Abstract
The main psychoactive component of marijuana, Δ9-tetrahydrocannabinol (THC), acts in the CNS via type 1 cannabinoid receptors (CB1Rs). The behavioral consequences of THC or synthetic CB1R agonists include suppression of motor activity. One explanation for movement suppression might be inhibition of striatal dopamine (DA) release by CB1Rs, which are densely localized in motor striatum; however, data from previous studies are inconclusive. Here we examined the effect of CB1R activation on locally evoked DA release monitored with carbon-fiber microelectrodes and fast-scan cyclic voltammetry in striatal slices. Consistent with previous reports, DA release evoked by a single stimulus pulse was unaffected by WIN55,212-2, a cannabinoid receptor agonist. However, when DA release was evoked by a train of stimuli, WIN55,212-2 caused a significant decrease in evoked extracellular DA concentration ([DA]o), implicating the involvement of local striatal circuitry, with similar suppression seen in guinea pig, rat, and mouse striatum. Pulse-train evoked [DA]o was not altered by either AM251, an inverse CB1R agonist, or VCHSR1, a neutral antagonist, indicating the absence of DA release regulation by endogenous cannabinoids with the stimulation protocol used. However, both CB1R antagonists prevented and reversed suppression of evoked [DA]o by WIN55,212-2. The effect of WIN55,212-2 was also prevented by picrotoxin, a GABAA receptor antagonist, and by catalase, a metabolizing enzyme for hydrogen peroxide (H2O2). Furthermore, blockade of ATP-sensitive K+ (KATP) channels by tolbutamide or glybenclamide prevented the effect of WIN55,212-2 on DA release. Together, these data indicate that suppression of DA release by CB1R activation within striatum occurs via a novel nonsynaptic mechanism that involves GABA release inhibition, increased generation of the diffusible messenger H2O2, and activation of KATP channels to inhibit DA release. In addition, the findings suggest a possible physiological substrate for the motor effects of cannabinoid agonist administration.
The main psychoactive component of marijuana, Δ9-tetrahydrocannabinol (THC), exerts its CNS effects via activation of type 1 cannabinoid receptors (CB1Rs), which are highly expressed in the brain, including the basal ganglia (Herkenham et al., 1990, 1991; Mailleux and Vanderhaeghen, 1992). Consistent with effects on basal-ganglia function, CB1R activation by THC or other agonists alters motor performance, with dose-dependent effects ranging from increased activity (Sulcova et al., 1998; Sañudo-Peña et al., 2000) to inhibition of spontaneous activity, including decreased and/or irregular locomotion (Gough and Olley, 1978; Navarro et al., 1993; Onaivi et al., 1996; Sañudo-Peña et al., 2000; Shi et al., 2005), and ultimately immobility or catalepsy (Gough and Olley, 1978; Onaivi et al., 1996; Sulcova et al., 1998). Moreover, other studies suggest that THC or cannabinoid agonists also show promise as therapeutic agents to ameliorate hyperkinetic movement, including the tics of Tourette's syndrome (Muller-Wahl et al., 2003) and DOPA-induced dyskinesia that can accompany the treatment of Parkinson's disease (Sieradzan et al., 2001, Segovia et al., 2003, Ferrer et al., 2003 Venderova et al., 2004; but see Carroll et al., 2004). Given the key role of the nigrostriatal dopamine (DA) system in basal-ganglia mediated movement (Albin et al., 1989; Olanow and Tatton, 1999), a likely contributing factor to these motor effects is decreased DA release in the dorsolateral striatum.
