Abstract
Background
In early type 1 diabetes mellitus, changes in proximal reabsorption influence glomerular filtration rate (GFR) through tubuloglomerular feedback (TGF). Due to TGF, a primary increase in proximal reabsorption causes early diabetic hyperfiltration, while a heightened sensitivity of the proximal tubule to dietary salt leads to the so-called salt paradox, where a change in dietary salt causes a reciprocal change in GFR (‘tubulocentric principle’). Here, experiments were performed in adenosine A1 receptor knockout mice (A1R–/–), which lack an immediate TGF response, to determine whether A1Rs are essential for early diabetic hyperfiltration and the salt paradox.
Methods
GFR was measured by inulin disappearance in conscious A1R–/– and wild-type (WT) mice after 4 weeks of streptozotocin diabetes on a control NaCl diet (1%), and measurements were repeated after 6 days of equilibration on a low-NaCl (0.1%) or a high-NaCl (4%) diet.
Results
A1R–/– and WT were similar with respect to blood glucose, dietary intakes and body weight changes on a given diet. Diabetic hyperfiltration occurred in WT, but was blunted in A1R–/–. A reciprocal relationship between GFR and dietary salt was found in WT diabetics, but not A1R–/– diabetics or nondiabetics of either strain.
Conclusion
A1Rs determine glomerular hyperfiltration and the salt paradox in early diabetes, which is consistent with the tubulocentric principle.
Key Words: Diabetes, Diabetic nephropathy, Glomerular hyperfiltration, Tubuloglomerular feedback, Salt paradox, Adenosine A1 receptor, Proximal tubule, Tubular hypothesis
Introduction
Over the past decade, we have noted several circumstances in which glomerular filtration in early diabetes was inordinately influenced by feedback from the proximal tubule and have proposed this as a general tubulocentric principle for the control of GFR in early diabetes [reviewed in ref. [1]]. One example is glomerular hyperfiltration, which is a cardinal feature of early diabetes mellitus and may predispose to the eventual development of diabetic nephropathy [2, 3]. The underlying mechanism(s) of diabetic hyperfiltration include(s) a primary increase in proximal reabsorption, which reduces delivery of NaCl to the macula densa [4, 5]. The macula densa senses this decline in salt delivery as an error signal and elicits an increase in glomerular filtration rate (GFR), which offsets a portion of the original error signal through negative tubuloglomerular feedback (TGF). The tubulocentric principle explains diabetic hyperfiltration in the rat because no other explanation can be given for the residual decrease in macula densa NaCl, which is consistently observed in hyperfiltering diabetic rats [4,5,6]. Tubular control of GFR has also been demonstrated in dogs where acute hyperglycemia causes GFR to increase, but only if TGF is intact [7]. Finally, evidence for a primary hyperreabsorption upstream of the macula densa and a potential role in glomerular hyperfiltration was also proposed in diabetic patients [8,9,10]. The primary increase in proximal reabsorption appears to originate with increased Na+/glucose cotransport, which is driven by excess glucose in the glomerular filtrate; rapid growth of the early diabetic proximal tubule also contributes to hyperreabsorption [4, 5]. The chain of events responsible for this growth remains obscure.
Another success of the tubulocentric principle is in explaining the so-called salt paradox of early diabetes. In normal subjects, GFR is either insensitive to dietary NaCl or changes in the same direction as dietary NaCl [11,12,13]. But in early diabetes, changing the dietary NaCl causes GFR to change in the opposite direction. Since this response is at odds with NaCl homeostasis, it is referred to as the salt paradox. The salt paradox was first demonstrated in diabetic rats [14, 15], and was later confirmed in young type I diabetic patients who responded to a low-salt diet with renal vasodilation and a rise in GFR [16]. We previously surmised by deduction and confirmed by experimentation that the salt paradox occurs because proximal reabsorption in early diabetes is inordinately sensitive to suppression by dietary salt [11]. Hence, an increase in dietary salt suppresses proximal reabsorption to the extent that TGF becomes activated and GFR declines. The opposite series of events follows a decrease in dietary salt. The unique feature of diabetes is that the effect of salt on the proximal tubule is strong enough to override the effect on GFR of other systems that might compete with it [reviewed in ref. 1, 17].
