Abstract
The roles of insulin-like growth factors (IGFs) in regulating growth and their modulation by six IGF binding proteins (IGFBP) are well established. IGFBP-5, the most abundant IGFBP stored in bone, is an important regulator of bone formation via IGF-dependent and -independent mechanisms. Two new proteins, four and a half lim (FHL)-2, a transcription modulator that interacts with IGFBP-5, and a disintegrin and metalloprotease (ADAM)-9, an IGFBP-5 protease, have been identified as potential regulators of IGFBP-5 action in bone. We tested the hypothesis that agents which modulate bone formation by regulating IGFBP-5 expression would also regulate FHL-2 and ADAM-9 expression in a coordinated manner. We evaluated the expression of IGFBP-5, FHL-2, and ADAM-9 by real-time reverse transcriptase (RT)-PCR during differentiation of mouse bone marrow stromal cells into osteoblasts and in response to treatment with bone formation modulators in the LSaOS human osteosarcoma cell line. IGFBP-5 and FHL-2 increased 4.3- and 3.0-fold (P ≤ 0.01), respectively, during osteoblast differentiation. Dexamethasone (Dex), an inhibitor of bone formation, decreased IGFBP-5 and FHL-2 and increased ADAM-9 in LSaOS cells (P ≤ 0.05). Bone morphogenic protein (BMP)-7, a stimulator of bone formation, increased IGFBP-5 and decreased ADAM-9 (P < 0.01). To determine if BMP-7 would eliminate Dex inhibition of IGFBP-5, cells were treated with Dex + BMP-7. The BMP-7-induced increase in IGFBP-5 was reduced, but not eliminated, in the presence of Dex (P ≤ 0.01), indicating that BMP-7 and Dex may regulate IGFBP-5 via different mechanisms. Transforming growth factor (TGF)-β, a stimulator of bone formation, increased IGFBP-5 and FHL-2 expression (P ≤ 0.01). IGF-I and TNF-α decreased expression of ADAM-9 (P < 0.05). In conclusion, our findings are consistent with the hypothesis that FHL-2 and ADAM-9 are important modulators of IGFBP-5 actions and are, in part, regulated in a coordinated manner in bone.
Keywords: IGFBP-5, FHL-2, ADAM-9, Bone
1. Introduction
The insulin-like growth factors (IGFs) are important regulators of growth and development. The IGFs are the most abundant growth factors produced by osteoblasts and are regulated by six high affinity IGF binding proteins (IGFBP). In general, IGFBP-1, -2, -4 and -6 inhibit IGF action, while IGFBP-3 and -5 stimulate IGF action in bone. Specifically, IGFBP-5 is the most abundant IGFBP stored in bone and several in vivo and in vitro experiments have confirmed its important role in regulating bone formation [1]. For example, treatment with IGFBP-5 in vitro stimulates proliferation and differentiation of osteoblasts [2–4] and systemic administration of IGFBP-5 increases bone formation parameters [osteocalcin (Oc); alkaline phosphatase (ALP)] in mice [5,6].
It is well accepted that many of IGFBP-5’s actions are dependent on IGF since IGFBP-5 can bind to hydroxyapatite and extracellular matrix protein to aid in binding IGFs to bone [3,4,7]. However, recent in vitro and in vivo data have suggested that IGFBP-5 may also function as an IGF-independent growth factor in bone [8,9]. Specifically, IGFBP-5 stimulates proliferation and ALP activity in osteoblasts derived from IGF-I knockout mice [9].
The important role of IGFBP-5 in regulating bone formation is further demonstrated by the recent findings that IGFBP-5 action in bone is controlled by several potential control mechanisms. First, the findings that the expression of IGFBP-5 in osteoblasts is modulated by bone formation regulators suggest that transcriptional regulation of IGFBP-5 is an important control point for regulating IGFBP-5 actions [5,10]. Second, it is known that IGFBP-5 proteases such as a disintegrin and metalloprotease (ADAM)-9 produced by osteoblasts in response to osteoregulatory agents could act to regulate the effective concentration and activity of IGFBP-5 in bone microenvironment [11]. Third, IGFBP-5 has been shown to interact with transcription modulators such as four and a half lim (FHL)-2 to mediate its IGF-independent effects in osteoblasts [12]. If FHL2 and/or ADAM-9 were to be rate limiting mediators of IGFBP-5 action in osteoblasts, we would expect the expression of these regulatory molecules to be modulated in a coordinated manner along with IGFBP-5 by systemic and local agents that regulate bone formation via IGFBP-5. As a means of testing this hypothesis, we evaluated the expression of IGFBP-5, FHL-2 and ADAM-9 during differentiation of bone marrow stromal cells into osteoblasts and in response to osteoregulatory agents that are known to modulate bone formation.
2. Materials and methods
2.1. Tissue culture analysis
2.1.1. Tissue culture reagents
α-Minimal essential medium (α-MEM), Dulbecco’s modified Eagle’s medium (DMEM), penicillin–streptomycin suspension, and calf serum were purchased from Gibco-BRL (Gaithersburg, MD). Bovine serum albumin (BSA) was purchased from Fluka (Buchs, Switzerland). Human IGF-I was purchased from GroPep (Adelaide SA 5000, Australia). Human bone morphogenic protein (BMP)-7 was a gift from Creative Biomolecules (Hopkinton, MA). Human transforming growth factor (TGF)-β was purchased from R&D Systems (Minneapolis, MN). Recombinant mouse tumor necrosis factor (TNF)-α was purchased from PeproTech Inc. (Rocky Hill, NJ). All other chemicals were purchased from Fisher Scientific (Tustin, CA) or Sigma (St. Louis, MO).
