Abstract
The metabotropic glutamate receptors (mGluRs) are family C G-protein-coupled receptors that participate in the modulation of synaptic transmission and neuronal excitability throughout the central nervous system. The mGluRs bind glutamate within a large extracellular domain and transmit signals through the receptor protein to intracellular signaling partners. A great deal of progress has been made in determining the mechanisms by which mGluRs are activated, proteins with which they interact, and orthosteric and allosteric ligands that can modulate receptor activity. The widespread expression of mGluRs makes these receptors particularly attractive drug targets, and recent studies continue to validate the therapeutic utility of mGluR ligands in neurological and psychiatric disorders such as Alzheimer’s disease, Parkinson’s disease, anxiety, depression, and schizophrenia.
Keywords: G-protein-coupled receptors, neuromodulation, allosterism, synaptic plasticity
INTRODUCTION
L-glutamate serves as the neurotransmitter at the majority of excitatory synapses in the mammalian central nervous system (CNS). The existence of neuromodulatory glutamate receptors, called metabotropic glutamate receptors (mGluRs), provides a mechanism by which glutamate can modulate cell excitability and synaptic transmission via second messenger signaling pathways. The widespread distribution of mGluR proteins suggests that these neuromodulatory receptors have the ability to participate in numerous functions throughout the CNS and may represent ideal targets for therapeutic intervention in a wide variety of CNS disorders.
STRUCTURAL FEATURES OF mGluRs
mGluRs are members of the G-protein-coupled receptor (GPCR) superfamily, the most abundant receptor gene family in the human genome. GPCRs are membrane-bound proteins that are activated by extracellular ligands such as light, peptides, and neurotransmitters, and transduce intracellular signals via interactions with G proteins. The resulting change in conformation of the GPCR induced by ligand binding activates the G protein, which is composed of a heterotrimeric complex of α, β, and γ subunits. In their inactive state, G proteins are bound to guanosine 5/ - diphosphate (GDP); activation of the G protein causes the exchange of guanosine 5/-triphosphate (GTP) for GDP within the α subunit. Activated G protein subunits then modulate the function of various effector molecules such as enzymes, ion channels, and transcription factors. Inactivation of the G protein occurs when the bound GTP is hydrolyzed to GDP, resulting in reassembly of the heterotrimer.
The GPCR family contains several subgroupings, and the majority of classical neurotransmitter GPCRs belong to family A. These receptors are often termed the rhodopsin-like GPCRs and are structurally similar in that they consist of an extracellular N-terminal domain, seven transmembrane-spanning domains, and an intracellular C-terminus. In contrast to family A receptors, mGluRs belong to class C GPCRs. These receptors are distinguished from their family A relatives by the presence of a large extracellular N-terminal domain that contains the endogenous ligand-binding site, discussed in more detail below. Family C GPCRs also include GABAB receptors, calcium-sensing receptors, pheromone receptors, and taste receptors (1). Genes encoding eight mGluR subtypes have been identified, many with multiple splice variants that are differentially expressed in distinct cell types throughout the CNS. mGluRs are subclassified into three groups based on sequence homology, G-protein coupling, and ligand selectivity. Group I includes mGluRs 1 and 5, Group II includes mGluRs 2 and 3, and Group III includes mGluRs, 4, 6, 7, and 8 (Table 1).
Table 1.
Key features of mGluRs
| Group | Receptor/splice variants |
CNS expression | Synaptic localization | Signaling pathways of group |
|---|---|---|---|---|
| Group I | mGluR1 a,b,c,d,e,f Taste mGluR1 |
Widespread in neurons Taste buds |
Predominantly postsynaptic |
Phospholipase C stimulation Stimulation of adenylyl cyclase (some systems) MAP kinase phosphorylation |
|
| ||||
| mGluR5 a,b |
Widespread in neurons, astrocytes |
|||
|
| ||||
| Group II | mGluR2 | Widespread in neurons | Presynaptic and postynaptic |
Inhibition of adenylyl cylcase Activation of K+ channels Inhibition of Ca++ channels |
|
| ||||
| mGluR3 GRM3A2 GRM3A4 GRM3A2A3 |
Widespread in neurons, astrocytes |
|||
|
| ||||
| Group III | mGluR4 | Widespread in neurons, High in cerebellum |
Predominantly presynaptic |
Inhibition of adenylyl cylcase Activation of K+ channels Inhibition of Ca++ channels |
| Taste mGluR4 | ||||
| Taste buds | ||||
|
|
||||
| mGluR6 a,b,c |
Retina | Postsynaptic in ON-bipolar retinal cells |
Stimulation of cGMP phosphodiesterase (mGluR6) |
|
|
| ||||
| mGluR7 a,b,c,d,e |
Widespread in neurons | Active zone of presynaptic terminals |
||
|
| ||||
| mGluR8 a,b,c |
Lower and more restricted expression than mGluR4/7 |
Predominantly presynaptic |
||
The Venus Flytrap Domain
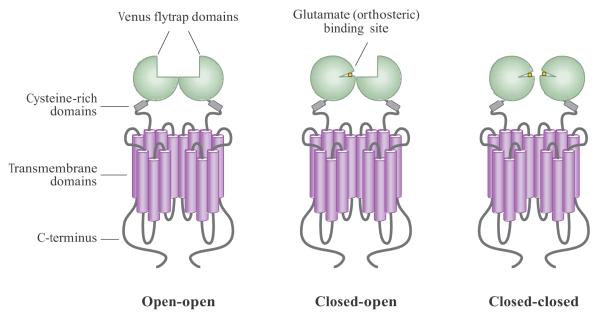
As mentioned above, mGluRs contain a large extracellular N-terminal domain, termed the Venus flytrap domain (VFD), which contains the glutamate-binding site (1) (Figure 1). The examination of crystal structures of N-terminal domains of mGluR1 (2, 3), mGluR3, and mGluR7 (4) reveals that each VFD consists of two lobes that sit one atop the other and bind glutamate in a cleft between them. Evidence suggests that two VFDs dimerize together, back to back, and large conformational changes are induced when agonists bind to one or both VFDs (5). Three main states of the VFD dimer exist: open-open, open-closed, and closed-closed (Figure 1). The open-open (inactive) conformation is stabilized by antagonists; the open-closed and closed-closed conformations are induced by the binding of ligand to one or two protomers. The mutation of residues that prevent closure of the VFD can switch the pharmacology of antagonists to agonists (6), indicating that the relative orientation of these domains is important for receptor activation. For glutamate binding, several conserved residues span lobes 1 and 2 and make critical contacts with the glutamate molecule (1, 7, 8). In addition to binding glutamate, VFDs also bind divalent cations such as magnesium and calcium, which can potentiate or activate the receptor (3, 9, 10).
Figure 1.
Schematic diagram of the mGluR dimer in different activity states. mGluR dimers contain two large extracellular domains called the Venus flytrap domains (VFDs), which bind glutamate and other orthosteric ligands. The cysteine-rich domain links the VFDs to seven transmembrane-spanning domains; the C-terminus faces intracellularly and is often subject to alternative splicing to generate different C-terminal protein tails. The open-open state (left) is the inactive state and can be stabilized by antagonists. Either one or two VFDs can then bind glutamate, resulting in active receptor conformations.
Cysteine-Rich Domains
Conformational changes induced by ligand binding are propagated from the VFD via cysteine-rich domains (CRDs) to the hepatahelical domain (HD)–C-terminal tail. The CRD contains nine critical cysteine residues, eight of which are linked by disulfide bridges (4). Crystallization and mutagenesis studies have shown that the signal induced by ligand binding is transmitted from the VFDs through the CRDs, in part because of a disulfide bridge formed by the ninth CRD cysteine with a cysteine in lobe 2 of the VFD (4, 11). Rondard et al. (11) recently showed that mutation of Cys234 in the VFD of mGluR2 resulted in a receptor that could be expressed at the surface, dimerize, and bind ligands appropriately but could not induce intracellular signaling. The receptor was still functional, however, as shown by normal signal transduction induced by an allosteric ligand that bound within the HD rather than the VFD. Further studies identified the CRD-located Cys518 as the partner of Cys234, and similar results were shown using homologous cysteines within mGluR5. These results suggest that a disulfide bridge linking the CRD and the VFD may be globally involved in propagation of signals induced by orthosteric agonist binding to mGluRs.
Heptahelical Domain and Intracellular Loops
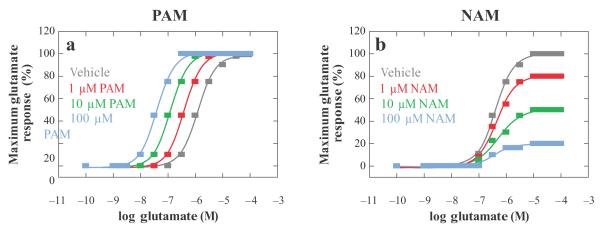
mGluR HDs share very low homology to family A GPCRs, and it has been proposed that the second intracellular loop (i2) of mGluRs may play a role similar to that of the i3 loop in rhodopsin-related receptors in regulating G protein coupling specificity (12, 13). The i2 loop is also a site for regulation by kinases, including G-protein-coupled receptor kinase 2 (14). The majority of characterized allosteric modulators of mGluRs that positively and negatively affect glutamate activity bind within the HD (reviewed in 15). Positive allosteric modulators (PAMs) do not activate the receptor directly in most systems but potentiate the response of the receptor to orthosteric agonists (Figure 2, left). Allosteric agonists bind to a site other than the orthosteric site and directly activate the receptor. Negative allosteric modulators (NAMs) antagonize the activity of agonists in a noncompetitive fashion by binding to a site other than the agonist, in this case glutamate, binding site (Figure 2, right). Interestingly, truncation of the N-terminal domain of mGluR5 results in a receptor that can be directly activated by PAMs (16). This suggests that there is a conformational restraint induced by the VFD-CRD region that prevents PAMs from acting as agonists until glutamate is bound.
Figure 2.
Simple schematic representation of the positive allosteric regulator (PAM) and negative allosteric regulator (NAM) activity using an in vitro functional assay. (a) Monitoring of a functional assay such as calcium mobilization shows that increasing PAM concentrations progressively shift the glutamate concentration response for an mGluR to the left. Depending on the assay used, PAMs can also cause an increase in the maximal agonist response. (b) Increasing NAM concentrations progressively shift the magnitude of the concentration response curve and produce little change in potency, indicating a noncompetitive form of antagonism.
C-Terminus
The C-termini of mGluRs are important regions for modulating G protein coupling. Additionally, this region of several of the mGluRs is subject to alternative splicing, regulation by phosphorylation, and modulatory protein-protein interactions. These issues are discussed in more detail in the following sections.
SIGNAL TRANSDUCTION
mGluR Activation
mGluRs, and other family C GPCRs, are constitutive dimers (reviewed in 1). There is some controversy as to whether binding of glutamate to only one protomer within the dimer is sufficient to activate the entire complex. In the case of the GABAB receptor, which is a heterodimer consisting of GABAB1 and GABAB2 subunits, ligand binding to GABAB1 is sufficient to activate the receptor (17). This may not be the case for mGluRs, however. For example, glutamate was unable to activate dimers formed by one wild-type and one mutant form of mGluR1, suggesting that glutamate binding to one protomer is not sufficient for activation (18). In contrast, Kniazeff et al. (19) showed that one glutamate molecule can activate mGluR5 homodimers but that occupation of both VFDs with ligand is more effective. Suzuki et al. showed that glutamate binding to one protomer exerts negative cooperativity for binding to the second protomer (20), suggesting that glutamate binds to both dimers but that this binding can induce complexities in receptor pharmacology.
General Signaling Profiles
In general, group I mGluRs couple to Gq /G11 and activate phospholipase Cβ , resulting in the hydrolysis of phosphotinositides and generation of inositol 1,4,5-trisphosphate (IP3 ) and diacyl- glycerol (Table 1). This classical pathway leads to calcium mobilization and activation of protein kinase C (PKC). However, it is now recognized that these receptors can modulate additional signaling pathways including other cascades downstream of Gq as well as pathways stemming from Gi/o , Gs , and other molecules independent of G proteins (21). Depending on the cell type or neuronal population, group I mGluRs can activate a range of downstream effectors, includ- ing phospholipase D, protein kinase pathways such as casein kinase 1, cyclin-dependent protein kinase 5, Jun kinase, components of the mitogen-activated protein kinase/extracellular receptor kinase (MAPK/ERK) pathway, and the mammalian target of rapamycin (MTOR)/p70 S6 kinase pathway (22–25). The latter pathways, MAPK/ERK and MTOR/p70 S6 kinase, are thought to be particularly important for the regulation of synaptic plasticity by group I mGluRs.