The tubulocentric principle invokes signaling from the macula densa to the glomerular microvessels by a mechanism that certainly resembles traditional TGF and papers on the tubulocentric principle invoke the term ‘TGF’ to explain it. However, it remains to be determined whether the mechanism of traditional TGF is also the mechanism for maintaining diabetic hyperfiltration or mediating the diabetic salt paradox. For present purposes, we define traditional TGF as control of the single nephron GFR that occurs from minute-to-minute and is mediated through adenosine A1 receptors (A1Rs) on the afferent arteriole [18]. Traditional TGF is known to operate from minute to minute because of the way it is normally studied, in microperfusion experiments lasting 1–5 min. But it is careless to assume that, because A1Rs mediate the immediate TGF response, they also account for the tonic influence over GFR that the tubule must exert for days or weeks at a time in order to account for diabetic hyperfiltration or the salt paradox. Furthermore, adenosine is not the only vasoactive moiety regulated by changes in macula densa salt. The macula densa responds to NaCl by also releasing ATP, making nitric oxide, suppressing renin, and making less prostaglandin.
Being confident in the tubulocentric principle, but not that traditional TGF accounts for it, we undertook to examine the role of A1R signaling in the early diabetic glomerulus vis-à-vis both hyperfiltration and the salt paradox. To this end, we induced the low-dose streptozotocin (STZ) diabetes model described by the Animal Model of Diabetic Complications Consortium [19] in mice lacking A1R (A1R–/–), which also lack an immediate TGF response [20,21,22]. In the absence of TGF, the diabetes-induced changes in proximal reabsorption should not lead to the TGF-mediated responses in GFR. Thus, the main hypothesis was that neither hyperfiltration nor a salt paradox would occur in mice lacking A1Rs.
Methods
Animals
All animal experimentation was conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, Md., USA) and was approved by the local Institutional Animal Care and Use Committee. Only male mice were used in the outlined experiments. Mice were housed in a 12-hour:12-hour light-dark cycle in standard rodent cages with free access to standard rodent chow (Harlan Teklad, 1% NaCl, Madison, Wisc., USA) and tap water. A1R–/– and WT littermates were from a subcolony of the original strain generated by Sun et al. [20] that is kept at the VA San Diego Healthcare System [23,24,25]. Mice have been reproduced by heterozygous crossing, and the genetic background of the animals was a mix of 129Sv/J and C57BL/6.
Induction of Diabetes
Mice were made diabetic using the protocol recommended by the Animal Model of Diabetic Complications Consortium (http://www.amdcc.org/shared/showProtocol.aspx?id=19) [19]. Briefly, mice were injected with STZ (50 mg/kg i.p.) on 5 consecutive days. Nondiabetic control mice received vehicle injections (Na+ citrate buffer with pH adjusted to 4.5). Three weeks after the last injection, blood glucose was measured by tail nick and only mice with levels greater than 300 mg/dl were included in further studies. Subsequently, daily food and fluid intake was determined over 3 days while the mice were maintained in their regular cages.
Measurement of GFR in Awake Mice
Four weeks after STZ or placebo, GFR measurements were performed in conscious mice using the plasma kinetics of FITC-inulin following a single-dose intravenous injection as described by Qi et al. [26]. Briefly, FITC-inulin (5% in 0.85% NaCl) was dialyzed for 24 h against 0.85% NaCl (resulting in a 2.5–3% solution, which also served to establish the standard curve). The dialyzed FITC-inulin solution was sterile filtered and injected into the retro-orbital plexus (2 μl/g/body weight) during brief isoflurane anesthesia. At 3, 7, 10,15, 20, 40, and 60 min after injection, blood was collected from the end of the tail into an Na+-heparinized 10-μl microcap (Hirschmann Laborgeräte, Germany). After centrifugation, 1 μl of plasma was diluted 1:10 in 0.5 mol/l HEPES (pH 7.4) and fluorescence determinedin 2-μl samples using a Nanodrop ND-3300 fluorospectrometer (NanodropTechnologies, Wilmington, Del., USA). GFR was calculated using a two-compartmentmodel of two-phase exponential decay (GraphPad Prism, San Diego, Calif., USA).
Low- and High-Salt Diet
Three–five days after initial assessment of GFR (see above), the mice were randomly assigned to either a low-salt diet (Harlan Teklad, 0.1% NaCl) or a high-salt diet (Harlan Teklad, 4% NaCl). Diets were otherwise identical in composition to the standard chow. Food and fluid intake was determined daily while the mice were maintained in their regular cages. After 7 days on the new diet, GFR measurements were repeated as described above. Three days later, the mice were anesthetized with isoflurane; blood was taken by puncturing the retro-orbital plexus, and a urine specimen was obtained by puncturing the bladder. The kidneys were removed to determine kidney weight.