2.1.2. Osteoblast differentiation
To evaluate changes in gene expression during the differentiation of bone marrow stromal cells into osteoblasts, mouse bone marrow cells were isolated, counted with a hemocytometer, and plated as 20 × 106 cells/90-mm petri dish. Bone marrow cells were cultured with α-MEM containing 10% calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin and culture media were changed every two days until 75% confluency was reached (about 6 days). This was considered day 0, at which point a differentiation medium (α-MEM containing 10% calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL ascorbic acid, and 10 mM β-glycerophosphate) was added to the cells to induce differentiation into osteoblast. Osteoblast differentiation was confirmed by nodule formation by day 30 of culture [13]. During the 30 days of culture, half of the media were changed every three days. At days 0, 6, 12, 18, and 24, six plates were washed with PBS and cells were extracted with 1 mL of Tri-Reagent (Molecular Research Center Inc., Cincinnati, OH) and stored at −70 °C until RNA extraction was performed.
2.1.3. Effects of osteoregulatory agents in osteoblasts
To evaluate the effects of osteoregulatory agents on gene expression in osteoblasts, we used LSaOS cells, a human osteosarcoma cell line which has been reported to display characteristics of osteoblasts [14]. LSaOS cells (HTB85, ATCC, Manassas, VA) were cultured in 90-mm petri dishes in DMEM media containing 10% calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin until they reached 70% confluency. Six plates per treatment were used for each experiment. Once the cells were 70% confluent, media were changed to serum-free media (DMEM, 0.1% BSA, 100 U/mL penicillin, and 100 μg/mL streptomycin) twice over a 24-h period. Cells were treated with DMEM media containing 0.1% BSA, 100 U/mL penicillin, 100 μg/mL streptomycin, and osteoregulatory agent [dexamethasone (Dex; 0.1–100 nM), BMP-7 (30 ng/mL), TGF-β (1 ng/mL), IGF-I (30 ng/mL), or TNF-α (20 ng/mL)] for 24 h based on previous reports [9,15–17]. Twenty-four hours after treatment, cells were washed with PBS and extracted with 1 mL Tri-Reagent (Molecular Research Center Inc.) and stored at −70 °C until RNA extraction was performed.
2.2. RNA extraction
RNA was extracted from the cell pellets using Tri-Reagent (Molecular Research Center Inc.) and an RNeasy Mini Kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s protocol. Following extraction, residual DNA was removed from up to 10 μg of RNA with a DNA-free Kit (Ambion, Austin, TX). RNA quality was determined using a 2100 Bioanalyzer (Agilent, Palo Alto, CA) and RNA was quantified using a Nano-Drop Spectrophotometer (Wilmington, DE).
2.3. Gene expression analysis
Quantitative real-time reverse transcriptase (RT)-PCR analysis was used to determine the expression levels of Oc, type 1 collagen (Col1), ALP, IGF-I, IGF-II, IGFBP-3, IGFBP-5, FHL-2, ADAM-9, PPIA, and Actin. Primer sequences are located in Table 1. Primers used were validated by four methods: (1) Specificity of primer sequences was determined using NCBI Blast [18]. (2) Amplification of the correct product size was verified on a 2% agarose gel. (3) Primers were designed for a melting temperature around 60 °C, and the appropriate melting temperature for each primer set was determined using a temperature gradient program on a PTC-225 Peltier Thermal Cycler (MJ Research Inc., Waltham, MA). (4) Serial dilutions of different RNA concentrations were used to determine the appropriate dilution and accuracy of the primer amplification. Specifically, RNA samples were normalized to 200 or 300 ng per sample and reverse transcribed with oligo(dT) primers and Superscript II (Invitrogen, Carlsbad, CA) to produce cDNA at a total volume of 20 μL. Real-time RT-PCR analysis was performed using a Stratagene Brilliant SYBRGreen Master Mix (Stratagene, La Jolla, CA) and a 7000 ABI Prism sequence detection system (Applied Biosystems, Foster City). Briefly, a total volume of 25 μL was loaded onto each well on a 96-well plate (1 μL of product, 0.125 μL of 10 μM forward and reverse primer, and 12.5 μL of 1 × SYBRGreen Master Mix). The PCR conditions were 95 °C for 10 min, 40 cycles at 95 °C for 15 s and 60 °C for 1 min, 95 °C for 15 s, 60 °C for 15 s, and 95 °C for 15 s. PPIA or Actin RNA were used as endogenous controls. Delta CT values were determined (CT value for gene of interest minus CT value for control gene) and comparisons of the CT values were used for relative quantification of gene expression [19].
Table 1.
