Abstract
Pregnancy-associated plasma protein (PAPP)-A, a protease for IGF binding protein (IGFBP)-2, -4, and -5, may enhance IGF action by increasing its bioavailability. Here we have determined the role and mechanism of action of PAPP-A in the regulation of osteoblast proliferation in vitro and bone metabolism in vivo. Recombinant PAPP-A (100 ng/ml) significantly increased osteoblast proliferation and free IGF-I concentration. These effects were abolished by noncleavable IGFBP-4, suggesting that PAPP-A promotes osteoblast proliferation by increasing IGF bioavailability. To determine whether PAPP-A exerts an anabolic effect on bone in vivo, we developed transgenic mice that overexpress PAPP-A in osteoblasts using the 2.3-kb rat type I collagen promoter. Consistent with the increase in IGFBP-4 proteolysis, free IGF-I concentration was significantly increased in the conditioned medium of cultured osteoblasts derived from transgenic mice compared with the wild-type littermates. Calvarial bone thickness, bone marrow cavity, and skull bone mineral density were significantly increased in transgenic mice. Bone size-related parameters in femur and tibia such as total bone area and periosteal circumference as determined by peripheral quantitated computed tomography and histological analysis were significantly increased in transgenic mice. Bone formation rate and osteoid surface were increased by more than 2-fold, whereas bone resorbing surface was unaffected. These anabolic effects were sustained with aging. These findings provide strong evidence that PAPP-A acts as a potent anabolic factor in the regulation of bone formation. Thus, enhancing IGF bioavailability by PAPP-A can be a powerful strategy in the treatment of certain metabolic diseases such as osteoporosis.
Pregnancy-associated plasma protein (PAPP)-A is a macromolecular glycoprotein identified in the blood of pregnant women (1). The human PAPP-A cDNA predicted a signal peptide of 22 residues and 58 residues of prepeptide preceding the mature circulating form consisting of 1547 residues (2). PAPP-A contains a putative Zn motif (amino acids 482–492), which is essentially identical to the active-site Zn motif of the matrix metalloproteases (2). However, the substrates for this putative protease were not identified until PAPP-A was determined to be the IGF-II-dependent IGF-binding protein (IGFBP)-4 protease (3). Subsequent studies from our laboratory suggest that PAPP-A is also the predominant IG-FBP-4 protease present in human pregnancy serum (4) and is produced by human and mouse osteoblasts (5). Although PAPP-A was initially identified as the IGFBP-4 protease, recent studies from our laboratory and others clearly demonstrate that PAPP-A also serves as a potent IGFBP-2 and IGFBP-5 protease (4, 6–8).
Among the three PAPP-A-cleavable IGFBPs, IGFBP-2 and IGFBP-4 are consistent inhibitors of the mitogenic activity of IGFs in a number of cell types including osteoblasts (9–11). In vivo, overexpression of IGFBP-2 or IGFBP-4 in mice caused a severe impairment in bone growth and development (12, 13). Although IGFBP-5 has been shown to stimulate osteoblast proliferation via both an IGF-dependent and IGF-independent fashion in vitro (11, 14), the in vivo role of IGFBP-5 in bone has been controversial. Local or systemic administration of recombinant IGFBP-5 alone or together with IGF-I has been shown to promote bone formation in mice (14, 15). Conversely, systemic or targeted overexpression of IGFBP-5 to the osteoblasts of mice led to impaired osteoblastic function and osteopenia (16, 17). This discrepancy could be, in part, explained by the intermittent vs. continuous supply of IGFBP-5 to the target tissues in these two models. Overall, these in vitro and in vivo studies suggest that PAPP-A-cleavable IGFBPs generally are inhibitory to the biological activity of IGFs in bone. Because all three PAPP-A-cleavable IGFBPs are produced by osteoblasts (18), it is conceivable that degradation of these IGFBPs could significantly enhance the biological activity of IGFs in bone. In support of this contention, we have previously demonstrated that a PAPP-A-resistant IGFBP-4 analog is much more potent than the wild-type IGFBP-4 in blocking the mitogenic activity of IGF-II in human osteoblasts (19). This finding suggests that the PAPP-A endogenously produced by osteoblasts is an important regulator for IGFBP-4 bioavailability.
Based on our in vitro studies that normal osteoblasts produce abundant PAPP-A (5, 20), which may enhance IGF bioavailability through degradation of selected IGFBPs, we proposed the hypothesis that transgenic overexpression of PAPP-A in osteoblasts should exert anabolic effects on bone in vivo. Our data show that PAPP-A transgenic mice overexpressing PAPP-A in osteoblasts exhibit a dramatic increase in bone size. These data, together with the most recent finding showing that inactivation of the PAPP-A gene in mice caused a severe global growth retardation (21), provide strong evidence that PAPP-A represents a potent anabolic factor in the regulation of growth and development of various tissues, especially bone.
Materials and Methods
Materials
Recombinant FLAG-tagged human PAPP-A was purified to homogeneity from the conditioned medium (CM) of HT1080 cells transfected with the PAPP-A (1–1547)/pFLAG plasmid DNA (7). The 6xHis-tagged recombinant IGFBP-4 peptides were prepared as described (10). M1 FLAG antibody and fetal calf serum (FCS) were from Sigma Chemical Co. (St. Louis, MO). Recombinant human IGF-I and IGF-II were purchased from GroPep (Adelaide, Australia).
Cell proliferation assay
Proliferation of human osteosarcoma MG63 cells, which do not produce PAPP-A, or normal mouse osteoblasts, which produce PAPP-A, was determined using alamarBlue dye (BioSource International, Camarillo, CA), which measures the metabolic activity of live cells (7, 14). Cells were seeded in 24-well plates in DMEM containing 10% FCS (20,000 cells per well). After 24 h, the cells were cultured for an additional 72–96 h in DMEM containing 0.1% BSA or 5% FCS supplemented with effectors. After CM collection, 0.5 ml of 10% alamarBlue in phenol-red-free DMEM was added to each well and fluorescence was determined (7). Cell lysates were subject to total protein quantitation using Bradford reagent as previously described (7) to confirm proliferation results derived from the alamarBlue assay.
