Abstract
Background and Purpose
The efficacy of both habituation and adaptation exercise interventions in the treatment of unilateral vestibular hypofunction has been demonstrated by prior studies. The purpose of this paper is to describe the preliminary results of an ongoing study that compares the effects of these two different exercise approaches on outcomes related to vestibular function.
Methods
Seven participants with unilateral vestibular hypofunction have completed a 6-week exercise intervention after randomize assignment to either habituation (H) exercises, or gaze-stability (GS) adaptation exercises. The following measures were taken pre-treatment and post-treatment: Dizziness Handicap Inventory (DHI) to measure the symptom impact, motion sensitivity quotient (MSQ) to assess sensitivity to head movements, and the dynamic visual acuity test (DVA) as a measure of gaze-stability during head movements.
Results
Following the 6-week intervention there was an overall improvement in the DHI, the MSQ, and both the active and passive DVA. The H and GS intervention group participants each demonstrated similar improvements in both the MSQ, as well as the active and passive DVA measures.
Discussion and Conclusions
The improvement in the MSQ for the GS group and the improvement in the DVA measures for the H group were unexpected findings. Head movement, which is required by both exercise interventions, rather than the specific type of exercise may be the critical factor underlying the observed improvements in motion sensitivity and dynamic visual acuity.
BACKGROUND AND PURPOSE
There are numerous studies that have documented the efficacy of exercises to alleviate the symptoms and physical limitations associated with loss of vestibular function.1–4 The exercise approaches have generally fallen into one of two categories, either adaptation or habituation exercises. The adaptation exercises are based on the demonstrated ability of the vestibular system to modify the magnitude of the vestibulo-ocular reflex (VOR) in response to a given input (head movement). The adaptation of the VOR has been demonstrated in individuals with normal vestibular function and those with unilateral vestibular hypofunction.5, 6 One of the signals that induces adaptation of the VOR is retinal slip combined with head movement.7 This is the basis for what has traditionally been considered adaptation exercises. These exercises require the individual to perform rapid, active head rotations while watching a visual target, with the stipulation that the target remains in focus during the head movements.8 If the target is stationary, then the exercises are referred to as x1 viewing exercises. If the target is moving in the opposite direction of the head movement, then these exercises are referred to as x2 viewing exercises. While these exercises have been shown to improve dynamic visual acuity, the actual mechanism behind this improvement is not known.9 As such, it may be more appropriate to refer to these exercises as gaze-stability exercises.
In contrast with adaptation exercises, habituation exercises are based on the idea that repeated exposure to a provocative stimulus (e.g. head movements) will lead to a reduction of the motion-provoked symptoms.3, 10 This change is thought to be due to long-term changes within the nervous system, and there is clinical evidence indicating that the habituation exercises can lead to long-term changes in symptoms.11 The actual neural mechanism behind the effectiveness of the habituation exercises is not understood.
While there is support for the two different intervention approaches, there are no studies to date that have compared the two interventions in terms of changes in symptoms, motion sensitivity, and dynamic visual acuity. What is reported herein are the preliminary results of a study designed to investigate this issue in individuals with an identified unilateral vestibular loss. The underlying hypotheses for this study are that: 1) individuals who perform gaze stability exercises will have a greater improvement in dynamic visual acuity compared to those who perform the habituation exercises, and 2) individuals who perform habituation exercises will have a greater reduction in their motion sensitivity compared to those who perform the gaze stability exercises.
METHODS
Participants in this study were recruited from the clinical practice of the author. All participants had a documented unilateral vestibular hypofunction based on surgical history, caloric test results, or clinical examination, and had an abnormal clinical dynamic visual acuity test.12, 13 Individuals were excluded from the study if they had a central nervous system disease or cause of dizziness, orthopaedic problems that precluded performance of the exercises, if they were legally blind, or if they suffered from dementia. Informed consent was obtained, and the institutional review board for human subjects protection at Duke University approved all aspects of the study.
