or after the lever press. Therefore, we examined some of the larger (e.g., > two-fold) changes in firing rates by VP neurons for illustration purposes.
Figure 2.
Frequency distributions of standardized change (B/A+B) in firing rates during the pre-press (A) and post-press (B) firing windows. Values greater than or less than 0.5 reflect increases or decreases from baseline firing rate, respectively. Values of 0.5 indicate no change from baseline firing rate. C. Pre-press and post-press standardized changes in firing rate for all neurons comprising panels A and B.
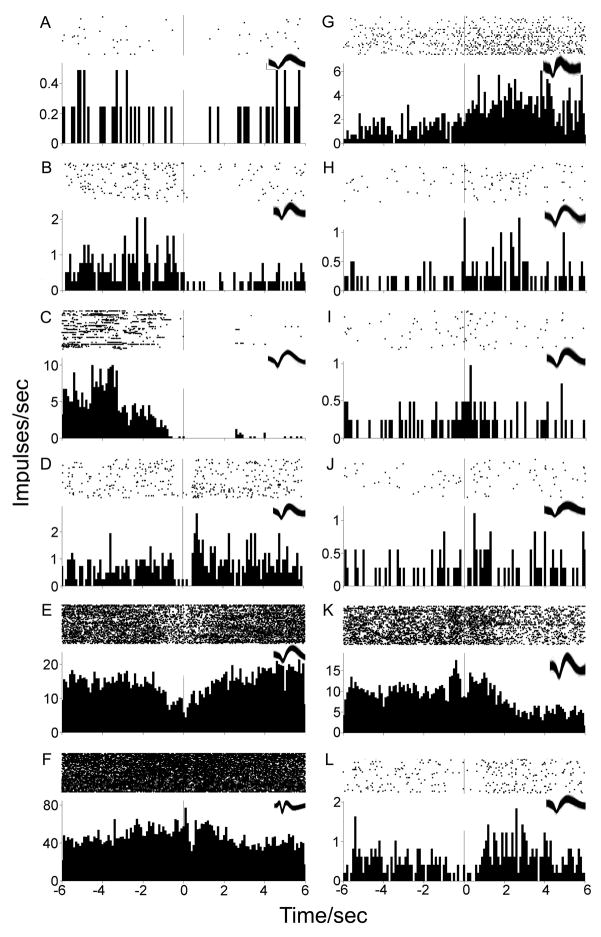
One population of neurons exhibited greater than two-fold phasic decreases in firing rate either prior to or following the lever press (10/54, 18.52%). Of these, four (40%) neurons exhibited decreases solely during the pre-press firing window (example: Fig 3A) while three (30%) exhibited decreases exclusive to the post-press firing window (example: Fig 3B). Three (30%) neurons exhibited decreases in firing rates during both pre and post-press firing windows (examples: Fig 3C, Fig 3D). Other neurons that did not exhibit greater than two-fold decreases nonetheless exhibited phasic changes (example: Fig 3E). While some neurons exhibited shorter suppressions of firing rate after the lever press (example: Fig 3F), the average post-press suppression of firing rates lasted 3.40 ± 0.37 sec, ranging between 0.4 to the maximum analyzed 6 sec.
Figure 3.
Examples of phasic firing patterns. Each PETH is centered (abscissa time 0, vertical line) at the cocaine-reinforced lever press in 100 msec bins with the raster display above it. X-axes span twelve seconds total, six seconds prior to and following the lever press. Y-axes display average firing rate (impulses/sec). Waveforms of each neuron are presented in the top-right inset of each histogram. Scale of calibration bar (panel A inset) applies to all waveforms: 160 μsec; 0.2 mV.
A second population of neurons exhibited a greater than two-fold phasic increase in firing rate either prior to or following the lever press (9/54, 16.67%). Of these, one (11.11%) neuron exhibited an increase solely during the pre-press firing window while three (33.33%) neurons exhibited increases solely during the post press firing window (examples: Fig 3G, Fig 3H). Five (55.56%) neurons exhibited increases in firing rate during both pre and post-press firing windows (example: Fig 3I). Other neurons that did not exhibit two fold increases nonetheless exhibited phasic changes (examples: Fig 3J, Fig 3K, Fig 3L). The average duration of post-press increases in firing rate was 2.91 ± 0.26 s, ranging between 0.7 to the maximum analyzed 6 sec.
In order to determine whether phasic firing changes were organized as a function of individual microwire positions, self-administration history of animals, or baseline firing rates of individual neurons, these variables were correlated with pre-press and post-press changes in firing rate. There were no significant correlations between baseline firing rate, pre-press, or post-press standardized changes in firing rate with anatomical positions of the microwires or with cocaine self-administration history (cumulative prior infusions, drug intake, or unreinforced presses), p > .05.