Construction of PAPP-A transgene
The osteoblast-specific human PAPP-A transgenic plasmid was prepared by modification of the PAPP-A (1–1547)/pFLAG-CMV1 plasmid (22). The 2.3-kb rat type I collagen promoter (rCol2.3) (provided by Dr. David W. Rowe, University of Connecticut Health Center) was first ligated to a fragment containing the pre-protrypsin secretion signal and the FLAG coding sequence and then cloned into the CMV promoter-deleted PAPP-A/pFLAG plasmid. A 619-bp rabbit β-globin intron 2 was inserted between the rCol2.3 promoter and the start codon to enhance transgene expression, which has been previously shown (23) and has been used to construct transgenic plasmids (24). The resulting construct was designated as rCol2.3-PAPP-A/pFLAG.
Generation and maintenance of PAPP-A transgenic mice
Development of PAPP-A transgenic mice and subsequent tissue collection/phenotypic analysis were carried out according to protocols approved by the Institutional Animal Care and Use Committee. The rCol2.3-PAPP-A/pFLAG plasmid was digested with XbaI and ScaI, and the 8-kb fragment containing the PAPP-A expression cassette was purified and injected into the pronuclei of fertilized zygotes from C57BL/6J × CBA/CA mice at the Transgenic Mouse Facility of University of Southern California. F1 generation mice were produced by breeding the transgenic male founders with C57BL/6J female mice. F2 generation mice were produced by breeding a male F1 PAPP-A transgenic mouse (with confirmed anabolic phenotype) with female C57BL/6J mice. Male and female pups were separated and genotyped at 3–4 wk of age. Genotyping of mice was carried out using DNA isolated from tail tissue using QIAGEN Dneasy purification columns and protocol (QIAGEN, Valencia, CA). PCR was then used to amplify a midregion of the human PAPP-A transgene using 100 ng genomic DNA and the following primers: 5′-ggcattgctggtcttgatga-3′ and 5′-tcgccagaatgcactgttac-3′. Conditions for the PCR were as follows: melting temperature of 94 C for 45 sec, annealing temperature of 58 C for 45 sec, and extension temperature of 72 C for 45 sec, repeated for 32 cycles.
Analysis of FLAG-PAPP-A production in osteoblasts derived from PAPP-A transgenic mice
Primary mouse osteoblasts were isolated from calvarial bones of 2-wk-old mice (25). Third-passage osteoblasts with proliferative potential designated normal osteoblasts were cultured in DMEM/10% FCS. Upon confluence, cells were washed with DMEM and cultured in DMEM/0.01% BSA for an additional 48 h. FLAG-PAPP-A in serum-free CM (6 ml) was concentrated by heparin-agarose resin and subjected to Western immunoblot analysis with the monoclonal M1 FLAG antibody (22). IGFBP proteolytic activity in serum-free CM was evaluated by an in vitro IGFBP-4 protease assay (4).
Assessment of IGF bioavailability in the CM of osteoblasts derived from PAPP-A transgenic mice
Primary osteoblasts (passage 1) isolated from 2-wk-old transgenic or wild-type pups of founder 26 were seeded in a six-well plate containing DMEM/10% FCS at a density of 300,000 cells per well. Cells were allowed to grow to 90% confluence, at which time culture medium was replaced by differentiation medium (DMEM containing 5% FCS, 50 μg/ml ascorbic acid, and 10 mM β-glycerol phosphate) to induce osteoblast differentiation. After a 24-h incubation, fresh differentiation medium was added. After 7 and 19 h, CM (200 μl) was collected and subjected to analysis of free IGF-I using a free IGF-I ELISA kit (Diagnostic Systems Laboratories, Webster, TX). Free IGF-I concentration was adjusted for total cellular proteins.
Measurement of skull bone mineral density (BMD) by dual-energy x-ray absorptiometry (DEXA)
Areal BMD (mg/cm2) of whole skulls in live mice was determined under general anesthesia by DEXA using the PIXImus instrument (Lunar Corp., Madison, WI) as described (26). A transgenic mouse and a wild-type littermate were analyzed simultaneously for each scan. Entire individual skull area was selected for calculation of BMD.
Analysis of volumetric BMD and geometric parameters of long bones by peripheral quantitated computed tomography (pQCT)
Volumetric BMD (mg/cm3) and geometric parameters in the mid-diaphysis of femur or tibia were measured by pQCT (Stratec XCT 960M; Norland Medical System, Ft. Athinson, WI). Length of isolated femurs was measured by a digital caliper. Length of tibia in live animals was estimated by using the bone scan from the pQCT software. A voxel size of 0.07 mm and a slice thickness of 0.5 mm were set for analysis (17). Nine slices symmetrically distributed from each other were scanned. Each mid-diaphyseal parameter represents the average of data obtained from the three midpoint slices.
Static histomorphometric analysis
Calvarial bones were isolated, fixed in 10% formalin, embedded in methyl methacrylate, and then cross-sectioned (5 μm). Bone sections were then subjected to either Goldner staining for measurement of osteoid surface and sinusoid area (27) or titrate-resistant acid phosphatase staining for measurement of bone-resorbing surface (28). All bone histomorphometric parameters were measured with the Osteomeasure software (Osteometrics, Atlanta, GA).
Dynamic histomorphometric analysis
Animals were injected with tetracycline and demeclocycline (40 mg/kg body weight) at the age of 4 and 5 wk, respectively. At 48 h after demeclocycline injection, femurs were isolated, fixed in 10% formalin overnight, embedded in methyl methacrylate, and sectioned (5 μm). Mineralizing surface was photographed under UV light and measured on sections under magnification of ×10 (25). Mineral apposition rate (μm/d) was determined by dividing the mean of the widths of the double labels by the interlabel time (7 d). The bone formation rate (BFR) (μm3/day) was calculated by multiplying the mineral apposition rate value by the labeled new bone area surface value.
Western [125I]IGF-II ligand blot analysis
[125I]IGF-II Western ligand blot analysis was performed as previously described (4). Briefly, proteins were separated on SDS-PAGE gels under nonreducing conditions and electrically transferred to Transblot nitro-cellulose membranes (catalog no. 162-0097; Bio-Rad Laboratories, Inc., Hercules, CA). The membrane was first washed with 50 ml buffer A (150 mmol/liter NaCl and 20 mmol/liter Tris, pH 7.4) containing 0.1% Triton X-100 for 15–30 min and then blocked with 50 ml buffer A containing 0.1% BSA for 1 h. Each BSA-treated membrane was incubated with 10 ml buffer A containing 0.1% BSA, 0.1% Tween 20, and 1 × 106 cpm [125I]IGF-II tracers (200–300 μCi/μg protein) for 2 h. All incubations were undertaken at room temperature with gentle shaking. The membranes were then washed with buffer A containing 0.1% Tween 20, five times each for 20–30 min each time. The membranes were exposed to x-ray film for 24 h.