Blood and Urine Analysis
Blood glucose was determined using the Ascensia Elite XL glucometer (Bayer Corporation, Mishawaka, Ind., USA). Plasma and urine Na+ and K+ were measured using a flame photometer (ELEX 6361, Eppendorf, Hamburg, Germany). Plasma aldosterone was determined by radioimmunoassay (Diagnostic Systems Laboratories, Webster, Tex., USA). Concentrations of albumin and creatinine in urine were measured using commercial assays (Exocell, Philadelphia, Pa., USA, and Thermo Fisher Scientific, Waltham, Mass., USA).
Statistical Analysis
Data are expressed as means ± SEM. Statistical analysis was by analysis of variance (ANOVA), repeated-measures ANOVA, or analysis of covariance (ANCOVA) using proprietary software (Systat Inc.). Post-hoc intergroup comparisons were adjusted for multiple comparisons by Tukey or Bonferroni methods. p < 0.05 has been considered statistically significant.
Results
Diabetic Hyperfiltration Is Blunted in A1R–/– Mice
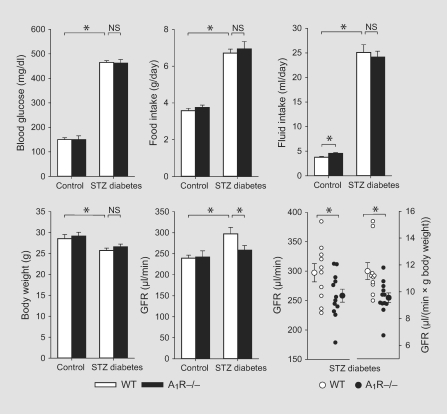
A1R–/– and littermate WT diabetic mice were well matched for blood glucose levels, food and fluid intake, and for a modest reduction in body weight when compared with nondiabetic control mice (fig. 1). GFR was not different between nondiabetic WT and A1R–/– mice (240 ± 7 vs. 242 ± 15 μl/min; n = 10/group). GFR was significantly higher in WT diabetic mice than in WT nondiabetics (297 ± 15 vs. 240 ± 7 μl/min, n = 10/group, p < 0.005). Among A1R–/–, however, diabetes was not associated with higher GFR (258 ± 11 vs. 242 ± 15 μl/min, n = 10–12/group). In other words, A1R–/– differed from WT mice in that WT mice manifested diabetic hyperfiltration whereas A1R–/– did not (p = 0.05 for ANCOVA cross-term with body weight as a covariate) (fig. 1). In accordance, GFR of diabetic WT mice was significantly greater compared to diabetic A1R–/– mice in absolute terms (p < 0.05) and when controlled for body weight [11.5 ± 0.6 vs. 9.5 ± 0.4 μl/(min·g body weight), p < 0.01] (fig. 1).
Fig. 1.
Diabetes-induced glomerular hyperfiltration is blunted in A1R–/– mice. Parameters were assessed in WT and littermate A1R–/– mice on a standard NaCl diet (1%) at 4 weeks after STZ or vehicle injection. GFR was assessed in awake mice. The absence of A1Rs did not affect the STZ-diabetes-induced increase in blood glucose, food and fluid intake, or the modest reduction in body weight. The lack of A1Rs, however, blunted the STZ-diabetes-induced increase in GFR. n = 10–12/group. * p < 0.05. NS = Not significant.
Response to Varying NaCl Intake in Nondiabetic Mice
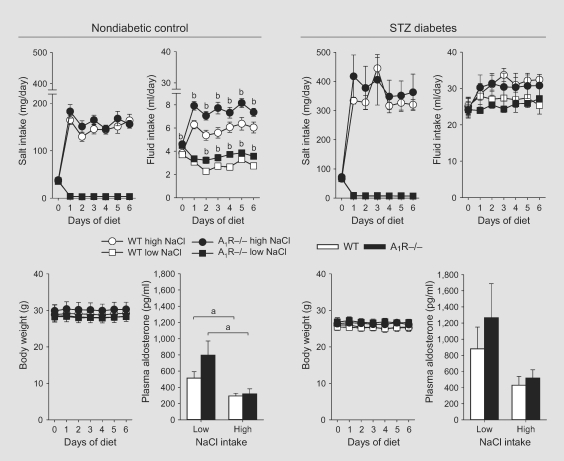
Switching to a low- or high-NaCl diet resulted in similar reductions and increases in NaCl intake in nondiabetic WT and A1R–/– mice (fig. 2, left panels). Fluid intake was greater in mice on high compared with low NaCl intake. Among nondiabetics, A1R–/– drank slightly more than WT littermates, irrespective of dietary salt content (fig. 1, 2, left panels). Body weight was not measurably sensitive to changes in NaCl intake in either WT or A1R–/– mice. Nor were hematocrit or plasma concentrations of Na+ or K+ affected by genotype or dietary salt intake (table 1). As expected, mice on the low-NaCl diet had significantly greater plasma aldosterone concentrations compared to mice on the high-NaCl diet (fig. 2, left panels).