In contrast to group I mGluRs, group II and III mGluRs are coupled predominantly to Gi/o proteins. Gi/o linked receptors are classically coupled to the inhibition of adenylyl cyclase and directly regulate ion channels and other downstream signaling partners via liberation of Gβγ subunits. As with group I mGluRs, it is becoming increasingly appreciated that group II and group III mGluRs also couple to other signaling pathways, including activation of MAPK and phosphatidyl inositol 3-kinase PI3 kinase pathways (26), providing further complexity regarding the mechanisms by which these receptors can regulate synaptic transmission.
ALTERNATIVE SPLICING
Several mGluR subtypes undergo alternative splicing; in many cases, this generates different C-terminal tails (Table 1). The gene for mGluR1a encodes four main distinct C-terminal splice variants: mGluR1a, b, c, and d; of these, mGluR1a is the longest (reviewed in 21) (Table 1). mGluR1e encodes an inactive form of mGluR1 that terminates before the transmembrane domains (27). In rats, another splice variant termed mGluR1f encodes a protein with a sequence identical to that of mGluR1b (28). mGluR5 exists as two main splice variants: mGluR5a and mGluR5b (29, 30). Whereas no splicing events have been reported for mGluR2, mGluR3 RNA has recently been shown to undergo alternative splicing to yield at least four variants: full length mGluR3, GRM3A2 (missing exon 2), GRM3A4 (missing exon 4), and GRM3A2A3 (missing exons 2 and 3) (31); GRM3A4 appears to be the most abundant. This variant lacks the transmembrane domain of the receptor but can be translated in cell lines and in vivo, suggesting that it may function as a unique glutamate receptor. As with mGluR3, three mGluR6 splice variants can be generated, and two of these terminate within the N-terminal domain [mGluR6b and mGluR6c (32)]. Additionally, an isoform of mGluR8 in human brain, mGluR8c, is also predicted to encode only the N-terminal region of the receptor (33). It has been postulated that these short N-terminal proteins may be secreted and could serve as soluble receptors for glutamate or as dominant negative receptor variants (32, 33).
The C-terminal tails of mGluRs 7 and 8 also serve as key regions for alternative splicing. For example, five C-terminal isoforms of mGluR7 (mGluR7a-e) have been identified, and two distinct isoforms of mGluR8 have been cloned (mGluR8a and mGluR8b) that differ at their C-termini (34, 35). Although an mGluR4b splice variant has been reported (36), other accounts have been unable to replicate this finding (37). Interestingly, there are isoforms of mGluR1 and 4 that lack approximately half of the amino terminus. These receptor variants, known as taste mGluR1 and taste mGluR4, are located in taste buds (38–40). Owing to the lack of much of the traditional glutamate-binding domain, glutamate exhibits lower potency when inducing signaling via these truncated receptors compared with their full-length counterparts; nevertheless, these isoforms have an emerging role in mediating the taste of monosodium glutamate, or umami (reviewed in (41).
PROTEIN-PROTEIN INTERACTIONS
Multiple proteins interact directly with the C-terminal tails of each of the mGluR subtypes and play important roles in regulating mGluR signaling. The most well-characterized are Homer proteins, which contain PDZ 1 (post-synaptic density 95, discs large, zona occludens 1) domains that interact with the last several amino acids, PPxxF, of mGluR1a, mGluR5a, and mGluR5b (42–44). Distinct Homer gene and splice variants can differentially regulate localization of mGluR1 and mGluR5 receptors in transfected cells and neurons (42, 45). Homer proteins also participate in the assembly of protein complexes at the C-terminal tails of mGluRs that are critical for receptor activity or that mediate functional responses downstream of receptors. For example, the long isoform of protein PI3 kinase enhancer (PIKE-L) associates with mGluR5 via Homer interactions, allowing agonist activity at mGluR5 to prevent apoptosis when certain Homer isoforms are present (46).
Although protein-protein interactions between group II receptors and other proteins have not been as extensively characterized as interactions occurring with group I mGluRs, calmodulin and protein phosphatase 2C (PP2C) have recently been shown to interact with the C-terminus of mGluR3 (47). Interestingly, binding of PP2C is inhibited by phosphorylation of serine 845 by protein kinase A (PKA), and PP2C can dephosphorylate this site (47). This suggests that there is a dynamic regulation of phosphorylation/dephosphorylation of mGluR3 that is regulated by binding of PKA/PP2C. Another protein, Ran binding protein in the microtubule-organizing center (RanBPM), binds to group II and certain group III mGluR splice variants in the retina and may localize these mGluRs to specific synaptic locations in neurons (48). Isoforms of mGluR8 also interact with proteins in the retina called Band 4.1 proteins, and this interaction facilitates cell surface expression of receptors and reduces their ability to inhibit cAMP accumulation (49).
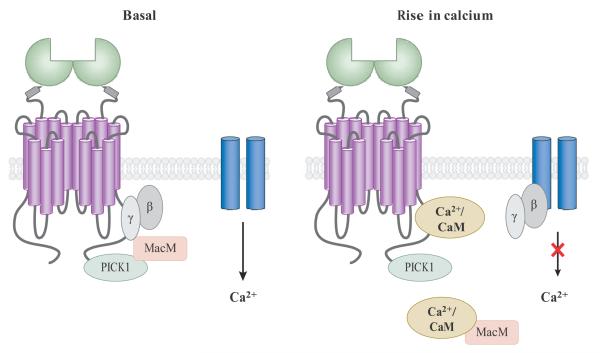
C-terminal tails of mGluR7 splice variants bind to a variety of interacting proteins such as the PDZ domain clustering protein PICK1 (protein interacting with C kinase 1) (50–53). PICK1 also binds protein kinase Cα (PKCα), and the three proteins form a complex at active zones of presynaptic terminals (52). The C-terminus of mGluR7a also interacts with Ca2+-calmodulin, G protein βγ subunits, and the protein MacMARCKS (macrophage myristoylated alanine rich C kinase substrate; MacM) (54), creating a complex interplay of signaling downstream of mGluR7 (Figure 3). To add further complexity, it has been shown that PKC phosphorylation of serine 862 inhibits the binding of Gβγ subunits and Ca2+-calmodulin. This complex signaling system may provide a way in which mGluR7, which is highly localized to presynaptic active zones, has extremely low affinity for glutamate and is predicted to only be activated during periods of intense synaptic activity, can serve as an integrator of multiple presynaptic signaling events including rises in intracellular calcium.
Figure 3.
Protein-protein interactions mediating the activity of mGluR7. It is proposed that in the resting state, mGluR7 binds PICK1, MacMARCKs (MacM), and Gβγ subunits; this binding prevents Gβγ subunits from inhibiting voltage-sensitive calcium channels (VSCCs; blue). Rises in calcium induce displacement of MacMARCKs and Gβγ subunits by calcium-calmodulin (Ca2+-CaM), promoting Gβγ subunit inhibition of VSCCs. Adapted from (52, 54).
Recently, an elegant set of studies was performed in which the mGluR7a-PICK1 interaction was disrupted by employing a viral vector in which the last nine amino acids of the C-terminus of mGluR7 were used as bait to compete with full-length mGluR7 for the binding of PICK1 (55). Infection of neurons with this construct in vivo inhibited mGluR7-PICK1 interactions and led to absence seizures and waveform changes specifically within thalamo-cortical brain regions, areas thought to mediate absence epilepsy phenotypes. Furthermore, knock-in mice in which the amino acids within the mGluR7 C-terminus that interact with PICK1 were mutated (56) exhibited spontaneous absence seizures (55), further substantiating that the interaction of mGluR7 and PICK1 is important for regulating mGluR7 signaling and that this protein-protein interaction may underlie certain disease states.
PHARMACOLOGICAL PROFILES OF METABOTROPIC GLUTAMATE RECEPTORS
Group I mGluRs
The first selective orthosteric agonist at group I mGluRs, (S)-3,5-dihydroxyphenylglycine [(S)-3,5-DHPG], has similar potencies at mGluR1 and mGluR5 and remains the most selective group I mGluR agonist. Most other group I mGluR agonists have activity at ionotropic glutamate receptors (i.e., quisqualate) or other mGluR subtypes [(1S,3R)-ACPD] (57). A related compound, 2-chloro-5-hydroxyphenylglycine (CHPG), is a selective orthosteric agonist of mGluR5 but has limited utility because of its relatively weak potency and efficacy. Multiple orthosteric antagonists for group I mGluRs, including MCPG and related phenylglycine derivatives, are of low potency but have been widely used in electrophysiology and other studies. More recently, more potent and selective orthosteric antagonists of group I mGluRs have emerged from further optimization of the 4-carboxyphenylglycine scaffold. These include compounds with improved potencies such as LY367385, which is highly useful as a selective antagonist of mGluR1 relative to mGluR5 (57).
A major advance in the identification of highly selective ligands for group I mGluRs came with the discovery of CPCCOEt as the first highly selective mGluR1 NAM (58). CPCCOEt acts in the HD and provided a major breakthrough in demonstrating the utility of targeting allosteric sites for discovery of highly subtype-selective mGluR antagonists. A large number of structurally distinct mGluR1-selective NAMs have become available, including Bay36–7620 (59), JNJ16259685 (60), FTIDC (61), YM 298198 (62), and others (63). Many of these compounds have nanomolar potencies and are useful for in vivo studies.
Varney et al. reported the discovery of two highly selective mGluR5 NAMs: SIB-1757 and SIB-1893 (64). Subsequent structural analogs MPEP (65) and MTEP (66) provided increased potency, selectivity, and brain penetration (67). Many structurally distinct and highly selective mGluR5 NAMs have now been reported, including compounds that have unique properties such as selective partial antagonists of mGluR5 (68). MTEP and MPEP, however, remain the most commonly used and selective mGluR5 antagonists for probing the function of this receptor in the CNS.
Highly selective PAMs have also been developed for each of these group I mGluR subtypes. These compounds do not activate the mGluR directly but act at an allosteric site in the HD to potentiate the response to glutamate, inducing robust leftward shifts of the glutamate concentration response relationship. Ro 67–7476, Ro 67–4853, and VU71 are selective mGluR1 PAMs (69, 70). Additionally, multiple mGluR5-selective PAMs have been identified including DFB, CPPHA, CDDPB, VU29, and ADX47273 (reviewed in 71). Whereas DFB and CPPHA are not sufficiently potent or soluble in physiological buffers to make them useful for studies of mGluR5 function, other mGluR5 PAMs are proving highly useful for assessment of the functional roles of mGluR5 in the CNS (72, 73).
Group II mGluRs
DCG-IV and (2R,4R)-APDC (abbreviations of all compounds, along with targets and mode of pharmacology, are defined in Table 2) were the first selective group II mGluR agonists (57). More recently, systemically active and highly selective agonists of group II mGluRs have been developed, providing valuable insights into the in vitro and in vivo functions of these receptors. The prototypical molecule in this series is LY354740, which has been followed by more recent compounds, including LY379268, now a commonly used tool for studies of group II mGluR function (57). These compounds are highly selective for group II mGluRs relative to other mGluR subtypes but do not differentiate between mGluR2 and mGluR3. Recently, an analog of LY354740 was reported that had mGluR2 agonist and mGluR3 antagonist activity (74). Thus far, no orthosteric antagonists have been discovered that are entirely specific for group II mGluRs among other family members, although LY341495 provides relatively high selectivity with nanomolar potency as a group II mGluR antagonist with submicromolar to micromolar potencies at all other mGluR subtypes (57).
Table 2.