Statistical analysis
Results are expressed as mean ± SEM and statistically analyzed by Student’s t test or ANOVA. A value of P < 0.05 was considered statistically significant.
Results
PAPP-A enhances osteoblast proliferation through an IGF-dependent mechanism
The effect of PAPP-A on osteoblast proliferation was first evaluated using human osteosarcoma MG63 cells, which do not produce detectable levels of PAPP-A (5). PAPP-A treatment significantly increased osteosarcoma cell proliferation as determined by cellular metabolic activity and total cellular protein (Fig. 1A). IGF-II ligand blot analysis did not detect a significant amount of IGFBPs in 40 μl serum-free CM (Fig. 1B, lanes 2 and 3). The predominant IGFBP in the serum-containing CM is the 34-Kda IGFBP (Fig. 1B, indicated by arrow) from the added FCS and was previously determined to be IGFBP-2 (29, 30). IGFBP-2 in the serum-containing CM of preosteoblast cultures treated with PAPP-A was completely degraded (Fig. 1B, lane 5 vs. lane 4).
Fig. 1.

Effect of PAPP-A treatment on cell proliferation, IGFBP degradation, and free IGF-I concentrations in human osteosarcoma MG63 cell cultures. A, Cellular proliferation, determined by alamarBlue dye and Bradford reagent, was significantly increased in response to PAPP-A in the absence or presence of serum; B, 40 μl of CM collected in A was subjected to IGF-II ligand blot analysis to evaluate degradation of IGFBPs (arrow indicates bovine IGFBP-2); C, PAPP-A-induced cell proliferation was completely abolished by PR-BP4; D, 50 μl of CM collected in C was subjected to free IGF-I measurement by a commercial kit (Diagnostic Laboratory Systems). The PAPP-A-induced increase in free IGF-I concentrations was completely abolished by PR-BP4. *, P < 0.05; **, P < 0.01, ***, P < 0.001 compared with untreated cultures.
To determine whether PAPP-A acts to promote osteoblast proliferation through an IGF-dependent mechanism, we evaluated whether blocking the activity of IGFs could abolish the mitogenic activity of PAPP-A. We have previously shown that the IGFBP-4 analog lacking the cleavage site (Met135-Lys136) binds to IGFs with similar affinity as exhibited by the wild-type IGFBP-4 (10, 19) but cannot be cleaved by PAPP-A (7). Thus, this protease-resistant IGFBP-4 analog, designated as PR-BP4, can serve as an ideal IGF inhibitor. The PAPP-A-induced increase in cell proliferation was completely abolished by PR-BP4 (Fig. 1C). Consistent with these results, the PAPP-A-induced increase in free IGF-I concentration did not occur in the presence of PR-BP4 (Fig. 1D).
Next, we determined whether the mitogenic effect of PAPP-A observed in the culture of MG63 osteosarcoma cells, which do not produce PAPP-A, could be reproduced using normal osteoblasts, which are known to produce PAPP-A. As shown in Fig. 2, treatment with recombinant PAPP-A significantly increased osteoblast proliferation (Fig. 2A), accompanied by a significant increase in free IGF-I (Fig. 2B).
Fig. 2.
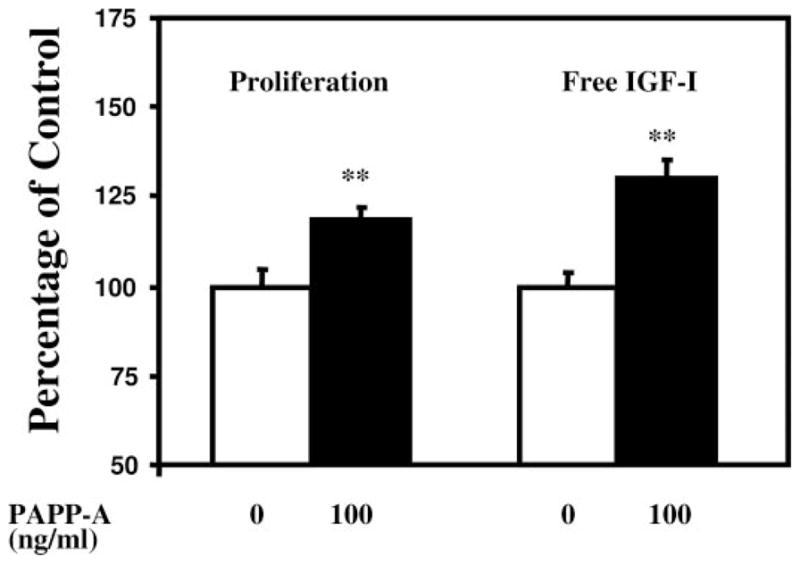
Effect of PAPP-A treatment on cell proliferation and free IGF-I concentration in normal mouse osteoblast cultures. Cellular proliferation determined by alamarBlue dye and free IGF-I concentrations in the culture medium (Material and Methods) were significantly increased in response to PAPP-A in the presence of 5% FCS.
Development and characterization of osteoblast-specific PAPP-A transgenic mouse founders
Human osteosarcoma MG63 and nonosteoblast cell line, human hepatoma HepG2 were transfected with the rCol2.3-PAPP-A/pFLAG transgenic plasmid. Biologically active FLAG-PAPP-A was detected in the CM of transfected osteoblasts but not HepG2 liver cells (data not shown). These data are consistent with past success in using the rCol2.3 promoter to target transgene to bone (31–35). After this in vitro characterization, the linearized rCol2.3-PAPP-A/pFLAG plasmid (Fig. 3A) was used to develop PAPP-A transgenic founders. Among 28 pups, three of them (founders 1, 2, and 26) carried the human PAPP-A cDNA as determined by PCR using isolated tail DNA (Fig. 3B). Founders 2 and 26 expressed human PAPP-A mRNA as determined by RT-PCR analysis of tail RNAs (Fig. 3C). In situ localization of transgene expression in bone was not determined because PAPP-A was secreted and little was retained intracellularly. However, immunoblot analysis with FLAG antibody revealed the presence of FLAG-PAPP-A protein in the CM of osteoblasts derived from PAPP-A transgenic mice but not the wild-type littermates (Fig. 3D). Consistent with these data, CM of cultured transgenic mouse osteoblasts exhibited more than 70% increase in IGFBP-4 proteolysis compared with the CM of wild-type mouse osteoblasts (Fig. 3E). Although FLAG-hPAPP-A mRNA was expressed in founder 2 (Fig. 3C), no FLAG-PAPP-A protein was detected in the CM of primary osteoblasts derived from the F1 generation of this founder (Fig. 3D). Furthermore, no human PAPP-A mRNA was detected by RT-PCR in osteoblasts or bone of founder 2 offspring (data not shown). There are several possibilities that could account for the loss of the PAPP-A transgene expression in the F1 offspring of this founder. First, the lack of transgene expression in F1 mice may be explained by lack of germline transmission by this founder. Second, the transgene may have been relocated to a region of the genome that leads to genomic imprinting and initiation of transgene silencing (36). Third, the PAPP-A transgene may have been integrated into a region of active recombination, which led to fragmentation of the transgene. To exclude the possibility that the observed phenotypic changes are artifacts caused by random insertion of the transgene, additional transgenic lines were subsequently developed. Among the 19 pups, four founders (founder 10 died at the age of 4 wk) were identified to be positive for the transgene by PCR analysis of tail DNA (Fig. 3F). Subsequently, progeny of founder 12, one of the new lines developed, was chosen for phenotypic analysis by pQCT and DEXA.