Fig. 2.
Plasma aldosterone concentrations and changes in salt and fluid intake and body weight in WT and A1R–/– mice in response to low and high NaCl intake. WT and littermate A1R–/– mice were switched from a control NaCl diet (1% NaCl) to a low- (0.1% NaCl) or high- (4%) NaCl diet at about 5 weeks after STZ or vehicle injection. The absence of A1Rs did not affect salt intake or the ability to maintain body weight in response to low or high NaCl intake. n = 5–6/group. a p < 0.05 vs. low NaCl; b p < 0.05 vs. WT on same diet.
Table 1.
Responses in plasma and urine parameters to low or high NaCl intake
| Control |
STZ diabetes |
|||||||
|---|---|---|---|---|---|---|---|---|
| WT |
A1R−/− |
WT |
A1R−/− |
|||||
| low NaCl | high NaCl | low NaCl | high NaCl | low NaCl | high NaCl | low NaCl | high NaCl | |
| [Na+]pl, mmol/l | 158 ± 2 | 156 ± 2 | 154 ± 1 | 154 ± 1 | 155 ± 2 | 151 ± 2 | 150 ± 1 | 153 ± 2 |
| [K+]pl, mmol/l | 5.1 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.2 | 5.2 ± 0.2 | 4.2 ± 0.2 | 4.4 ± 0.1 | 4.3 ± 0.1 | 4.7 ± 0.1 |
| [Gluc]bl, mg/dl | 156 ± 22 | 155 ± 21 | 152 ± 17 | 147 ± 17 | 461 ± 25 | 449 ± 9 | 461 ± 12 | 475 ± 11 |
| Hct, % | 48 ± 2 | 48 ± 1 | 48 ± 2 | 48 ± 2 | 53 ± 3 | 53 ± 2 | 53 ± 2 | 52 ± 1 |
| [Gluc]/[Crea]u, mg/mg | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.1 | 1.6 ± 0.3 | 190 ± 48 | 147 ± 27 | 206 ± 75 | 149 ± 69 |
| [Alb]/[Crea]u, (μg/mg | 0.21 ± 0.01 | 0.21 ± 0.04 | 0.30 ± 0.06 | 0.24 ± 0.09 | 1.36 ± 0.30 | 1.68 ± 0.37 | 0.91 ± 0.21 | 0.64 ± 0.11 |
Data are means ± SE; n = 5–6 per group. [Na+]pl, [K+]pl = plasma concentration of Na+ and K+, respectively; [Gluc]bl = blood glucose concentration; Hct = hematocrit; Crea = creatinine; u = urine; Alb = albumin.
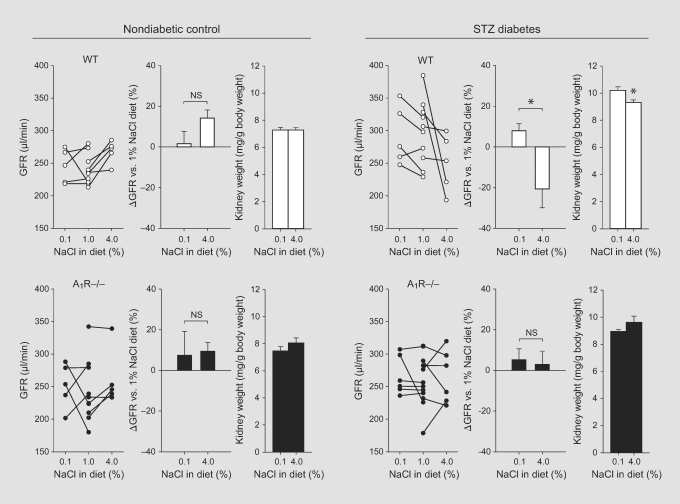
In nondiabetic WT mice, switching from control to low NaCl intake did not alter GFR, but changing to a high NaCl intake significantly increased GFR (p < 0.05, paired comparison) (fig. 3, left panels). In non-diabetic A1R–/– mice an overall similar response was observed, although the increase in GFR in response to switching from control to high NaCl intake did not reach statistical significance (p = 0.09, paired comparison). Kidney weight was not significantly different between low and high NaCl intake and was unaffected by genotype (fig. 3, left panels). Neither urinary glucose/creatinine nor urinary albumin/creatinine ratios were significantly affected by genotype or NaCl intake (table 1).