Although CB1R activation has been shown to inhibit release of striatal transmitters, including GABA and glutamate (e.g., Szabo et al., 1998; Gerdeman and Lovinger, 2001; Huang et al., 2001; Gerdeman et al., 2002; Ronesi et al., 2004; Kőfalvi et al., 2005; Freiman et al., 2006; Kreitzer and Malenka, 2005, 2007), few studies have addressed consequences of CB1R activation on DA release. Using fast-scan cyclic voltammetry to monitor evoked extracellular DA concentration ([DA]o) in rat striatal slices, Szabo et al. (1999) found no effect of CB1R agonists or antagonists on single-pulse evoked [DA]o in either dorsal striatum or ventral striatum (nucleus accumbens). In other in vitro studies, in which prolonged electrical stimulation (3-min stimulation at 0.5 to 10 Hz) was used to elicit release of [3H]DA from striatal slices, cannabinoid agonist application produced either a decrease or no effect on DA release (Cadogan et al., 1997; Kőfalvi et al., 2005). In vivo voltammetric data from the nucleus accumbens indicate that systemic administration of the cannabinoid agonist WIN55,212-2 leads to inhibition of [DA]o evoked by pulse-train stimulation of the median forebrain bundle (Cheer et al., 2004), although the frequency of spontaneous [DA]o transients increased. However, whether these observations reflect a direct effect within the nucleus accumbens or the involvement of long feedback pathways between the accumbens and midbrain dopamine neurons in the ventral tegmental area was not addressed.
It is important to note that modulation of single-pulse evoked [DA]o by a given receptor agonist implies a direct effect on receptors located on DA axons, e.g., D2 autoreceptors (Palij et al. 1990; Benoit-Marand et al., 2001) or nicotinic acetylcholine (ACh) receptors (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004). On the other hand, pulse-train stimulation can reveal indirect effects of striatal microcircuitry and concurrently released transmitters on DA release, including the release of GABA and glutamate (Avshalumov et al., 2003; 2007). The lack of effect of CB1R agonists on DA release elicited by a single stimulus in previous studies (Szabo et al., 1999), therefore, suggests the absence of direct presynaptic modulation of axonal DA release. This implies that any effect of CB1Rs on striatal DA release must be indirect.
The aim of the present work was to resolve whether CB1R activation within striatum modulates axonal DA release. Using striatal slices to eliminate confounding effects of long feedback pathways, we compared the effect of WIN55,212-2 on endogenous [DA]o evoked by single-pulse or pulse-train stimulation to assess direct versus indirect effects of CB1R activation on DA release. Evoked [DA]o was monitored in real-time using fast-scan cyclic voltammetry and carbon-fiber microelectrodes. Pharmacological agents were used as appropriate to determine the mechanism of DA release suppression seen during pulse-train stimulation.
Methods
Brain slice preparation
For most studies, coronal forebrain slices were prepared from young adult male guinea pigs; however, in some experiments, slices were prepared from adult rats (male, Wistar) or mice (male, C57/BL6). All animals were deeply anesthetized with 40 mg/kg pentobarbital (intraperitoneal) and decapitated. After removal, the brain was cooled for 1-2 min in ice-cold HEPES-buffered ACSF, containing (in mM): 120 NaCl; 5 KCl; 20 NaHCO3; 6.7 HEPES acid; 3.3 HEPES salt; 2 CaCl2; 2 MgSO4; and 10 glucose, saturated with 95% O2/5% CO2 (Rice et al., 1997). Striatum was blocked and coronal slices (400 μm thickness for guinea-pig slices; 350 μm for rat and mouse) were cut on a Vibratome; slices were maintained for at least 1 h at room temperature in HEPES-buffered ACSF. For recording, slices were transferred to a submersion chamber at 32°C and superfused at 1.2 mL/min with recording ACSF containing the following (in mM): 124 NaCl, 3.7 KCl, 26 NaHCO3, 2.4 CaCl2, 1.3 MgSO4, 1.3 KH2PO4, and 10 glucose, equilibrated with 95% O2/5% CO2 (Rice et al., 1997).
