Abstract
28 synovial effusions (SE) were obtained from 24 patients, paired samples of peripheral blood (PB) from 10 of these patients, and PB from 36 healthy individuals for analysis of CD146 on T-lymphocytes by flow cytometry. CD146+ or CD146− T-lymphocytes were sorted from 3 SE to study gene expression profiles and selected genes revalidated using QPCR assays. We found more CD3+CD146+ and CD4+CD146+ T-lymphocytes in PB from patients compared to PB of healthy individuals (4.71%±2.48% vs 2.53%±1.08%, p=0.028) and (6.29%±2.74% vs 2.41%±0.96%, p=0.0017) respectively, whereas CD8+CD146+ T-lymphocytes were not significantly different (2.55%±1.65% vs 3.18%±2.59%, p=0.5008). SE displayed CD146 staining on 16.32%±6.06% of CD3+ cells. This expression was skewed towards CD4+ T-lymphocytes, with CD146 present on 24.06%±8.20% of the CD4+ T-lymphocytes compared to 6.19%±5.22% of the CD8+ T-lymphocytes. CD146 on CD3+, CD4+ and CD8+ T-lymphocytes in SE was significantly higher compared to PB in patients (p<0.0001, p<0.0001 and p=0.0036 respectively). Gene expression profiles of sorted CD146+CD4+CD3+ vs CD146−CD4+CD3+ T-lymphocytes (n=2) and CD2+CD146+ vs CD2+CD146− (n=1) from SE, displayed increased CD146, LAIR2, CXCL13, CD109, IL6ST, IL6R, TNFRsf18, and TNFRsf4 genes, whereas decreased CCR7, CCL5, and cytotoxicity-associated genes including granzymes b, h and k, perforin were found with the CD146− T-lymphocytes. By QPCR higher mRNA expression of CXCL13, CD146 and CD109 was also noted in the CD146+ subset, compared to the CD146− subset, in PB of healthy individuals and in PB and SE from patients. Our study establishes increased CD146+ T-lymphocytes in diseases with joint effusions, and demonstrates pro-inflammatory gene profiles in these cells.
INTRODUCTION
CD146 is an adhesion molecule expressed on endothelial cells, as well as a limited number of other cell types including activated lymphocytes (1–4). Although the function of this molecule on endothelial cells has been well-described, its role in lymphocyte biology has not been examined in depth. Recent studies suggest that this molecule plays a role in lymphocyte binding to the endothelium (5,6). Lymphocytes from healthy individuals expressing CD146 have an effector memory phenotype and display proinflammatory gene profiling characteristics (5).
Synovial fluid from healthy individuals contains fewer than two hundred cells, consisting primarily of synoviocytes. The number of cells increases in inflammatory states such as rheumatoid arthritis, and, in inflammatory conditions, lymphocytes infiltrate into the inflamed synovia. These infiltrating lymphocytes have been repeatedly described but much remains unknown about the recruitment, regulation, and pathogenesis of these synovial lymphocytes (7–8).
The expression of CD146 on lymphocytes in synovial fluids has been previously studied, but the data are inconsistent, and often contradictory. Pickl and colleagues (3) reported the presence of CD146+ T lymphocytes in synovial fluid from rheumatoid arthritis patients, but a subsequent study by Neidhart reported no substantial CD146 expression on mononuclear infiltrates in RA synovium (9). However, the latter study described high levels of soluble CD146 in these synovial fluids and ascribed this to increased activity of endothelial cells and angiogenesis. Using immunohistochemistry, Middleton and colleagues reported some weak staining of CD146 in mononuclear infiltrates of RA synovia but did not offer further characterization (10).
The current study was undertaken to definitively establish whether or not T lymphocytes in synovial fluids express CD146 and, if so, to better understand the nature of these cells. To these ends, synovial fluids from a variety of different diseases, and in several instances, peripheral blood from the same patients, were studied.
METHODS AND MATERIALS
Specimens
Twenty eight synovial effusions from twenty four patients with both inflammatory and noninflammatory joint effusions were studied for the expression of CD146 on T lymphocytes (Table 1). Peripheral blood was drawn from 10 patients at the same times the synovial effusion was obtained, including 6 patients with RA, 1 with polyarthritis, 1 with a meniscal tear, 1 with SLE, and 1 with sarcoid. Additionally, peripheral blood was drawn from 36 healthy volunteers. Specimens were obtained under protocols approved by the NHLBI and NIAMS Institutional Review Boards.
TABLE 1.