The computerized Dynamic Visual Acuity Testing apparatus was similar to devices reported previously in the literature.14 An optotype (the letter E) was displayed on 25-inch, high resolution computer monitor; the orientation and size of the optotype was under computer control. The orientation of the optotype was randomly altered, and the size of the optotype was progressively decreased in 0.1 logMAR increments (logarithm of the minimal angle of resolution). For each optotype size, five orientations of the optotype were presented. Static visual acuity was determined first. When the participant was unable to correctly identify all trials at a given acuity level, the test was stopped, and the number of missed optotypes was recorded. For determination of dynamic visual acuity (DVA), the optotype was presented every time the horizontal head velocity was between 120 deg/s and 180 deg/s (determined with a rate sensor) Within each trial, the optotype was presented 5 times before the participant was forced to determine its orientation. When the participant was unable to correctly identify all trials at a given acuity level, the test was stopped. The participant’s DVA was determined by subtracting the number of optotypes missed under the static visual acuity test from the number of optotypes missed under the dynamic component of the test. The DVA test was performed under both active (self-generated head movements) and passive (examiner generated head movements) conditions. The test was performed separately for ipsilesional and contralesional head rotation. Since the ipsilesional DVA test was the item of interest in this study, the contralesional passive DVA test was always run first to account for any learning effects that might occur. This method of DVA testing has been shown to be reliable with good sensitivity and specificity.14
The Dizziness Handicap Inventory (DHI) was administered to measure the participant’s perception of how the symptoms are interfering with their lives.15 This tool is used routinely in clinical settings, and has been shown to have greater sensitivity to change than global health surveys.16 Motion-provoked dizziness was measured using the Motion Sensitivity Test (MST). The MST measures the perceived intensity and duration of symptoms provoked by16 rapid changes in head or body position. An overall score, the motion sensitivity quotient (MSQ), is determined from the results of each of the movements. The MST has been shown to be both a reliable and valid measure of motion-provoked dizziness.17
Participants were randomly assigned to either the gaze-stabilization (GS) exercise group, or the habituation (H) exercise group using a program that generated a randomized block assignment to group based on the order in which the participant was enrolled in the study. The participants in the GS group performed a series of exercises designed to improve gaze-stability during head movements (Table 1) as well as balance and gait exercises. The participants in the H group performed a series of exercises designed to decrease their sensitivity to head movements (Table 1), as well as balance and gait exercises similar to those performed by the GS group. The exercises used in each group are used routinely in clinical practice and are often referred to as either adaptation exercises (the GS group), or habituation exercises (the H group). The participants were instructed to perform the exercises three times a day over a 6-week period. The participants returned to the lab once a week for clinical assessment and progression of the exercise program. The exercise progression was based on clinical experience treating patients with acute and subacute vestibular disorders. The exercise program was devised so that the participants could successfully complete each phase of the program. Static and dynamic visual acuity tests, MST, and the DHI were administered at the start of the program and at the end of the 6-week intervention period. The author performed all testing and treatment interventions.
Table 1.
Exercise Progression
| Gaze-Stabilization Exercises | Week | Habituation Exercises |
|---|---|---|
| Horizontal and vertical x1 viewing exercise with near target, 1 minute duration, sitting |
1 | Large amplitude, rapid cervical rotation (horizontal or vertical), each set of exercise consisted of 5 complete movements (cycles) and the individual performed 3 sets, sitting |
| Horizontal and vertical x1 viewing exercise with near target, 2 minute duration, sitting |
2 | Large amplitude, rapid horizontal cervical rotation (seated) and standing pivots, or large amplitude, rapid vertical cervical rotation (seated) and seated trunk flexion- extension,3 sets of 5 cycles |
| Horizontal and vertical x1 viewing exercise with near and far targets, 2 minute duration, standing |
3 | Large amplitude, rapid horizontal and vertical cervical rotation (seated) and standing pivots, or large amplitude, rapid horizontal and vertical cervical rotation (seated) and seated trunk flexion- extension,3 sets of 5 cycles |
| Horizontal and vertical x1 viewing exercise with near and far targets, and targets located in front of a busy background, 2 minute duration, standing |
4 | Large amplitude, rapid horizontal and vertical cervical rotation (seated), standing pivots, and seated trunk flexion-extension, 3 sets of 5 cycles |