Voltammetric recording
Axon-terminal DA release was elicited in dorsolateral striatum using a surface concentric bipolar stimulating electrode and detected at a carbon-fiber microelectrode positioned ~100 μm away. Single-pulse or pulse-train (10 Hz, 30 pulses) stimulation was used with pulse duration of 100 μs and amplitude of 0.6-0.8 mA; DA release under these conditions is tetrodotoxin-sensitive and Ca2+-dependent (Chen and Rice, 2001). Evoked DA]o was monitored using fast-scan cyclic voltammetry (Millar Voltammeter; Dr. Julian Millar, Queen Mary, University of London, UK) with 8-μm carbon fiber microelectrodes that were made in-house using methods modified from Millar and Pelling (2001) or obtained commercially (MPB Electrodes, London, UK; or WPI, Sarasota, FL, USA). Scan rate was 800 V/s, voltage range was -0.7 to +1.3 V versus Ag/AgCl, and sampling interval was 100 ms. Data acquisition and analysis were as described previously (Chen and Rice, 2001). Released DA was identified by characteristic oxidation and reduction peak potentials (e.g. Fig. 1B, inset); [DA]o was calculated from post-experiment electrode calibration in the recording chamber at 32°C in all media used in a given experiment.
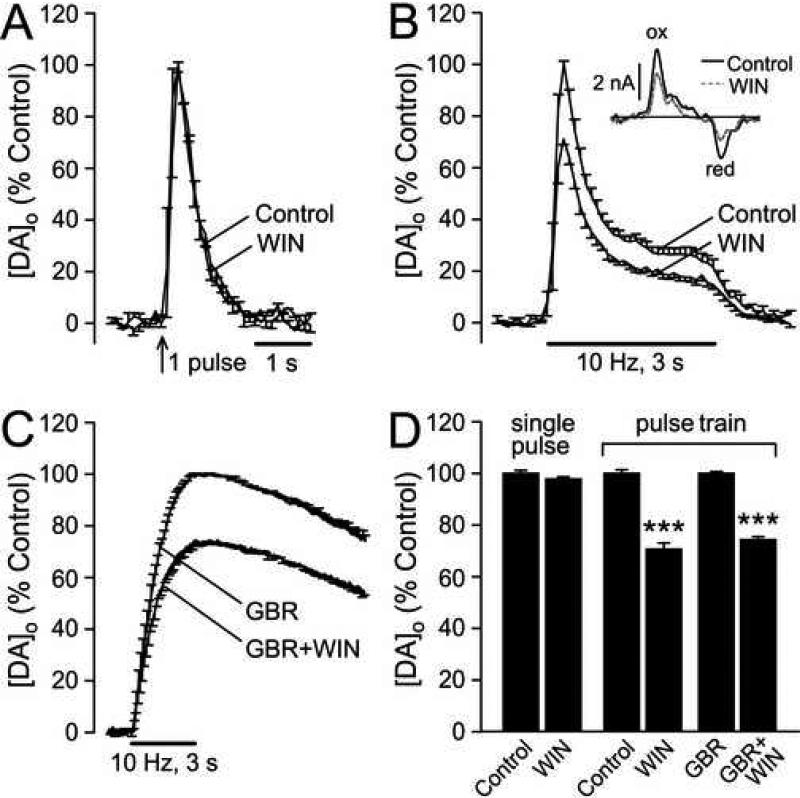
Figure 1. Effect of CB1R activation on DA release in dorsolateral striatum.
A) Averaged DA release records after single-pulse stimulation under control conditions and in the presence of WIN55,212-2 (WIN; 5 μM) indicate no effect of WIN on either the amplitude or the time-course of evoked [DA]o (n = 3). B) Effect of WIN (1-5 μM; see Results) on averaged [DA]o evoked by pulse-train stimulation (30 pulses, 10 Hz) (n = 13). Inset, representative cyclic voltammograms for peak evoked [DA]o at a given site under control conditions and in the presence of WIN; DA was identified by characteristic oxidation (ox) and reduction (red) peak potentials (typically +600 and -200 mV vs. Ag/AgCl). C) The effect of WIN (5 μM) persisted when the DA transporter (DAT) was inhibited by GBR-12909 (GBR; 2 μM) (n = 3). D) Activation of CB1Rs by WIN had no effect on single-pulse evoked [DA]o (p > 0.05 WIN vs. same-site control; n = 3), but suppressed evoked [DA]o during pulse-train stimulation (***p < 0.001 WIN vs. same-site control; n = 13) in ACSF alone, as well as when the DAT was inhibited by GBR (***p < 0.001 GBR + WIN vs. GBR alone; n = 3).