Fig. 3.
The NaCl paradox of the diabetic kidney is present in WT mice but absent in A1R–/– mice. Paired GFR measurements were performed in awake WT and littermate A1R–/– mice under the control NaCl diet (1% NaCl, single measurements are shown from the experiments summarized in fig. 1) and after 1 week of low (0.1% NaCl) or high (4%) NaCl intake. Measurements on the 1% NaCl diet were taken at 4 weeks after STZ or vehicle injection. One week later, the mice were switched to a low- or high-NaCl diet. In STZ-diabetic WT mice, switching from control to low or high NaCl intake induced significantly different and opposing responses in GFR, i.e. a rise in response to low NaCl and a fall in response to high NaCl intake. Moreover, the kidney weight was greater in STZ-diabetic WT mice on low versus high NaCl intake. In STZ-diabetic A1R–/– mice, the NaCl paradox of GFR and the greater kidney weight in mice on the low-NaCl diet were not detectable. n = 5–6/group. * p < 0.05.
The Salt Paradox of GFR Is Blunted in STZ-Diabetic A1R–/– Mice
STZ-diabetic WT and A1R–/– mice were well matched for NaCl intake in the respective diets (fig. 2, right panels). Fluid intake was not different between genotypes and tended to be greater in mice on high-NaCl diet (fig. 2, right panels). In comparison, a previous study reported that STZ-diabetic but not control rats responded to a low-NaCl diet with a pronounced increase in urine flow rate [14]. Further studies are necessary to explain these different responses. Notably, the present study used diets that only differed in NaCl content and were otherwise identical in composition, whereas the control and low-NaCl diets used in the previous study were not identical. Thus, it is possible that differences in the basic diet composition caused this previous phenomenon.
Mice on a low-NaCl diet tended to have greater plasma aldosterone concentrations compared with mice on a high-NaCl diet but in the STZ-diabetic mice of both genotypes this did not reach statistical significance (fig. 2, right panels). Body weights were not significantly altered by changes in NaCl intake in both STZ-diabetic WT and A1R–/– mice (fig. 2, right panels). Likewise, hematocrit and plasma concentrations of glucose, Na+ and K+ were not different between mice on low compared with high NaCl intake (table 1).
In STZ-diabetic WT mice, switching from a control to a low-salt diet caused GFR to increase and changing to a high-salt diet caused GFR to decrease (fig. 3, right panels). Hence, these WT mice exhibited the same diabetic salt paradox as previously described in rats and humans [11,14,15,16, 27]. Moreover, kidney weight was greater in STZ-diabetic WT mice on low compared with high NaCl intake, consistent with previous studies in the STZ-diabetic rat [11, 14]. In contrast, the salt paradox of GFR was not apparent in STZ-diabetic A1R–/– mice, and kidney weight was not significantly greater in mice on the low-versus high-NaCl diet (fig. 3, right panels).
The urinary glucose/creatinine ratios were not significantly different between genotypes or between low and high NaCl intake (table 1), arguing against major differences in renal glucose reabsorption. Likewise, the urinary albumin/creatinine ratios were not significantly different between low and high NaCl intake (table 1). Pooling the data per genotype, however, revealed that the urinary albumin/creatinine ratio was significantly lower in STZ-diabetic A1R–/– compared with WT mice (0.77 ± 0.12 vs. 1.54 ± 0.24 μg/mg; n = 10–12; p = 0.006).
Discussion
In the present studies, diabetic mice lacking A1Rs differed from their WT littermates in that they manifested blunted glomerular hyperfiltration. The most straightforward interpretation of these findings is that proximal hyperreabsorption in diabetes alters the TGF/A1R-mediated afferent arteriolar tone, thereby increasing GFR. In the absence of TGF/A1R such a response is missing. The studies were not designed to address the influence of A1R on clinically relevant long-term alterations in kidney function and morphology (fibrosis). However, the present study shows another relevant consequence of lacking TGF/ A1Rs in diabetes, namely the absence of the salt paradox, which we proposed is based on hyperresponsiveness of proximal tubular reabsorption that affects GFR through TGF [1, 11, 17]. The present finding is mortar for the tubulocentric construct although the construct would not have collapsed without it since its basic validity rests on error signals in macula densa salt concentration [4,5,6] that are in the wrong direction to be explained by a primary problem with the afferent arteriole, notwithstanding that several defects in vasoactivity have been ascribed to the diabetic afferent arteriole.