Fig. 3.

Development of osteoblast-specific PAPP-A transgenic mice. A, Expression of the full-length human PAPP-A is under control of the 2.3-kb rat type I collagen (rCol2.3) promoter. B, PCR was conducted with Taq DNA polymerase, 100 ng tail DNA, and a pair of primers that amplifies a 1-kb sequence in the midregion of the human PAPP-A cDNA. Three pups (1, 2, and 26) carried the PAPP-A transgene. C, Expression of human PAPP-A mRNA was determined by PCR using the cDNAs derived from tail RNAs. Founders 2 and 26 expressed FLAG-PAPP-A mRNA in bone. hOB, Human osteoblast cDNA; hPL, human placenta cDNA; mOB, mouse osteoblast cDNA; P.C., plasmid control. D, Production of FLAG-PAPP-A protein was determined by immunoblot analysis of osteoblast CM with monoclonal M1 PLAG antibody (Material and Methods). Founder 26 but not founder 2 inherited PAPP-A transgene expression to the next negation. E, A 100-ng IGFBP-4 and 50-ng of IGF-II mix was incubated for 20 h at 37 C with 2 or 10 μl of 15-fold concentrated CM. Proteolysis of IGFBP-4 was evaluated by IGF-II ligand blot analysis (4). F, A second micro-injection of pronuclei of fertilized zygotes from C57BL/6J × CBA/CA mice was performed, and 19 pups were born from four recipients. Four pups (two females and two males) were identified to be transgenic founders by PCR analysis of tail DNA as described in Materials and Methods. One transgenic male (no. 10) died at an age of 4 wk.
IGF bioavailability was increased in the CM of osteoblasts derived from PAPP-A transgenic mice
Our in vitro studies demonstrate that treatment of osteoblasts with recombinant PAPP-A increased free IGF-I concentration in the CM (Figs. 1 and 2). Consistent with these data, we found that free IGF-I concentration was significantly increased in the CM of osteoblasts derived from calvarial bone of 2-wk-old PAPP-A transgenic mice compared with the wild-type littermates (Fig. 4). The increase in IGF bio-availability is consistent with the increase in the rate of IG-FBP-4 proteolysis in the CM of transgenic osteoblasts (Fig. 3E).
Fig. 4.
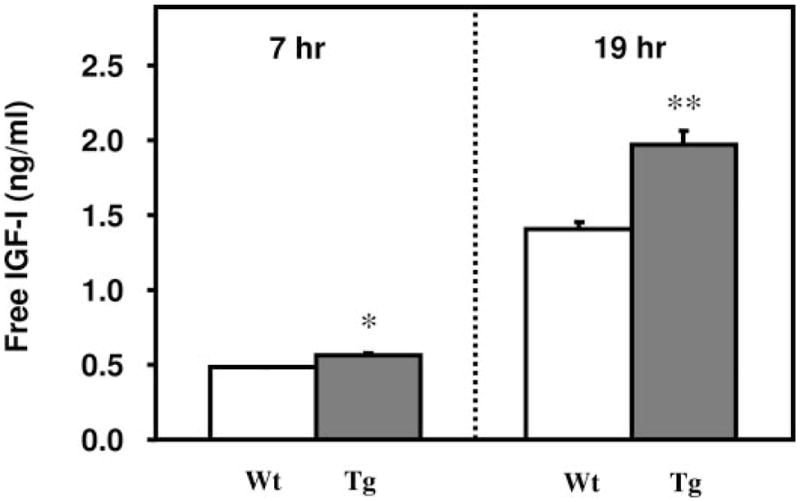
Cultured transgenic osteoblasts overexpressing PAPP-A exhibit an increase in free IGF-I when compared with wild-type littermate osteoblasts. CM collected from confluent cultured osteoblasts derived from 2-wk-old PAPP-A transgenic and wild-type littermate mice was subjected to determination of free IGF-I concentration (Materials and Methods). Free IGF-I levels are corrected for total protein as measured by Bradford reagent (n = 3).
Effect of PAPP-A overexpression in osteoblasts on postnatal growth
Body weights of PAPP-A transgenic mice and wild-type littermates in F1 and F2 generations of founder 26 were compared at various ages (Fig. 5). At 5 wk of age, male transgenic mice showed a slight increase in body weight compared with wild-type littermates. Regardless of gender, no significant difference in body weight between transgenic and wild-type mice was observed at an age of 3 or 6 months.
Fig. 5.

Effect of osteoblast-specific PAPP-A overexpression in transgenic mice on postnatal growth. Body weights of F1 and F2 generation mice derived from founder 26 were determined at different ages. The body weight of PAPP-A transgenic mice was significantly higher than that of the wild-type littermates at 5 wk of age (P < 0.05; n = 3). No significant difference in body weight between transgenic mice and wild-type littermates was noticed at an age of 3 or 6 months (P > 0.05).
Evidence that PAPP-A transgenic mice exhibit dramatic increase in bone size at multiple sites
It was recently reported that overexpression of IGF-I in osteoblasts using the rat type I collagen promoter led to a dramatic increase in calvarial bone thickness and marrow cavity (33). If PAPP-A’s effect on bone is mediated via increasing free IGF-I as predicted, we would predict the skeletal phenotype of PAPP-A transgenic mice to be similar to that of IGF-I transgenic mice. Therefore, we performed histological analysis on isolated calvarial bones using Goldner staining (Fig. 6). Wild-type calvarial bones contained small bone marrow cavities, which are separated by bone columns that bridge the two cortical bone plates (Fig. 6). In transgenic mice, bone marrow cavity and calvaria thickness were dramatically increased. These observed differences were magnified with age (Fig. 6).