Names, abbreviations, and actions of the drugs mentioned in text
| Nonselective Ligands | |||
|---|---|---|---|
| Compound | Chemical name | Target | Activity |
| L-glutamate | (S )-1-Aminopropane-1,3-dicarboxylic acid | Group I/Group II>Group III | Orthosteric agonist |
| (1S,3R)-ACPD | (1S,3R)-1-Aminocyclopentane-1,3-dicarboxylic acid | Group I/II>Group III | Orthosteric agonist |
| LY341495 | (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid | Group II>Group III>Group I | Orthosteric antagonist |
| Group I preferring ligands | |||
|---|---|---|---|
| Compound | Chemical name | Target | Activity |
| (S)-3,5-DHPG | (S)-3,5-dihydroxyphenylglycine | mGluR1/5 | Orthosteric agonist |
| L-quisqualate | (L)-(+)-a-Amino-3,5-dioxo-1,2,4-oxadiazolidine-2-propanoic acid | mGluR1/5/glutamatergic ion channels |
Orthosteric agonist |
| CHPG | (RS)-2-chloro-5-hydroxyphenylglycine | mGluR5 | Orthosteric agonist |
| (S)-MCPG | (S)-a-Methyl-4-carboxyphenylglycine | Group I/II | Orthosteric antagonist |
| LY367385 | (S)-(+)-a-Amino-4-carboxy-2-methylbenzeneacetic acid | mGluR1 | Orthosteric antagonist |
| CPCCOEt | 7-(Hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester | mGluR1 | NAM |
| Bay 36–7620 | (3aS,6aS)-Hexahydro-5-methylene-6a-(2-naphthalenylmethy l)- 1H-cyclopenta[c]furan-1-one |
mGluR1 | NAM |
| JNJ16259685 | 3-4-Dihydro-2H-pyrano[2,3-b]quinolin-7-yl-(cis-4-methoxycyclohexyl)-methanone | mGluR1 | NAM |
| FTIDC | 4-[1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2, 3-triazol-4-yl]-N-isopropyl-N-methyl-3,6-dihydropyridine-1(2H)-carboxamide |
mGluR1 | NAM |
| YM 298198 | 6-Amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimi dazole-2-carboxamide | mGluR1 | NAM |
| SIB-1757 | 6-Methyl-2-(phenylazo)-3-pyridinol | mGluR5 | NAM |
| SIB-1893 | 2-Methyl-6-(2-phenylethenyl)pyridine | mGluR5/mGluR4 | NAM/PAM |
| MPEP | 2-Methyl-6-(phenylethynyl)pyridine | mGluR5/mGluR4 | NAM/PAM |
| MTEP | 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]pyridine | mGluR5 | NAM |
| Ro 67–7476 | (S)-2-(4-fluorophenyl)-1-(toluene-4-sulfonyl)pyrrolidine | mGluR1 | PAM |
| Ro 67–4853 | Butyl (9H-xanthene-9-carbonyl)carbamate | mGluR1 | PAM |
| VU71 | 4-nitro-N-(1,4-diphenyl-1H-pyrazol-5-yl)benzamide | mGluR1 | PAM |
| DFB | [(3-Fluorophenyl)methylene]hydrazone-3-fluorobenzaldehyde | mGluR5 | PAM |
| CPPHA |
N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}- 2-hydroxybenzamide |
mGluR5 | PAM |
| CDDPB | 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide | mGluR5 | PAM |
| VU29 | 4-nitro-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide | mGluR5 | PAM |
| ADX47273 | [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazol-5-yl]-piperidin-1-yl}- methanone] |
mGluR5 | PAM |
| Group II preferring ligands | |||
|---|---|---|---|
| Compound | Chemical name | Target | Activity |
| DCG-IV | (2S,2/ R,3/ R)-2-(2/ ,3/ )-Dicarboxycyclopropyl)glycine | Group II | Orthosteric agonist |
| (2R,4R)-APDC | (2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate | Group II | Orthosteric agonist |
| LY354740 | (1S,2S,5R,6S)-2-Aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid | Group II | Orthosteric agonist |
| LY379268 | (1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid | Group II | Orthosteric agonist |
| LY487379 | 2,2,2-Trifluoro-N-[4-(2-methoxyphenoxy)phenyl]-N- (3-pyridinylmethyl)ethanesulfonamide |
mGluR2 | PAM |
| BINA | Biphenyl-indanone A | mGluR2 | PAM |
| Group III preferring ligands | |||
|---|---|---|---|
| Compound | Chemical name | Target | Activity |
| L-AP4 | L-(+)-2-Amino-4-phosphonobutyric acid | mGluR4,6,8>mGluR7 | Orthosteric agonist |
| (S)-3,4-DCPG | (S)-3,4-Dicarboxyphenylglycine | mGluR8>mGluR4/6 | Orthosteric agonist |
| Z-cyclopentyl AP4 | cis-( ± )-1-Amino-3-phosphonocyclopentane carboxylic acid | mGluR4>other Group III receptors | Orthosteric agonist |
| CPPG | (RS)-a-Cyclopropyl-4-phosphonophenylglycine | Group III | Orthosteric antagonist |
| MAP4 | (S)-2-Amino-2-methyl-4-phosphonobutanoic acid | Group III | Orthosteric antagonist |
| PHCCC | N-Phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide | mGluR4 | PAM |
| VU0155041 | cis-2-[[(3,5-Dichlorophenyl)amino]carbonyl] cyclohexanecarboxylic acid | mGluR4 | PAM |
| AMN082 | N,N/ -Bis(diphenylmethyl)-1,2-ethanediamine | mGluR7 | Allosteric agonist |
| MMPIP | 6-(4-Methoxyphenyl)-5-methyl-3-(4-pyridinyl)-isoxazolo [4,5-c]pyridin-4(5H)-one hydrochloride |
mGluR7 | NAM |
Multiple selective PAMs of mGluR2 have now been identified, with the majority being structurally related to either LY487379 or BINA, two prototypical mGluR2 PAMs (71, 75). Many of these compounds are highly selective for mGluR2 and do not potentiate responses to activation of mGluR3 or any other mGluR subtype. In addition, group II mGluR NAMs have been reported that block both mGluR2 and mGluR3 (76–78). As with group I mGluR allosteric modulators, mutation analysis and molecular pharmacology studies have revealed that these compounds interact with the receptor in the HD (76, 79, 80).
Group III mGluRs
The prototypical orthosteric agonist of group III mGluRs is L-AP4, which is highly selective for group III mGluRs relative to other mGluRs or ionotropic glutamate receptors (57). L-AP4 has submicromolar to low micromolar potencies at mGluRs 4, 6, and 8 but submillimolar to millimolar potency at mGluR7 (57). Multiple related compounds have provided additional group III mGluR agonists, but most have the same selectivity profile as L-AP4 (57, 81, 82). (S)-3,4-DCPG has recently emerged as a novel agonist with 100-fold selectivity for mGluR8 over mGluR4 (83, 84). Additionally, several L-AP4 analogs, including Z-cyclopentyl AP4, have been shown to have some selectivity for mGluR4 compared with other group III mGluRs (85–87). Several orthosteric antagonists have been reported to have high selectivity for group III mGluRs (i.e., CPPG, MAP4, and others) (57). However, these compounds exhibit low potencies for antagonizing group III mGluRs, and in some cases have no effect even at high concentrations (88). In cell lines expressing the cloned rat receptors, the group II–preferring antagonist LY341495 has been shown to be the most potent antagonist across group III mGluRs, followed by CPPG (88).
As with group I and group II mGluRs, exciting progress has been made in identifying selective allosteric modulators of group III mGluRs. PHCCC is an mGluR4 PAM (89, 90) that has no direct agonist activity at mGluR4 but increases the potency of glutamate at mGluR4. Two mGluR5 NAMs, SIB-1893 and MPEP, also possess mGluR4 PAM activity (91), although these compounds have relatively low potency and efficacy at mGluR4. Recently, a high-throughput screening approach identified VU0155041 and VU0080421 as structurally distinct mGluR4 PAMs (92, 93). VU0155041 exhibits significant improvements in aqueous solubility, potency, and selectivity for mGluR4 relative to PHCCC. Additionally, in contrast to PHCCC, VU155041 exhibits allosteric agonist activity at mGluR4 when assessed in vitro (92), suggesting that these two ligands may induce potentiation of mGluR4 in mechanistically distinct ways.
AMN082 has been reported as a selective allosteric agonist of mGluR7 (94). Whereas AMN082 is becoming widely used as a putative mGluR7 agonist in vivo, subsequent studies suggest that the activity of this compound is complex. For instance, in contrast to L-AP4, AMN082 does not induce calcium mobilization downstream of mGluR7 in a cell line coexpressing the receptor with a promiscuous G protein (95), does not induce mGluR7-mediated activation of GIRK potassium channels in human embryonic kidney (HEK) cells (87), and does not activate mGluR7 at the Shaffer collateral-CA1 synapse (87). Thus, activity of AMN082 on mGluR7 may be context-specific and may only be observed in some systems, perhaps depending on the specific signaling pathways engaged.
Similar complexities have been observed with recently discovered mGluR7 NAMs. MMPIP is an mGluR7-selective NAM that inhibits L-AP4-induced calcium mobilization in cells expressing mGluR7 and Gα15 (95, 96). Interestingly, MMPIP does not appear to effectively block coupling of mGluR7 to inhibition of cAMP accumulation, activation of G-protein-activated inwardly rectifying potassium channels (GIRK) potassium channels, or L-AP4-mediated inhibition of synaptic transmission at the Shaffer collateral-CA1 synapse (96). Thus, as with AMN082, MMPIP may only have activity on mGluR7 in some cellular contexts or may block specific signaling pathways while permitting signaling through others, an idea recently coined permissive antagonism (97).
FUNCTIONAL ROLES OF mGluRs
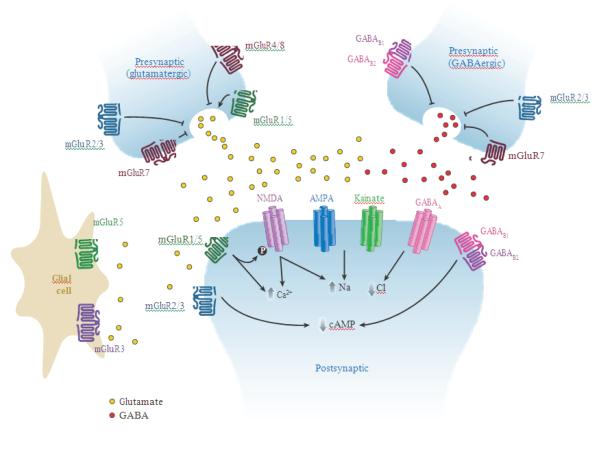
mGluRs are broadly distributed throughout the CNS and are specifically localized at discrete synaptic and extrasynaptic sites in both neurons and glia in virtually every major brain region. Activation of mGluRs results in diverse actions on neuronal excitability and synaptic transmission by modulation of a variety of ion channels and other regulatory and signaling proteins. Discussion of the physiological roles of mGluRs has been presented in detail in multiple previous reviews (98–103). While broad generalizations should be avoided, there are common roles for the major subgroups of mGluRs. Group I mGluRs are often localized postsynaptically, and their activation often leads to cell depolarization and increases in neuronal excitability (Table 1; Figure 4). Modulation of neuronal excitability results from modulation of a number of ion channels and can range from robust excitation to more subtle changes in patterns or frequency of cell firing or responses to excitatory inputs (98–101). In contrast, group II and group III mGluRs are often localized on presynaptic terminals or preterminal axons where they inhibit neurotransmitter release (Figure 4). This occurs at excitatory (glutamatergic), inhibitory (GABAergic), and neuromodulatory (i.e., monoamines, ACh, peptides) synapses.
Figure 4.
Schematic representation of mGluRs at the synapse. In general, group I mGluRs (green) are localized postsynaptically, and group II (blue) and III (red) receptors are localized in presynaptic locations, although exceptions occur. In presynaptic locations, mGluRs 2, 3, 4, and 8 are generally found in extrasynaptic locations, and mGluR7 is highly localized to the active zone (136). Group II and III receptors inhibit release of glutamate (left, yellow circles) or GABA (right, red circles), whereas group I receptors promote release when present. At the postsynaptic terminal, the glutamate gated ion channels N-methyl-D-asparate (NMDA), α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) and kainate respond to glutamate with increases in intracellular sodium or calcium, promoting cell excitability. Group I mGluRs signal via Gq proteins to increase intracellular calcium; additionally, mGluR5 and NMDA receptors are closely linked signaling partners reciprocally regulated by phosphorylation (black circle) (71). Postsynaptic mGluR2/3 and GABAB1/2 receptors couple to cAMP inhibition. GABAA chloride channels (pink) modulate intracellular chloride. Expression of mGluR3 and mGluR5 on glia is now emerging as another key site for mGluR regulation of synaptic activity, although the signaling pathways and consequences of receptor activation on these cells are not presently well understood.