But even those who are anxious to incorporate traditional TGF into the tubulocentric principle should recognize problems with this straightforward interpretation. To begin with, this interpretation implies that diabetic hyperfiltration must disappear when single- nephron GFR (SNGFR) is compared between diabetic and nondiabetic nephrons while flow past the macula densa is eliminated. Since diabetic hyperfiltration is easily demonstrated by measuring SNGFR from the late proximal tubule, this certainly does not happen. The explanation is TGF resetting. The juxtaglomerular apparatus of each nephron has a capacity to adjust its own TGF response and tends to invoke this capacity to align the steep portion of its TGF curve with the ambient tubular flow. A primary diabetes-induced increase in NaCl reabsorption upstream of the macula densa is expected to lower the NaCl concentration at the macula densa, increase GFR and shift the operating point to the flatter part of the TGF curve. This would reduce TGF efficiency. In order to operate at a maximum TGF efficiency, the TGF curve is expected to reset such that the operating point is restored to the steepest part of the TGF curve [28]. In accordance, the entire TGF curve in diabetes resets leftward and upward [6], and the greatest TGF efficiency resides at the ambient operating point [6]. As a consequence, the SNGFR values for minimal TGF signals at the macula densa, which are achieved when SNGFR is determined from proximal tubular collection, are enhanced in STZ diabetic compared with control rats [5, 6].
The tubulocentric principle invokes feedback from the tubule as the dominant controller of GFR in early diabetes, but does not require feedback to be the only controller. The theory still allows for additional primary defects in afferent arteriolar vasoconstriction and predicts such defects will be unmasked when feedback from the tubule is eliminated. In such a case, some degree of hyperfiltration would persist in the absence of A1Rs while the macula densa salt would convert from subnormal to supranormal. While distal salt delivery has not been measured in A1R–/– diabetic mice and A1R–/– diabetic mice did not hyperfilter in our hands, two other studies have reported hyperfiltration in A1R–/– diabetic mice [29, 30]. However, in one study the nondiabetic A1R–/– but not the alloxan-diabetic A1R–/– mice were hypotensive compared with their WT controls during measurements of GFR [29]. This is in contrast to two previous reports on the same mouse strain, which concluded that blood pressure under isoflurane anesthesia as well as in conscious mice is higher in A1R–/– compared with WT control mice [21, 31]. As a consequence, the higher GFR in normotensive alloxan-diabetic A1R–/– may have been the reflection of impaired renal autoregulation, which is a known trait of the TGF-less mouse [32]. The other study used Akita diabetic A1R–/– mice, which have blood glucose levels of 600–900 mg/dl, and are thus severely hyperglycemic [30]. In this model, the resulting excessive glucose load to the proximal tubule may inhibit proximal reabsorption [33], in contrast to the primary increase in proximal tubular reabsorption with modest hyperglycemia [4, 5]. As a consequence, TGF activation may serve to limit glomerular hyperfiltration during severe hyperglycemia. Especially under pathophysiological conditions, adenosine derived from sources outside of the juxtaglomerular apparatus can in addition activate renal vascular A1Rs and thus influence GFR independent of the TGF mechanism [for a review, see ref. [34]]. It is thus also possible that knockdown of A1Rs in the severely diabetic Akita animals induced renal vasodilation independent of TGF. Or in other words, the diabetes model and severity may determine the A1R-dependent contribution of primary vascular and TGF influences on GFR, and thus determine the net response to A1R blockade or knockout. The present data indicate that intact A1Rs and TGF are essential to glomerular hyperfiltration under conditions of modest hyperglycemia.
A1R antagonists have been reported to affect cardiorenal interactions and are currently under clinical development in patients with decompensated heart failure to enhance salt excretion while increasing or preserving GFR [35,36,37,38]. One might predict that A1R antagonists also increase or preserve GFR in the subgroup of diabetic patients with decompensated heart failure, since TGF resetting in diabetes restores the vasoconstrictor influence of A1R/TGF. In comparison, compounds like dipyridamole, which inhibit cellular adenosine uptake and increase renal interstitial adenosine concentrations, thereby mimicking normal or enhanced NaCl concentrations at the macula densa, reduce glomerular hyperfiltration in STZ-diabetic rats [39]. Further studies are necessary to better understand the influence of A1R on the acute and chronic control of GFR in diabetic patients.