Sequences for primers used in real-time RT-PCR
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| Osteocalcin (m) | CTCTCTCTGCTCACTCTGCT | TTTGTAGGCGGTCTTCAAGC |
| Type 1 collagen (m) | GCCTCCCAGAACATCACCTA | AGTTCCGGTGTGACTCGTG |
| Alkaline phosphatase (m) | ATGGTAACGGGCCTGGCTACA | AGTTCTGCTCATGGACGCCGT |
| IGF-I (m) | CATCTCCAGTCTCCTCAGATC | GTCCACACACGAACTGAAGAGC |
| IGF-II (m) | AGCGGCCTCCTTACCCAA | CGCGGTCCGAACAGACAA |
| IGFBP-3 (m) | CCAGAACTTCTCCTCCGAGTCTAAG | CTCAGCACATTGAGGAACTTCAGAT |
| IGFBP-5 (m) | ATACAACCCAGAACGCCAGCT | ACCTGGGCTATGCACTTGATG |
| IGFBP-5 (h) | GAGGCAAGTTGGATGAACAG | TGTCCATACTGCTGGCTAGA |
| FHL-2 (m) | AAAGCCTATCACCACAGGAGGT | CCCAGCACATTTCTTAGCGTAC |
| FHL-2 (h) | CTTCACAGCTCGCGATGACT | CAAGCAGCAACTTCTCTGTG |
| ADAM-9 (m) | CCAGACCCAGGGATGGTGAAT | GGCCATGACATTTTCCCTGAA |
| ADAM-9 (h) | GCATTAACCTCACCGATGAC | GGAGTGTTCCTCGACATGTT |
| PPIA (m) | TCCTGGACCCAAAACGCTCC | CCATGGCAAATGCTGGACCA |
| Actin (h) | GAGGCATTGCTGACAGGATG | TGCTGATCCACATCTGCTGG |
h = primer specific for human; m = primer specific for mouse; IGF = insulin-like growth factor; IGFBP = IGF binding protein; FHL = four and a half lim; ADAM = a disintegrin and metalloprotease.
2.4. Statistical analysis
Osteoblast differentiation and dose response data were analyzed using ANOVA, and post hoc analysis for dose response was analyzed using the Dunnett test (Statistica 6, StatSoft, Tulsa, OK). Data for response to osteoregulatory agents were analyzed using Student’s t-test (Microsoft Excel). Data are presented as means ± standard error and a significant difference was determined at P ≤ 0.05.
3. Results
3.1. Expression of IGFBP-5 and FHL-2 increase during osteoblast differentiation
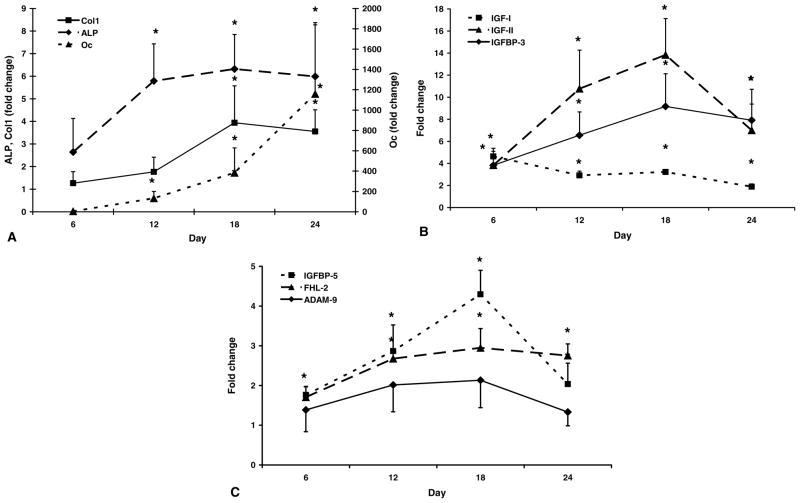
During differentiation of mouse bone marrow stromal cells into osteoblasts, gene expression of Oc, Col1, and ALP, markers of osteoblast differentiation, increased (P < 0.05) between day 0 and day 24 of cell culture (Fig. 1A). Specifically, Oc increased over 1000-fold (P < 0.01) by day 24 and ALP and Col1 increased 6- and 4-fold (P < 0.05), respectively, by day 18 of culture compared with day 0. Similar to markers of differentiation, components of the IGF axis increased during osteoblast differentiation (P < 0.01; Fig. 1B). Specifically, expression of IGF-I increased 5-fold (P < 0.01) by day 6, IGF-II increased 11-fold (P < 0.01) by day 12, and IGFBP-3 increased 9-fold by day 18 compared with day 0 (P < 0.01). There was a steady increase in IGFBP-5 expression such that there was a 4-fold increase (P < 0.01) by day 18 compared with day 0 of culture (Fig. 1C). Similar to IGFBP-5, FHL-2 expression increased 3-fold (P < 0.01) by day 18 of culture (Fig. 1C). ADAM-9 expression did not change significantly during osteoblast differentiation (P > 0.10; Fig. 1C).
Fig. 1.
Gene expression during differentiation of mouse bone marrow stromal cells into osteoblast cells. Data are presented as fold change from day 0 (means ± standard error). *A significant difference (P ≤ 0.05) compared with day 0. (A) Oc = osteocalcin; Col1 = type 1 collagen; ALP = alkaline phosphatase. (B) IGF = insulin-like growth factor; IGFBP = IGF binding protein. (C) FHL-2 = four and a half lim-2; ADAM-9 = a disintegrin and metalloprotease-9.
3.2. IGFBP-5, FHL-2, and ADAM-9 expression in response to osteoregulatory agents
Increasing concentrations of Dex resulted in a biphasic response in terms of IGFBP-5 expression. At 0.1 nM, Dex treatment caused a 2.4-fold increase (P < 0.05) in IGFBP-5 expression, while at 100 nM, Dex decreased IGFBP-5 expression 5-fold (P < 0.001). Dex at both 10 and 100 nM doses decreased FHL-2 expression (P < 0.05). All four doses of Dex increased expression of ADAM-9 2-fold (P < 0.05) (Fig 2).
Fig. 2.