Discussion
Given the neuroanatomical position of the VP within basal forebrain circuitry, coupled with recent published findings implicating ventral pallidal throughput in drug-seeking behavior, we hypothesized that single VP neurons would exhibit firing rate changes during self-administering lever presses. Indeed, the present examination found single VP neurons that exhibited rapid-phasic increases or decreases in firing rate during the seconds prior to and/or following cocaine reinforced lever presses. Since lever-press related firing rate changes occurred in animals that voluntarily increased drug intake over weeks of training, consistent with preclinical observations of “addiction-like behavior” in rats (Ahmed and Koob, 1998; Deroche-Gamonet et al, 2004), our results support the notion that VP has a role in established drug-seeking behavior.
Phasic firing rate increases or decreases in VP neurons, especially those that occurred prior to the drug-reinforced lever press, were not likely the result of pharmacological changes nor were they direct locomotor correlates. Based on the pharmacokinetic profile as determined using the group mean asymptotic self-administered drug level, cocaine level decays by 0.0027 mg/kg over the average 1.7 sec pre-press firing window, which is less than one-tenth the intravenous cocaine dose that produces half maximal firing of VP neurons (Johnson and Napier, 1996). Therefore, it is improbable that such a miniscule decay in drug level could have accounted for observed phasic firing rate changes. With regard to whether locomotor activity is correlated with phasic patterns, locomotion is greatest during the thirty seconds prior to the reinforced lever press using this dose and FR1 schedule (Peoples et al, 1998) yet changes in firing rate were observed within three seconds of the press but not during the baseline (−9 to −3 sec pre-press). Since locomotor activity is elevated during both periods, locomotion alone does not explain the changes in firing rate around the lever press. Furthermore, because VP neurons do not exhibit locomotor “step” correlations (Tindell et al, 2004) the observed changes in firing rate prior to the cocaine reinforced lever press may have been related to drug-seeking behavior. Given the monosynaptic projection from NAcc to VP (Haber and Nauta, 1983; Zahm and Heimer, 1990) and because lever-press related firing of NAcc neurons during cocaine self-administration is specifically correlated with drug-seeking behavior (Peoples et al, 1997), VP firing rate changes prior to the self-administering lever press may represent a cocaine-seeking correlate. It is likely that post-press changes in firing rate were also related to self-administration behavior given their significant correlation with pre-press changes in firing rate, which have the same time constraints on pharmacological influences as pre-press changes. Although related, several VP neurons exhibited selective changes in firing rate during pre-press or post-press firing windows, suggesting that differential processing of self-administration behavior occurs over the self-administering lever press. Pre-press changes may be associated with drug-seeking behavior while post-press changes may be related to processing the successful completion of drug-seeking behavior or the anticipation of forthcoming cocaine. However, it is possible that post-press firing was influenced by the presence of the conditioned stimulus during the post-press firing window.
The fact that phasic activity in the VP is similar to that found in NAcc is not surprising, especially since NAcc is the main afferent of the VP (Haber and Nauta 1983; Zahm and Heimer, 1990; O’Donnell et al, 1997). This raises the question of the extent to which pallidal firing contributes to the role of the NAcc in drug-seeking behavior (Roberts et al, 1977; McFarland and Kalivas, 2001, 2004). As in the NAcc, the direction and durations of VP firing rate changes at the reinforced lever press varied across neurons (Carelli and Deadwyler, 1994; Ghitza et al, 2004), suggesting that similar to the proposed functional organization of NAcc neurons (Pennartz et al, 1994; Groenewegen et al, 1996; O’Donnell 2003), VP information processing may be subserved by neuronal ensembles. Furthermore, it appears that both within-NAcc and within-VP firing rate directionality is characteristically heterogeneous at the cocaine reinforced lever press. The differential contribution of rapid phasic increase and decrease firing patterns in VP neurons is not understood but likely involves NAcc throughput and perhaps pharmacological compensatory mechanisms as well.