| Horizontal and vertical x1 viewing exercise with near and far targets, and targets located in front of a busy background. Horizontal and vertical x2 viewing exercise, plain background. All exercises 2 minute duration, standing |
5 | Large amplitude, rapid horizontal and vertical cervical rotation (standing), standing pivots, and seated trunk flexion- extension,3 sets of 5 cycles |
| Horizontal and vertical x1 viewing exercise with near and far targets, and targets located in front of a busy background. Horizontal and vertical x2 viewing exercise, busy background. All exercises 2 minute duration, standing |
6 | Large amplitude, rapid horizontal and vertical cervical rotation (standing), standing pivots (180 degrees), seated trunk flexion-extension, and Brandt- Daroff exercise 3 sets of 5 cycles |
Given the preliminary nature of this report, between-group statistical analyses were not performed. With the small sample size, pre-treatment to post-treatment changes across all participants were assessed with the Wicoxon Signed Rank Test. Based on the initial studies of the DHI, an 18-point change is considered to be statistically significant.15 This value was based on test-retest results of a small sample (n=14), and raises the question of how to measure improvement in individuals whose pre-treatment DHI score is <18. Despite these limitations, the DHI changes of each individual will be compared to this 18-point value. While there have been demonstrated changes in computerized DVA with treatment, neither a mimial clinically significant difference, nor statistically significant change scores have been reported for computerized DVA.9 Earlier work by Herdman and colleagues14 documented the normal values of the DVA by age. In subsequent studies, they refer to a reference value for documenting individual improvement in DVA.9 This is reported to be a change of 6 or more optotypes (personal communication). The DVA changes of each participant were compared to these values. To our knowledge, there are no reports that have determined significant clinical or statistical change scores for the MSQ.
RESULTS
Eight participants with unilateral vestibular hypofunction have been enrolled in the study to date. Seven of the participants have completed the study. One participant was unable to complete the study due to having to return to work. The mean age of the participants was 43.9 years (range 27–65 years). Six of the seven participants were female. The time since onset of the symptoms ranged from 2 weeks to 6 months, and the causes of the unilateral vestibular hypofunction included vestibular nerve resection, labyrinthectomy, and vestibular neuritis. Three of the participants were enrolled in the GS group and four were enrolled in the H group. These data are summarized in Table 2. Compliance with the exercise program (based on weekly exercise logs) averaged 69.7% (range 34% – 90%).
Table 2.
Key: The DHI measures reflect the scores on the Dizziness Handicap Inventory, with lower scores indicating improvements in perceived handicap. The DVA scores reflect the number of missed optotypes during the dynamic test condition (minus the number of optotypes missed during the static visual acuity test). A decrease in the number of missed optotypes reflects improvements in the DVA. The MSQ values represent the results of the Motion Sensitivity Test. These values can range from 0 (no motion sensitivity) to 100 (severe symptoms in all test positions). A decrease in MSQ reflects improvement. Note: S7 was 3 years status post acoustic neuroma resection; she had an exacerbation of symptoms following childbirth.
| ID | Age | Diagnosis | Symptom Duration |
Intervention | DHI Pre- / Post- |
Active Ipsilesional DVA Pre- / Post- |
Passive Ipsilesional DVA Pre- / Post- |
MSQ Pre- / Post- |
|---|---|---|---|---|---|---|---|---|
| S1 | 40 | Vestibular Neuronitis | 2 mos | GS | 60 / 0 | 1 / 1 | 3 / 1 | |
| S3 | 65 | Vestibular Neuronitis | 3 mos | GS | 36 / 0 | 28 / 27 | 27 / 28 | 0.05 / 0.44 |
| S4 | 54 | Labyrinthectomy | 1 mos | GS | 68 / 14 | 15 / 4 | 22 / 14 | 10.98 / 0.73 |
| S2 | 56 | Vestibular Neuronitis | 1 mos | Habituation | 76 / 2 | 21 / 5 | 13 / 5 | 67.38 / 1.17 |
| S5 | 32 |
Acoustic Neuroma Resection |
1 mos | Habituation | 64 / 18 | 14 / 6 | 24 / 9 | |
| S7 | 33 |
Acoustic Neuroma Resection |
4 mos | Habituation | 22 / 12 | 9 / 7 | 6 / 3 | 4.44 / 5.47 |
| S8 | 27 | Vestibular Neuronitis | 1 mos | Habituation | 64 / 10 | 5 / 1 | 43.21 / 1.46 |
Taken as a group, the participants demonstrated improvements in DHI scores, MSQ, and both active and passive ipsilesional DVA. The DHI scores improved with intervention (pretreatment mean=55.71, sd=19.34; post-treatment mean = 8.0, sd=7.30). This change was significant based on the Wilcoxon Signed Rank Test (p < 0.01). The number of optotypes missed during the active ipsilesional DVA also decreased (pre-treatment mean=14.7, sd= 9.35, post-treatment mean=8.3, sd= 9.37), and this change was also significant (p < 0.05). In a similar manner the number of optotypes missed during the passive ipsilesional DVA decreased (pretreatment mean=14.3, sd=10.0, post-treatment mean=8.7, sd=9.71), which was also a significant change (p < 0.05). There was a marked reduction in the measure of motion sensitivity post-treatment (pre-treatment mean=25.21; post-treatment mean=1.85), but due to the small sample size and the large variability, this was not statistically significant.