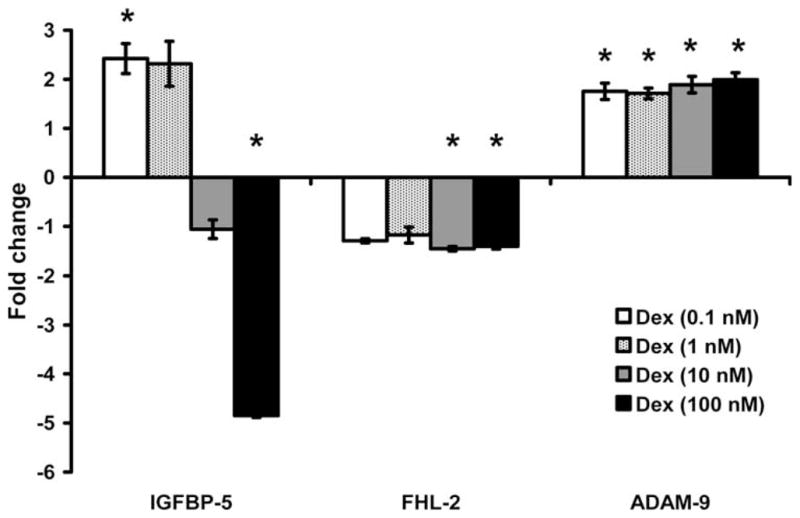
Effect of different doses of dexamethasone (Dex) on gene expression. Data are presented as fold change from corresponding control (means ± standard error). *A significant difference (P < 0.05) compared with corresponding control. IGFBP = insulin-like growth factor binding protein; FHL-2 = four and a half lim-2; ADAM-9 = a disintegrin and metalloprotease-9.
Bone morphogenic protein-7, a stimulator of bone formation, increased IGFBP-5 expression 6-fold (P < 0.01; Fig. 3), but no significant changes in FHL-2 or ADAM-9 were observed (P > 0.1). To determine if BMP-7 would eliminate Dex inhibition of IGFBP-5, cells were treated with a combination of Dex and BMP-7. In terms of IGFBP-5 expression, the combination of Dex + BMP-7 resulted in an intermediate response compared with BMP-7 or Dex treatment alone (P ≤ 0.01; Fig. 3). While the combined treatment abolished the Dex increase in ADAM-9 expression, it did not modify the reduction in FHL-2 expression caused by Dex treatment. To determine if other osteoregulatory agents would regulate IGFBP-5, FHL-2, and ADAM-9 in a coordinated manner, cells were treated with TGF-β, IGF-I, and TNF-α. TGF-β increased IGFBP-5 and FHL-2 expression 12- and 2-fold, respectively (P < 0.01; Fig. 4). Interestingly, IGF-I and TNF-α treatment decreased ADAM-9 expression (P < 0.05), but did not significantly change IGFBP-5 or FHL-2 expression (P > 0.1; Fig. 4).
Fig. 3.
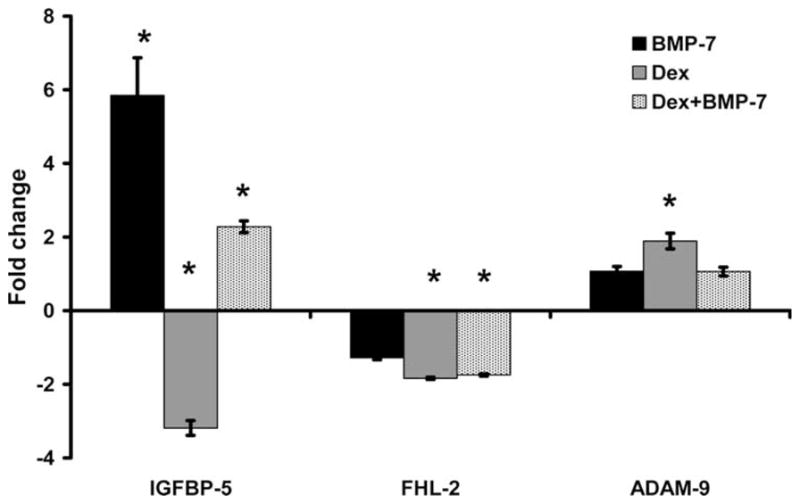
Effect of bone morphogenic protein (BMP)-7 and dexamethasone (Dex) treatment on gene expression. Data are presented as fold change from corresponding control (means ± standard error) and a representation of three different experiments. *A significant difference (P ≤ 0.05) compared with corresponding control. IGFBP = insulin-like growth factor binding protein; FHL-2 = four and a half lim-2; ADAM-9 = a disintegrin and metalloprotease-9.
Fig. 4.
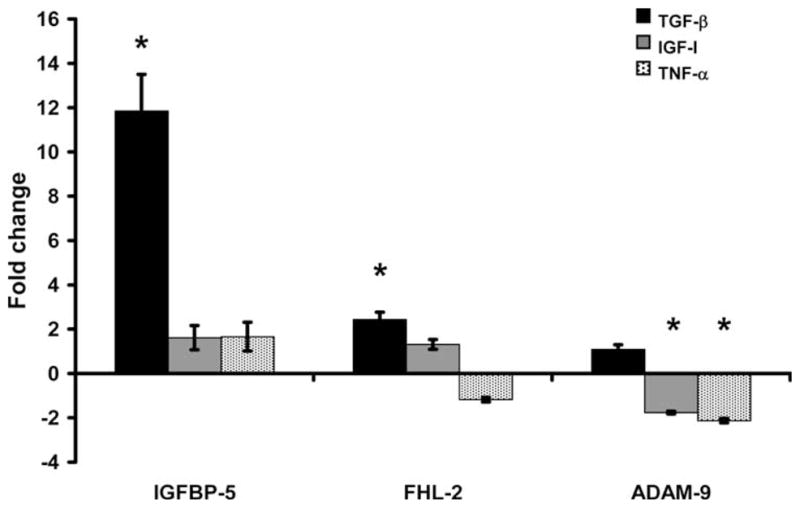
Effect of transforming growth factor (TGF)-β, insulin-like growth factor (IGF)-I, and tumor necrosis factor (TNF)-α treatment on gene expression. Data are presented as fold change from corresponding control (means ± standard error). *A significant difference (P ≤ 0.05) compared with corresponding control. IGFBP = insulin-like growth factor binding protein; FHL-2 = four and a half lim-2; ADAM-9 = a disintegrin and metalloprotease-9.