Characteristics of Patients and Synovial Fluids
| Gend | Age | Inflam | Dx | RF | CCP | ESR | CRP | MEDs | Other | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 66 | ND | Yes | Polyarthritis | 19 | 126 | 66 | 0.74 | MTX, Pred | Marginal zone lymphoma |
| 2 | F | 25 | Syn WBC 5540, 6 PMN, 24 L | Yes | Reactive | 19 | 19 | ND | ND | ||
| 3 | F | 25 | ND | Yes | Reactive | 19 | 19 | ND | ND | ||
| 4 | M | 55 | Syn WBC 6778, 15% L, 57% PMN | Yes | Cppd | 206 | 19 | ND | ND | NSAID | OA, meniscal tear |
| 5 | F | 51 | Syn WBC 9 | No | RA | 19 | 19 | 6 | ND | HCQ | |
| 6 | M | ND | Yes | Chr Int Rearr | ND | ND | ND | ND | |||
| 7 | F | 35 | ND | Yes | RA | 24 | 126 | 13 | 0.454 | MTX, Pred | |
| 8 | F | 65 | Syn 370, 13L, 87PMN | No | OA | 19 | 19 | 5 | 0.39 | NSAID, Acet | |
| 9 | F | 24 | Syn 28375 3L, 91 PMN | Yes | RA | 609 | 126 | 46 | 1.47 | MTX, Pred, HCQ | |
| 10 | M | 56 | Syn WBC 2430 18% L, 34% PMN | Yes | Psor Arth | 19 | 19 | 28 | 1.3 | AZA | |
| 11 | F | 45 | WBC 8340 14%L 33%N 53%Other | yes | RA | 912 | 56 | 15 | 0.845 | NSAID | |
| 12 | F | 38 | WBC 10580 12%L 83%N 5%Other | yes | RA | 184 | >125 | 43 | 4.2 | ||
| 13 | F | 35 | WBC 6722 0%L 76%N 24%Other | yes | SLE + RA | 39 | 60 | 97 | 11.9 | MMT, MTX, PLAQ, NSAID | |
| 14 | F | 24 | WBC 8700 5%L 45%N 50%Other | yes | RA | 278 | >125 | 8 | 2.61 | MTX, ENBREL, PRED | |
| 15 | F | 51 | WBC 717 23%L 0%N 74%Other | No | OA | ND | ND | 29 | NSAID | ||
| 16 | M | 55 | WBC 22138 6%L 72%N 22%Other | yes | LYME | 21 | <20 | 26 | 7.58 | DOXYCYCLINE | |
| 17 | F | 76 | WBC 24200 5%L 84%N 11%Other | yes | RA | 166 | >125 | 81 | 2.18 | NSAID | TUBERCULOSIS |
| 18 | M | 34 | WBC 5444 37%L 2%N 61%Other | yes | SARCOID | 93 | ND | 44 | 3.63 | NSAID | |
| 19 | F | 33 | ND | Unk | RA | <20 | <20 | 23 | 0.246 | NONE | PREGNANT |
| 20 | F | 58 | WBC 10725 58%L 9%N 33%Other | Yes | RA | 35 | 58 | 10 | 0.805 | NSAID | |
| 21 | F | 32 | WBC 10222 2%L 7%N 91%Other | yes | RA | <20 | <20 | 25 | 1.42 | ENBREL, PRED | |
| 22 | F | 61 | ND | Yes | PA or SLE | 19 | 19 | 28 | 0.102 | Pred, HCQ | |
| 23 | F | 55 | WBC 36, 12L,4N,84O | No | Torn Meniscus | 19 | 19 | 7 | 0.39 | NSAID | |
| 24 | F | 33 | WBC 1837, 1L, 79N. 20 other | Yes | RA | 19 | 19 | 53 | 1.05 | ||
| 25 | F | 26 | WBC 4467, 6L,31N, 63O | yes | JRA | 19 | 19 | 38 | 0.09 | NSAID | |
| 26 | F | 34 | WBC 10060, 6L,89N,5 O | yes | RA | 19 | 19 | 17 | 0.46 | Pred | |
| 27 | F | 60 | WBC 9611, 18L,55N,27 O | yes | RA | 19 | 19 | 94 | 11.3 | Pred, HCQ, MTX | |
| 28 | F | 23 | WBC 17550, 6L,87N,7 O | yes | SLE | 19 | ND | 37 | 1.24 | Pred, HCQ, Imuran |
WBC – total synovial white blood cell count/mm3, N - % synovial neutrophils, L % synovial lymphocytes, RF - rheumatoid factor (normal < 19 IU/ml), CCP – anti-CCP antibody (< 19 units/ml), ESR – erythrocyte sedimentation rate (male < 25 mm/hr, female < 40 mm/hr), CRP – c-reactive protein (< 0.8 mg/dl), MTX – methotrexate, NSAID – nonsteroidal anti-inflammatory drug, HCQ – hydroxycholorquine, Pred – prednisone, Acet – acetaminophen, AZA – azathioprine, MMT - mycophenolate mofetil, Plaq – plaquenil, ND – not done.
Inflammatory effusion status was determined by either gross physical examination of the synovial effusion or by the number of total synovial white blood cells.
Flow Cytometry
The synovial effusion samples were processed within two hours after collection for flow cytometry. Samples were centrifuged at 540×g for 5 min and supernatants were removed. Cells were stained immediately. The cells were incubated in the following antibodies (all from Becton Dickinson, San Jose, CA): anti-CD3, anti-CD146, anti-CD45, anti-CD4, anti-CD8, anti-CD45RA, anti-CD45RO, anti-CCR7, anti-CD62L, anti-CCR5 (CD195), and anti-CXCR4 (CD184). The fluorochromes used varied with the experiment performed, however, these use of PE CD146 remained invariant. Mouse anti-isotypic (IgG1) antibodies were used as controls. After 30 min incubation, the cells were placed in lysing suspension (Cat No.349202, FACSLyse, Becton Dickinson). After 10 min incubation, cells were centrifuged then supernatants were discarded. The cell pellet was washed in phosphate buffered saline (Cat No. 10010, Invitrogen). The cells were pelleted again, the supernatant discarded and the cells resuspended by vortexing and fixed with 1% formaldehyde/PBS before analysis. Peripheral blood specimens were stained after removal of red blood cells for 30 minutes with the antibodies described above. Expression of cell surface markers was analyzed on a FACSCalibur flow cytometer using Cellquest software (Becton Dickinson) or on an LSR II using FACSDiva software (BDIS). Between 25,000 and 100,000 cells were measured in each sample, depending on the number of cells present in the synovial effusion specimen. The number of markers examined also depended on the cell number of the original synovial effusion. Two tail p values were calculated using a nonpaired T test assuming unequal variance using Welch's correction.