Whereas these generalizations hold in many instances, there are many exceptions, and the physiological roles of different mGluR subtypes are highly specific to the neuronal population and even subcellular localization. For instance, activation of mGluR1 induces hyperpolarization of some neuronal populations such as midbrain dopamine neurons (101). Also, group I mGluRs can act presynaptically to either increase or decrease neurotransmitter release. In some cases, this is mediated by postsynaptic group I mGluRs and release of retrograde messengers, such as endocannabinoids. However, in others, it is likely mediated by group I mGluRs that are local- ized presynaptically (100, 102, 103). Also, different group I mGluR subtypes often have different physiological roles in a single neuronal population. For instance, in CA1 pyramidal cells mGluR1 activation leads to somatic calcium transients and neuronal depolarization, whereas mGluR5 activation inhibits IAHP (slow after hyperpolarization potential) potassium currents and potentiates NMDA receptor currents (104). Group I mGluRs also play important roles in induction of long-lasting forms of synaptic plasticity, including long-term depression (LTD) and long-term potentiation (LTP) of transmission at multiple glutamatergic synapses and induce long-lasting changes in neuronal excitability (87, 100, 102, 105).
All of the mGluRs have been genetically deleted in mice. Results of studies with these animals have revealed potential roles for each of the receptors in cell function and in various disease states. mGluR1 was the first mGluR to be knocked out in mice. These animals show normal gross brain morphology but exhibit several phenotypes. For example, the high expression of mGluR1 in the hippocampus points to a potential role in learning and memory. Indeed, electrophysiological recordings from mGluR1 –/– mice show that these animals exhibit abnormal induction of long- term potentiation and that this phenotype correlates with deficits in context-specific learning (106). Recently, Gil-Sanz et al. recorded activity occurring at hippocampal CA3-CA1 synapses in mice as the animals were learning an associative task (107). These studies revealed that mGluR1 knockout mice were unable to learn this task and showed significant impairments in the ability to induce LTP.
Additionally, these mice showed cerebellar gait problems; in contrast to the effects seen in the hippocampus, mGluR1 deficiency leads to deficits in long-term depression in the cerebellum (108). A recent extension of this initial work showed that mice deficient in mGluR1 showed abnormal levels of regression of climbing fibers from cerebellar Purkinje cells (109). Normally, these fibers regress during development such that one fiber maintains a strong excitatory input to an individual Purkinje cell, and the remaining cells regress. The authors suggest a critical role for mGluR1 in maintaining proper levels of innervations of cerebellar neurons. Interestingly, it has recently been shown that several spontaneous mutations giving rise to spontaneous ataxia in mice genetically map to the mGluR1 locus (110). These mutations alter the ligand-binding domain of mGluR1, suggesting that mGluR1 function is critical for this ataxic phenotype.
mGluR5 knockout mice have been intensely studied in models of learning and memory, addiction, motor regulation, and obesity. Most recently, mGluR5 has emerged as a novel player in the treatment of fragile X syndrome based, in part, on studies with knockout animals (discussed further in the following section). Both mGluR1 and mGluR5 knockout animals show deficits in prepulse inhibition (111, 112), which is a measure of sensorimotor gating that is impaired in schizophrenic patients and can be reversed by antipsychotic agents. In models of drug abuse and addiction, mGluR5 knockout mice do not self-administer cocaine or increase their locomotor activity after cocaine administration (113). mGluR5 knockout mice weigh less than littermate controls, eat less than control mice when challenged with a starvation and refeeding paradigm, and are resistant to effects induced by a high-fat diet such as weight gain and increases in plasma insulin and leptin levels (114).
The physiological roles of group II and group III mGluRs are also more complex than a simple role in acute presynaptic regulation of neurotransmitter release. Group II mGluRs can be localized postsynaptically where they can induce hyperpolarization (115), and mGluR4 and -8 have also both been shown to be expressed postynaptically in certain areas such as the hippocampus and retina (116, 117). Both group II and group III mGluRs play important roles in induction of LTD (102, 103). In addition to their roles in regulating neuronal excitability and synaptic transmission, mGluRs play other important roles, including regulation of metabolism, gene transcription, and multiple aspects of glial function and glial-neuronal communication.
Both mGluR2 and mGluR3 have been separately deleted from the mouse genome, and these animals are critically important for several reasons. For example, the high conservation of mGluR2 and mGluR3 makes it difficult to develop selective ligands that can be used to differentiate the function of one receptor versus the other, although the emergence of more selective allosteric modulators is helping to define the roles of these receptors. In terms of functional correlates of learning and memory, mGluR2 knockout mice show normal regulation of basal synaptic transmission and LTP at mossy fiber-CA3 synapses in the hippocampus, but these animals are severely impaired in terms of LTD induced by low-frequency stimulation at this synapse (118). In other studies using knockout mice combined with other pharmacological tools, mGluR2 activation has been postulated to result in cognitive impairment, suggesting a potential role for mGluR2 antagonists in cognitive disorders (119). mGluR2 has also been implicated in addiction to drugs of abuse because mGluR2 knockout mice show increased responsiveness to cocaine (120), and this receptor has also been shown to selectively mediate the beneficial effects of group II agonists in rodent models of psychosis (121, 122).
A potentially important role for mGluR3 in astrocytes is emerging from studies with knockout animals. For example, mixed cultures of cortical neurons and astrocytes from wild-type, mGluR2, and mGluR3 knockout mice have shown that an agonist of group II receptors, LY379268, is neuroprotective when cultures are challenged with NMDA (123). This neuroprotection is lost, however, if mGluR3 is missing from atrocytes in the culture. In these same experiments, the authors found that activation of mGluR2 might actually be harmful in terms of excitotoxicity (123).
Each of the group III mGluRs has also been genetically deleted in mice, and these animals show several interesting phenotypes. mGluR6 exhibits the most restricted expression of all of the mGluRs and is found primarily in retinal ON bipolar cells (124). The mGluR6 activation cascade has been shown to involve Go protein subunits (125). When mGluR6 is deleted from mice, bipolar cells from homozygous mutants show deficits in ON responses to light, which appears to be manifested by a delayed and decreased ON response to light stimulation (126, 127).
mGluR4 is very highly expressed in the cerebellum, with lower levels of expression in the hippocampus, basal ganglia, and olfactory bulb (reviewed in 128 and references therein). mGluR4 is predominantly expressed presynaptically (37, 129). As mentioned above, a splice variant of mGluR4, termed taste mGluR4, is located in taste buds, and the longer, brain version of mGluR4 is also expressed in this location(130). mGluR4 protein is also expressed peripherally in tissues such as pancreatic islets (131). Consistent with the high expression of mGluR4 in cerebellum, mice lacking this receptor show impairments in cerebellar synaptic plasticity and in learning complicated motor tasks (132). These animals also show impaired abilities in spatial memory performance (133). mGluR4 has also been shown to modulate GABA(A) receptor-mediated seizure activity (134), and mGluR4–/– mice also lack motor stimulatory effects induced by ethanol (135).
mGluR7 exhibits a wide distribution throughout the entire brain. mGluR7 has an extremely low affinity for glutamate and is highly localized to the active zones of synapses (136, 137). It has been proposed that mGluR7 serves a low pass filter role in neurotransmission, only becoming active when glutamate levels are very high and thus serving as a brake for overstimulation by glutamate. In support of this hypothesis, knockout of mGluR7 results in animals with an epileptic phenotype (138). mGluR7–/– mice also show abnormalities in learning tasks (139–144), and mGluR7 function appears to be particularly important in mediating amygdala-dependent learning (139). Additionally, these mice have revealed roles for mGluR7 in CNS disorders such as anxiety and depression (144–146).
mGluR8 is expressed at lower levels than mGluR4 and mGluR7 but is widely distributed in the brain. As for mGluR4, mGluR8 has been localized predominantly presynaptically but has been identified in some postsynaptic locations and in the periphery (reviewed in 128). The mGluR8 gene is exceptionally large, spanning approximately 1000 kilobases of genomic DNA in a region that maps to the same location as mutations causing two human disorders, Smith-Lemli-Optiz syndrome and retinitis pigmentosa (147). mGluR8 knockout mice show enhanced anxiety and weight gain compared to controls (148–150), suggesting that mGluR8 activators may be of benefit in anxiety disorders. Additionally, expression and function of mGluR8 in peripheral locations such as the gut and pancreas suggests that this receptor is involved in gastrointestinal motility and insulin secretion in vivo (151, 152).
THERAPEUTIC POTENTIAL OF mGluRs
The wide diversity and heterogeneous distribution of mGluR subtypes provides an opportunity for selectively targeting individual mGluR subtypes involved in only one or a limited number of CNS functions for the development of novel treatment strategies for psychiatric and neurological disorders. A large body of preclinical studies now suggests that ligands for specific mGluR subtypes have potential for the treatment of multiple CNS disorders, including depression (153), anxiety disorders (154), schizophrenia (71, 155), pain syndromes (156), epilepsy (157), Alzheimer’s disease (158), and Parkinson’s disease (159), among others. Data from clinical studies with mGluR ligands are beginning to emerge and are providing strong proof of concept for clinical efficacy of some of these compounds. Whereas each of these therapeutic areas has been extensively reviewed, several exciting advances are especially noteworthy and are discussed here.
Allosteric Modulators of mGluR5
A major focus of many efforts to discover novel mGluR5 ligands has been the discovery of mGluR5 antagonists for the treatment of anxiety disorders. This receptor increases neuronal excitability and NMDA receptor currents in brain regions thought to be involved in anxiety, such as the amygdala (160), leading to the hypothesis that an antagonist of mGluR5 might dampen the hyperactivity of glutamatergic transmission believed to underlie anxiety disorders. Consistent with this, MPEP and related mGluR5 NAMs have robust efficacy in several animal models of anxiolytic activity (154), and the clinically validated anxiolytic agent fenobam (161) acts as a selective mGluR5 NAM (162). Animal studies suggest that selective mGluR5 NAMs also have potential utility in the treatment of fragile X syndrome (FXS) (163–165) as well as chronic pain, addictive disorders, depression, gastroesophogeal reflux (GERD), migraine, and some neurodegenerative disorders (166). Exciting new clinical studies have now established the efficacy of mGluR5 NAMs in treatment of GERD (167) and migraine (168).
Potential for mGluR5 NAMs for the treatment of FXS also has a strong clinical basis because the primary mutation giving rise to the disorder leads to increased mGluR5 signaling (163, 169). FXS is the most common inherited form of mental retardation and a leading cause of autism (170). FXS is caused by genetic defects in the gene encoding the fragile X mental retardation protein (FMRP), a translational repressor that regulates local protein translation in neuronal dendrites; these proteins promote LTD. In mouse models of FXS, the group I mGluR-dependent LTD is excessive, suggesting that antagonists of mGluR1 or mGluR5 might represent novel mechanisms for FXS treatment (163). Consistent with this, studies examining the phenotypes of FXS mice interbred with mGluR5 knockout animals have revealed that many of the phenotypes of FXS can be rescued when mGluR5 levels are reduced (164). Early work with the mGluR5 NAM MPEP has also validated the use of mGluR5 antagonists as a possible treatment for FXS; MPEP administration can suppress several phenotypes, such as seizures and anxiety, present in FXS mice (165). Intense efforts are now underway to develop highly selective mGluR5 antagonists for clinical trials in FXS patients.
In addition to the utility of mGluR5 NAMs, mGluR5 PAMs may have potential utility in the treatment of schizophrenia and other disorders that involve impaired cognitive function (71, 155). Multiple mGluR5 PAMs have been identified and can potentiate mGluR5-mediated electrophysiological responses in midbrain and forebrain circuits, including potentiation of NMDA receptor currents (68, 171, 172), a response that is thought to be relevant for schizophrenia and cognitive function. Furthermore, two mGluR5 PAMs, CDPPB and ADX47273, have robust antipsychotic-like effects and improve cognitive function in animal models (72, 73, 173). Although lacking the clinical validation of mGluR5 NAMs, these exciting findings suggest that that mGluR5 PAMs have potential utility as novel antipsychotic and cognition-enhancing agents.