Since the number of participants was low, between-group statistical comparisons could not be performed. Observation of the individual data points for the different measures, however, is illuminating, as there are clear trends in the preliminary data. Figure 1A and 1B demonstrate the individual changes in DHI and MSQ, respectively, by intervention group across the six week intervention session. For the DHI, all participants demonstrated a reduction in their scores (Fig 1A). The three participants in the GS group each had decreases in the DHI score of >18 points. Three of the four participants in the H group also demonstrated decreases in their DHI scores of >18 points. The one individual that did not demonstrate an 18-point change had a 10-point decrease from 22 to 12. Regardless of intervention group, the majority of the participants had improvements in DHI that are considered to be significant based on current understanding of the test metrics.15
Fig 1.
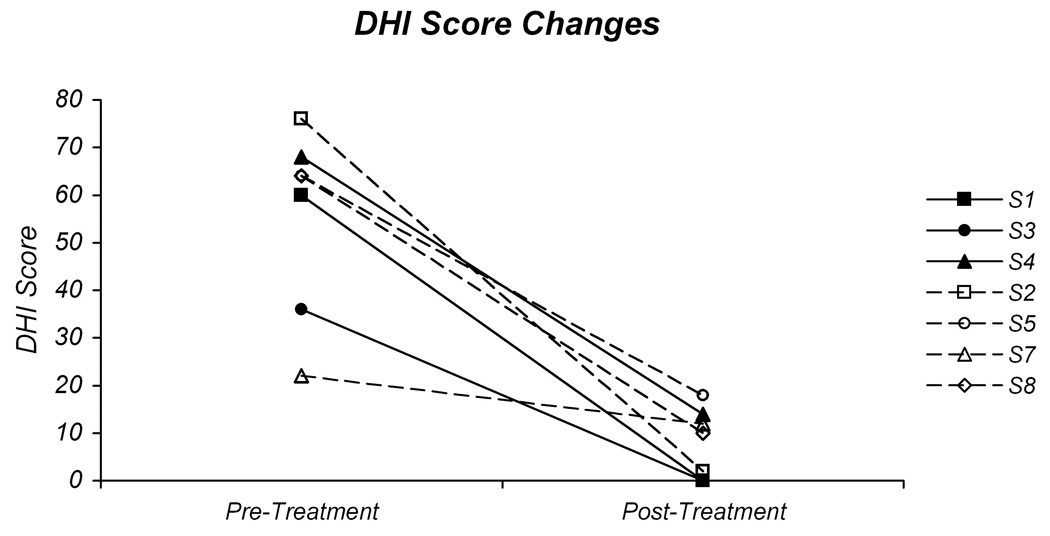
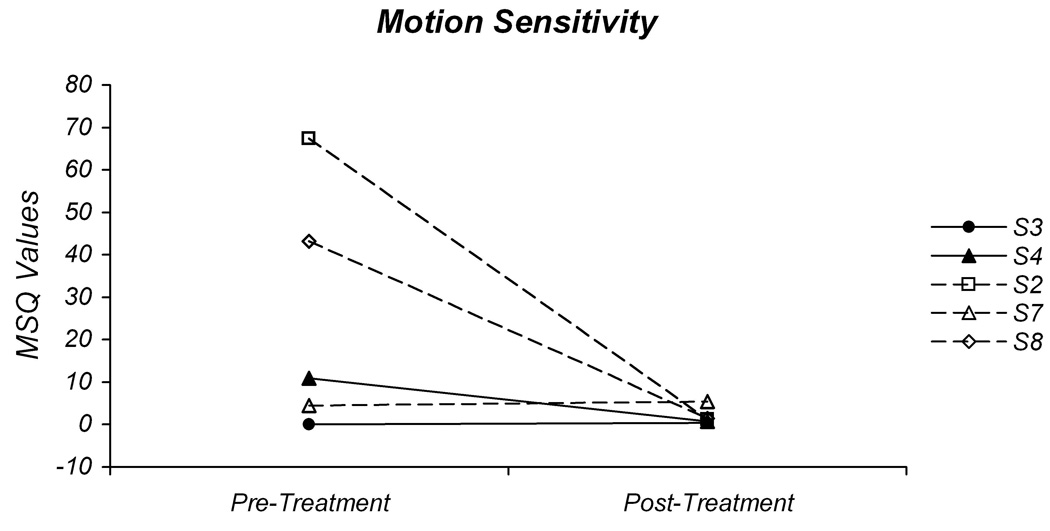
Pre-treatment and post-treatment values for the Dizziness Handicap Inventory (A) and motion sensitivity (B). The individual DHI scores for the participants in the gaze-stability intervention group (GS) are plotted in 1A with solid lines and those in the habituation intervention group (H) are plotted with dashed lines. The individual motion sensitivity quotient (MSQ) values are plotted in 1B.
Due to symptom severity at the time of the initial assessment, not all participants were able to complete the MST. Three of the five participants who were able to complete this test demonstrated a reduction in the MSQ (Fig 1B). For two of the individuals in the H group, there was a marked reduction in their motion sensitivity from values >40 to values <2. The other individual in the H group had a pre-treatment MSQ score below 10 and demonstrated a mild increase in MSQ score; the post-treatment value, however, was still <10. For the two participants in the GS group, the MSQ decreased from 10.98 to 0.73 in one individual, and increased from 0.05 to 0.44 in the other. For the first individual this represents a change from a motion sensitive condition to one with little motion sensitivity. For the other individual, there was little motion sensitivity either pre-treatment or post-treatment.