4. Discussion
4.1. Increased expression of FHL-2 and IGFBP-5 during mouse osteoblast differentiation
Expression of IGFBP-5, an important regulator of bone formation [5,6,9,20], increased during osteoblast differentiation. In previous studies, we and others [5,6,9,10] have found that intermittent administration of IGFBP-5 increases bone formation. We found that IGFBP-5 treatment (systemic and local) increases osteocalcin and alkaline phosphatase levels. Bauss et al. [10] demonstrated that a complex of IGFBP-5/IGF-I increased cortical thickness and BMD in femoral midshaft and tibial metaphysis. Andress [6] has shown that IGFBP-5 systemic treatment increased bone formation rate and BMD in ovariectomized rats. These in vivo findings are consistent with the in vitro findings that IGFBP-5 treatment increases osteoblast proliferation and/or differentiation [2]. In contrast to these anabolic effects, two recent studies on overexpression of IGFBP-5 in transgenic models have shown that overexpression of IGFBP-5 decreased bone formation at the periosteum [8,21]. It remains to be determined whether continuous exposure of osteoblasts to high levels of IGFBP-5 produces inhibitory effects, while intermittent exposure to low doses produces anabolic effects as in the case of PTH [22]. The increased expression of IGFBP-5 during osteoblast differentiation suggests a potential anabolic role for IGFBP-5 in the current model.
Similar to IGFBP-5, other members of the somatotropic axis, IGF-I, IGF-II, and IGFBP-3, that have previously been demonstrated to stimulate cell differentiation [23–25], increased during osteoblast differentiation. Since IGFBP-5 is an important regulator of bone cells, binds with FHL-2, and both proteins translocate into the nucleus [12], we were not surprised that FHL-2 expression followed a similar pattern to IGFBP-5 during differentiation. Previous experiments have demonstrated that FHL-2 can bind to several proteins including β-catenin, androgen receptor, AP-1, CREB, and CREM [12,26–29] and can function as a transcriptional coactivator. In particular, its interaction with β-catenin [30], a key mediator of the Wnt signaling pathway, suggests that it may also play an important role in bone formation. In addition, the FHL2 interaction with β-catenin promotes myogenic differentiation and it has been reported to bind with CREB and CREM, important regulators of proliferation and differentiation [26,29]. Based on these data and previous reports, we hypothesize that FHL-2 may, in part, mediate IGFBP-5 action during osteoblast differentiation.
4.2. Coordinated expression of IGFBP-5, FHL-2, and ADAM-9 in response to osteoregulatory agents in vitro
The decreased expression of IGFBP-5 following Dex treatment is similar to previous reports that glucocorticoids, such as Dex, have growth suppressing effects, inhibit the somatotropic axis, and long-term treatment leads to bone loss and osteoporosis [31–34]. Our data are similar to previous observations that Dex treatment decreased expression of IGF-I, IGFBP-3, and IGFBP-5, stimulatory factors, and increased expression of IGFBP-4, an inhibitory factor, in human osteoblast-like cells and decreased IGFBP-5 expression in preosteoblastic human bone marrow stromal cells [35,36]. In addition, similar to our findings, other studies in differentiating rat osteoblasts found that Dex treatment at 10 nMdoses did not change IGFBP-5 expression [37]. These data indicate that, in our model, the members of the somatotropic axis respond to Dex treatment in a predicted manner. In addition to decreased expression of IGFBP-5, the decreased expression of FHL-2, which binds to IGFBP-5, and the increased ADAM-9, an IGFBP-5 protease, support our hypothesis that these three factors are regulated in a coordinated manner in bone.
Similar to our previous reports, BMP-7, a known stimulator of bone formation, was observed to stimulate IGFBP-5 expression [16], however no significant change in FHL-2 was observed. These data suggest that BMP-7 may regulate IGFBP-5 and FHL-2 through different mechanisms in bone. In support of this hypothesis, BMP-7 was able to abolish the Dex inhibition of IGFBP-5, but not FHL-2. Interestingly, the combined treatment of Dex and BMP-7 reduced, but did not eliminate, the BMP-7-induced increase in IGFBP-5 expression, suggesting that BMP-7 and Dex may regulate IGFBP-5 via different mechanisms. Further analysis is needed to determine the mechanisms by which BMP-7 and Dex regulate IGFBP-5 and FHL-2 in bone. In contrast to our observed increase in IGFBP-5 expression in human osteosarcoma cells following BMP-7 treatment, previous data have been reported that IGFBP-5 mRNA expression decreased in primary rat calvaria cells following BMP-7 treatment [38–40]. These data suggest that IGFBP-5 response to BMP-7 treatment may be species and/or cell-type specific.