Agonists and PAMs of Group II mGluRs
Preclinical and clinical studies provide strong evidence that agonists of group II mGluRs have potential as a novel approach for the treatment of anxiety disorders and schizophrenia. Selective group II mGluR agonists such as LY354740 and related compounds have robust anxiolytic (154, 174) and antipsychotic-like effects in animal models (175), and clinical studies reveal that group II mGluR agonists have efficacy in treatment of both anxiety and schizophrenia (71, 154). Group II mGluR agonists also have robust efficacy in human models of panic attacks and fear-potentiated startle and improve symptoms of generalized anxiety disorder (154, 176). Furthermore, a selective group II mGluR agonist improves positive and negative symptoms in patients suffering from schizophrenia (177). These clinical findings represent a major breakthrough and could ultimately lead to the introduction of group II mGluR activators as a fundamentally novel approach to the treatment of anxiety disorders and schizophrenia. Interestingly, the effects of mGluR2 and -3 agonists in animal models of anxiety disorders and schizophrenia can be observed with highly selective mGluR2 PAMs (reviewed in 71). These studies raise the possibility that selective mGluR2 PAMs may also provide a novel approach to the treatment of these disorders.
Recently, a direct interaction between mGluR2 and the serotonin 5-HT2A receptor has been proposed as providing a mechanism for antipsychotic effects of mGluR2 agonists (178). 5-HT2A receptors are activated by hallucinogens such as lysergic acid diethylamide (LSD) and inhibited by atypical antipsychotics such as clozapine. Activation of 5-HT2A receptors induces a dramatic increase in spontaneous excitatory postsynaptic potentials at thalamo-cortical synapses in the medial prefrontal cortex (179), and increased spontaneous activity at this synapse has been postulated to participate in some aspects of schizophrenia (155, 179). Interestingly, it has been shown that 5HT2A receptors can heterodimerize with mGluR2 (178), and activation of mGluR2 dramatically reduces serotonin-stimulated increases in spontaneous excitatory postysynaptic potentials at thalamocortical synapses (179, 180). Based on this, it is possible that heterodimerization and functional interactions between 5HT2A receptors and mGluR2 may be important for the antipsychotic activity of group II mGluR agonists. Additionally, this raises the possibility that more examples of heterodimers between mGluRs and other GPCRs exist, providing further complexity to mGluR biology.
In addition to schizophrenia and anxiety, ligands at group II mGluRs have also been suggested as therapeutic directions for other CNS disorders, including mGluR2 and mGluR3 agonists and mGluR2 PAMs for treatment of pain, addictive disorders, depression, and epilepsy (reviewed in 181). Whereas less is known about the potential utility of mGluR3-selective compounds, agonists of these receptors appear to be neuroprotective, and this effect has been attributed to mGluR3 with the use of knockout animals (123). The future development of more selective compounds that differentiate between mGluR2 and mGluR3 is anticipated to advance the understanding of the individual roles of these receptors in various disease states.
mGluR4 PAMs
Another mGluR subtype, mGluR4, has emerged as an exciting new target for the treatment of Parkinson’s disease (PD) (159). Activation of mGluR4 reduces GABAergic transmission at the striato-pallidal synapse (182–184), a synapse that is overactive after the loss of dopamine neurons in patients suffering from PD (185). Agonists of group III mGluRs have robust efficacy in rodent models of PD (182, 186–190). Furthermore, a highly selective mGluR4 PAM, PHCCC, potentiates mGluR4 responses at the striato-pallidal synapse (90), and intracerebroventricular injection of PHCCC as well as several other mGluR4 PAMs have antiparkinsonian effects in rodent models (90, 92, 191, 192). In addition to providing symptomatic relief for PD patients, a number of studies suggest that mGluR4 activation could also provide a neuroprotective effect and slow disease progression by reducing excessive excitatory drive onto dopamine neurons and potential excitotoxicity, which may contribute to loss of these neurons (193, 194).
Group III mGluRs also have potential for the treatment of other CNS disorders. For example, mGluR4 knockout animals appear to be resistant to several of the features of alcohol addiction (135), and mGluR8 levels are regulated by cocaine and amphetamine administration, suggesting roles for group III receptors in drug abuse (195, 196). mGluR7 appears to have a role in mediating seizures based on studies with knockout mice (138) as well as mouse models examining interacting proteins (55). Additionally, emerging studies suggest roles of group III mGluRs in depression and anxiety (153), neuroblastoma (197), and neuronal differentiation (198).
SUMMARY AND CONCLUSIONS
Our understanding of the mGluR field has grown exponentially within the past decade. Novel insights into the mechanism of activation of receptors, detailed studies regarding interacting proteins that modulate receptor function, and the identification of many new ligands available to probe receptor biology have all contributed to the growth of this area from both basic science and therapeutic perspectives. Advances in selective compound discovery, spanning orthosteric and allosteric binding sites, have promoted the development of highly selective tools and, in some cases, compounds that can be tested in the clinical setting. It is anticipated that the fast-paced development in the mGluR field will continue to enhance our understanding of CNS function and will soon open additional possibilities for treatments of neurological and psychiatric disorders.
ACKNOWLEDGMENTS
The authors would like to thank Douglas Sheffler, Kari Johnson, Jennifer Ayala, and Gregory Digby for advice and comments on the manuscript. They were supported by grants from NINDS, NIMH, the Stanley Foundation, NARSAD, and MJFF, Seaside Therapeutics, and Janssen Pharmaceuticals.
Footnotes
DISCLOSURE STATEMENT Dr. Conn has served as a consultant over the past year for Epix Pharmaceuticals, Evotec Inc., Seaside Therapeutics, Merck Serono, AstraZeneca USA, NeurOp Inc., Millipore Corp., Genentech, Abbott Laboratories, AMRI, and PureTech. Dr. Conn and Dr. Niswender receive research support that includes salary support from The Michael J. Fox Foundation, Seaside Therapeutics, and Johnson and Johnson.
LITERATURE CITED
- 1.Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 2003;98(3):325–54. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchiya D, et al. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+ Proc. Natl. Acad. Sci. USA. 2002;99(5):2660–65. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407(6807):971–77. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 4.Muto T, Tsuchiya D, Morikawa A, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA. 2007;104(10):3759–64. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jingami H, Nakanishi S, Morikawa K. Structure of the metabotropic glutamate receptor. Curr. Opin. Neurobiol. 2003;13(3):271–78. doi: 10.1016/s0959-4388(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 6.Bessis AS, Rondard P, Gaven F, Brabet I, Triballeau N, et al. Closure of the Venus flytrap module of mGlu8 receptor and the activation process: insights from mutations converting antagonists into agonists. Proc. Natl. Acad. Sci. USA. 2002;99(17):11097–102. doi: 10.1073/pnas.162138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuang D, Yao Y, Wang M, Pattabiramann N, Kotra LP, Hampson DR. Molecular similarities in the ligand binding pockets of an odorant receptor and the metabotropic glutamate receptors. J. Biol. Chem. 2003;278(43):42551–59. doi: 10.1074/jbc.M307120200. [DOI] [PubMed] [Google Scholar]
- 8.Acher FC, Bertrand HO. Amino acid recognition by Venus flytrap domains is encoded in an 8-residue motif. Biopolymers. 2005;80(2–3):357–66. doi: 10.1002/bip.20229. [DOI] [PubMed] [Google Scholar]
- 9.Kubo Y, Miyashita T, Murata Y. Structural basis for a Ca2+ -sensing function of the metabotropic glutamate receptors. Science. 1998;279(5357):1722–25. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- 10.Francesconi A, Duvoisin RM. Divalent cations modulate the activity of metabotropic glutamate receptors. J. Neurosci. Res. 2004;75(4):472–79. doi: 10.1002/jnr.10853. [DOI] [PubMed] [Google Scholar]
- 11.Rondard P, et al. Coupling of agonist binding to effector domain activation in metabotropic glutamate-like receptors. J. Biol. Chem. 2006;281(34):24653–61. doi: 10.1074/jbc.M602277200. [DOI] [PubMed] [Google Scholar]
- 12.Gomeza J, Joly C, Kuhn R, Knöpfel T, Bockaert J, Pin J-P. The second intracellular loop of metabotropic glutamate receptor 1 cooperates with the other intracellular domains to control coupling to G-proteins. J. Biol. Chem. 1996;271(4):2199–205. doi: 10.1074/jbc.271.4.2199. [DOI] [PubMed] [Google Scholar]
- 13.Pin JP, Joly C, Heinemann SF, Bockaert J. Domains involved in the specificity of G protein activation in phospholipase C-coupled metabotropic glutamate receptors. EMBO J. 1994;13(2):342–48. doi: 10.1002/j.1460-2075.1994.tb06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhami GK, Babwah AV, Sterne-Marr R, Ferguson SSG. Phosphorylation-independent regulation of metabotropic glutamate receptor 1 signaling requires g protein-coupled receptor kinase 2 binding to the second intracellular loop. J. Biol. Chem. 2005;280(26):24420–27. doi: 10.1074/jbc.M501650200. [DOI] [PubMed] [Google Scholar]
- 15.Hampson DR, Rose EM, Antflick JE. The structures of metabotropic glutamate receptors. In: Gereau RW, Swanson GT, editors. The Glutamate Receptors. Human Press; Totowa, NJ: 2008. pp. 363–86. [Google Scholar]
- 16.Goudet C, Kniazeff J, Vol C, Liu J, Cohen-Gonsaud M, et al. Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors. Proc. Natl. Acad. Sci. USA. 2004;101(1):378–83. doi: 10.1073/pnas.0304699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galvez T, Urwyler S, Prézeau L, Mosbacher J, Joly C, et al. Ca2+ requirement for high-affinity gamma-aminobutyric acid (GABA) binding at GABA(B) receptors: involvement of serine 269 of the GABA(B)R1 subunit. Mol. Pharmacol. 2000;57(3):419–26. doi: 10.1124/mol.57.3.419. [DOI] [PubMed] [Google Scholar]
- 18.Kammermeier PJ, Yun J. Activation of metabotropic glutamate receptor 1 dimers requires glutamate binding in both subunits. J. Pharmacol. Exp. Ther. 2005;312(2):502–8. doi: 10.1124/jpet.104.073155. [DOI] [PubMed] [Google Scholar]
- 19.Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prézeau L, Pin JP. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat. Struct. Mol. Biol. 2004;11(8):706–13. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki Y, Moriyoshi E, Tsuchiya D, Jingami H. Negative cooperativity of glutamate binding in the dimeric metabotropic glutamate receptor subtype 1. J. Biol. Chem. 2004;279(34):35526–34. doi: 10.1074/jbc.M404831200. [DOI] [PubMed] [Google Scholar]
- 21.Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 2001;359(Pt 3):465–84. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li XM, Li CC, Yu SS, Chen JT, Sabapathy K, Ruan DY. JNK1 contributes to metabotropic glutamate receptor-dependent long-term depression and short-term synaptic plasticity in the mice area hippocampal CA1. Eur. J. Neurosci. 2007;25(2):391–96. doi: 10.1111/j.1460-9568.2006.05300.x. [DOI] [PubMed] [Google Scholar]
- 23.Saugstad JA, Ingram SL. Group I metabotropic glutamate receptors (mGluR1 and mGluR5) 2008. pp. 387–464. See Ref. 15.