The individual changes in ipsilesional active and passive DVA by intervention group are depicted in Figure 2A and B. For the majority of the participants there was an improvement in both active and passive DVA. For the participants in the GS intervention group the results were mixed. For participant S1, even though the clinical DVA test was abnormal, the computerized DVA was normal. There was an improvement of 2 optotypes during the passive DVA; however, this is not a significant change. Participant S3 had a markedly abnormal active and passive DVA at intial testing, neither of which showed improvement with the exercise intervention. There were significant improvements in the active and passive DVA for participant S4. The active DVA improved by 11 optotypes, and returned to a normal level. The passive DVA improved by 8 optotypes, but did not return to a normal level.
Fig 2.
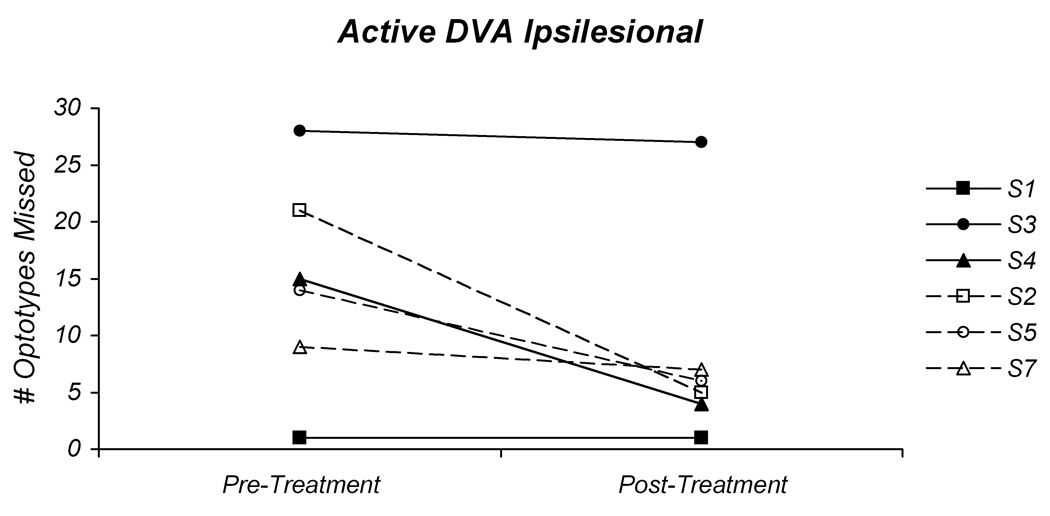
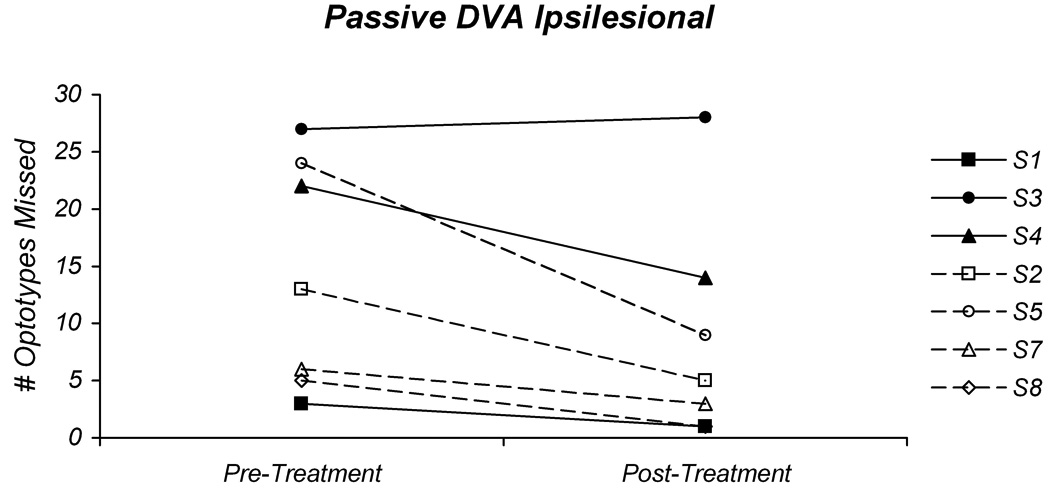
Pre-treatment and post-treatment values for the active (A) and passive (B) ipsilesional dynamic visual acuity tests. In both graphs the individual values for the participants in the gaze-stability intervention group (GS) are plotted with solid lines and those in the habituation intervention group (H) are plotted with dashed lines.
Surprisingly, there were substantial reductions in the number of missed optotypes for the participants in the H intervention group. Participant S2 demonstrated an improvement of 16 optotypes for the active DVA and an improvement of 8 optotypes for the passive DVA. This also represents a change from an abnormal pre-treatment DVA to a normal result post-treatment. Participant S5 had an improvement of 8 optotypes for the active DVA, and an improvement of 15 optotypes for the passive DVA. While this change is significant, this individual did not attain normal levels for either active or passive DVA post-treatment. The third participant in the H group, S7, did not demonstrate a significant in improvement in active DVA and