Since NAcc neurons exhibit increasing or decreasing firing rate changes at the reinforced lever press, the most parsimonious explanation of different firing rate change directionalities of VP neurons during the same behavioral event is through GABAergic inhibition or disinhibition of VP neurons, respectively. However, much of the GABAergic NAcc projection to VP is opioid colocalized (Haber and Nauta 1983; Zahm et al, 1985). Since opioids are antagonistic to GABA (Chrobak and Napier, 1993; Kalivas et al, 2001), perhaps due to presynaptic μ opioid receptors (Olive et al, 1997), NAcc firing may produce heterogeneous firing rate directionalities within VP. That is, VP neurons which coexpress GABAergic and enkephalinergic terminals may differ in their responsiveness to NAcc throughput from other neurons selectively expressing GABAergic terminals (Zahm et al, 1985). In addition, NAcc projects substance P to VP (Haber and Nauta, 1983; Napier et al, 1995), which elicits increases in firing rate in VP neurons (Napier et al, 1995). Since substance P antagonism blocks increased firing rates of VP neurons following NAcc stimulation (Mitrovic and Napier, 1998) and substance P is rarely colocalized with GABAergic or enkephalinergic VP terminals (Zahm et al, 1985), substance P projected from NAcc is likely an additional contributor to heterogeneous firing patterns within VP during the self-administering lever press.
Neuronal transmission within VP may also be altered by chronic cocaine exposure, which renders VP neurons less sensitive to GABA-induced decreases in firing rate and more sensitive to opioid- and glutamate-induced increases in firing rate, compared with saline treated animals (McDaid et al, 2005). Indeed, opioid receptor expression is increased 48% in rat VP in response to two week experimenter administered 10 mg/kg but not 1 mg/kg cocaine (Hammer, 1989). Furthermore, dynorphin concentrations are increased by 346% in postmortem human cocaine user’s VP (Frankel et al, 2008). Although confirmation of such a mechanism is not demonstrated in the present data, it is plausible that GABAergic and opioidergic signals enhance VP neuronal firing patterns heterogeneity in the cocaine-experienced animal. Given that intra-VP GABA agonists as well as opioid antagonists block reinstatement of cocaine-seeking behavior (McFarland et al, 2001, 2004; Tang et al, 2005), the possible influence of colocalized peptides and their interaction with endogenous VP neurotransmitters on drug-seeking behavior warrants further investigation.
Neurochemical innervation of the VP arising from nonaccumbal regions also likely influenced VP firing rates during self-administration. Dopamine is a robust modulator of VP firing rates (Napier et al, 1991, 1994) and extracellular dopamine concentrations in this region increase during cocaine self-administration (Sizemore et al, 2000). Cocaine’s principle mechanism of action is the inhibition of the dopamine transporter, which has been strongly linked with cocaine’s reinforcing properties (Ritz et al, 1987). Dopamine generally attenuates GABA-induced decreases as well as glutamate-induced increases in VP firing (Johnson and Napier, 1997), perhaps directly affecting the observed lever-press related firing. In turn, VP is likely to alter several other dopaminoceptive targets which are themselves key components of reward circuitry. Among the various pallidal efferent projections associated with appetitive processing, two main projection systems are likely to have widespread impact upon dopaminoceptive targets. First, the pathways from the VP to the medial prefrontal cortex are segregated into two circuits, each of which may project discrete signals to NAcc. Ventromedial VP neurons project GABA to the ventromedial prefrontal cortex via the medial-MD thalamus, while dorsolateral VP neurons project GABA to the dorsomedial prefrontal cortex via the central-MD thalamus (Zahm et al, 1996; Groenewegen et al, 1993; O’Donnell et al, 1997; Churchill et al, 1996). The prefrontal projections to NAcc (Sesack et al, 1989) and back to VP (Zahm and Heimer, 1990) likely form independent parallel loops of information processing related to motivated behaviors (Alexander et al, 1986). Although this experiment did not reveal significant correlations between firing rate changes around the lever press and anatomical microwire positions, future investigations dichotomously comparing ventromedial VP and dorsolateral VP neuronal firing patterns, which requires additional histochemical techniques than those used presently (Zahm et al, 1996), may reveal topographic VP information processing. Second, VP is positioned to alter the efficacy of the “limbic/cognitive/motor” serial striatomesencephalic and mesencephalostriatal spiral loop circuit from shell to putamen (Haber et al, 2000), via GABAergic as well as glutamatergic pallidomesencephalic projections (Kalivas et al, 1993; Geisler et al, 2007). The efferent projections of the VP are therefore positioned to influence dopamine output to medial prefrontal cortex, NAcc, and dorsal striatum, which have all been linked to cocaine self-administration (Roberts et al, 1977; Goeders and Smith, 1983; McGregor et al 1996; Belin and Everitt, 2008).