- 24.Page G, Khadir FA, Pain S, Barrier L, Fauconneau B, et al. Group I metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochem. Int. 2006;49(4):413–21. doi: 10.1016/j.neuint.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 2004;24(28):6352–61. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iacovelli L, Bruno V, Salvatore L, Melchiorri D, Gradini R, et al. Native group-III metabotropic glutamate receptors are coupled to the mitogen-activated protein kinase/phosphatidylinositol-3-kinase pathways. J. Neurochem. 2002;82(2):216–23. doi: 10.1046/j.1471-4159.2002.00929.x. [DOI] [PubMed] [Google Scholar]
- 27.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34(1):1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 28.Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Identification, cloning and analysis of ex- pression of a new alternatively spliced form of the metabotropic glutamate receptor mGluR1 mRNA1. Biochim. Biophys. Acta. 1999;1446(1–2):161–66. doi: 10.1016/s0167-4781(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 29.Joly C, Gomeza J, Brabet I, Curry K, Bockaert J, Pin J-P. Molecular, functional, and pharmacological characterization of the metabotropic glutamate receptor type 5 splice variants: comparison with mGluR1. J. Neurosci. 1995;15(5 Pt 2):3970–81. doi: 10.1523/JNEUROSCI.15-05-03970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minakami R, Yamamoto T, Nakamura K, Sugiyama H. Molecular cloning and the functional expression of two isoforms of human metabotropic glutamate receptor subtype 5. Biochem. Biophys. Res. Commun. 1994;199(3):1136–43. doi: 10.1006/bbrc.1994.1349. [DOI] [PubMed] [Google Scholar]
- 31.Sartorius LJ, Nagappan G, Lipska BK, Lu B, Sei Y, et al. Alternative splicing of human metabotropic glutamate receptor 3. J. Neurochem. 2006;96(4):1139–48. doi: 10.1111/j.1471-4159.2005.03609.x. [DOI] [PubMed] [Google Scholar]
- 32.Valerio A, Ferraboli S, Paterlini M, Spano PF, Barlati S. Identification of novel alternatively-spliced mRNA isoforms of metabotropic glutamate receptor 6 gene in rat and human retina. Gene. 2001;262(1–2):99–106. doi: 10.1016/s0378-1119(00)00547-3. [DOI] [PubMed] [Google Scholar]
- 33.Malherbe P, Kratzeisen C, Lundstrom K, Richards JG, Faull RL, Mutel V. Cloning and functional expression of alternative spliced variants of the human metabotropic glutamate receptor 8. Mol. Brain Res. 1999;67(2):201–10. doi: 10.1016/s0169-328x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 34.Corti C, Restituito S, Rimland M, Brabet I, Corsi M, et al. Cloning and characterization Of alternative mRNA forms for the rat metabotropic glutamate receptors mGluR7 and mGluR8. Eur. J. Neurosci. 1998;10(12):3629–41. doi: 10.1046/j.1460-9568.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- 35.Duvoisin RM, Zhang C, Ramonell K. A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J. Neurosci. 1995;15(4):3075–83. doi: 10.1523/JNEUROSCI.15-04-03075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomsen C, Pekhletski R, Haldeman B, Gilbert TA, O’Hara P, Hampson DR. Cloning and characterization of a metabotropic glutamate receptor, mGluR4b. Neuropharmacology. 1997;36(1):21–30. doi: 10.1016/s0028-3908(96)00153-0. [DOI] [PubMed] [Google Scholar]
- 37.Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110(3):403–20. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat. Neurosci. 2000;3(2):113–19. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- 39.San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am. J. Clin. Nutr. 2009;90:743S–46S. doi: 10.3945/ajcn.2009.27462I. [DOI] [PubMed] [Google Scholar]
- 40.San Gabriel A, Uneyama H, Yoshie S, Torii K. Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for L-glutamate stimuli. Chem. Senses. 2005;30(Suppl 1):i25–26. doi: 10.1093/chemse/bjh095. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am. J. Clin. Nutr. 2009;90:738S–42S. doi: 10.3945/ajcn.2009.27462H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrengruber MU, Kato A, Inokuchi K, Hennou S. Homer/Vesl proteins and their roles in CNS neurons. Mol. Neurobiol. 2004;29(3):213–27. doi: 10.1385/MN:29:3:213. [DOI] [PubMed] [Google Scholar]
- 43.Beneken J, Tu JC, Xiao B, Nuriya M, Yuan JP, et al. Structure of the Homer EVH1 domain-peptide complex reveals a new twist in polyproline recognition. Neuron. 2000;26(1):143–54. doi: 10.1016/s0896-6273(00)81145-9. [DOI] [PubMed] [Google Scholar]
- 44.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, et al. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21(4):717–26. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 45.Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8(2):206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J. Neurosci. 2005;25(10):2741–52. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flajolet M, Rakhilin S, Wang H, Starkova N, Nuangchamnong N, et al. Protein phosphatase 2C binds selectively to and dephosphorylates metabotropic glutamate receptor 3. Proc. Natl. Acad. Sci. USA. 2003;100(26):16006–11. doi: 10.1073/pnas.2136600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seebahn A, Rose M, Enz R. RanBPM is expressed in synaptic layers of the mammalian retina and binds to metabotropic glutamate receptors. FEBS Lett. 2008;582(16):2453–57. doi: 10.1016/j.febslet.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Rose M, Dutting E, Enz R. Band 4.1 proteins are expressed in the retina and interact with both isoforms of the metabotropic glutamate receptor type 8. J. Neurochem. 2008;105:2375–87. doi: 10.1111/j.1471-4159.2008.05331.x. [DOI] [PubMed] [Google Scholar]
- 50.Boudin H, Doan Z, Xia J, Shigemoto R, Huganir RL, et al. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron. 2000;28(2):485–97. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- 51.El Far O, Airas J, Wischmeyer E, Nehring RB, Karschin A, Betz H. Interaction of the C-terminal tail region of the metabotropic glutamate receptor 7 with the protein kinase C substrate PICK1. Eur. J. Neurosci. 2000;12(12):4215–21. doi: 10.1046/j.1460-9568.2000.01309.x. [DOI] [PubMed] [Google Scholar]
- 52.El Far O, Betz H. G-protein-coupled receptors for neurotransmitter amino acids: C-terminal tails, crowded signalosomes. Biochem. J. 2002;365(Pt 2):329–36. doi: 10.1042/BJ20020481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dev KK, Nakanishi S, Henley JM. Regulation of mglu(7) receptors by proteins that interact with the intracellular C-terminus. Trends Pharmacol. Sci. 2001;22(7):355–61. doi: 10.1016/s0165-6147(00)01684-9. [DOI] [PubMed] [Google Scholar]
- 54.Bertaso F, Lill Y, Airas JM, Espeut J, Blahos J, et al. MacMARCKS interacts with the metabotropic glutamate receptor type 7 and modulates G protein-mediated constitutive inhibition of calcium channels. J. Neurochem. 2006;99(1):288–98. doi: 10.1111/j.1471-4159.2006.04121.x. [DOI] [PubMed] [Google Scholar]
- 55.Bertaso F, Zhang C, Scheschonka A, de Bock F, Fontanaud P, et al. PICK1 uncoupling from mGluR7a causes absence-like seizures. Nat. Neurosci. 2008;11(8):940–48. doi: 10.1038/nn.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang CS, Bertaso F, Eulenburg V, Lerner-Natoli M, Herin GA, et al. Knock-in mice lacking the PDZ-ligand motif of mGluR7a show impaired PKC-dependent autoinhibition of glutamate release, spatial working memory deficits, and increased susceptibility to pentylenetetrazol. J. Neurosci. 2008;28(34):8604–14. doi: 10.1523/JNEUROSCI.0628-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38(10):1431–76. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 58.Annoura H, Fukunaga A, Uesugi M, Tatsuoka T, Horikawa Y. A novel class of antagonists for metabotropic glutamate receptors, 7-(hydroxyimino)cyclopropa[b]chromen1a-carboxylates. Bioorg. Med. Chem. Lett. 1996;6:763–66. [Google Scholar]
- 59.Carroll FY, Stolle A, Beart PM, Voerste A, Brabet I, et al. BAY36–7620: a potent non-competitive mGlu1 receptor antagonist with inverse agonist activity. Mol. Pharmacol. 2001;59(5):965–73. [PMC free article] [PubMed] [Google Scholar]
- 60.Lavreysen H, Pereira SN, Leysen JE, Langlois X, Lesage AS. Metabotropic glutamate 1 receptor distribution and occupancy in the rat brain: a quantitative autoradiographic study using [3H]R214127. Neuropharmacology. 2004;46(5):609–19. doi: 10.1016/j.neuropharm.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki G, Kimura T, Satow A, Kaneko N, Fukuda J. Pharmacological characterization of a new, orally active and potent allosteric metabotropic glutamate receptor 1 antagonist, 4-[1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-triazol-4-yl]-N-isopropyl-N-methyl-3,6- dihydropyridine-1(2H)-carboxamide (FTIDC) J. Pharmacol. Exp. Ther. 2007;321(3):1144–53. doi: 10.1124/jpet.106.116574. [DOI] [PubMed] [Google Scholar]
- 62.Kohara A, Toya T, Tamura S, Watabiki T, Nagakura Y, et al. Radioligand binding properties and pharmacological characterization of 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide (YM-298198), a high-affinity, selective, and noncompetitive antagonist of metabotropic glutamate receptor type 1. J. Pharmacol. Exp. Ther. 2005;315(1):163–69. doi: 10.1124/jpet.105.087171. [DOI] [PubMed] [Google Scholar]
- 63.Schkeryantz JM, Kingston AE, Johnson MP. Prospects for metabotropic glutamate 1 receptor antagonists in the treatment of neuropathic pain. J. Med. Chem. 2007;50(11):2563–68. doi: 10.1021/jm060950g. [DOI] [PubMed] [Google Scholar]
- 64.Varney MA, Cosford ND, Jachec C, Rao SP, Sacaan A, et al. SIB-1757 and SIB-1893: selec- tive, noncompetitive antagonists of metabotropic glutamate receptor type 5. J. Pharmacol. Exp. Ther. 1999;290(1):170–81. [PubMed] [Google Scholar]
- 65.Gasparini F, Lingenhö hl K, Stoehr N, Flor PJ, Heinrich M, et al. 2-Methyl-6-(phenylethynyl)- pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharma- cology. 1999;38(10):1493–503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 66.Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, et al. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem. 2003;46(2):204–6. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- 67.Lea PM, 4th, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12(2):149–66. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez AL, Nong Y, Sekaran NK, Alagille D, Tamagnan GD, Conn J. A close structural analog of 2-methyl-6-(phenylethynyl)-pyridine acts as a neutral allosteric site ligand on metabotropic glutamate receptor subtype 5 and blocks the effects of multiple allosteric modulators. Mol. Pharmacol. 2005;68(6):1793–802. doi: 10.1124/mol.105.016139. [DOI] [PubMed] [Google Scholar]
- 69.Hemstapat K, de Paulis T, Chen Y, Brady AE, Grover VK, et al. A novel class of positive allosteric modulators of metabotropic glutamate receptor subtype 1 interact with a site distinct from that of negative allosteric modulators. Mol. Pharmacol. 2006;70(2):616–26. doi: 10.1124/mol.105.021857. [DOI] [PubMed] [Google Scholar]
- 70.Knoflach F, Mutel V, Jolidon S, Kew JNC, Malherbe P, et al. Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc. Natl. Acad. Sci. USA. 2001;98(23):13402–7. doi: 10.1073/pnas.231358298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conn PJ, Lindsley CW, Jones C. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 2008;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kinney GG, O’Brien JA, Lemaire W, Burno M, Bickel DJ, et al. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J. Pharmacol. Exp. Ther. 2005;313(1):199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- 73.Liu F, Grauer S, Kelley C, Navarra R, Graf R, et al. ADX47273 [S-(4-fluoro-phenyl)-{3-[3- (4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piper idin-1-yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J. Pharmacol. Exp. Ther. 2008;327(3):827–39. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- 74.Dominguez C, Prieto L, Valli MJ, Massey SM, Bures M, et al. Methyl substitution of 2- aminobicyclo[3.1.0]hexane 2,6-dicarboxylate ( LY354740) determines functional activity at metabotropic glutamate receptors: identification of a subtype selective mGlu2 receptor agonist. J. Med. Chem. 2005;48(10):3605–12. doi: 10.1021/jm040222y. [DOI] [PubMed] [Google Scholar]
- 75.Johnson MP, Schoepp DD. Group II metabotropic glutamate receptors (mGluR2 and 3) 2008. pp. 465–88. See Ref. 15.