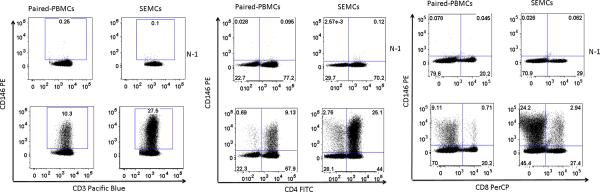
Lymphocyte populations were gated using light scatter and T cells were identified using CD3. CD146 staining was identified using either isotype controls or fluorescence minus one controls, with similar results (Figure 1).
Figure 1.
Determination of CD146 staining on lymphocytes from peripheral blood (PBMC) and synovial effusions (SEMC) from patients. Lymphocytes were gated by light scatter and for CD3 staining. Left panel: CD146 vs CD3+; middle panel CD146 vs CD8; right panel CD146 vs CD4. Upper dotplots in each groups display the N-1 controls while the lower dotplot display staining with CD146.
Gene profiling studies were conducted on 2 synovial effusion specimens (one with RA and one with sarcoid) containing adequate numbers of cells for these studies and CD4+CD146+ cells and CD4+CD146− cells were sorted from these SF samples. SE from an additional patient (with SLE), were sorted for CD2+CD146+ cells and CD2+CD146− cells. Both isotype controls and fluorescence minus one controls were used to assist in determining positive staining. Cell sorting was performed using either a MoFlo cell sorter (Beckman Coulter, Ft Collins, CO) or a FACSAria (BDIS, San Jose, CA).
RNA isolation
Total RNA from sorted cells was extracted using an RNAqueous micro kit (Ambio, Austin, TX) following the manufacturer's directions. The concentration of the isolated RNA was determined using the Nanodrop ND-100 spectrophotometer (Nanodrop Technologies, Wilmington, DE). Quality and integrity of the total RNA isolated was assessed on the Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA).
Target preparation and hybridization to genechips
T7 based RNA amplification was carried out on 20ng of the isolated total RNA using the messageamp premier amplification kit as suggested by the manufacturer (Ambion, Austin, TX). Briefly, total RNA was incubated with oligo dT/T7 primers and reverse transcribed into double stranded cDNA. In vitro transcription and biotin labeling of the purified cDNA was performed using T7 RNA polymerase at 37°C for 16hrs using the IVT labeling kit following the manufacturer's directions. The yield and integrity of the biotin labeled cRNA were determined using the nanodrop ND-1000 spectrophotometer and the Agilent 2100 bioanalyzer. 15μg of biotin labeled RNA was fragmented to ~200 bp size by incubating in fragmentation buffer containing 200 mM Tris-Acetate pH 8.2, 500 mM Potassium Acetate and 500 Magnesium Acetate for 35 minutes at 94°C prior to hybridization. Fragmented RNA was assessed for the fragment size on Agilent 2100 bioanalyzer and hybridized to Affymetrix U133+PM peg arrays for 16 hours, washed, stained and scanned on Affymetrix Genchip HT array plate scanner.
Microarray data processing and analysis
Affymetrix Expression Console version 1.1 was used to calculate the signal intensity. The signal intensity values obtained for probe sets in the microarrays were transformed using RMA (Robust Multichip Average). The processed data from all the chips were subjected to a principal component analysis (PCA) to detect outliers. Probesets were selected with more than two fold-change. JMP7 software (SAS Institute, Cary, NC) was used to cluster sets of probesets/genes with similar expression patterns. Pathway analysis was performed using Ingenuity Pathway Analysis (Ingenuity Systems Inc., Redwood City, CA).
Quantitative RT-PCR analysis
Total RNA was isolated from sorted cells from peripheral blood and synovial effusions of patients and from peripheral blood of healthy individuals using MicroRNA aquos kit (Ambion, Texas, USA) as per the manufacturers' instruction. First-strand cDNA was synthesized using Invitrogen's Superscript cDNA synthesis kit (Invitrogen, Carlsbad, CA) following the manufacturer's directions. Quantitative real-time PCR assays were carried out with the use of gene-specific double fluorescently labeled probes in a 7900 Sequence Detector (PE Applied Biosystems, Norwalk, CT). Probes and primers for CXCL13, CD109, CD146 and β-actin were obtained from Applied Biosystems. In brief, PCR amplification was performed in a 384 well plate with a 20-μl reaction mixture containing 300 nm of each primer, 200 nm probe, 200nm dNTP in 1× real time PCR buffer and passive reference (ROX) fluorochrome. The thermal cycling conditions were 2 min at 50° C and 10 min at 95° C, followed by 40 cycles of 15 sec denaturation at 95° C and 1 min annealing and extension at 60° C. Samples were analyzed in duplicate and the Ct (cycle threshold) values obtained were normalized to the housekeeping gene β-actin.