Fig. 6.
PAPP-A transgenic mice show an age-dependent increase in calvarial bone thickness and bone marrow cavity. Calvarial bones isolated from mice at indicated ages were sectioned and subjected to Goldner staining (Materials and Methods). The thickness and bone marrow cavity in calvarial bones are increased in PAPP-A transgenic mice. This difference between transgenic and wild-type mice was intensified with age.
Consistent with histological analysis (Fig. 6), the thickness of the calvarial bones measured by a caliper was significantly increased in transgenic mice (Fig. 7). The skull BMD measured by DEXA in transgenic mice was approximately 15 and 19% higher than the skull BMD in wild-type littermates at 3 and 8 months of age, respectively (P < 0.05; Fig. 7). The skull BMD of founder 2 mouse measured at the age of 8 months was increased by approximately 36% compared with the mean value of skull BMD measured in three wild-type mice of similar age and genetic background (Fig. 7). To exclude the possibility that the observed phenotypic changes are artifacts caused by random insertion of the transgene, additional PAPP-A transgenic founders were developed and their skeletal phenotype analyzed. Consistent with the results obtained from founder 2 and founder 26, an increase in skull BMD (a hallmark of PAPP-A overexpression in osteoblasts) was observed in all three new founders at 6 wk of age (data not shown) and subsequently confirmed using the F1 progeny of one of the three new lines (line 12) at 5 months of age (Fig. 7).
Fig. 7.

PAPP-A transgenic mice show increased calvarial bone thickness and skull BMD. The thickness of the isolated calvarial bones was determined by a digital caliper. Skull BMD was measured by DEXA on anesthetized animals (Materials and Methods). Eight-month-old F1 females [three wild-type (Wt.) and three transgenic (Tg.) mice] and 3-month-old F2 males (five wild-type and five transgenic mice) were derived from founder 26. The in vivo skull BMD of the founder 2 mouse (male) measured at an age of 8 months was compared with the average BMD of three wild-type male mice with similar age and genetic background. The in vivo skull BMD of the female F1 progeny of line 12 (one of the three new lines subsequently developed) was analyzed at the age of 5 months of age (n = 3). *, P < 0.05; **, P < 0.01 compared with values of wild-type mice.
In addition to an increase in calvarial bone thickness, a dramatic color change in calvarial bone surface was observed in transgenic mice. The wild-type calvarial bone surface exhibited a normal pink translucent appearance compared with an intense red opaque appearance observed in transgenic mice (Fig. 8). This difference is not caused by residual blood on the surface of bone because 1) all bones were extensively washed with PBS before photography and 2) intense red opaque appearance was observed in all PAPP-A transgenic mice. This color change is evident at 3 months (Fig. 8A), with a dramatic contrast observed at 8 months (Fig. 8B). Similar results were obtained in the 22-month-old founder 2 mouse when compared with a wild-type mouse of similar age and genetic background (Fig. 8C).
Fig. 8.

PAPP-A transgenic mice show an age-dependent manifestation of an intense red opaque appearance on the calvarial bone surface. Calvarial bones were isolated, washed with PBS, and photographed. The PAPP-A transgenic (Tg.) mouse calvarial bone exhibited an intense red opaque appearance in contrast to a pink translucent appearance observed in wild-type (Wt.) mice. The contrast in bone color is evident at 3 months (A), magnified at 8 months (B), and sustained at an advanced age of 22 months (C).
The effect of PAPP-A overexpression in osteoblasts on BMD and geometric parameters of long bones (femur and tibia) was evaluated by pQCT. The majority of bone-size-related parameters including femur length, bone mineral content (BMC), bone area, cortical BMC, cortical area, and bone circumferences were significantly increased in 3-month-old F1 female transgenic mice compared with the female wild-type littermates (Table 1) (F1 male transgenic mice were kept for breeding). Transgenic mice also exhibited a marginal increase in femur cortical bone thickness (P = 0.059). This observed phenotype was intensified at 8 months of age (data not shown). No significant difference in total volumetric BMD was observed (Table 1). Increase in bone size was also observed in the tibia of F2 male transgenic mice (Table 1), suggesting that the effect of PAPP-A overexpression on bone is gender independent. In vivo pQCT analysis of the tibia in all three new founders (data not shown) and F1 progeny of one of the three new founders (line 12) again revealed a significant increase in tibia bone size (Table 1). It is important to note that the changes in size of bone cannot be explained on the basis of differences in body weight, which was similar in transgenic and control mice at 3 and 6 months of age (Fig. 5).
TABLE 1.
PAPP-A transgenic mice exhibit increased size of long bones as determined by pQCT
| Line 26 |
Line 12 |
||
|---|---|---|---|
| Femur | Tibia | Tibia | |
| Bone length | 107 ± 1.5a | 98 ± 1.6 | 105 ± 1.5 |
| BMC | 126 ± 3.5c | 122 ± 0.4b | 121 ± 6.5a |
| Bone area | 126 ± 2.8c | 127 ± 7.1c | 118 ± 4.8a |
| Cortical BMC | 125 ± 4.3b | 122 ± 1.4b | 122 ± 6.7a |
| Cortical area | 123 ± 3.4c | 126 ± 9.3b | 118 ± 5.9a |
| Periosteal circumference | 112 ± 1.3c | 113 ± 3.1c | 108 ± 2.2a |
| Endosteal circumference | 114 ± 1.9c | 113 ± 0.8c | 106 ± 1.2 |
| Cortical thickness | 108 ± 2.6 | 112 ± 5.7 | 110 ± 4.1 |
| Total BMD | 100 ± 2.0 | 97 ± 5.9 | 103 ± 1.2 |
Femurs isolated from 3-month-old female F1 offspring of founder 26 (nine transgenic mice and five wild-type mice) were subjected to ex vivo pQCT analysis. The 3-month-old F2 male offspring of founder 26 (n = 3) were anesthetized by ip ketamine/xylazine injection and subjected to in vivo pQCT analysis of the tibia. The 5-month-old female F1 progeny of line 12 was subjected to in vivo pQCT analysis of the tibia (n = 3). Measurements of wild-type mice were defined as 100%.
P < 0.05.
P < 0.01.
P < 0.001.
