Abstract
Introduction
Ischemic heart disease (IHD) is common in heart failure (HF), yet the association between incident coronary revascularization and mortality in these patients has not been examined in a propensity-matched study.
Methods
In the Digitalis Investigation Group trial, 2853 patients without coronary revascularization and 120 patients with coronary revascularization during the first three years were alive at the end of three years. We used propensity scores to match 119 and 357 patients with and without coronary revascularization. Matched Cox regression models were used to estimate hazard ratio (HR) and 95% confidence interval (CI) for mortality during the fourth year of follow-up, for all patients and by the mean left ventricular ejection fraction (LVEF) of 35%.
Results
Coronary revascularization was associated with higher mean LVEF (36 % versus 32 %; p<0.0001) and prevalence of angina pectoris (48% versus 32%; p<0.0001) but fewer prior myocardial infarction (80% versus 87%; p=0.023), all of which were balanced post-match. All-cause mortality occurred in 5.9% and 6.2% patients respectively with and without coronary revascularization (HR for coronary revascularization, 0.95; 95% CI, 0.39–2.32; p=0.910). HR for mortality associated with coronary revascularization for patients with LVEF ≤35% and >35% were respectively 1.34 (95% CI, 0.48–3.71; p=0.578) and 0.61 (95% CI, 0.13–2.87; p=0.532).
Conclusion
Chronic HF patients with IHD receiving coronary revascularization were more likely to have angina and higher LVEF. However, in a balanced propensity-matched cohort, there was no association between coronary revascularization and mortality. The LVEF-associated variation in mortality needs to be prospectively studied.
Keywords: heart failure, revascularization, mortality, outcomes
1. Introduction
Ischemic heart disease (IHD) is the leading cause of heart failure (HF), and coronary revascularization is a major treatment option for HF patients with IHD [1]. Yet, the role of coronary revascularization in chronic HF patients with IHD remains controversial [2–5]. To the best of our knowledge the effect of incident coronary revascularization on subsequent mortality has not been examined in a propensity-matched population of ambulatory chronic HF patients with low and normal left ventricular ejection fraction (LVEF).
2. Materials and methods
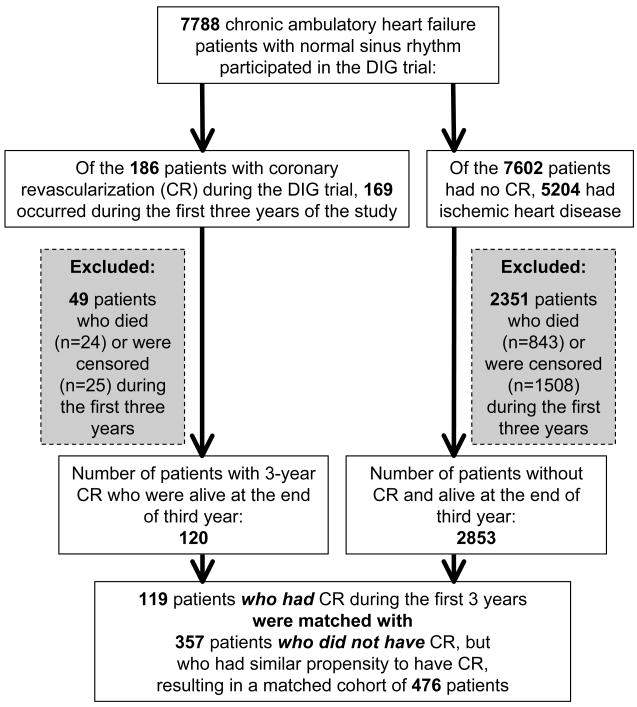
The Digitalis Investigator Group (DIG) trial enrolled 7788 ambulatory patients with chronic HF in normal sinus rhythm from 302 clinical centers in the United States and Canada from 1991 to 1993 [6, 7]. Overall, 120 patients who underwent coronary revascularization during the first three years of the study and 2853 patients with IHD who did not undergo coronary revascularization were alive at the end of three years (Figure 1). IHD and hospitalization due to coronary revascularization were ascertained by study investigators and were not centrally adjudicated. The primary endpoints were mortality due to all causes, cardiovascular causes, and worsening HF. Data on vital status were 99% complete [8].
Figure 1.