In summary, the glomerular hyperfiltration observed in the low-dose STZ-induced diabetes model in WT mice is blunted in the absence of A1Rs. Moreover, the salt paradox of GFR, i.e. the inverse relationship between NaCl intake and GFR, is present in this diabetes model in WT mice, but absent in mice lacking A1R. The results indicate the importance of A1Rs for GFR control in early diabetes, which is consistent with the tubulocentric principle.
Acknowledgements
Heterozygote A1R breeder mice were kindly provided by Jurgen Schnermann, NIH, Bethesda, Md., USA. This work was supported by the National Institutes of Health (DK56248, DK070667, DK28602, DK070123, P30DK079337), the Department of Veterans Affairs, the National Kidney Foundation, and the Deutsche Forschungsgemeinschaft (RI 1535/3-1 and 3-2).
References
- 1.Vallon V, Blantz RC, Thomson S. Glomerular hyperfiltration and the salt paradox in early type 1 diabetes mellitus: a tubulo-centric view. J Am Soc Nephrol. 2003;14:530–537. doi: 10.1097/01.asn.0000051700.07403.27. [DOI] [PubMed] [Google Scholar]
- 2.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 3.Mogensen CE. Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand J Clin Lab Invest. 1986;46:201–206. doi: 10.3109/00365518609083660. [DOI] [PubMed] [Google Scholar]
- 4.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- 5.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest. 2001;107:217–224. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallon V, Blantz RC, Thomson S. Homeostatic efficiency of tubuloglomerular feedback is reduced in established diabetes mellitus in rats. Am J Physiol. 1995;269:F876–F883. doi: 10.1152/ajprenal.1995.269.6.F876. [DOI] [PubMed] [Google Scholar]
- 7.Woods LL, Mizelle HL, Hall JE. Control of renal hemodynamics in hyperglycemia: possible role of tubuloglomerular feedback. Am J Physiol. 1987;252:F65–F73. doi: 10.1152/ajprenal.1987.252.1.F65. [DOI] [PubMed] [Google Scholar]
- 8.Vervoort G, Veldman B, Berden JH, Smits P, Wetzels JF. Glomerular hyperfiltration in type 1 diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur J Clin Invest. 2005;35:330–336. doi: 10.1111/j.1365-2362.2005.01497.x. [DOI] [PubMed] [Google Scholar]
- 9.Brochner-Mortensen J, Stockel M, Sorensen PJ, Nielsen AH, Ditzel J. Proximal glomerulo-tubular balance in patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1984;27:189–192. doi: 10.1007/BF00273804. [DOI] [PubMed] [Google Scholar]
- 10.Hannedouche TP, Delgado AG, Gnionsahe DA, Boitard C, Lacour B, Grunfeld JP. Renal hemodynamics and segmental tubular reabsorption in early type 1 diabetes. Kidney Int. 1990;37:1126–1133. doi: 10.1038/ki.1990.95. [DOI] [PubMed] [Google Scholar]
- 11.Vallon V, Huang DY, Deng A, Richter K, Blantz RC, Thomson S. Salt-sensitivity of proximal reabsorption alters macula densa salt and explains the paradoxical effect of dietary salt on glomerular filtration rate in diabetes mellitus. J Am Soc Nephrol. 2002;13:1865–1871. doi: 10.1097/01.asn.0000016441.41118.57. [DOI] [PubMed] [Google Scholar]
- 12.Thomson SC, Blantz RC. Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J Am Soc Nephrol. 2008;19:2272–2275. doi: 10.1681/ASN.2007121326. [DOI] [PubMed] [Google Scholar]
- 13.Thomson SC, Deng A, Wead L, Richter K, Blantz RC, Vallon V. An unexpected role for angiotensin II in the link between dietary salt and proximal reabsorption. J Clin Invest. 2006;116:1110–1116. doi: 10.1172/JCI26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallon V, Wead LM, Blantz RC. Renal hemodynamics and plasma and kidney angiotensin II in established diabetes mellitus in rats: effect of sodium and salt restriction. J Am Soc Nephrol. 1995;5:1761–1767. doi: 10.1681/ASN.V5101761. [DOI] [PubMed] [Google Scholar]
- 15.Vallon V, Kirschenmann D, Wead LM, Lortie MJ, Satriano J, Blantz RC, Thomson SC. Effect of chronic salt loading on kidney function in early and established diabetes mellitus in rats. J Lab Clin Med. 1997;130:76–82. doi: 10.1016/s0022-2143(97)90061-5. [DOI] [PubMed] [Google Scholar]
- 16.Miller JA. Renal responses to sodium restriction in patients with early diabetes mellitus. J Am Soc Nephrol. 1997;8:749–755. doi: 10.1681/ASN.V85749. [DOI] [PubMed] [Google Scholar]