RESULTS
Immunophenotyping of peripheral blood specimens from healthy donors
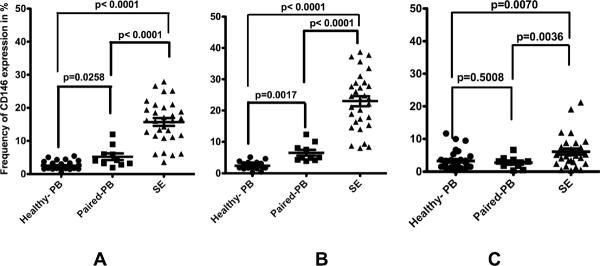
Peripheral blood from 36 healthy individuals displayed CD146 staining on 2.53% ± 1.08% of the CD3+ T lymphocytes, 2.41% ± 0.96% of the CD3+CD4+ T lymphocytes, and 3.18% ± 2.59% of the CD3+CD8+ T lymphocytes (Figure 2).
Figure 2.
The expression of CD146 on (left) CD3+ (middle) CD3+CD4+ and (right) CD3+CD8+ T lymphocyte subsets on peripheral blood from 36 healthy patients, peripheral blood from 10 patients with paired SE samples, and 28 SE samples. Data are shown as the percentages of CD146+CD3+ divided by total CD3 cells (left) and as CD146+CD3+CD4+ divided by total CD3+CD4+ cells (middle) and CD146+CD3+CD8+ divided by total CD3+CD8+ (right). The mean values for levels in peripheral blood from healthy individuals are shown by the hatched line, and non-paired T-test indicates significance as shown.
Immunophenotyping of synovia effusions
All synovial effusions examined showed CD146 expression on T lymphocytes (16.32±6.06%), which was highest (26.6%) on a patient with chronic internal derrangement (Figure 2). Expression of CD146 was highly skewed towards CD3+CD4+ T lymphocytes (24.06% ± 8.20%) as compared to that of CD3+CD8+ T lymphocytes (6.19% ± 5.22%) within synovial effusions (p < 0.0001). Interestingly, 2 of the SF from RA patients displayed ratios of the percentages of CD146+CD4+CD3+:CD146+CD8+CD3+ of approximately 50:1, higher than the remaining SF samples. For the synovial effusions examined, the CD146 expression was significantly greater than found in normal blood for CD3+ cells (p<0.0001), CD4+ T cells (p<0.0001), and CD8+ T cells (p=0.0070). SE lymphocytes bearing CD146+ were found to be predominantly CD45RO+ (96.28%±4.33%), whereas only 13.86%±14.56% coexpressed CCR7. 28% of the CD146+ T lymphocytes also coexpressed CD62L (n=22). Furthermore, we also found 62.27% ± 17.64% of CD146+ T lymphocytes coexpressing CXCr4, whereas CCR5 coexpression was 71.68% ± 31.36 [(n=11), 1 polyarthritis, 2 reactive arthritis (two samples, same patient), seven rheumatoid arthritis]. Interestingly, among these, one of the specimens, from a patient with psoriatic arthritis, had less than 1% of the CD146+CD3+ lymphocytes coexpressing CCR5.
Immunophenotyping of peripheral blood specimens from patients
The peripheral blood from 10 patients with various musculoskeletal diseases revealed that 4.71 ± 2.48% of the CD3+ lymphocytes expressed CD146 (p=0.0258 compared to the healthy peripheral blood cohort), 6.29 ± 2.74% of the CD3+ CD4+ lymphocytes expressed CD146 (p<0.0017 compared to the healthy peripheral blood cohort), and 2.55 ± 1.65 of the CD3+CD8+ lymphocytes expressed CD146 (p=0.5008 compared to the healthy peripheral blood cohort) (Figure 2).
No significant differences were observed among the CD146+ lymphocytes subsets in patients suffering from various diseases, nor were any correlations observed between the percentages of CD146+ cells detected in the synovial effusions and serum markers of inflammation such as CRP and ESR. There was a positive correlation between the percentages of CD3+ lymphocytes expressing CD146+ cells and of CD4+ CD146+ lymphocytes in the circulation and CRP (r2=0.602 and r2=0.676, respectively), while the correlation was very weak between the percentages of CD8+ T lymphocytes expressing CD146 and CRP. A weak correlation was also observed between the percentage of CD4+CD146+ and of CD3+CD146+ T lymphocytes and ESR (r2 = 0.481 and r2 = 0.431, respectively).