We next examined histologically the distal growth plate and mid-diaphysis of the femurs. The growth plate (indicated by yellow arrows) appears to be wider in the transgenic mice compared with that of the control mice (Fig. 9A). Accordingly, femur length was significantly increased in transgenic mice. Consistent with pQCT analysis, histological analysis of femur mid-diaphysis sections revealed a significant increase in bone-size-related parameters in transgenic mice vs. wild-type littermates (Fig. 9, B and C).
Fig. 9.

Histological analysis of the growth plate and mid-diaphysis of femur. Femur sections of line 26 progeny were subjected to Goldner staining. A, The growth plate (indicated by arrows) was wider in transgenic (Tg) mice compared with wild-type (Wt) littermates at 6 months of age; B, transgenic mice show increased inner and outer diameters in the mid-diaphysis of the femurs at 6 wk of age; C, size-related bone parameters were significantly increased in transgenic mice compared with the wild-type littermates at the age of 6 wk measured by histological analysis (n = 3, mean ± SEM). B. Area, Cortical bone area; Ec. Pm, endocoritcal perimeter; Me. Area, medullary area (bone marrow cavity area); Ps. Pm, periosteal perimeter; Tt.Area, total area (bone marrow cavity area plus cortical bone area). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Evidence that increase in bone size in PAPP-A transgenic mice is primarily caused by increased bone formation
To determine whether increased bone size in PAPP-A transgenic mice is caused by enhanced bone formation, we compared BFR (μm3/d) in the femur of transgenic mice vs. wild-type littermates by in vivo tetracycline labeling. PAPP-A transgenic mice showed a dramatic increase in the width of newly formed bone (between the labeled yellow surfaces) (Fig. 10A) and exhibited more than a 2-fold increase in BFR at both the periosteal and endosteal surfaces (Fig. 10B). The surface area of osteoid (stained red, indicated by arrow in Fig. 10C), which is unmineralized bone matrix and an indicator of osteoblast number and activity, was also significantly increased in transgenic mice (Fig. 10D). Bone resorption, as indicated by bone-resorbing area, was not significantly affected (Fig. 10D).
Fig. 10.
PAPP-A transgenic mice show increased BFR without a change in bone resorbing area. A and B, Newly formed bone in femur double-labeled with tetracycline (Materials and Methods) was laid between the two fluorescence-labeled bone surfaces (A, indicated by arrows). BFR (μm3/d) was significantly increased in transgenic mice (B) (n = 3; P < 0.05). C and D, Calvaria sections of 3-month-old F2 males were subjected to Goldner staining. Percentage of osteoid surface (stained red, indicated by arrows in C) was significantly increased in transgenic mice (n = 4, P < 0.05). Bone-resorbing surface area (percentage of total bone area) measured in TRAP-stained sections (data not shown) was not significantly different in transgenic (Tg.) vs. wild-type (Wt.) littermates.
Discussion
Our findings demonstrate that targeted overexpression of PAPP-A to osteoblasts in mice resulted in a significant increase in bone size. These in vivo data provide strong evidence that multiple PAPP-A-cleavable IGFBPs regulate IGF bioavailability in skeletal tissue and that PAPP-A acts as a potent anabolic factor in the regulation of bone metabolism.
Our data clearly demonstrate that PAPP-A transgenic mice exhibit a dramatic increase in bone size at multiple skeletal sites (Figs. 6, 7, and 9 and Table 1). The net increase in bone volume depends on the relative rate of bone formation mediated by osteoblasts and the rate of bone resorption mediated by osteoclasts. Our data suggest that the PAPP-A-induced increase in bone size is mainly attributed to increased bone formation rather than reduced bone resorption. This uncoupling of bone formation to resorption can be explained by the increase in osteoblast number and/or activity rather than a decrease in osteoclast number and/or activity. Evidence to support this contention includes the following. 1) BFR determined by double tetracycline labels or quantitation of osteoid surface is significantly increased in transgenic mice (Fig. 10). Both parameters are determined by the number and activity of osteoblasts, which form new bone. 2) Osteoblast proliferation in vitro is enhanced by PAPP-A treatment (Figs. 1 and 2). 3) Bone resorbing surface is not significantly affected (Fig. 10).
PAPP-A transgenic mice also exhibit an age-dependent manifestation of an intense red calvarial bone surface (Fig. 8). This unique phenotype can be explained by either an increase in the total volume of blood trapped in the enlarged bone marrow cavity per bone and/or an increase in bone vascular density (number or area of blood vessels divided by volume of bone marrow) in transgenic mice. Efforts were made but unsuccessful in developing an in situ immunostaining for CD31 or von Willebrand factor in bone specimens to quantitate vascular density. Therefore, future studies are needed to identify the precise cause for this important phenotype.
The mechanism by which PAPP-A increases bone formation can only be speculated at this moment. The similarity in major bone phenotypes observed in PAPP-A transgenic mice and IGF-I transgenic mice (33) using an identical promoter (the 2.3-kb rat type I collagen promoter) as described below suggests that PAPP-A may increase bone formation through an IGF-dependent mechanism. First, both PAPP-A transgenic mice and IGF-I transgenic mice exhibited a significant increase in calvarial bone thickness. This phenotype was observed in young adult mice and sustained with age in both transgenic mouse models. Second, calvarial bone marrow cavity was dramatically enlarged in both PAPP-A and IGF-I transgenic mice. Third, the primary bone-size-related parameters in long bones were increased in both PAPP-A and IGF-I transgenic mice.
Additional support for an IGF-dependent mechanism of PAPP-A action in bone arises from recent studies on characterization of PAPP-A knockout mice (21, 37). Functional disruption of the PAPP-A gene in mice resulted in proportional dwarfism and a delay in embryonic skeletal ossification. These phenotypic changes are similar to those observed in IGF-II knockout mice (38). Moreover, cross-breeding PAPP-A knockout mice with ΔH19 mutant mice (which have increased IGF-II expression and fetal overgrowth because of disruption of IgfII imprinting) corrected the dwarf phenotype of the PAPP-A-null mice (37). These findings, together with our in vitro data showing that PAPP-A stimulates osteoblast proliferation solely via an IGF-dependent mechanism (Fig. 1) and our ex vivo data showing that PAPP-A overexpression in osteoblasts increases IGFBP degradation and IGF bioavailability, suggest that PAPP-A may regulate bone formation by enhancing IGF bioavailability in the local bone environment. However, our findings do not exclude the possibility that PAPP-A may promote bone formation through an IGF-independent mechanism because PAPP-A contains multiple functional domains and interacts with a number of functional proteins (2, 22, 39–41). Evaluation of whether overexpressing an IGF inhibitor, such as a PAPP-A-resistant IGFBP-4 analog, in PAPP-A transgenic mice can abolish some or all of the PAPP-A-induced phenotypic changes may provide a more definitive answer to this important issue.