- 17.Vallon V, Blantz R, Thomson S. The salt paradox and its possible implications in managing hypertensive diabetic patients. Curr Hypertens Rep. 2005;7:141–147. doi: 10.1007/s11906-005-0089-x. [DOI] [PubMed] [Google Scholar]
- 18.Thomson S, Bao D, Deng A, Vallon V. Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest. 2000;106:289–298. doi: 10.1172/JCI8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breyer MD, Bottinger E, Brosius FC, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 20.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 22.Vallon V, Richter K, Huang DY, Rieg T, Schnermann J. Functional consequences at the single-nephron level of the lack of adenosine A1 receptors and tubuloglomerular feedback in mice. Pflügers Arch. 2004;448:214–221. doi: 10.1007/s00424-004-1239-8. [DOI] [PubMed] [Google Scholar]
- 23.Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther. 2005;313:403–409. doi: 10.1124/jpet.104.080432. [DOI] [PubMed] [Google Scholar]
- 24.Rieg T, Schnermann J, Vallon V. Adenosine A1 receptors determine effects of caffeine on total fluid intake but not caffeine appetite. Eur J Pharmacol. 2007;555:174–177. doi: 10.1016/j.ejphar.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Rieg T, Pothula K, Schroth J, Satriano J, Osswald H, Schnermann J, Insel PA, Bundey RA, Vallon V. Vasopressin regulation of inner medullary collecting ducts and compensatory changes in mice lacking adenosine A1 receptors. Am J Physiol Renal Physiol. 2008;294:F638–F644. doi: 10.1152/ajprenal.00344.2007. [DOI] [PubMed] [Google Scholar]
- 26.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 27.Birk C, Richter K, Huang DY, Piesch C, Luippold G, Vallon V. The salt paradox of the early diabetic kidney is independent of renal innervation. Kidney Blood Press Res. 2003;26:344–350. doi: 10.1159/000073941. [DOI] [PubMed] [Google Scholar]
- 28.Thomson SC, Vallon V, Blantz RC. Resetting protects efficiency of tubuloglomerular feedback. Kidney Int Suppl. 1998;67:S65–S70. doi: 10.1046/j.1523-1755.1998.06713.x. [DOI] [PubMed] [Google Scholar]
- 29.Sallstrom J, Carlsson PO, Fredholm BB, Larsson E, Persson AE, Palm F. Diabetes-induced hyperfiltration in adenosine A1-receptor deficient mice lacking the tubuloglomerular feedback mechanism. Acta Physiol (Oxf) 2007;190:253–259. doi: 10.1111/j.1748-1716.2007.01705.x. [DOI] [PubMed] [Google Scholar]
- 30.Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol. 2008;19:722–730. doi: 10.1681/ASN.2007060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown RD, Thoren P, Steege A, Mrowka R, Sallstrom J, Skott O, Fredholm BB, Persson AE. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1324–R1329. doi: 10.1152/ajpregu.00313.2005. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto S, Huang Y, Briggs J, Schnermann J. Reduced autoregulatory effectiveness in adenosine 1 receptor-deficient mice. Am J Physiol Renal Physiol. 2006;290:F888–F891. doi: 10.1152/ajprenal.00381.2005. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein AM. Osmotic diuresis in a mathematical model of the rat proximal tubule. Am J Physiol. 1986;250:F874–F884. doi: 10.1152/ajprenal.1986.250.5.F874. [DOI] [PubMed] [Google Scholar]
- 34.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 35.Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC. The effects of KW-3902, an adenosine A1-receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol. 2007;50:1551–1560. doi: 10.1016/j.jacc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb SS. Adenosine A1 antagonists and the cardiorenal syndrome. Curr Heart Fail Rep. 2008;5:105–109. doi: 10.1007/s11897-008-0017-x. [DOI] [PubMed] [Google Scholar]
- 37.Vallon V, Miracle C, Thomson S. Adenosine and kidney function: potential implications in patients with heart failure. Eur J Heart Fail. 2008;10:176–187. doi: 10.1016/j.ejheart.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalk P, Eggert B, Relle K, Godes M, Heiden S, Sharkovska Y, Fischer Y, Ziegler D, Bielenberg GW, Hocher B. The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol. 2007;151:1025–1032. doi: 10.1038/sj.bjp.0707319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallon V, Osswald H. Dipyridamole prevents diabetes-induced alterations of kidney function in rats. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:217–222. doi: 10.1007/BF00169840. [DOI] [PubMed] [Google Scholar]