Transforming growth factor-β, a known stimulator of bone formation and member of the same superfamily as BMP-7 [41], increased expression of IGFBP-5, as well as FHL-2, thus suggesting that BMP-7 and TGF-β may mediate FHL-2 expression through different mechanisms in bone. In addition, the lack of change in ADAM-9 expression following TGF-β or BMP-7 treatment suggests a mechanism by which intact IGFBP-5 is made available by other growth factors for autocrine/paracrine actions. In contrast to our findings in osteoblasts, it has been reported that TGF-β inhibits IGFBP-5 synthesis through the JNK pathway in muscle [42]. Therefore, similar to BMP-7, TGF-β regulation of IGFBP-5 may be tissue-specific and mediated through different pathways in different cell types. Further investigation is needed to determine the pathway(s) through which TGF-β and BMP-7 mediate their effects on IGFBP-5 and FHL-2 in bone.
Interestingly, while IGF-I did not alter IGFBP-5 and FHL-2 expression, it did significantly decrease ADAM-9 expression. In some cell models, it has been demonstrated that IGF-I treatment increases IGFBP-5 mRNA levels [43,44]. In addition, IGF-I treatment also modulates proteolysis of IGFBP-5 in osteoblast cell culture media [45,46], which is suggested by the increased expression of ADAM-9 in the current model. Thus, the effect of IGF-I treatment on IGFBP-5 protein level is regulated by both transcriptional and post-transcriptional mechanisms. In this regard, Rutter et al. [44] found that IGFBP-5 protein level increased 4-fold in the bones of transgenic mice overexpressing IGF-I in osteoblasts. However, the IGFBP-5 mRNA level was increased only 1.5-fold. In our study, we found that mRNA level of IGFBP-5 was increased nearly 2-fold, but it was not significant. Thus, the magnitude of effects of IGF-I on IGFBP-5 mRNA level may vary depending on cell type and the increased ADAM-9 expression may indicate post-transcriptional regulation of IGFBP-5 by IGF-I treatment.
A recent study determined that treatment of osteoblasts with TNF-α increased pregnancy-associated plasma protein (PAPP)-A expression, thereby increasing IGFBP-4 proteolytic activity and, in turn, increasing IGF-I-induced cell proliferation [42]. These findings combined with our data suggest that in LSaOS cells, these osteoregulatory agents may allow more IGFBP-5 availability by limiting the amount of ADAM-9.
In conclusion, the IGFBP-5 molecular pathway, specifically FHL-2 and ADAM-9, is regulated by agents which regulate bone formation. In addition, the similar pattern of expression between IGFBP-5 and FHL-2 and the opposite pattern of ADAM-9 suggest that coordinated regulation of FHL-2 and ADAM-9 may provide efficient regulation of IGFBP-5 in bone. Further experiments are in progress to determine the mechanisms by which FHL-2 and ADAM-9 regulate IGFBP-5 action in bone.
Acknowledgments
The authors thank Zenay Bakhsh for technical assistance and Sean Belcher for secretarial assistance. This work was supported by National Institute of Health Grant AR31062.
Abbreviations
- α
Alpha
- β
Beta
References
- 1.Govoni KE, Baylink DJ, Mohan S. The multi-functional role of insulin-like growth factor binding proteins in bone. Pediatr Nephrol. 2004 doi: 10.1007/s00467-004-1658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andress DL, Loop SM, Zapf J, Kiefer MC. Carboxytruncated insulin-like growth factor binding protein-5 stimulates mitogenesis in osteoblast-like cells. Biochem Biophys Res Commun. 1993;195(1):25–30. doi: 10.1006/bbrc.1993.2004. [DOI] [PubMed] [Google Scholar]
- 3.Bautista CM, Baylink DJ, Mohan S. Isolation of a novel insulin-like growth factor (IGF) binding protein from human bone: a potential candidate for fixing IGF-II in human bone. Biochem Biophys Res Commun. 1991;176(2):756–763. doi: 10.1016/s0006-291x(05)80249-9. [DOI] [PubMed] [Google Scholar]
- 4.Mohan S, Nakao Y, Honda Y, Landale E, Leser U, Dony C, Lang K, Baylink DJ. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem. 1995;270(35):20424–20431. doi: 10.1074/jbc.270.35.20424. [DOI] [PubMed] [Google Scholar]
- 5.Richman C, Baylink DJ, Lang K, Dony C, Mohan S. Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology. 1999;140(10):4699–4705. doi: 10.1210/endo.140.10.7081. [DOI] [PubMed] [Google Scholar]
- 6.Andress DL. IGF-binding protein-5 stimulates osteoblast activity and bone accretion in ovariectomized mice. Am J Physiol Endocrinol Metab. 2001;281(2):E283–E288. doi: 10.1152/ajpendo.2001.281.2.E283. [DOI] [PubMed] [Google Scholar]
- 7.Nicolas V, Mohan S, Honda Y, Prewett A, Finkelman RD, Baylink DJ, Farley JR. An age-related decrease in the concentration of insulin-like growth factor binding protein-5 in human cortical bone. Calcif Tissue Int. 1995;57(3):206–212. doi: 10.1007/BF00310260. [DOI] [PubMed] [Google Scholar]