Flow chart for the assembly of study cohort
Using propensity scores for coronary revascularization, we assembled a balanced cohort of 119 patients who underwent coronary revascularization and 357 patients who were treated medically (Figure 1) [9, 10]. Propensity scores for incident coronary revascularization for each of the 2973 patients with IHD were estimated using a non-parsimonious, multivariate logistic regression model, adjusting for key baseline covariates presented in Table 1. The propensity scores were then used for matching, and the details of the matching protocol have been described elsewhere [11–14]. Absolute standardized differences were estimated to examine covariate balance and were presented as a Love plot, developed by Thomas E. Love [11–13, 15, 16].
Table 1.
Baseline patient characteristics by coronary revascularization during first three years, before and after propensity score matching
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| No coronary revascularization (n=2853) | Coronary revascularization (n=120) | P value | No coronary revascularization (n=357) | Coronary revascularization (n=119) | P value | |
| Age, years | 64 (±10) | 62 (±9) | 0.074 | 62 (±10) | 62 (±9) | 0.571 |
| Age ≥65 years | 1465 (51%) | 51 (43%) | 0.057 | 156 (44%) | 51 (43%) | 0.873 |
| Female | 624 (22%) | 28 (23%) | 0.705 | 76 (21%) | 27 (23%) | 0.748 |
| Non-white | 232 (8%) | 11 (9%) | 0.685 | 38 (11%) | 11 (9%) | 0.663 |
| Body mass index, kg/m2 | 27 (±5) | 28 (±4) | 0.046 | 28 (±5) | 28 (±4) | 0.427 |
| Ejection fraction, % | 32 (±11) | 36 (±12) | <0.0001 | 36 (±12) | 36 (±11) | 0.820 |
| Ejection fraction > 45% | 299 (11%) | 19 (16%) | 0.063 | 66 (19%) | 18 (15%) | 0.405 |
| Comorbid conditions | ||||||
| Prior myocardial infarction | 2486 (87%) | 96 (80%) | 0.023 | 293 (82%) | 96 (81%) | 0.732 |
| Current angina pectoris | 904 (32%) | 58 (48%) | <0.0001 | 167 (47%) | 57 (48%) | 0.832 |
| Hypertension | 1184 (42%) | 61 (51%) | 0.042 | 169 (47%) | 60 (50%) | 0.560 |
| Diabetes mellitus | 781 (27%) | 33 (28%) | 0.976 | 95 (27%) | 32 (27%) | 0.952 |
| Chronic kidney disease | 1276 (45%) | 44 (37%) | 0.082 | 135 (38%) | 44 (37%) | 0.870 |
| Medications | ||||||
| Digoxin (pre-trial use) | 1177 (41%) | 43 (36%) | 0.237 | 135 (38%) | 43 (36%) | 0.743 |
| Digoxin (trial use) | 1396 (49%) | 72 (60%) | 0.018 | 185 (52%) | 71 (60%) | 0.137 |
| ACE inhibitors | 2667 (94%) | 111 (93%) | 0.671 | 330 (92%) | 110 (92%) | 1.000 |
| Diuretics | 2029 (71%) | 85 (71%) | 0.946 | 243 (68%) | 84 (71%) | 0.608 |
| NYHA class III-IV | 757 (27%) | 31 (26%) | 0.865 | 79 (22%) | 31 (26%) | 0.379 |
| Heart rate, beats per minute | 76 (±12) | 75 (±13) | 0.306 | 75 (±12) | 75 (±13) | 0.942 |
| Systolic blood pressure, mm Hg | 127 (±19) | 128 (±19) | 0.336 | 127 (±18) | 128 (±19) | 0.475 |
| Serum creatinine, mg/dL | 1.3 (±0.3) | 1.2 (±0.3) | 0.275 | 1.2 (±0.3) | 1.2 (±0.3) | 0.967 |
(ACE=angiotensin-converting enzyme; NYHA=New York Heart Association)
The baseline characteristics of patients who underwent coronary revascularization and those who were treated medically were compared using Pearson’s chi-square and Wilcoxon’s rank-sum tests. Kaplan-Meier analysis and matched Cox regression analyses were used to determine the association of incident coronary revascularization with subsequent mortality during the fourth year of follow-up. All statistical tests were done using SPSS-15 for Windows [17].
3. Results
Patients had a mean (±SD) age of 62 (±10) years, 22% were women, and 10% were nonwhites. Before matching, patients with (versus without) coronary revascularization had a higher mean baseline LVEF (36 {±12} % versus 32 {±11} %; p<0.0001), were less likely to have prior myocardial infarction (80% versus 87%; p=0.023), but more likely to have unstable angina pectoris (48% versus 32%; p<0.0001). However, there were no differences in NYHA class symptoms. Pre-match imbalances (>10% indicate substantial imbalance) and post-match balances in baseline covariates are displayed in Table 1 and Figure 2.