Acknowledgments
We thank Nancy Lowen, Carolyn Hargrave, Arun Sivanandam, Christopher Sexton, Tina Boykin, Daniela Guilliam, Blake Bonafede, Diana Hou, Dr. Georgy Bagi, and Dr. Weilong Xing for their excellent technical help. We thank Dr. David W. Rowe (University of Connecticut Health Center) for providing us the 2.3-kb rat type I collagen promoter. All work was performed at facilities provided by the Jerry L. Pettis Memorial Veterans Affairs Medical Center in Loma Linda, CA.
This study was mainly supported by grants from Department of Veterans Affair (Merit Review to X.Q.) and National Institutes of Health (R01 AR45210 to X.Q.; AR310622 to S.M.).
Abbreviations
- BFR
Bone formation rate
- BMC
bone mineral content
- BMD
bone mineral density
- CM
conditioned medium
- DEXA
dual-energy x-ray absorptiometry
- FCS
fetal calf serum
- IGFBP
IGF-binding protein
- PAPP
pregnancy-associated plasma protein
- pQCT
peripheral quantitated computed tomography
- PR-BP4
PAPP-A-resistant IGFBP-4
Footnotes
Disclosure summary: all authors have nothing to disclose.
References
- 1.Lin TM, Galbert SP, Kiefer D, Spellacy WN, Gall S. Characterization of four human pregnancy-associated plasma proteins. Am J Obstet Gynecol. 1974;118:223–236. doi: 10.1016/0002-9378(74)90553-5. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen T, Oxvig C, Sand O, Moller NP, Sottrup-Jensen L. Amino acid sequence of human pregnancy-associated plasma protein-A derived from cloned cDNA. Biochemistry. 1994;33:1592–1598. doi: 10.1021/bi00172a040. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, 3rd, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci USA. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byun D, Mohan S, Yoo M, Sexton C, Baylink DJ, Qin X. Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J Clin Endocrinol Metab. 2001;86:847–854. doi: 10.1210/jcem.86.2.7223. [DOI] [PubMed] [Google Scholar]
- 5.Qin X, Byun D, Lau KH, Baylink DJ, Mohan S. Evidence that the interaction between insulin-like growth factor (IGF)-II and IGF binding protein (IGFBP)-4 is essential for the action of the IGF-II-dependent IGFBP-4 protease. Arch Biochem Biophys. 2000;379:209–216. doi: 10.1006/abbi.2000.1872. [DOI] [PubMed] [Google Scholar]
- 6.Monget P, Mazerbourg S, Delpuech T, Maurel MC, Maniere S, Zapf J, Lalmanach G, Oxvig C, Overgaard MT. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation. Biol Reprod. 2003;68:77–86. doi: 10.1095/biolreprod.102.007609. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Mohan S, Newton J, Rehage M, Tran K, Baylink DJ, Qin X. Pregnancy-associated plasma protein-A regulates myoblast proliferation and differentiation through an insulin-like growth factor-dependent mechanism. J Biol Chem. 2005;280:37782–37789. doi: 10.1074/jbc.M505278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504:36–40. doi: 10.1016/s0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- 9.Feyen JH, Evans DB, Binkert C, Heinrich GF, Geisse S, Kocher HP. Recombinant human [Cys281]insulin-like growth factor-binding protein 2 inhibits both basal and insulin-like growth factor I-stimulated proliferation and collagen synthesis in fetal rat calvariae. J Biol Chem. 1991;266:19469–19474. [PubMed] [Google Scholar]
- 10.Qin X, Strong DD, Baylink DJ, Mohan S. Structure-function analysis of the human insulin-like growth factor binding protein-4. J Biol Chem. 1998;273:23509–23516. doi: 10.1074/jbc.273.36.23509. [DOI] [PubMed] [Google Scholar]
- 11.Mohan S, Nakao Y, Honda Y, Landale E, Leser U, Dony C, Lang K, Baylink DJ. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem. 1995;270:20424–20431. doi: 10.1074/jbc.270.35.20424. [DOI] [PubMed] [Google Scholar]
- 12.Eckstein F, Pavicic T, Nedbal S, Schmidt C, Wehr U, Rambeck W, Wolf E, Hoeflich A. Insulin-like growth factor-binding protein-2 (IGFBP-2) overexpression negatively regulates bone size and mass, but not density, in the absence and presence of growth hormone/IGF-I excess in transgenic mice. Anat Embryol (Berl) 2002;206:139–148. doi: 10.1007/s00429-002-0282-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Faugere MC, Malluche H, Rosen CJ, Chernausek SD, Clemens TL. Paracrine overexpression of IGFBP-4 in osteoblasts of transgenic mice decreases bone turnover and causes global growth retardation. J Bone Miner Res. 2003;18:836–843. doi: 10.1359/jbmr.2003.18.5.836. [DOI] [PubMed] [Google Scholar]
- 14.Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest. 2001;107:73–81. doi: 10.1172/JCI10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauss F, Lang K, Dony C, Kling L. The complex of recombinant human insulin-like growth factor-I (rhIGF-I) and its binding protein-5 (IGFBP-5) induces local bone formation in murine calvariae and in rat cortical bone after local or systemic administration. Growth Horm IGF Res. 2001;11:1–9. doi: 10.1054/ghir.2000.0181. [DOI] [PubMed] [Google Scholar]
- 16.Devlin RD, Du Z, Buccilli V, Jorgetti V, Canalis E. Transgenic mice overexpressing insulin-like growth factor binding protein-5 display transiently decreased osteoblastic function and osteopenia. Endocrinology. 2002;143:3955–3962. doi: 10.1210/en.2002-220129. [DOI] [PubMed] [Google Scholar]
- 17.Salih DA, Mohan S, Kasukawa Y, Tripathi G, Lovett FA, Anderson NF, Carter EJ, Wergedal JE, Baylink DJ, Pell JM. Insulin-like growth factor-binding protein-5 induces a gender-related decrease in bone mineral density in transgenic mice. Endocrinology. 2005;146:931–940. doi: 10.1210/en.2004-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang E, Wang J, Chin E, Zhou J, Bondy CA. Cellular patterns of insulin-like growth factor system gene expression in murine chondrogenesis and osteogenesis. Endocrinology. 1995;136:2741–2751. doi: 10.1210/endo.136.6.7750499. [DOI] [PubMed] [Google Scholar]