- 8.Salih DA, Mohan S, Kasukawa Y, Tripathi G, Lovett FA, Anderson NF, Carter EJ, Wergedal JE, Baylink DJ, Pell JM. Insulin-like growth factor-binding protein-5 induces a gender-related decrease in bone mineral density in transgenic mice. Endocrinology. 2005;146(2):931–940. doi: 10.1210/en.2004-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest. 2001;107(1):73–81. doi: 10.1172/JCI10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauss F, Lang K, Dony C, Kling L. The complex of recombinant human insulin-like growth factor-I (rhIGF-I) and its binding protein-5 (IGFBP-5) induces local bone formation in murine calvariae and in rat cortical bone after local or systemic administration. Growth Horm IGF Res. 2001;11(1):1–9. doi: 10.1054/ghir.2000.0181. [DOI] [PubMed] [Google Scholar]
- 11.Mohan S, Thompson GR, Amaar YG, Hathaway G, Tschesche H, Baylink DJ. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41(51):15394–15403. doi: 10.1021/bi026458q. [DOI] [PubMed] [Google Scholar]
- 12.Amaar YG, Thompson GR, Linkhart TA, Chen ST, Baylink DJ, Mohan S. Insulin-like growth factor-binding protein 5 (IGFBP-5) interacts with a four and a half LIM protein 2 (FHL2) J Biol Chem. 2002;277(14):12053–12060. doi: 10.1074/jbc.M110872200. [DOI] [PubMed] [Google Scholar]
- 13.Chung YS, Baylink DJ, Srivastava AK, Amaar Y, Tapia B, Kasukawa Y, Mohan S. Effects of secreted frizzled-related protein 3 on osteoblasts in vitro. J Bone Miner Res. 2004;19(9):1395–1402. doi: 10.1359/JBMR.040412. [DOI] [PubMed] [Google Scholar]
- 14.Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, Milz S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004;24(6):3743–3748. [PubMed] [Google Scholar]
- 15.Harbour ME, Gregory JW, Jenkins HR, Evans BA. Proliferative response of different human osteoblast-like cell models to proinflammatory cytokines. Pediatr Res. 2000;48(2):163–168. doi: 10.1203/00006450-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Knutsen R, Honda Y, Strong DD, Sampath TK, Baylink DJ, Mohan S. Regulation of insulin-like growth factor system components by osteogenic protein-1 in human bone cells. Endocrinology. 1995;136(3):857–865. doi: 10.1210/endo.136.3.7532581. [DOI] [PubMed] [Google Scholar]
- 17.Tremollieres FA, Strong DD, Baylink DJ, Mohan S. Insulin-like growth factor II and transforming growth factor beta 1 regulate insulin-like growth factor I secretion in mouse bone cells. Acta Endocrinol (Copenh) 1991;125(5):538–546. doi: 10.1530/acta.0.1250538. [DOI] [PubMed] [Google Scholar]
- 18.N.B. http://www.ncbi.nlm.nih.gov/BLAST/.
- 19.Dorak MT. REAL-TIME PCR. 2005 Available from: < http://dorakmt.tripod.com/genetics/realtime.html>.
- 20.Malpe R, Baylink DJ, Linkhart TA, Wergedal JE, Mohan S. Insulin-like growth factor (IGF)-I, -II, IGF binding proteins (IGFBP)-3, -4, and -5 levels in the conditioned media of normal human bone cells are skeletal site-dependent. J Bone Miner Res. 1997;12(3):423–430. doi: 10.1359/jbmr.1997.12.3.423. [DOI] [PubMed] [Google Scholar]
- 21.Durant D, Pereira RM, Canalis E. Overexpression of insulin-like growth factor binding protein-5 decreases osteoblastic function in vitro. Bone. 2004;35(6):1256–1262. doi: 10.1016/j.bone.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Lotinun S, Sibonga JD, Turner RT. Differential effects of intermittent and continuous administration of parathyroid hormone on bone histomorphometry and gene expression. Endocrine. 2002;17(1):29–36. doi: 10.1385/ENDO:17:1:29. [DOI] [PubMed] [Google Scholar]
- 23.Boutinaud M, Shand JH, Park MA, Phillips K, Beattie J, Flint DJ, Allan GJ. A quantitative RT-PCR study of the mRNA expression profile of the IGF axis during mammary gland development. J Mol Endocrinol. 2004;33(1):195–207. doi: 10.1677/jme.0.0330195. [DOI] [PubMed] [Google Scholar]
- 24.Birnbaum RS, Wiren KM. Changes in insulin-like growth factor-binding protein expression and secretion during the proliferation, differentiation, and mineralization of primary cultures of rat osteoblasts. Endocrinology. 1994;135(1):223–230. doi: 10.1210/endo.135.1.8013356. [DOI] [PubMed] [Google Scholar]
- 25.Birnbaum RS, Bowsher RR, Wiren KM. Changes in IGF-I and -II expression and secretion during the proliferation and differentiation of normal rat osteoblasts. J Endocrinol. 1995;144(2):251–259. doi: 10.1677/joe.0.1440251. [DOI] [PubMed] [Google Scholar]
- 26.Martin B, Schneider R, Janetzky S, Waibler Z, Pandur P, Kuhl M, Behrens J, von der Mark K, Starzinski-Powitz A, Wixler V. The LIM-only protein FHL2 interacts with beta-catenin and promotes differentiation of mouse myoblasts. J Cell Biol. 2002;159(1):113–122. doi: 10.1083/jcb.200202075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller JM, Isele U, Metzger E, Rempel A, Moser M, Pscherer A, Breyer T, Holubarsch C, Buettner R, Schule R. FHL2, a novel tissue-specific coactivator of the androgen receptor. EMBO J. 2000;19(3):359–369. doi: 10.1093/emboj/19.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purcell NH, Darwis D, Bueno OF, Muller JM, Schule R, Molkentin JD. Extracellular signal-regulated kinase 2 interacts with and is negatively regulated by the LIM-only protein FHL2 in cardiomyocytes. Mol Cell Biol. 2004;24(3):1081–1095. doi: 10.1128/MCB.24.3.1081-1095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fimia GM, De Cesare D, Sassone-Corsi P. A family of LIM-only transcriptional coactivators: tissue-specific expression and selective activation of CREB and CREM. Mol Cell Biol. 2000;20(22):8613–8622. doi: 10.1128/mcb.20.22.8613-8622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y, Renard CA, Labalette C, Wu Y, Levy L, Neuveut C, Prieur X, Flajolet M, Prigent S, Buendia MA. Identification of the LIM protein FHL2 as a coactivator of beta-catenin. J Biol Chem. 2003;278(7):5188–5194. doi: 10.1074/jbc.M207216200. [DOI] [PubMed] [Google Scholar]