Figure 2.
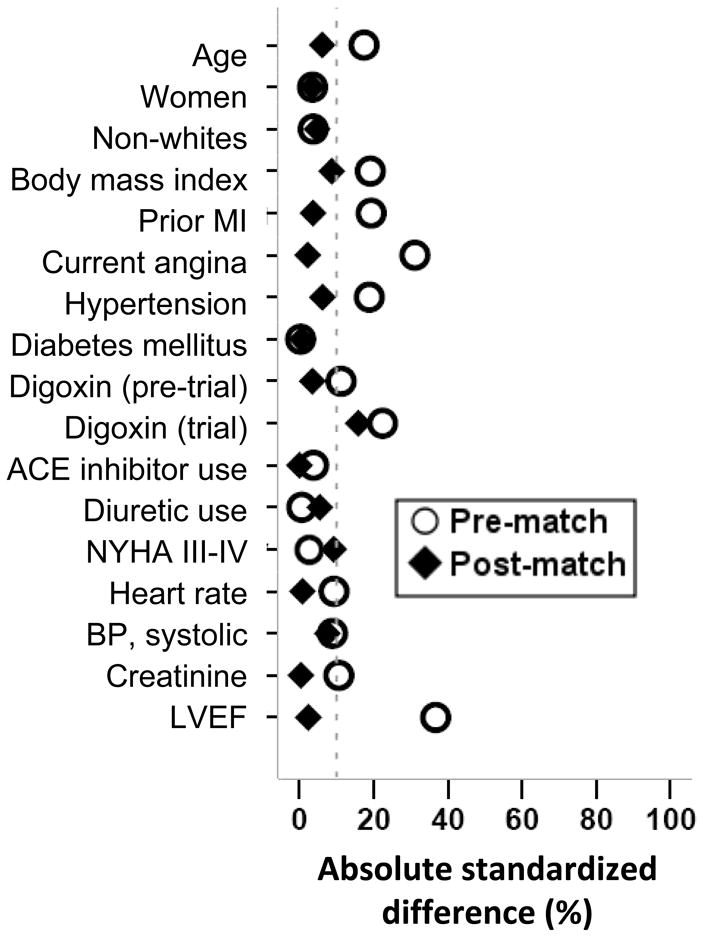
Love plot for absolute standardized differences before and after propensity score matching comparing key covariate values for patients with and without coronary revascularization
(MI= myocardial infarction; ACE=angiotensin-converting enzyme; NYHA=New York Heart Association; BP=blood pressure; LVEF=left ventricular ejection fraction)
Overall, 29 patients (6%) died, including 24 (5%) due to cardiovascular causes and 13 (3%) due to progressive HF, during 392 patient-years of subsequent follow-up. Kaplan-Meier plots for all-cause and cardiovascular mortality are displayed in Figure 3. All-cause mortality occurred in 5.9% (rate, 154/10000 person-years) of patients who underwent coronary revascularization and in 6.2% (rate, 161/10000 person-years) of matched patients treated medically (hazard ratio {HR}, 0.95; 95% confidence interval {CI}, 0.39–2.32; p=0.910; Table 2). Coronary revascularization was not associated with mortality caused by cardiovascular causes or progressive HF (Table 2). HR for mortality by coronary revascularization for patients with LVEF ≤35% and >35% were respectively 1.34 (95% CI, 0.48–3.71; p= 0.578) and 0.61 (95% CI, 0.13–2.87; p= 0.532; Table 3).
Figure 3.