Gene expression studies on CD146+ cells
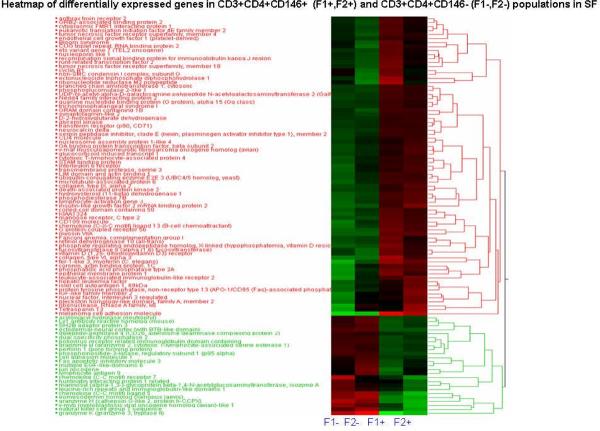
Sufficient numbers of cells were present in the synovial effusions from 2 patients for sorting of CD3+CD4+CD146+ and CD3+ CD4+ CD146− populations. These cells were then used for RNA extraction and subsequent gene expression profiling as described above. Two hundred twenty-nine probe sets representing 158 unique genes displayed the most pronounced differences between these populations and these genes by Ingenuity Pathway analysis and were found to be involved in TGF-b signaling, thrombopoietin signaling, T cell receptor signaling and cytotoxic T lymphocyte-mediated apoptosis. (Figure 3). As would be expected, the CD3+CD4+CD146+ cells displayed the greatest increase in the expression of MelCAM (CD146) compared to the CD146− sorted cells, giving confirmation to the fidelity of these experiments. Of particular interest was the finding that LAIR2, CXCL13, CD109, IL-6R, IL6ST (gp130), TNFRsf18, and TNFR sf4 genes were upregulated in the CD146+ population. Conversely CD3+CD4+CD146+ lymphocytes displayed decreased expression of CCR7 and cytotoxicity-associated genes such as granzymes b, h and k, perforin, and CCL5. A strikingly similar expression pattern was observed in the additional SE sorted using CD2 andCD146 (data not shown). While immunophenotyping of the synovial effusions confirmed the observed differences in gene expression of CD146 and CCR7, such confirmation was not available for CXCL13, CD109, CD130, IL-6R, TNFRsf18, and TNFR sf4 due to inadequate cell numbers from these specimens.
Figure 3.
Heat map of differential gene expression in CD3+CD4+ CD146+ (F1+, F2+) and CD3+CD4+ CD146− (F1−, F2−) populations from synovial effusions (one RA and one sarcoid). Cluster analysis was applied to gene expression data derived from all probes on HG-U133+ PM gene chips at FC >2.0. The level of expression for the top 100 genes in each sample relative to the median level of expression of that gene across all samples is represented using a red, black and green color scale (green – below median; black – equal to median; red - above median).
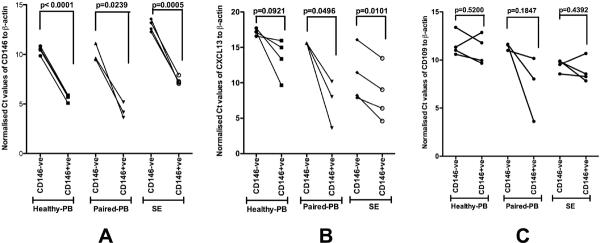
Using QPCR, mRNA expression of CXCL13 was found to be significantly higher in CD146+ T-cells from SF compared to CD146− T lymphocytes from the same patients (p=0.0101). Similar differences for CXCL13 mRNA expression were also noted for peripheral bloods from these same patients in terms of CD146+ T lymphocytes compared to CD146− T-cells (p=0.0496). mRNA levels of CXCL13 from peripheral blood CD146+ T lymphocytes of healthy individuals were found to be higher than those from CD146− T lymphocytes, but this difference was not statistically significant (p=0.0921) (Figure 4). The QPCR data validate the findings of microarray experiments, where we found increased expression of CXCL13 in CD146+ T lymphocytes compared to CD146− T cells in SF. CD109 upregulation in SE was similarly confirmed by QPCR, although upregulation of this gene in peripheral blood specimens was more variable (Figure 4).
Figure 4.
Normalized Ct (ΔcT) values for CD146 (A), CXCL13 (B), and CD109 (C). Lines indicate the change in Ct values between CD146+ cells and CD146− cells from one patient specimen. CD146, as a control, showed strong upregulation in CD146 positive cells for all samples. Similar results were obtained for CXCL13. CD109 upregulation was observed in all blood samples from arthritis patients, and in 3 out of four blood samples from healthy individuals as well as 3 out of four samples from synovial effusions.