- 76.Hemstapat K, Da Costa H, Nong Y, Brady AE, Luo Q, et al. A novel family of potent negative allosteric modulators of group II metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 2007;322(1):254–64. doi: 10.1124/jpet.106.117093. [DOI] [PubMed] [Google Scholar]
- 77.Woltering TJ, Adam G, Wichmann J, Goetschi E, Kew JN, et al. Synthesis and characterization of 8-ethynyl-1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives. Part 2. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg. Med. Chem. Lett. 2008;18(3):1091–95. doi: 10.1016/j.bmcl.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 78.Woltering TJ, Wichmann J, Goetschi E, Adam G, Kew JN, et al. Synthesis and characterization of 1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives. Part 3. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists. Bioorg. Med. Chem. Lett. 2008;18(8):2725–29. doi: 10.1016/j.bmcl.2008.02.076. [DOI] [PubMed] [Google Scholar]
- 79.Rowe BA, Schaffhauser H, Morales S, Lubbers LS, Bonnefous C, et al. Transposition of three amino acids transforms the human metabotropic glutamate receptor (mGluR)-3-positive allosteric modulation site to mGluR2, and additional characterization of the mGluR2-positive allosteric modulation site. J. Pharmacol. Exp. Ther. 2008;326(1):240–51. doi: 10.1124/jpet.108.138271. [DOI] [PubMed] [Google Scholar]
- 80.Schaffhauser H, Rowe BA, Morales A, Chavez-Noriega LE, Yin R, et al. Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2. Mol. Pharmacol. 2003;64(4):798–810. doi: 10.1124/mol.64.4.798. [DOI] [PubMed] [Google Scholar]
- 81.Selvam C, et al. L-(+)-2-Amino-4-thiophosphonobutyric acid (L-thioAP4), a new potent agonist of group III metabotropic glutamate receptors: increased distal acidity affords enhanced potency. J. Med. Chem. 2007;50(19):4656–64. doi: 10.1021/jm070400y. [DOI] [PubMed] [Google Scholar]
- 82.Sibille P, Lopez S, Brabet I, Valenti O, Oueslati N, et al. Synthesis and biological evaluation of 1-amino-2-phosphonomethylcyclopropanecarboxylic acids, new group III metabotropic glutamate receptor agonists. J. Med. Chem. 2007;50(15):3585–95. doi: 10.1021/jm070262c. [DOI] [PubMed] [Google Scholar]
- 83.Thomas NK, Wright RA, Howson PA, Kingston AE, Schoepp DD, Jane DE. (S)-3,4-DCPG, a potent and selective mGlu8a receptor agonist, activates metabotropic glutamate receptors on primary afferent terminals in the neonatal rat spinal cord. Neuropharmacology. 2001;40(3):311–18. doi: 10.1016/s0028-3908(00)00169-6. [DOI] [PubMed] [Google Scholar]
- 84.Zhai J, Tian MT, Wang Y, Yu JL, Köster A, et al. Modulation of lateral perforant path excitatory responses by metabotropic glutamate 8 (mGlu8) receptors. Neuropharmacology. 2002;43(2):223–30. doi: 10.1016/s0028-3908(02)00087-4. [DOI] [PubMed] [Google Scholar]
- 85.Kroona HB, Peterson NL, Koerner JF, Johnson RL. Synthesis of the 2-amino-4-phosphonobutanoic acid analogues (E)- and (Z)-2-amino-2,3-methano-4-phosphonobutanoic acid and their evaluation as inhibitors of hippocampal excitatory neurotransmission. J. Med. Chem. 1991;34(5):1692–99. doi: 10.1021/jm00109a023. [DOI] [PubMed] [Google Scholar]
- 86.Johansen PA, Chase LA, Sinor AD, Koerner JF, Johnson RL, Robinson MB. Type 4a metabotropic glutamate receptor: identification of new potent agonists and differentiation from the L-(+)-2-amino-4-phosphonobutanoic acid-sensitive receptor in the lateral perforant pathway in rats. Mol. Pharmacol. 1995;48(1):140–49. [PubMed] [Google Scholar]
- 87.Ayala JE, Niswender CM, Luo Q, Banko JL, Conn PJ. Group III mGluR regulation of synaptic transmission at the SC-CA1 synapse is developmentally regulated. Neuropharmacology. 2008;54(5):804–14. doi: 10.1016/j.neuropharm.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, et al. A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol. Pharmacol. 2008;73(4):1213–24. doi: 10.1124/mol.107.041053. [DOI] [PubMed] [Google Scholar]
- 89.Maj M, Bruno V, Dragic Z, Yamamoto R, Battaglia G, et al. (−)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology. 2003;45(7):895–906. doi: 10.1016/s0028-3908(03)00271-5. [DOI] [PubMed] [Google Scholar]
- 90.Marino MJ, Williams DL, Jr, O’Brien JA, Valenti O, McDonald TP, et al. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson’s disease treatment. Proc. Natl. Acad. Sci. USA. 2003;100(23):13668–73. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mathiesen JM, Svendsen N, Bräuner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br. J. Pharmacol. 2003;138(6):1026–30. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, et al. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol. Pharmacol. 2008;74(5):1345–58. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Niswender CM, Lebois EP, Luo Q, Kim K, Muchalski H, et al. Positive allosteric modulators of the metabotropic glutamate receptor subtype 4 (mGluR4). Part I. Discovery of pyrazolo[3,4-d]pyrimidines as novel mGluR4 positive allosteric modulators. Bioorg. Med. Chem. Lett. 2008;18(20):5626–30. doi: 10.1016/j.bmcl.2008.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitsukawa K, Yamamoto R, Ofner S, Nozulak J, Pescott O, et al. A selective metabotropic glutamate receptor 7 agonist: activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc. Natl. Acad. Sci. USA. 2005;102(51):18712–17. doi: 10.1073/pnas.0508063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suzuki G, Tsukamoto N, Fushiki H, Kawagishi A, Nakamura M, et al. In vitro pharmacological characterization of novel isoxazolopyridone derivatives as allosteric metabotropic glutamate receptor 7 antagonists. J. Pharmacol. Exp. Ther. 2007;323(1):147–56. doi: 10.1124/jpet.107.124701. [DOI] [PubMed] [Google Scholar]
- 96.Niswender CM, et al. Permissive antagonism induced by negative allosteric modulators of mGluR7. Neuropharmacology. 2008;55:615. (Abstr.) [Google Scholar]
- 97.Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat. Rev. Drug Discov. 2005;4(11):919–27. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- 98.Coutinho V, Knopfel T. Metabotropic glutamate receptors: electrical and chemical signaling properties. Neuroscientist. 2002;8(6):551–61. doi: 10.1177/1073858402238514. [DOI] [PubMed] [Google Scholar]
- 99.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 100.Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Rev. 1999;29(1):83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 101.Valenti O, Conn PJ, Marino MJ. Distinct physiological roles of the G-coupled metabotropic glutamate receptors co-expressed in the same neuronal populations. J. Cell. Physiol. 2002;191(2):125–37. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- 102.Bellone C, Luscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell. Mol. Life Sci. 2008;65(18):2913–23. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat. Rev. Neurosci. 2008;9(6):423–36. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- 104.Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci. 2001;21(16):5925–34. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kullmann DM, Lamsa K. Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation. J. Physiol. 2008;586(6):1481–86. doi: 10.1113/jphysiol.2007.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79(2):365–75. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 107.Gil-Sanz C, Delgado-Garcıa JM, Fairén A, Gruart A. Involvement of the mGluR1 receptor in hippocampal synaptic plasticity and associative learning in behaving mice. Cereb. Cortex. 2008;18(7):1653–63. doi: 10.1093/cercor/bhm193. [DOI] [PubMed] [Google Scholar]
- 108.Aiba A, Kano M, Chen C, Stanton ME, Fox DG, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79(2):377–88. [PubMed] [Google Scholar]
- 109.Levenes C, Daniel H, Jaillard D, Conquet F, Crépel F. Incomplete regression of multiple climbing fibre innervation of cerebellar Purkinje cells in mGLuR1 mutant mice. Neuroreport. 1997;8(2):571–74. doi: 10.1097/00001756-199701200-00038. [DOI] [PubMed] [Google Scholar]
- 110.Sachs AJ, Schwendinger JK, Yang AW, Haider NB, Nystuen AM. The mouse mutants recoil wobbler and nmf373 represent a series of Grm1 mutations. Mamm. Genome. 2007;18(11):749–56. doi: 10.1007/s00335-007-9064-y. [DOI] [PubMed] [Google Scholar]
- 111.Brody SA, Conquet F, Geyer MA. Disruption of prepulse inhibition in mice lacking mGluR1. Eur. J. Neurosci. 2003;18(12):3361–66. doi: 10.1111/j.1460-9568.2003.03073.x. [DOI] [PubMed] [Google Scholar]
- 112.Brody SA, Dulawa SC, Conquet F, Geyer MA. Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol. Psychiatry. 2004;9(1):35–41. doi: 10.1038/sj.mp.4001404. [DOI] [PubMed] [Google Scholar]
- 113.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat. Neurosci. 2001;4(9):873–74. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 114.Bradbury MJ, Campbell U, Giracello D, Chapman D, King C, et al. Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J. Pharmacol. Exp. Ther. 2005;313(1):395–402. doi: 10.1124/jpet.104.076406. [DOI] [PubMed] [Google Scholar]
- 115.Muly EC, Mania I, Guo JD, Rainnie DG. Group II metabotropic glutamate receptors in anxiety circuitry: correspondence of physiological response and subcellular distribution. J. Comp. Neurol. 2007;505(6):682–700. doi: 10.1002/cne.21525. [DOI] [PubMed] [Google Scholar]
- 116.Bradley SR, Levey AI, Hersch SM, Conn PJ. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J. Neurosci. 1996;16(6):2044–56. doi: 10.1523/JNEUROSCI.16-06-02044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koulen P, Brandstatter JH. Pre- and postsynaptic sites of action of mGluR8a in the mammalian retina. Invest. Ophthalmol. Vis. Sci. 2002;43(6):1933–40. [PubMed] [Google Scholar]
- 118.Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, et al. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273(5275):645–47. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 119.Higgins GA, Ballard TM, Kew JN, Richards JG, Kemp JA, et al. Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology. 2004;46(7):907–17. doi: 10.1016/j.neuropharm.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 120.Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc. Natl. Acad. Sci. USA. 2005;102(11):4170–75. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid ( LY404039) J. Pharmacol. Exp. Ther. 2008;326(1):209–17. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- 122.Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl.) 2008;196(3):431–40. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- 123.Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, et al. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegen- eration/neuroprotection. J. Neurosci. 2007;27(31):8297–308. doi: 10.1523/JNEUROSCI.1889-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, et al. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J. Biol. Chem. 1993;268(16):11868–73. [PubMed] [Google Scholar]
- 125.Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr, Birnbaumer L, et al. The light response of ON bipolar neurons requires G[alpha]o. J. Neurosci. 2000;20(24):9053–58. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80(5):757–65. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 127.Sugihara H, Inoue T, Nakanishi S, Fukuda Y. A late ON response remains in visual response of the mGluR6-deficient mouse. Neurosci. Lett. 1997;233(2–3):137–40. doi: 10.1016/s0304-3940(97)00656-3. [DOI] [PubMed] [Google Scholar]
- 128.Lavreysen H, Dautzenberg FM. Therapeutic potential of group III metabotropic glutamate receptors. Curr. Med. Chem. 2008;15(7):671–84. doi: 10.2174/092986708783885246. [DOI] [PubMed] [Google Scholar]
- 129.Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, et al. Immunohistochemical localiza- tion of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J. Comp. Neurol. 1999;407(1):33–46. [PubMed] [Google Scholar]
- 130.Toyono T, Seta Y, Kataoka S, Harada H, Morotomi T, et al. Expression of the metabotropic glutamate receptor, mGluR4a, in the taste hairs of taste buds in rat gustatory papillae. Arch. Histol. Cytol. 2002;65(1):91–96. doi: 10.1679/aohc.65.91. [DOI] [PubMed] [Google Scholar]
- 131.Uehara S, Muroyama A, Echigo N, Morimoto R, Otsuka M, et al. Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by alpha-cells of islet of Langerhans. Diabetes. 2004;53(4):998–1006. doi: 10.2337/diabetes.53.4.998. [DOI] [PubMed] [Google Scholar]
- 132.Pekhletski R, Gerlai R, Overstreet LS, Huang XP, Agopyan N, et al. Impaired cerebellar synaptic plasticity and motor performance in mice lacking the mGluR4 subtype of metabotropic glutamate receptor. J. Neurosci. 1996;16(20):6364–73. doi: 10.1523/JNEUROSCI.16-20-06364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gerlai R, Roder JC, Hampson DR. Altered spatial learning and memory in mice lacking the mGluR4 subtype of metabotropic glutamate receptor. Behav. Neurosci. 1998;112(3):525–32. doi: 10.1037//0735-7044.112.3.525. [DOI] [PubMed] [Google Scholar]
- 134.Snead OC, 3rd, Banerjee PK, Burnham M, Hampson D. Modulation of absence seizures by the GABA(A) receptor: a critical role for metabotropic glutamate receptor 4 (mGluR4) J. Neurosci. 2000;20(16):6218–24. doi: 10.1523/JNEUROSCI.20-16-06218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blednov YA, Walker D, Osterndork-Kahanek E, Harris RA. Mice lacking metabotropic glutamate receptor 4 do not show the motor stimulatory effect of ethanol. Alcohol. 2004;34(2–3):251–59. doi: 10.1016/j.alcohol.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 136.Shigemoto R, Kinoshita A, Wada E, Nomura A, Ohishi H, et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997;17(19):7503–22. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kinoshita A, Shigemoto R, Ohishi H, van der Putten H, Mizuno N. Immunohistochemical local- ization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: a light and electron microscopic study. J. Comp. Neurol. 1998;393(3):332–52. [PubMed] [Google Scholar]