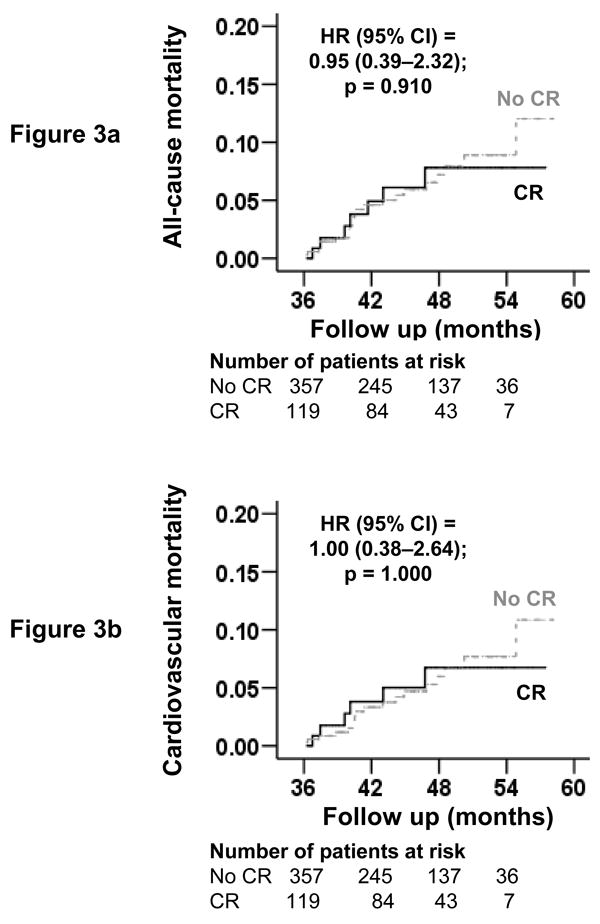
Kaplan-Meier plots for mortality due to (a) all-causes and (b) cardiovascular causes
(HR=hazard ratio; CI=confidence interval; CR=coronary revascularization)
Table 2.
Mortality in chronic heart failure patients, before and after matching by propensity scores for coronary revascularization
| Rate, per 10,000 person-years (Events/total follow up years) | Absolute rate difference* (per 10,000 person-years) | Hazard ratio (95% confidence interval) | P value | ||
|---|---|---|---|---|---|
| No coronary revascularization | Coronary revascularization | ||||
| Pre-match | N=2853 | N=120 | |||
| All-cause | 250 (273/10931) | 153 (7/457) | −97 | 0.63 (0.30–1.33) | 0.226 |
| Cardiovascular | 188 (206/10931) | 131 (6/457) | −57 | 0.72 (0.32–1.62) | 0.422 |
| Worsening heart failure | 95 (104/10931) | 67 (3/457) | −28 | 0.71 (0.23–2.24) | 0.557 |
| Post-match | N=357 | N=119 | |||
| All-cause | 161 (22/1367) | 154 (7/454) | −7 | 0.95 (0.39–2.32) | 0.910 |
| Cardiovascular | 132 (18/1367) | 132 (6/454) | 0 | 1.00 (0.38–2.64) | 1.000 |
| Worsening heart failure | 73 (10/1367) | 66 (3/454) | −7 | 0.80 (0.21–3.06) | 0.744 |
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the no coronary revascularization group from the event rates in the coronary revascularization group (before values were rounded)
Table 3.
All-cause mortality in matched chronic heart failure patients, by median left ventricular ejection fraction (LVEF) of 35%
| Rate, per 10,000 person-years (Events/total follow up years) | Absolute rate difference* (per 10,000 person-years) | Hazard ratio (95% confidence interval) | P value | ||
|---|---|---|---|---|---|
| No coronary revascularization | Coronary revascularization | ||||
| LVEF ≤35% (range, 10% to 35%) | 201 (14/697) | 241 (5/207) | + 40 | 1.34 (0.48–3.71) | 0.578 |
| LVEF >35% (range, 36% to 74%) | 119 (8/670) | 81 (2/247) | − 38 | 0.61 (0.13–2.87) | 0.532 |
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the no coronary revascularization group from the event rates in the coronary revascularization group (before values were rounded)
4. Discussion
The findings of our study demonstrate that chronic HF patients who underwent coronary revascularization were less likely to have a history of prior myocardial infarction but were more likely to have symptoms of unstable angina pectoris, and were also more likely to have a higher LVEF. However, when these and other key measured baseline characteristics were balanced after matching, incident coronary revascularization was not associated with subsequent mortality in patients with chronic systolic and diastolic HF with IHD.