DISCUSSION
The current study definitively demonstrates elevated numbers of CD146+ T lymphocytes in both the synovial fluids and peripheral blood of patients with a variety of inflammatory and non-inflammatory joint effusions. Of particular interest is the significant increase in the percentage of CD4 lymphocytes expressing CD146 in the peripheral blood compared to healthy individuals, as this is the major lymphocyte subset found in many joint effusions. Whether these circulating cells reflect cells primed to transmigrate to the synovial fluid of those which have emigrated from those fluids remains to be determined. CD146+ peripheral blood T lymphocytes have previously been identified by our group as CD45RO+, CD45RA−, and CCR7− consistent with an effector memory immunophenotype and similar findings were made in the current study of synovial fluid T cells. CD146+ CD4+ lymphocytes were found to be predominantly CD45RO+, CCR7−, and with the exception of the psoriatic arthritis synovial fluid, CCR5+. These data are consistent with the findings of Gattorno et al (11) in which synovial fluids from juvenile idiopathic arthritis were found to be enriched with CD4+CCR7− memory T lymphocytes. The disparate results for CCR5 staining on the cells from the patient with psoriatic arthritis, as well as the finding of elevated CD146+ CD8+ lymphocytes only in the patient with a chronic internal derangement suggests a different role in pathogenesis for these cells in such conditions, and perhaps provide a method to assist in differential diagnosis. Many more patients with these conditions need to be studied before such speculation can be confirmed.CD4+ T lymphocytes are the predominant population of cells infiltrating the synovia and are thought to play a crucial role in inflammatory arthritis (12, 13), in contrast to noninflammatory joint disease, such as osteoarthritis, where fewer cells are found in the synovial fluid. Adhesion markers on synovial fluid T lymphocytes, other than CD146, have been studied in several previous reports (14, 15). Ueki et al (14) found that the expression of CD11a, CD18, CD49d, and CD49e on CD4+ T lymphocytes in synovial fluids from RA and OA patients was increased compared to peripheral blood from these patients, and only CD49d expression differed in RA and OA fluids. Agarwal and Brenner (15) have recently presented an excellent review of the role of adhesion markers in synovial inflammation. Amidst the numerous adhesion molecules implicated in arrest, transmigration, and retention of leukocytes into the synovium, no mention was made of CD146. Previous studies have demonstrated that CD146 expression on T lymphocytes can mediate or enhance binding of these cells to endothelial monolayers (5,6). On endothelial monolayers, CD146 is found at the endothelial junctions (2) and has been shown to bind via both homotypic and heterotypic mechanisms (1). It is therefore a logical hypothesis that lymphocytes expressing CD146 can bind preferentially to endothelial junctions via homotypic binding, and indeed Guezguez and colleagues have presented data demonstrating that CD146 promotes lymphocytes rolling on endothelium via microvilli induction and is an endothelial adhesion receptor on CD4 T lymphocytes (6). The current data suggest that CD146 may well play a role in T lymphocyte migration, particularly effector memory CD4+ T lymphocytes, into synovia during a wide assortment of arthritic conditions including but not limited to rheumatoid arthritis. Gene expression revealed a strong association of tensin 3 with CD146 among the SF CD4+T lymphocytes. Tensin 3 has been postulated to play a role in EGF-mediated cell migration and it is interesting to speculate that it may play a role in the migration of cells into the synovium (16).
The association of CD146+ CD4 T lymphocytes with CXCL13 and CD109 both in the synovial fluid and in the peripheral blood is intriguing. CXCL13 is a known B–cell chemoattractant and arrest chemokine for B lymphocytes in high endothelial venules (17). Recently Manzo et al (18) reported CXCL13 being synthesized by mature antigen-experienced T helper cells from the rheumatoid joint. In contrast to previous descriptions of follicular B helper T cells (19), Manzo reported the CXCL13-synthesizing cells from SF did not express CXCR5 and were largely negative for CCR7. In many ways the cells described by Manzo are similar to the CD146+ CD4 lymphocytes reported in this study, as CCR7 and CXCR5 are both lacking on the cells currently described. The upregulation of the CD109 gene, confirmed by RT-PCR, is also interesting, and, although the role of CD109 in hematopoietic cells remains largely unknown, it has been suggested that this antigen plays a role in antibody-inducing T helper function (20, 21). The negative correlations between CD146 expression on CD4+ T lymphocytes in synovial fluids and granzymes b, h, and k and CCL5 are not unexpected. The granzymes have been previously demonstrated to be associated with cytotoxic CD8 T lymphocytes (22), and, in juvenile idiopathic arthritis, CCL 5 has been demonstrated to be associated with CD8 T lymphocytes in synovial fluids (23).
Together, the expression of CD109 and CXCL13 on the CD4+CD146+ T lymphocytes in the synovial fluids suggest a potential role for these cells in facilitating B lymphocyte responses to this site consistent with the hypothesis put forward by Manzo et al (18). As a point of further speculation, CD146+ T lymphocytes, as discussed above, may have augmented ability to adhere to or migrate through endothelial monolayers, and thus may function as sentinels of inflammation for B lymphocytes. Clearly, much work remains to discern whether this speculation is warranted. Cell sorting of CD146+ and CD146− populations of CD4 lymphocytes from synovial fluids with subsequent direct examination of the populations to adhere to, and migrate through, the endothelium, and to synthesize CXCL13, would be invaluable. Unfortunately, as in the current study, the number of cells available in SE samples is variable and often a limiting factor in these studies.
The sorted CD4+CD146+ T lymphocytes demonstrated substantially higher gene expression of proinflammatory cytokine receptors CD130 (gp130), IL-6R, TNFRsf18 (tumor necrosis factor- receptor), and TNFR sf4 than the corresponding CD146− counterparts. A number of cytokines, including both TNF and IL-6, have been of great interest in the pathogenesis of arthritic diseases and therapies have been devised based on inhibiting TNF-α and IL-6 (24,25,26). The significance of this is uncertain, but the association of the pertinent cytokine receptors with an endothelial adhesion protein on a defined subpopulation of T lymphocytes is intriguing.
A recent report by Cayrol et al (27) identified effector memory CD4 and CD8 lymphocytes expressing CD146 in neuroinflammation. These cells were found to produce more inflammatory cytokines, interferon-gamma, and IL-17 than corresponding CD146 negative cells. The authors suggest the CD146+ T lymphocytes play a role in CNS inflammatory reactions, including those found in multiple sclerosis. Such data are highly consistent with the present observations, and strongly suggest that the CD146 T lymphocytes presently identified may play a role in various inflammatory processes other than diseases involving joint effusions.