did not attain normal levels for this test condition. For the passive DVA, however, there was an improvement of 3 optotypes, which improved performance to a normal level. Since participant S8 was too symptomatic to perform the active DVA tests at the pre-treatment assessment, only the passive DVA was measured. There was an improvement of 4 optotypes with the passive DVA, which represents a change from an abnormal DVA pre-treatment to a normal DVA post-treatment.
DISCUSSION
The improvements observed across participants were not unexpected based on prior studies.2–4 Both intervention groups demonstrated similar improvements in the DHI, which suggests that there was a decrease in the impact of the symptoms on the participants’ lives, and that the type of exercise intervention was not an important factor. Participant S3 presents unusual findings; there was a significant decrease in the DHI score, yet no change in either active or passive DVA. In addition, the MSQ was <1 on both the pre-treatment and post-treatment assessments. According to the self-report exercise log, participant S3 was compliant with the exercise program (73%). A possible explanation for the disparity between the DVA and DHI scores is that this participant avoided movements and activities for fear of symptom provocation, which would be reflected in the pre-treatment DHI score. With initiation of the exercise program, the participant may have discovered that he was able to move and function without symptoms (as reflected in the low MSQ scores), which would lead to decreases in the DHI scores. It is not clear why participant S3 demonstrated no improvement in DVA. It could be due to improper performance of the exercises at home, or some other factor. Exercise intervention in the treatment of vestibular hypofunction has been shown to not be beneficial in all cases.18 In that study, the percentage of participants who felt the rehabilitation program was of benefit was greater than the percentage of participants who demonstrated improvements in gait measures, which is consistent with the disparity between physiologic measures and patient reported outcome measures observed in participant S3.