- 31.Hammon HM, Zbinden Y, Sauerwein H, Breier BH, Blum JW, Donkin SS. The response of the hepatic insulin-like growth factor system to growth hormone and dexamethasone in calves. J Endocrinol. 2003;179(3):427–435. doi: 10.1677/joe.0.1790427. [DOI] [PubMed] [Google Scholar]
- 32.Ontsouka CE, Sauter SN, Blum JW, Hammon HM. Effects of colostrum feeding and dexamethasone treatment on mRNA levels of insulin-like growth factors (IGF)-I and -II, IGF binding proteins-2 and -3, and on receptors for growth hormone, IGF-I, IGF-II, and insulin in the gastrointestinal tract of neonatal calves. Domest Anim Endocrinol. 2004;26(2):155–175. doi: 10.1016/j.domaniend.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Huang TS, Yang RS, Tsai TW, Liu SH. Growth hormone cannot enhance the recovery of dexamethasone-induced osteopenia after withdrawal in young female wistar rats. Tohoku J Exp Med. 2004;204(4):257–266. doi: 10.1620/tjem.204.257. [DOI] [PubMed] [Google Scholar]
- 34.Allen DB. Growth suppression by glucocorticoid therapy. Endocrinol Metab Clin North Am. 1996;25(3):699–717. doi: 10.1016/s0889-8529(05)70348-0. [DOI] [PubMed] [Google Scholar]
- 35.Cheng SL, Zhang SF, Avioli LV. Expression of bone matrix proteins during dexamethasone-induced mineralization of human bone marrow stromal cells. J Cell Biochem. 1996;61(2):182–193. doi: 10.1002/(sici)1097-4644(19960501)61:2<182::aid-jcb3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 36.Chevalley T, Strong DD, Mohan S, Baylink D, Linkhart TA. Evidence for a role for insulin-like growth factor binding proteins in glucocorticoid inhibition of normal human osteoblast-like cell proliferation. Eur J Endocrinol. 1996;134(5):591–601. doi: 10.1530/eje.0.1340591. [DOI] [PubMed] [Google Scholar]
- 37.Jia D, Heersche JN. Expression of insulin-like growth factor system constituents in differentiating rat osteoblastic cell populations. Growth Horm IGF Res. 2002;12(6):399–410. doi: 10.1016/s1096-6374(02)00117-x. [DOI] [PubMed] [Google Scholar]
- 38.Yeh LC, Adamo ML, Duan C, Lee JC. Osteogenic protein-1 regulates insulin-like growth factor-I (IGF-I), IGF-II, and IGF-binding protein-5 (IGFBP-5) gene expression in fetal rat calvaria cells by different mechanisms. J Cell Physiol. 1998;175(1):78–88. doi: 10.1002/(SICI)1097-4652(199804)175:1<78::AID-JCP9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Yeh LC, Adamo ML, Kitten AM, Olson MS, Lee JC. Osteogenic protein-1-mediated insulin-like growth factor gene expression in primary cultures of rat osteoblastic cells. Endocrinology. 1996;137(5):1921–1931. doi: 10.1210/endo.137.5.8612532. [DOI] [PubMed] [Google Scholar]
- 40.Yeh LC, Adamo ML, Olson MS, Lee JC. Osteogenic protein-1 and insulin-like growth factor I synergistically stimulate rat osteoblastic cell differentiation and proliferation. Endocrinology. 1997;138(10):4181–4190. doi: 10.1210/endo.138.10.5465. [DOI] [PubMed] [Google Scholar]
- 41.Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19(Suppl 1):1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 42.Resch ZT, Chen BK, Bale LK, Oxvig C, Overgaard MT, Conover CA. Pregnancy-associated plasma protein a gene expression as a target of inflammatory cytokines. Endocrinology. 2004;145(3):1124–1129. doi: 10.1210/en.2003-1313. [DOI] [PubMed] [Google Scholar]
- 43.Dong Y, Canalis E. Insulin-like growth factor (IGF) I and retinoic acid induce the synthesis of IGF-binding protein 5 in rat osteoblastic cells. Endocrinology. 1995;136(5):2000–2006. doi: 10.1210/endo.136.5.7536661. [DOI] [PubMed] [Google Scholar]
- 44.Rutter MM, Marko E, Clayton L, Akeno N, Zhao G, Clemens TL, Chernausek SD. Osteoblast-specific expression of insulin-like growth factor-1 in bone of transgenic mice induces insulin-like growth factor binding protein-5. Bone. 2005;36(2):224–231. doi: 10.1016/j.bone.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Conover CA, Kiefer MC. Regulation and biological effect of endogenous insulin-like growth factor binding protein-5 in human osteoblastic cells. J Clin Endocrinol Metab. 1993;76(5):1153–1159. doi: 10.1210/jcem.76.5.7684391. [DOI] [PubMed] [Google Scholar]
- 46.Kanzaki S, Hilliker S, Baylink DJ, Mohan S. Evidence that human bone cells in culture produce insulin-like growth factor-binding protein-4 and -5 proteases. Endocrinology. 1994;134(1):383–392. doi: 10.1210/endo.134.1.7506211. [DOI] [PubMed] [Google Scholar]