In summary, CD146 identifies a unique subpopulation of T lymphocytes that may perform an important role in the pathogenesis of many joint diseases as well as other inflammatory diseases. The current data demonstrate that CD4+ CD146+ lymphocytes preferentially accumulate in synovial fluids and express distinct phenotypes and genotypes from the CD4+CD146−. Many of these differences include chemokines and cytokine receptors of known relevance in arthritis and strongly suggest that CD146 can be used to identify functionally important subsets of T lymphocytes in diseases with joint effusions. While the current data are preliminary in nature, they do identify a means of identifying a potentially important component of immune response in arthritic and other inflammatory diseases and thus warrant further investigation.
Acknowledgements
This work was supported by the Intramural Research Programs of the National Heart, Lung, and Blood Institute and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. We thank Peter J. Munson, PhD for his help and guidance with the gene profiling studies and Ms Kimberly Woodhouse for her technical assistance in QPCR analysis.
References
- 1.Shih IE. The Role of CD146 (MelCAM) in Biology and Pathology. J Pathol. 1999;189:4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Bardin N, Anfosso F, Masse JM, Cramer E, Sabatier F, Le Bivic A, Sampol J, Dignat-George F. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood. 2001;98(13):3677–3684. doi: 10.1182/blood.v98.13.3677. [DOI] [PubMed] [Google Scholar]
- 3.Pickl WF, Fischer GF, Petzelbauer P, Fae I, Waclavicek M, Stockl J, Scheinecker C, Vidicki T, Aschauer H, Johnson JP, Knapp W. MUC18/MCAM (CD146), an activation antigen of human T lymphocytes. Journal of Immunology. 1997;158(5):2107–2115. [PubMed] [Google Scholar]
- 4.Elshal MF, Khan SS, Takahashi Y, Solomon MA, McCoy JP. CD146 (Mel-CAM), an Adhesion Marker of Endothelial Cells, is A Novel Marker of Lymphocyte Subset Activation in Normal Peripheral Blood. Blood. 2005;106(8):2923–2924. doi: 10.1182/blood-2005-06-2307. [DOI] [PubMed] [Google Scholar]
- 5.Elshal MF, Khan SS, Raghavachari N, Takahashi Y, Barb J, Bailey JJ, Munson PJ, Solomon MA, Danner RL, McCoy JP. A Unique Population of Effector Memory Lymphocytes Identified by CD146 having a Distinct Immunophenotypic and Genomic Profile. BMC Immunology. 2007;8:29. doi: 10.1186/1471-2172-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guezguez B, Vigneron P, Lamerant N, Kieda C, Jaffredo T, Dunon D. Dual Role of Melanoma Cell Adhesion Molecule (MCAM)/CD146 in Lymphocyte Endothelium Interaction: MCAM/CD146 Promotes Rolling via Microvilli Induction in Lymphocyte and Is an Endothelial Adhesion Receptor. Journal of Immunology. 2007;179:6673–6685. doi: 10.4049/jimmunol.179.10.6673. [DOI] [PubMed] [Google Scholar]
- 7.Buckley CD. Why Do Leukocytes Accumulate within Chronically Inflamed Joints? Rheumatology. 2003;42:1433–1444. doi: 10.1093/rheumatology/keg413. [DOI] [PubMed] [Google Scholar]
- 8.Firestein GS. Evolving Concepts of Rheumatoid Arthritis. Nature. 2003;423(6937):356–61. doi: 10.1038/nature01661. 15. [DOI] [PubMed] [Google Scholar]
- 9.Neidhart M, Wehrli R, Bruhlmann P, Michel BA, Gay RE, Gay S. Synovial Fluid CD146 (MUC18, a Marker for Synovial Membrane Angiogenesis in Rheumatoid Arthritis. Arth Rheumatism. 1999;42(4):622–630. doi: 10.1002/1529-0131(199904)42:4<622::AID-ANR4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.Middleton J, Americh L, Gayon R, Julien D, Mansat M, Mansat P, Anract P, Cantagrel A, Cattan P, Reimund JM, Aguilar L, Amalric F, Girard JP. A Comaprative Study of Endothelial Cell Markers Expressed in Chronically Inflamed Human Tissues: MECA-79, Duffy antigen Receptor for Chemokines, von Willebrand factor, CD31, CD34, CD105, and CD146. J Pathology. 2005;206:260–268. doi: 10.1002/path.1788. [DOI] [PubMed] [Google Scholar]
- 11.Gattorno M, Prigione I, Morandi F, Gregorio A, Chiesa S, Ferlito F, Favre A, Uccelli A, Gambini C, Martini A, Pistoia V. Phenotypic and Functional Characterization of CCR7+ and CCR7− CD4+ Memory T Cells Homing to the Joints in Juvenile Idiopathic Arthritis. Arthritis Res Ther. 2005;7:R256–R267. doi: 10.1186/ar1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN. The Presence of Cytokine-Suppressive CD4+CD25+ T Cells in the Peripheral Blood and Synovial Fluid of Patients with Rheumatoid Arthritis. Scand J Immunology. 2005;62:312–317. doi: 10.1111/j.1365-3083.2005.01656.x. [DOI] [PubMed] [Google Scholar]