Given the small number of participants in this preliminary report, one must be cautious to not over-interpret the results. That said, the present results demonstrate two somewhat unexpected results that bear further discussion and exploration. The first result is the reduction of the MSQ in the Gaze-Stability group. This intervention group did not perform exercises designed to specifically alleviate their sensitivity to large amplitude, rapid head movements. The one participant in this group that had a pre-treatment MSQ score >10 had a marked reduction in the MSQ to a value <1. While there are several possible explanations for this, one must keep in mind that of the two participants in the Gaze-Stability group that completed the MST, only one had measurable motion sensitivity. One possible explanation for the reduction in motion sensitivity is that the gaze-stability and balance exercises, as well as the participant’s daily activities, included sufficient provocative stimuli to cause habituation of the motion-provoked symptoms. Another possible explanation for the reduction in the motion sensitivity is that the GS intervention led to adaptation of the vestibular system. This adaptation would result in resolution of the sensory mismatch between vestibular, visual and somatosensory inputs. With the loss of the sensory mismatch, which is thought to produce the symptoms of motion sensitivity, there would be no motion-provoked dizziness. The efficacy of the gaze stabilization exercises to reduce motion sensitivity needs to be confirmed with continued enrollment of participants in the study.
The most interesting finding in the study is the improvement in the active and passive ipsilesional DVA in the participants performing the habituation exercises. This improvement was seen across all participants in the H intervention group, who did not perform exercises designed to improve their visual acuity during head movements. The improvements in both the GS and H groups are similar (except for one participant in the GS group who showed little to no improvement). There are several possible explanations for this improvement in DVA in the H intervention group. One, it is possible that the balance exercises and the participants’ daily activities provided sufficient stimuli to induce improvements in DVA. However, in a study by Herdman and colleagues9, wherein the participants performed either gaze stabilization and balance exercises, or a placebo (saccadic eye movements) and balance exercises, the group performing the placebo and balance exercises showed no improvement in their DVA measures. Another possible explanation is that the head movements used in the H intervention enabled, or facilitated, the central compensations that resulted in the improvements in DVA. It may be that the head movements pose a challenge (sensory mismatch) to the central nervous system, which then attempts to resolve the challenge. As the CNS learns to compensate for the sensory mismatch, it may also learn strategies for improving visual acuity during head movements. The common factor in the exercises performed by both the GS and H groups is the head movement, which may be the enabling factor in the compensation process. A similar result was described by Cohen and Kimball.19 These investigators demonstrated improvements in measures of ataxia, as well as static and dynamic postural stability, in individuals with unilateral vestibular hypofunction following a rehabilitation program consisting solely of habituation exercises. This explanation, that head movement enables the compensation process, is also supported by the clinical observation that those individuals with a vestibular deficit who move tend to improve (decreased symptoms and increased functional levels) without intervention.
The actual mechanisms underlying the improved DVA for either intervention are not known. Analysis of the eye movements during the DVA test both pre- and post-intervention may help elucidate these mechanisms. These data are currently being collected, but are beyond the scope of this report.
CONCLUSIONS
Gaze-stability and habituation exercises have previously been shown to decrease symptoms of dizziness and increase function in individuals with vestibular disorders. The preliminary results of this study indicate that both exercise interventions lead to a reduction in the self-report measure of the impact of symptoms on the ability to function, a decrease in the sensitivity to movements, and an improvement in the ability to see clearly during head movements. Continued investigation is needed to determine if these results will hold, to determine if there are different effects of the two interventions, and to determine the mechanisms of improved visual acuity.