The substantial yet non-significant reduction in mortality in patients receiving coronary revascularization before matching may be explained by baseline covariate imbalances such as younger age, a better comorbidity profile and a higher mean LVEF among those receiving coronary revascularization. However, when these covariates were balanced after matching, there was no substantial difference in mortality between patients receiving and not receiving coronary revascularization. Interestingly, there was a substantial but non-significant reduction in mortality in matched patients with LVEF >35% who received coronary revascularization. This is consistent with the findings from the Coronary Artery Surgery Study and the Veteran Affairs Cooperative Study that suggested that coronary revascularization was associated with improved survival in patients with IHD and LVEF >35% [18, 19]. HF patients with normal or near normal LVEF are more likely to have viable ischemic myocardium, which has been shown to determine both the degree of improvement in LV function and the long-term outcomes after revascularization [20–25]. The association between a higher mean LVEF and the presence of viable ischemic myocardium may also explain the higher prevalence of unstable angina pectoris in patients receiving coronary revascularization in our study. One of the reasons for excluding patients with LVEF <35% from the Coronary Artery Surgery Study and the Veteran Affairs Cooperative Study was that they were considered high-risk for coronary revascularization. This notion is congruent with the findings from our subgroup analysis that indicate that among HF patients with IHD and LVEF ≤35%, the direction of association was toward an increased mortality in those receiving coronary revascularization. However, this association was not statistically significant and needs to be interpreted with caution. The association between coronary revascularization and outcomes in HF patients with IHD and LVEF <35% will be clarified when the findings from the currently ongoing Surgical Treatment for Ischemic Heart Failure (STICH) trial will be published [26].
The lack of a statistically significant association between coronary revascularization and mortality in our study may also be explained by the small sample size and lack of adequate power. However, the direction and the magnitude of the associations in our subgroup analysis are mechanistically plausible and suggest that coronary revascularization may not be safe in chronic HF patients with IHD and low LVEF. We also noted that HF patients who received coronary revascularization had lower prevalence of a history of prior myocardial infarction but a higher prevalence of angina pectoris. Patients with a history of prior myocardial infarction are less likely to have viable myocardium and thus less likely to have angina pectoris [22]. Therefore, angina pectoris may serve as marker of viable myocardium in HF patients with a history of prior myocardial infarction who should undergo tests for myocardial viability to identify those who might benefit from coronary revascularization. HF patients with non-viable ischemic myocardium may not benefit from coronary revascularization [27, 28].
The findings of the current study are consistent with findings from a previous study that also examined the association between coronary revascularization and mortality [29]. In that study, in HF patients with IHD and LVEF <45% (mean, 25%), 5-year all-cause mortality occurred in 43% of patients (n=10) in the revascularization group and in 60% of those (n=67) who did not undergo revascularization (p=0.257). To the best of our knowledge, this is the first report of an association between incident coronary revascularization and subsequent total and cause-specific mortality in a propensity-matched population of chronic systolic and diastolic HF patients with IHD.
Lack of data on the type or timing of coronary revascularization is a key limitation of our study [30]. DIG participants were mostly younger men in normal sinus rhythm from the pre-beta-blocker era of HF therapy, which may limit generalizability to contemporary HF patients. Our propensity matching was able to balance all key baseline covariates. However, imbalances in unmeasured covariates are possible and may potentially confound our findings.
In conclusion, in chronic systolic and diastolic HF patients with IHD, younger age, higher LVEF and the presence of angina pectoris had bivariate association with coronary revascularization. Despite a non-significant association between incident coronary revascularization and subsequent mortality, the directions of these associations in our subgroup analysis suggest that in HF patients with IHD and LVEF >35%, the symptom of angina pectoris may be used to identify patients for further evaluation for myocardial viability and to identify those who might benefit from coronary revascularization.
Acknowledgments
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through a grant from the National Heart, Lung, and Blood Institute (5-R01-HL085561-02) and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Footnotes
Presentation: An abstract based on the current analysis was presented at the Heart Failure society of America 12th Annual Scientific meeting in Toronto, Ontario, Canada, in September 2008.