- 13.Lundy SK, Sarkar S, Tesmer LA, Fox DA. Cells of the Synovium in Rheumatoid Arthritis: T Lymphocytes. Arthritis Res and Ther. 2007;9:202. doi: 10.1186/ar2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueki Y, Eguchi K, Shimada H, Nakashima M, Ida H, Miyake S, Nagataki S, Tominaga Y. Increase in adhesion molecules on CD4+ cells and CD4+ cell subsets in synovial fluid from patients with rheumatoid arthritis. J Rheumatology. 1994;21(6):1003–1010. [PubMed] [Google Scholar]
- 15.Agarwal SK, Brenner MB. Role of Adhesion Molecules in Synovial Inflammation. Curr Opin Rheumatol. 2006;18:268–276. doi: 10.1097/01.bor.0000218948.42730.39. [DOI] [PubMed] [Google Scholar]
- 16.Cui Y, Liao YC, Lo SH. Epidermal growth factor modulates tyrosine phosphorylation of a novel tensin family member, tensin3. Mol Cancer Res. 2004;2(4):225–32. [PubMed] [Google Scholar]
- 17.Kanemitsu N, Ebisuno Y, Tanaka T, Otani K, Hayasaka H, Kaisho T, Akira S, Katagiri K, Kinashi T, Fujita N, Tsuruo T, Miyasaka M. CXCL13 is an Arrest Chemokine for B Cells In High Endothelial Venules. Blood. 2005;106(8):2613–2618. doi: 10.1182/blood-2005-01-0133. [DOI] [PubMed] [Google Scholar]
- 18.Manzo A, Vitolo B, Humby F, Caporali R, Jarrossay D, Dell'Accio F, Ciardelli L, Uguccioni M, Montecucco C, Pitzalis C. Mature Antigen-Experienced T Helper Cells Synthesize and Secrete the B Cell Chemoattractant CXCL13 in the Inflammatory Environment of the Rheumatoid Joint. Arth Rheumatism. 2008;58(11):3377–3387. doi: 10.1002/art.23966. [DOI] [PubMed] [Google Scholar]
- 19.Rasheed AU, Rahn HP, Sallustro F, Lipp M, Muller G. Follicular B Helper T Cell Activity is Confined to CXCR5hi ICOShi CD4T Cells and is Independent of CD57 Expression. Europ J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 20.Lin M, Sutherland DR, Horsfall W, Totty N, Yeo E, Nayar R, Wu XF, Schuh AC. Cell Surface Antigen CD109 is a Novel Member of the α2 Macroglobulin/C3, C4, C5 Family of Thioester-containing Proteins. Blood. 2002;99(5):1683–1691. doi: 10.1182/blood.v99.5.1683. [DOI] [PubMed] [Google Scholar]
- 21.Suciu-Foca S, Reed E, Rubinstein P, MacKenzie W, Ng A, King DW. A Late-Differentiation Antigen Associated with the Helper Inducer Function of Human T Cells. Nature. 1985;318(5):465–467. doi: 10.1038/318465a0. [DOI] [PubMed] [Google Scholar]
- 22.Bade B, Boettcher HE, Lohrmann J, Hink-Schauer C, Bratke K, Jenne DE, Virchow JC, Jr, Luttmann W. Differential expression of the granzymes A, K and M and perforin in human peripheral blood lymphocytes. Int Immunol. 2005;17(11):1419–28. doi: 10.1093/intimm/dxh320. [DOI] [PubMed] [Google Scholar]
- 23.Pharoah DS, Varsani H, Tatham RW, Newton KR, de Jager W, Prakken BJ, Klein N, Wedderburn LR. Expression of the inflammatory chemokines CCL5, CCL3 and CXCL10 in juvenile idiopathic arthritis, and demonstration of CCL5 production by an atypical subset of CD8+ T cells. Arthritis Res Ther. 2006;8(2):R50. doi: 10.1186/ar1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan FM, McInnes IB. Evidence that Cytokines Play a Role in Rheumatoid Arthritis. J Clin Invest. 2008;118(11):3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choy EHS, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H, Cheung N, Williams B, Hazelman B, Price R, Yoshizaki K, Nishimoto N, Kishimoto T, Panayi GS. Therapeutic Benefit of Blocking Interleukin-6 Activity with an Anti-Interleukin-6 Receptor Monoclonal Antibody in Rheumatoid Arthritis. Arth Rheumatism. 2002;46(12):3143–3150. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 26.Hennigan S, Kavanaugh A. Interleukin-6 Inhibitors in the Treatment of Rheumatoid Arthritis. Ther Clin Risk Manag. 2008;4(4):767–775. doi: 10.2147/tcrm.s3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cayrol R, Kebir H, Ifergan I, Dodelet-Devillers A, Terouz S, Haqqani A, Poirier J, Stanimirovic D, Duquette P, Arbour N, Prat A. MCAM/CD146 is Expressed by Brain Endothelial Cells and Defines a Unique Effector Memory Lymphocyte Subset Involved in Neuroinflammation. Clin Immunol. 2009;131(Suppl):OR21. S12. [Google Scholar]