- 19.Qin X, Byun D, Strong DD, Baylink DJ, Mohan S. Studies on the role of human insulin-like growth factor-II (IGF-II)-dependent IGF binding protein (hIGFBP)-4 protease in human osteoblasts using protease-resistant IGFBP-4 analogs. J Bone Miner Res. 1999;14:2079–2088. doi: 10.1359/jbmr.1999.14.12.2079. [DOI] [PubMed] [Google Scholar]
- 20.Qin X, Sexton C, Byun D, Strong DD, Baylink DJ, Mohan S. Differential regulation of pregnancy associated plasma protein (PAPP)-A during pregnancy in human and mouse. Growth Horm IGF Res. 2002;12:359–366. doi: 10.1016/s1096-6374(02)00046-1. [DOI] [PubMed] [Google Scholar]
- 21.Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer EM, Oxvig C, van Deursen J. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- 22.Sivanandam AS, Mohan S, Kapur S, Kita H, Lau KH, Bagi G, Baylink DJ, Qin X. Covalent interaction between proform of eosinophil major basic protein (proMBP) and pregnancy-associated plasma protein-A (PAPP-A) is a cell-mediated event and required for proMBP inhibition of the catalytic activity of PAPP-A. Arch Biochem Biophys. 2004;423:343–350. doi: 10.1016/j.abb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Miller AD, Bender MA, Harris EA, Kaleko M, Gelinas RE. Design of retrovirus vectors for transfer and expression of the human β-globin gene. J Virol. 1988;62:4337–4345. doi: 10.1128/jvi.62.11.4337-4345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang TK, Tam KB, Huang SC. High-level and erythroid-specific expression of human glucose-6-phosphate dehydrogenase in transgenic mice. J Biol Chem. 1993;268:9522–9525. [PubMed] [Google Scholar]
- 25.Sheng MH, Lau KH, Beamer WG, Baylink DJ, Wergedal JE. In vivo and in vitro evidence that the high osteoblastic activity in C3H/HeJ mice compared to C57BL/6J mice is intrinsic to bone cells. Bone. 2004;35:711–719. doi: 10.1016/j.bone.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Stabnov L, Kasukawa Y, Guo R, Amaar Y, Wergedal JE, Baylink DJ, Mohan S. Effect of insulin-like growth factor-1 (IGF-1) plus alendronate on bone density during puberty in IGF-1-deficient MIDI mice. Bone. 2002;30:909–916. doi: 10.1016/s8756-3282(02)00738-x. [DOI] [PubMed] [Google Scholar]
- 27.Gruber HE. Adaptations of Goldner’s Masson trichrome stain for the study of undecalcified plastic embedded bone. Biotech Histochem. 1992;67:30–34. doi: 10.3109/10520299209110002. [DOI] [PubMed] [Google Scholar]
- 28.Gruber HE, Marshall GJ, Nolasco LM, Kirchen ME, Rimoin DL. Alkaline and acid phosphatase demonstration in human bone and cartilage: effects of fixation interval and methacrylate embedments. Stain Technol. 1988;63:299–306. doi: 10.3109/10520298809107604. [DOI] [PubMed] [Google Scholar]
- 29.Funston RN, Moss GE, Roberts AJ. Insulin-like growth factor-I (IGF-I) and IGF-binding proteins in bovine sera and pituitaries at different stages of the estrous cycle. Endocrinology. 1995;136:62–68. doi: 10.1210/endo.136.1.7530196. [DOI] [PubMed] [Google Scholar]
- 30.Skaar TC, Baumrucker CR, Deaver DR, Blum JW. Diet effects and ontogeny of alterations of circulating insulin-like growth factor binding proteins in newborn dairy calves. J Anim Sci. 1994;72:421–427. doi: 10.2527/1994.722421x. [DOI] [PubMed] [Google Scholar]
- 31.Rossert J, Eberspaecher H, de Crombrugghe B. Separate cis-acting DNA elements of the mouse pro-α1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol. 1995;129:1421–1432. doi: 10.1083/jcb.129.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dacic S, Kalajzic I, Visnjic D, Lichtler AC, Rowe DW. Col1a1-driven transgenic markers of osteoblast lineage progression. J Bone Miner Res. 2001;16:1228–1236. doi: 10.1359/jbmr.2001.16.7.1228. [DOI] [PubMed] [Google Scholar]
- 33.Jiang J, Lichtler AC, Gronowicz GA, Adams DJ, Clark SH, Rosen CJ, Kream BE. Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone. 2006;39:495–504. doi: 10.1016/j.bone.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 34.Marijanovic I, Jiang X, Kronenberg MS, Stover ML, Erceg I, Lichtler AC, Rowe DW. Dual reporter transgene driven by 2.3Col1a1 promoter is active in differentiated osteoblasts. Croat Med J. 2003;44:412–417. [PubMed] [Google Scholar]
- 35.Kalajzic Z, Liu P, Kalajzic I, Du Z, Braut A, Mina M, Canalis E, Rowe DW. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone. 2002;31:654–660. doi: 10.1016/s8756-3282(02)00912-2. [DOI] [PubMed] [Google Scholar]
- 36.Surani MA. Imprinting and the initiation of gene silencing in the germ line. Cell. 1998;93:309–312. doi: 10.1016/s0092-8674(00)81156-3. [DOI] [PubMed] [Google Scholar]
- 37.Bale LK, Conover CA. Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J Endocrinol. 2005;186:325–331. doi: 10.1677/joe.1.06259. [DOI] [PubMed] [Google Scholar]
- 38.DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 39.Sinosich MJ, Zakher A. Pregnancy-associated plasma protein A interaction with heparin: a critical appraisal. Gynecol Obstet Invest. 1991;32:72–77. doi: 10.1159/000292998. [DOI] [PubMed] [Google Scholar]
- 40.Oxvig C, Haaning J, Kristensen L, Wagner JM, Rubin I, Stigbrand T, Gleich GJ, Sottrup-Jensen L. Identification of angiotensinogen and complement C3dg as novel proteins binding the proform of eosinophil major basic protein in human pregnancy serum and plasma. J Biol Chem. 1995;270:13645–13651. doi: 10.1074/jbc.270.23.13645. [DOI] [PubMed] [Google Scholar]
- 41.Bischof P, Geinoz A, Herrmann WL, Sizonenko PC. Pregnancy-associated plasma protein A (PAPP-A) specifically inhibits the third component of human complement (C3) Placenta. 1984;5:1–7. doi: 10.1016/s0143-4004(84)80044-2. [DOI] [PubMed] [Google Scholar]