Acknowledgments
This work was supported by NIH (NCMRR) grant R03HD049885
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cawthorne T. The physiological basis for head exercises. J Chart Soc Physiother. 1944:106–107. [Google Scholar]
- 2.Horak FB, Jones-Rycewicz C, Black FO, Shumway-Cook A. Effects of vestibular rehabilitation on dizziness and imbalance. Otolaryngol.Head Neck Surg. 1992;106(2):175–180. [PubMed] [Google Scholar]
- 3.Shepard NT, Telian SA, Smith-Wheelock M, Raj A. Vestibular and balance rehabilitation therapy. Annals of Otology, Rhinology & Laryngology. 1993;102(3 Pt 1):198–205. doi: 10.1177/000348949310200306. [DOI] [PubMed] [Google Scholar]
- 4.Herdman SJ, Clendaniel RA, Mattox DE, Holliday MJ, Niparko JK. Vestibular adaptation exercises and recovery: acute stage after acoustic neuroma resection. Otolaryngol Head Neck Surg. 1995;113(1):77–87. doi: 10.1016/s0194-5998(95)70148-6. [DOI] [PubMed] [Google Scholar]
- 5.Pfaltz CR. Vestibular compensation. Physiological and clinical aspects. Acta Otolaryngol. 1983 May–Jun;95(5–6):402–406. doi: 10.3109/00016488309139422. [DOI] [PubMed] [Google Scholar]
- 6.Szturm T, Ireland DJ, Lessing-Turner M. Comparison of different exercise programs in the rehabilitation of patients with chronic peripheral vestibular dysfunction. Journal of Vestibular Research. 1994;4(6):461–479. -32676. [PubMed] [Google Scholar]
- 7.Shelhamer M, Tiliket C, Roberts D, Kramer PD, Zee DS. Short-term vestibulo-ocular reflex adaptation in humans. II. Error signals. Exp Brain Res. 1994;100(2):328–336. doi: 10.1007/BF00227202. [DOI] [PubMed] [Google Scholar]
- 8.Herdman SJ. Exercise strategies for vestibular disorders. Ear Nose Throat J. 1989;68(12):961–964. [PubMed] [Google Scholar]
- 9.Herdman SJ, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch.Otolaryngol Head Neck Surg. 2003;129(8):819–824. doi: 10.1001/archotol.129.8.819. [DOI] [PubMed] [Google Scholar]
- 10.Telian SA, Shepard NT, Smith-Wheelock M, Kemink JL. Habituation therapy for chronic vestibular dysfunction: preliminary results. Otolaryngol Head Neck Surg. 1990;103(1):89–95. doi: 10.1177/019459989010300113. [DOI] [PubMed] [Google Scholar]
- 11.Clement G, Tilikete C, Courjon JH. Retention of habituation of vestibulo-ocular reflex and sensation of rotation in humans. Exp Brain Res. 2008 Sep;190(3):307–315. doi: 10.1007/s00221-008-1471-0. [DOI] [PubMed] [Google Scholar]
- 12.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Archives of Neurology. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 13.Longridge NS, Mallinson AI. The dynamic illegible E-test. A technique for assessing the vestibulo- ocular reflex. Acta Otolaryngol (Stockh) 1987;103(3–4):273–279. [PubMed] [Google Scholar]
- 14.Herdman SJ, Tusa RJ, Blatt P, Suzuki A, Venuto PJ, Roberts D. Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am J Otol. 1998;19(6):790–796. [PubMed] [Google Scholar]
- 15.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Archives of Otolaryngology Head and Neck Surgery. 1990;116:424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 16.Enloe LJ, Shields RK. Evaluation of health-related quality of life in individuals with vestibular disease using disease-specific and general outcome measures. Physical Therapy. 1997;77(9):890–903. doi: 10.1093/ptj/77.9.890. [DOI] [PubMed] [Google Scholar]
- 17.Akin FW, Davenport MJ. Validity and reliability of the Motion Sensitivity Test. J.Rehabil.Res.Dev. 2003;40(5):415–421. doi: 10.1682/jrrd.2003.09.0415. [DOI] [PubMed] [Google Scholar]
- 18.Krebs DE, Gill-Body KM, Parker SW, Ramirez JV, Wernick-Robinson M. Vestibular rehabilitation: useful but not universally so. Otolaryngol Head Neck Surg. 2003;128(2):240–250. doi: 10.1067/mhn.2003.72. [DOI] [PubMed] [Google Scholar]
- 19.Cohen HS, Kimball KT. Decreased ataxia and improved balance after vestibular rehabilitation. Otolaryngol Head Neck Surg. 2004 Apr;130(4):418–425. doi: 10.1016/j.otohns.2003.12.020. [DOI] [PubMed] [Google Scholar]