- 138.Sansig G, Bushell TJ, Clarke VRJ, Rozov A, Burnashev N, et al. Increased seizure susceptibility in mice lacking metabotropic glutamate receptor 7. J. Neurosci. 2001;21(22):8734–45. doi: 10.1523/JNEUROSCI.21-22-08734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Masugi M, Yokoi M, Shigemoto R, Muguruma K, Watanabe Y, et al. Metabotropic glutamate receptor subtype 7 ablation causes deficit in fear response and conditioned taste aversion. J. Neurosci. 1999;19(3):955–63. doi: 10.1523/JNEUROSCI.19-03-00955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bushell TJ, Sansig G, Collett VJ, van der Putten H, Collingridge GL. Altered short-term synaptic plasticity in mice lacking the metabotropic glutamate receptor mGlu7. Sci. World J. 2002;2:730–37. doi: 10.1100/tsw.2002.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Holscher C, Schmid S, Pilz PK, Sansig G, van der Putten H, Plappert CF. Lack of the metabotropic glutamate receptor subtype 7 selectively impairs short-term working memory but not long-term memory. Behav. Brain. Res. 2004;154(2):473–81. doi: 10.1016/j.bbr.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 142.Holscher C, Schmid S, Pilz PKD, Sansig G, van der Putten H, Plappert CF. Lack of the metabotropic glutamate receptor subtype 7 selectively modulates Theta rhythm and working memory. Learn. Mem. 2005;12(5):450–55. doi: 10.1101/lm.98305. [DOI] [PubMed] [Google Scholar]
- 143.Goddyn H, Callaerts-Vegh Z, Stroobants S, Dirikx T, Vansteenwegen D, et al. Deficits in acquisition and extinction of conditioned responses in mGluR7 knockout mice. Neurobiol. Learn. Mem. 2008;90(1):103–11. doi: 10.1016/j.nlm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 144.Callaerts-Vegh Z, Beckers T, Ball SM, Baeyens F, Callaerts F, et al. Concomitant deficits in working memory and fear extinction are functionally dissociated from reduced anxiety in metabotropic glutamate receptor 7-deficient mice. J. Neurosci. 2006;26(24):6573–82. doi: 10.1523/JNEUROSCI.1497-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur. J. Neurosci. 2003;17(11):2409–17. doi: 10.1046/j.1460-9568.2003.02667.x. [DOI] [PubMed] [Google Scholar]
- 146.Stachowicz K, Brañski P, Klak K, van der Putten H, Cryan JF, et al. Selective activation of metabotropic G-protein-coupled glutamate 7 receptor elicits anxiolytic-like effects in mice by modulat- ing GABAergic neurotransmission. Behav. Pharmacol. 2008;19(5–6):597–603. doi: 10.1097/FBP.0b013e32830cd839. [DOI] [PubMed] [Google Scholar]
- 147.Scherer SW, Soder S, Duvoisin RM, Huizenga JJ, Tsui LC. The human metabotropic glutamate receptor 8 (GRM8) gene: a disproportionately large gene located at 7q31.3-q32.1. Genomics. 1997;44(2):232–36. doi: 10.1006/geno.1997.4842. [DOI] [PubMed] [Google Scholar]
- 148.Linden AM, Johnson BG, Peters SC, Shannon HE, Tian M, et al. Increased anxiety-related behavior in mice deficient for metabotropic glutamate 8 (mGlu8) receptor. Neuropharmacology. 2002;43(2):251–59. doi: 10.1016/s0028-3908(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 149.Duvoisin RM, Zhang C, Pfankuch TF, O’Connor H, Gayet-Primo J, et al. Increased measures of anxiety and weight gain in mice lacking the group III metabotropic glutamate receptor mGluR8. Eur. J. Neurosci. 2005;22(2):425–36. doi: 10.1111/j.1460-9568.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- 150.Robbins MJ, Starr KR, Honey A, Soffin EM, Rourke C, et al. Evaluation of the mGlu8 receptor as a putative therapeutic target in schizophrenia. Brain Res. 2007;1152:215–27. doi: 10.1016/j.brainres.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 151.Tong Q, Ouedraogo R, Kirchgessner AL. Localization and function of group III metabotropic glutamate receptors in rat pancreatic islets. Am. J. Physiol. Endocrinol. Metab. 2002;282(6):E1324–33. doi: 10.1152/ajpendo.00460.2001. [DOI] [PubMed] [Google Scholar]
- 152.Tong Q, Kirchgessner AL. Localization and function of metabotropic glutamate receptor 8 in the enteric nervous system. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285(5):G992–1003. doi: 10.1152/ajpgi.00118.2003. [DOI] [PubMed] [Google Scholar]
- 153.Pilc A, Chakib S, Nowaka G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem. Pharmacol. 2008;75(5):997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 154.Swanson CJ, Bures M, Johnson MP, Linden A-M, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005;4(2):131–44. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 155.Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl.) 2004;174(1):39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- 156.Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin. Cell. Dev. Biol. 2006;17(5):592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 157.Alexander GM, Godwin DW. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res. 2006;71(1):1–22. doi: 10.1016/j.eplepsyres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 158.Lee HG, Zhu X, O’Neill MJ, Webber K, Casadesus G, et al. The role of metabotropic glutamate receptors in Alzheimer’s disease. Acta. Neurobiol. Exp. (Wars.) 2004;64(1):89–98. doi: 10.55782/ane-2004-1494. [DOI] [PubMed] [Google Scholar]
- 159.Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat. Rev. Neurosci. 2005;6(10):787–98. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- 160.Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995;355(3):455–69. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 161.Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J. Clin. Psychopharmacol. 1982;2(2):129–33. [PubMed] [Google Scholar]
- 162.Porter RH, Jaeschke G, Spooren W, Ballard TM, Büttelmann B, et al. Fenobam: a clinically vali- dated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J. Pharmacol. Exp. Ther. 2005;315(2):711–21. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- 163.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–77. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 164.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–62. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49(7):1053–66. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 166.Slassi A, Isaac M, Edwards L, Minidis A, Wensbo D, et al. Recent advances in non-competitive mGlu5 receptor antagonists and their potential therapeutic applications. Curr. Top. Med. Chem. 2005;5(9):897–911. doi: 10.2174/1568026054750236. [DOI] [PubMed] [Google Scholar]
- 167.Lehmann A. Novel treatments of GERD: focus on the lower esophageal sphincter. Eur. Rev. Med. Pharmacol. Sci. 2008;12(Suppl 1):103–10. [PubMed] [Google Scholar]
- 168.Keywood C, Wakefield M. ADX10059, a negative allosteric modulator of mGluR5, demonstrates proof of concept for a role in the management of migraine, in a placebo controlled phase 2A study. Neuropharmacology. 2008;55:605. [Google Scholar]
- 169.Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J. Neurosci. 2008;28(2):543–47. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur. J. Hum. Genet. 2008;16(6):666–72. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.O’Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, et al. A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 in rat forebrain. J. Pharmacol. Exp. Ther. 2004;309(2):568–77. doi: 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- 172.Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, et al. Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol. Pharmacol. 2007;71(5):1389–98. doi: 10.1124/mol.106.032425. [DOI] [PubMed] [Google Scholar]
- 173.Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav. Pharmacol. 2008;19(3):225–34. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6(3):189–97. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- 175.Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J. Pharmacol. Exp. Ther. 1999;291(1):161–70. [PubMed] [Google Scholar]
- 176.Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tol- erability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. 2008;33(10):2549. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- 177.Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized phase 2 clinical trial. Nat. Med. 2007;13(9):1102–7. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 178.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, et al. Identification of a sero- tonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452(7183):93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105(2):379–92. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 180.Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 2007;72(2):477–84. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- 181.Brady AE, Conn PJ. Metabotropic glutamate receptor ligands as novel therapeutic agents. 2008. pp. 529–564. See Ref. 15.
- 182.Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, et al. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J. Neurosci. 2003;23(18):7218–26. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Macinnes N, Duty S. Group III metabotropic glutamate receptors act as hetero-receptors modulating evoked GABA release in the globus pallidus in vivo. Eur. J. Pharmacol. 2008;580(1–2):95–99. doi: 10.1016/j.ejphar.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 184.Matsui T, Kita H. Activation of group III metabotropic glutamate receptors presynaptically reduces both GABAergic and glutamatergic transmission in the rat globus pallidus. Neuroscience. 2003;122(3):727–37. doi: 10.1016/j.neuroscience.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 185.Wichmann T, DeLong MR. Functional neuroanatomy of the basal ganglia in Parkinson’s disease. Adv. Neurol. 2003;91:9–18. [PubMed] [Google Scholar]
- 186.MacInnes N, Messenger MJ, Duty S. Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat. Br. J. Pharmacol. 2004;141(1):15–22. doi: 10.1038/sj.bjp.0705566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lopez S, Turle-Lorenzo N, Acher F, De Leonibus E, Mele A, Amalric M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson’s disease. J. Neurosci. 2007;27(25):6701–11. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ossowska K, Konieczny J, Wardas J, Pietraszek M, Kuter K, et al. An influence of ligands of metabotropic glutamate receptor subtypes on parkinsonian-like symptoms and the striatopallidal pathway in rats. Amino Acids. 2007;32(2):179–88. doi: 10.1007/s00726-006-0317-y. [DOI] [PubMed] [Google Scholar]
- 189.Konieczny J, Wardas J, Kuter K, Pilc A, Ossowska K. The influence of group III metabotropic glutamate receptor stimulation by (1S,3R,4S)-1-aminocyclo-pentane-1,3,4- tricarboxylic acid on the parkinsonian-like akinesia and striatal proenkephalin and prodynorphin mRNA expression in rats. Neu- roscience. 2007;145(2):611–20. doi: 10.1016/j.neuroscience.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 190.Amalric M, Lopez S. Metabotropic glutamate receptors and Parkinson’s disease. Neuropharmacology. 2008;55:585. [Google Scholar]
- 191.Reynolds IJ. Metabotropic glutamate receptors as therapeutic targets in Parkinson’s disease. Neuropharmacology. 2008;55:620. [Google Scholar]
- 192.Ortuno D, Cheng C, Weiss Bergeron MM, Shanker Y. Identification and characterization of a potent and selective positive allosteric modulator of mGluR4. Presented at Annu. Meet. Soc. Neurosci..2008. [Google Scholar]
- 193.Battaglia G, Busceti CL, Molinaro G, Biagioni F, Traficante A, et al. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurosci. 2006;26(27):7222–29. doi: 10.1523/JNEUROSCI.1595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Valenti O, Mannaioni G, Seabrook GR, Conn PJ, Marino MJ. Group III metabotropic glutamate-receptor-mediated modulation of excitatory transmission in rodent substantia nigra pars compacta dopamine neurons. J. Pharmacol. Exp. Ther. 2005;313(3):1296–304. doi: 10.1124/jpet.104.080481. [DOI] [PubMed] [Google Scholar]
- 195.Parelkar NK, Wang JQ. Upregulation of metabotropic glutamate receptor 8 mRNA expression in the rat forebrain after repeated amphetamine administration. Neurosci. Lett. 2008;433(3):250–54. doi: 10.1016/j.neulet.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang GC, Vu K, Parelkar NK, Mao LM, Stanford IM, et al. Acute administration of cocaine reduces metabotropic glutamate receptor 8 protein expression in the rat striatum in vivo. Neurosci. Lett. 2008;449(3):224–27. doi: 10.1016/j.neulet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Iacovelli L, Arcella A, Battaglia G, Pazzaglia S, Aronica E, et al. Pharmacological activation of mGlu4 metabotropic glutamate receptors inhibits the growth of medulloblastomas. J. Neurosci. 2006;26(32):8388–97. doi: 10.1523/JNEUROSCI.2285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saxe JP, Wu H, Kelly TK, Phelps ME, Sun YE, et al. A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation. Chem. Biol. 2007;14(9):1019–30. doi: 10.1016/j.chembiol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]