Conflict of Interest Disclosures: None
References
- 1.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–9. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 2.Tarakji KG, Brunken R, McCarthy PM, et al. Myocardial viability testing and the effect of early intervention in patients with advanced left ventricular systolic dysfunction. Circulation. 2006;113:230–7. doi: 10.1161/CIRCULATIONAHA.105.541664. [DOI] [PubMed] [Google Scholar]
- 3.Mickleborough LL, Carson S, Tamariz M, Ivanov J. Results of revascularization in patients with severe left ventricular dysfunction. J Thorac Cardiovasc Surg. 2000;119:550–7. doi: 10.1016/s0022-5223(00)70135-8. [DOI] [PubMed] [Google Scholar]
- 4.Shanmugam G, Legare JF. Revascularization for ischaemic cardiomyopathy. Curr Opin Cardiol. 2008;23:148–52. doi: 10.1097/HCO.0b013e3282f43011. [DOI] [PubMed] [Google Scholar]
- 5.Samady H, Elefteriades JA, Abbott BG, Mattera JA, McPherson CA, Wackers FJ. Failure to improve left ventricular function after coronary revascularization for ischemic cardiomyopathy is not associated with worse outcome. Circulation. 1999;100:1298–304. doi: 10.1161/01.cir.100.12.1298. [DOI] [PubMed] [Google Scholar]
- 6.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 7.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 8.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–30. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 10.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 11.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99:549–53. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–9. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giamouzis G, Sui X, Love TE, Butler J, Young JB, Ahmed A. A propensity-matched study of the association of cardiothoracic ratio with morbidity and mortality in chronic heart failure. Am J Cardiol. 2008;101:343–7. doi: 10.1016/j.amjcard.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambassi G, Agha SA, Sui X, et al. Race and the natural history of chronic heart failure: a propensity-matched study. J Card Fail. 2008;14:373–8. doi: 10.1016/j.cardfail.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–98. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 17.SPSS for Windows, Rel. 15 program] SPSS Inc; 2008. [Google Scholar]
- 18.Kaiser GC, Davis KB, Fisher LD, et al. Survival following coronary artery bypass grafting in patients with severe angina pectoris (CASS). An observational study. J Thorac Cardiovasc Surg. 1985;89:513–24. [PubMed] [Google Scholar]
- 19.The VA Coronary Artery Bypass Surgery Cooperative Study Group. Circulation. Vol. 86. 1992. Eighteen-year follow-up in the Veterans Affairs Cooperative Study of Coronary Artery Bypass Surgery for stable angina; pp. 121–30. [DOI] [PubMed] [Google Scholar]
- 20.Iriarte M, Caso R, Murga N, et al. Microvascular angina pectoris in hypertensive patients with left ventricular hypertrophy and diagnostic value of exercise thallium-201 scintigraphy. Am J Cardiol. 1995;75:335–9. doi: 10.1016/s0002-9149(99)80549-9. [DOI] [PubMed] [Google Scholar]
- 21.Iriarte M, Murga N, Sagastagoitia D, et al. Congestive heart failure from left ventricular diastolic dysfunction in systemic hypertension. Am J Cardiol. 1993;71:308–12. doi: 10.1016/0002-9149(93)90796-f. [DOI] [PubMed] [Google Scholar]
- 22.Nijland F, Kamp O, Verhorst PM, de Voogt WG, Visser CA. In-hospital and long-term prognostic value of viable myocardium detected by dobutamine echocardiography early after acute myocardial infarction and its relation to indicators of left ventricular systolic dysfunction. Am J Cardiol. 2001;88:949–55. doi: 10.1016/s0002-9149(01)01968-3. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed A, Zile MR, Rich MW, et al. Hospitalizations due to unstable angina pectoris in diastolic and systolic heart failure. Am J Cardiol. 2007;99:460–4. doi: 10.1016/j.amjcard.2006.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagano D, Townend JN, Littler WA, Horton R, Camici PG, Bonser RS. Coronary artery bypass surgery as treatment for ischemic heart failure: the predictive value of viability assessment with quantitative positron emission tomography for symptomatic and functional outcome. J Thorac Cardiovasc Surg. 1998;115:791–9. doi: 10.1016/S0022-5223(98)70357-5. [DOI] [PubMed] [Google Scholar]
- 25.Bax JJ, Poldermans D, Elhendy A, Boersma E, van der Wall EE. Assessment of myocardial viability by nuclear imaging techniques. Curr Cardiol Rep. 2005;7:124–9. doi: 10.1007/s11886-005-0024-4. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez EJ, Lee KL, O’Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–7. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–8. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 28.Phillips HR, O’Connor CM, Rogers J. Revascularization for heart failure. Am Heart J. 2007;153:65–73. doi: 10.1016/j.ahj.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Miller WL, Tointon SK, Hodge DO, Nelson SM, Rodeheffer RJ, Gibbons RJ. Long-term outcome and the use of revascularization in patients with heart failure, suspected ischemic heart disease, and large reversible myocardial perfusion defects. Am Heart J. 2002;143:904–9. doi: 10.1067/mhj.2002.120153. [DOI] [PubMed] [Google Scholar]
- 30.Bax JJ, Schinkel AF, Boersma E, et al. Early versus delayed revascularization in patients with ischemic cardiomyopathy and substantial viability: impact on outcome. Circulation. 2003;108(Suppl 1):II39–42. doi: 10.1161/01.cir.0000089041.69175.9d. [DOI] [PubMed] [Google Scholar]