Integrating the present data with the known involvement of the dopamine system in drug-seeking, it is likely that the VP is a critical nucleus in regulating cocaine-seeking behavior by virtue of its capacity to influence the ventral and dorsal striatopallidal systems whether via thalamic or mesencephalic routes. This capacity for widespread influence may explain why VP lesions or pharmacological challenges block cocaine-induced conditioned place preference (Gong et al, 1997), self-administration (Hubner and Koob, 1990; Robledo and Koob, 1993), and reinstatement (McFarland et al, 2001, 2004; Tang et al, 2005). The observed changes in firing rate around the reinforced lever press provide insight into the activity of individual VP neurons during drug-seeking behavior. While the precise neurophysiological mechanisms underlying phasic changes in VP firing during appetitive behavior remain to be elucidated, demonstrating VP firing changes during cocaine self-administering behavior is an important initial step in studying the necessary role of ventral striatopallidal circuitry in cocaine-seeking behavior.
Acknowledgments
This study was supported by the National Institute on Drug Abuse Grants DA 006886 and DA 026252 and the National Institute of Mental Health Grant MH 0919957. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Linda King, Thomas Grace Sr., Smruti Patel, Karina Gotliboym, Dana Silagi, Samir Kumar, Alyssa Ames, and Abigail Klein for technical assistance.
Abbreviations
- NAcc
nucleus accumbens
- VP
ventral pallidum
- VTA
ventral tegmental area
Literature Cited
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bell K, Churchill L, Kalivas PW. GABAergic projection from the ventral pallidum and globus pallidus to the subthalamic nucleus. Synapse. 1995;20(1):10–18. doi: 10.1002/syn.890200103. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Smith AD, Bolam JP. The substantia nigra as a site of synaptic integration of functionally diverse information arising from the ventral pallidum and the globus pallidus in the rat. Neuroscience. 1996;75(1):5–12. doi: 10.1016/0306-4522(96)00377-6. [DOI] [PubMed] [Google Scholar]
- Carelli RA, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci. 1994;14(12):7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Sawyer SF, Lee RS, Woodward DJ. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J Neurosci. 1994;14:1224–1244. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC. Opioid and GABA modulation of accumbens-evoked ventral pallidal activity. J Neural Transm. 1993;93:123–143. doi: 10.1007/BF01245342. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC. Basal forebrain infusions impair delayed-non-match-to--sample radial arm maze performance. Pharmacology, Biochemistry and Behavior. 2002;72:209–212. doi: 10.1016/s0091-3057(01)00752-3. [DOI] [PubMed] [Google Scholar]
- Churchill L, Zahm DS, Kalivas PW. The mediodorsal nucleus of the thalamus in rats--I. forebrain gabaergic innervation. Neuroscience. 1996;70(1):83–102. doi: 10.1016/0306-4522(95)00351-i. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Fabbricatore AT, Ghitza UE, Prokopenko VF, West MO. Electrophysiological evidence of mediolateral functional dichotomy in the rat accumbens during cocaine self-administration: tonic firing patterns. Eur J Neurosci. 2009;30:2387–2400. doi: 10.1111/j.1460-9568.2009.07033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increaed in human chronic cocaine users. Neuropharmacology. 2008;55:41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258(3):317–38. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27(21):5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23(19):7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko VF, West MO. Differences between accumbens core and shell neurons exhibiting phasic firing patterns related to drug-seeking behavior during a discriminative stimulus task. J Neurophysiol. 2004;92:1608–1614. doi: 10.1152/jn.00268.2004. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopamine involvement in cocaine reinforcement. Science. 1983;221(4612):773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res. 1997;754(1–2):103–112. doi: 10.1016/s0006-8993(97)00059-0. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J Comp Neurol. 1990 Apr 22;294(4):607–22. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57(1):113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AVJ. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- Olive MF, Anton B, Micevych P, Evans CJ, Maidment NT. Presynaptic versus postsynaptic localization of μ and δ opioid receptors in dorsal and ventral striatopallidal pathways. J Neurosci. 1997;17(19):7471–7479. doi: 10.1523/JNEUROSCI.17-19-07471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Nauta WJ. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983;9(2):245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235(3):322–35. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigral pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer RP., Jr Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Heidenreich BA, Napier TC. Effects of serotonergic 5-HT1A and 5-HT1B ligands on ventral pallidal neuronal activity. Neuroreport. 2000;11(13):2849–2853. doi: 10.1097/00001756-200009110-00005. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Koob GF. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res. 1990;508(1):20–29. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Napier TC. Contribution of the nucleus accumbens to cocaine-induced responses of ventral pallidal neurons. Synapse. 1996;22(3):253–260. doi: 10.1002/(SICI)1098-2396(199603)22:3<253::AID-SYN8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Napier TC. GABA- and glutamate-evoked responses in the rat ventral pallidum are modulated by dopamine. Eur J Neurosci. 1997;9(7):1397–1406. doi: 10.1111/j.1460-9568.1997.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57(4):1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Jackson D, Romanidies A, Wyndham L, Duffy P. Involvement of pallidothalamic circuitry in working memory. Neuroscience. 2001;104(1):129–136. doi: 10.1016/s0306-4522(01)00054-9. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, Kalivas PW. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 1992;50(2):371–386. doi: 10.1016/0306-4522(92)90430-a. [DOI] [PubMed] [Google Scholar]
- McDaid J, Dallimore JE, Mackie AR, Mickiewicz AL, Napier TC. Cross-sensitization to morphine in cocaine-sensitized rats: behavioral assessments correlate with enhanced responding of ventral pallidal neurons to morphine and glutamate, with diminished effects of GABA. J Pharmacol Exp Ther. 2005;313(3):1182–1193. doi: 10.1124/jpet.105.084038. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Baker G, Roberts DC. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 1996;53(1):5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, Napier TC. Substance P attenuates and DAMGO potentiates amygdala glutamatergic neurotransmission within the ventral pallidum. Brain Res. 1998;792:193–206. doi: 10.1016/s0006-8993(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Napier TC, Mitrovic I, Churchill L, Klitenick MA, Lu XY, Kalivas PW. Substance P in the ventral pallidum: projection from the ventral striatum, and electrophysiological and behavioral consequences of pallidal substance P. Neuroscience. 1995;69(1):59–70. doi: 10.1016/0306-4522(95)00218-8. [DOI] [PubMed] [Google Scholar]
- Napier TC, Maslowski-Cobuzzi RJ. Electrophysiological verification of the presence of D1 and D2 dopamine receptors within the ventral pallidum. Synapse. 1994;17(3):160–166. doi: 10.1002/syn.890170304. [DOI] [PubMed] [Google Scholar]
- Napier TC, Simson PE, Givens BS. Dopamine electrophysiology of ventral pallidal/substantia innominata neurons: comparison with the dorsal globus pallidus. J Pharmacol Exp Ther. 1991;258(1):249–262. [PubMed] [Google Scholar]
- Nayak PK, Misra AL, Mule SJ. Physiological disposition and biotransformation of (3H) cocaine in acutely and chronically treated rats. J Pharmacol Exp Ther. 1976;196(3):556–69. [PubMed] [Google Scholar]
- O’Donnell P, Lavin A, Enquist LW, Grace AA, Card JP. Interconnected parallel circuits between rat nucleus accumbens and thalamus revealed by retrograde transynaptic transport of pseudorabies virus. J Neurosci. 1997;17(6):2143–2167. doi: 10.1523/JNEUROSCI.17-06-02143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Peoples LL, Uzwiak AJ, Gee F, West MO. Operant behavior during sessions of intravenous cocaine infusion is necessary and sufficient for phasic firing of single nucleus accumbens neurons. Brain Research. 1997;757:280–284. doi: 10.1016/s0006-8993(97)00299-0. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Gee F, Bibi R, West MO. Phasic firing time locked to cocaine self-infusion and locomotion: dissociable firing patterns of single nucleus accumbens neurons in the rat. J Neurosci. 1998;18(18):7588–7598. doi: 10.1523/JNEUROSCI.18-18-07588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of fucntionally distinct neuronal ensembles: an integration of behavioural, electrophysiologcal and anatomical data. Prog Neurobiol. 1994;42(6):719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6(6):615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17(5):901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12(5):781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Robledo P, Koob GF. Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Behav Brain Res. 1993;55(2):159–166. doi: 10.1016/0166-4328(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290(2):213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sizemore GM, Co C, Smith JE. Ventral pallidal extracellular fluid levels of dopamine, serotonin, gamma amino butyric acid, and glutamate during cocaine self-administration in rats. Psychopharmacology (Berl) 2000;150(4):391–398. doi: 10.1007/s002130000456. [DOI] [PubMed] [Google Scholar]
- Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci. 2005;25(18):4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24(5):1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel AR, Pickens R. Drug level of d- and l-amphetamine during intravenous self-administration. Psychopharmacologia. 1974;34:255–264. doi: 10.1007/BF00421966. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Zaborszky L, Alones VE, Heimer L. Evidence for the coexistence of glutamate decarboxylase and Met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res. 1985;325(1–2):317–321. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302(3):437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Williams E, Wohltmann C. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J Comp Neurol. 1996;364(2):340–362. doi: 10.1002/(SICI)1096-9861(19960108)364:2<340::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]