Chemicals and statistical analysis
The non-selective cannabinoid agonist WIN55,212-2 (R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate), the CB1R inverse agonist AM251 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide), and the DA uptake inhibitor GBR-12909 were from Tocris (Ellisville, MO, USA). The neutral antagonist VCHSR1 (5-(4-chlorophenyl)-3-[(E)-2-cyclohexylethenyl]-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole) was synthesized in the Reggio laboratory (Hurst et al., 2002). Picrotoxin, glybenclamide (glyburide, glibenclamide), tolbutamide, dimethylsulfoxide (DMSO), and components of ACSF were from Sigma (St. Louis, MO, USA). Catalase (bovine liver) was from Calbiochem (La Jolla, CA, USA). Individual stock solutions of WIN55,212-2, AM251, and VCHSR1 were made in DMSO. Final DMSO level was 0.01% in ACSF, which was used to obtain control data for these agents; 0.01% DMSO alone had no effect on evoked [DA]o. For studies with of the effect of WIN55,212-2 on DA uptake, slices were preincubated in GBR-12909 (2 μM) in HEPES-buffered ACSF for at least 1 h before experimentation; GBR-12909 was also included in the recording ACSF. For studies with catalase (500 U/mL), active or heat-inactivated enzyme was included in the recording ACSF throughout experimentation; catalase was inactivated as described previously (Avshalumov et al., 2003). In all studies, at least three consistent evoked [DA]o records were obtained to provide control data before drug application. To minimize the effect of inter-slice variation, data were normalized, with the average of the final 2-3 control records taken as 100%; the response in the presence of the drug(s) was evaluated as % same-site control. Data are given as means ± SEM, n = number of slices, with 1-2 slices per animal. Statistical evaluation was performed using paired Student's t-test or one-way ANOVA with appropriate post hoc analysis.
Results
Suppression of pulse-train evoked DA release by CB1R activation
We first tested the effect of the CB1 receptor agonist WIN55,212-2 on single-pulse-evoked [DA]o in dorsolateral striatum. Mean peak [DA]o was 1.3 ± 0.4 μM (n = 3). In agreement with previous studies (Szabo et al., 1999), WIN55,212-2 (5 μM) had no effect on single-pulse-evoked [DA]o (p > 0.05; n = 3) (Fig. 1A,D). We then examined DA release evoked by pulse-train stimulation (10 Hz, 30 pulses); mean peak evoked [DA]o was 1.4 ± 0.1 μM (n = 13). In contrast to single-pulse evoked [DA]o, pulse-train evoked [DA]o was suppressed by ~30% in the presence of WIN55,212-2 (Fig. 1B,D) (p < 0.001; n = 13). Maximal release suppression was achieved with 1-5 μM WIN, depending on the batch, consistent with the range of effective concentrations reported previously for in vitro brain-slice studies (Cadogan et al., 1997; Szabo et al., 1998, 1999; Hájos and Freund, 2002; Kőfalvi et al., 2005; Mátyás et al., 2006; Kreitzer and Malenka, 2005; 2007). In the present studies, the minimal effective concentration was determined and used for each new batch of drug.
Monitored [DA]o reflects the net effect of release and uptake, such that a decrease in evoked [DA]o could reflect either decreased DA release or increased DA uptake via the DA transporter (DAT). The complete lack of effect of WIN55,212-2 on the time course of single-pulse evoked [DA]o (Fig. 1A) argues against an effect of this drug on DA uptake. We then examined whether the effect of WIN55,212-2 (5 μM) on pulse-train evoked [DA]o was altered when the DAT was inhibited by GBR-12909 (2 μM). Inhibition of the DAT increased and prolonged evoked [DA]o, as reported previously (Chen and Rice, 2001; Avshalumov et al., 2003). However, the ~30% WIN-induced decrease in evoked [DA]o (from 4.6 ± 0.4 μM to 3.3 ± 0.5 μM; p < 0.001; n = 3) persisted in GBR-12909 (Fig. 1C,D), confirming that WIN55,212-2 alters DA release rather than uptake.
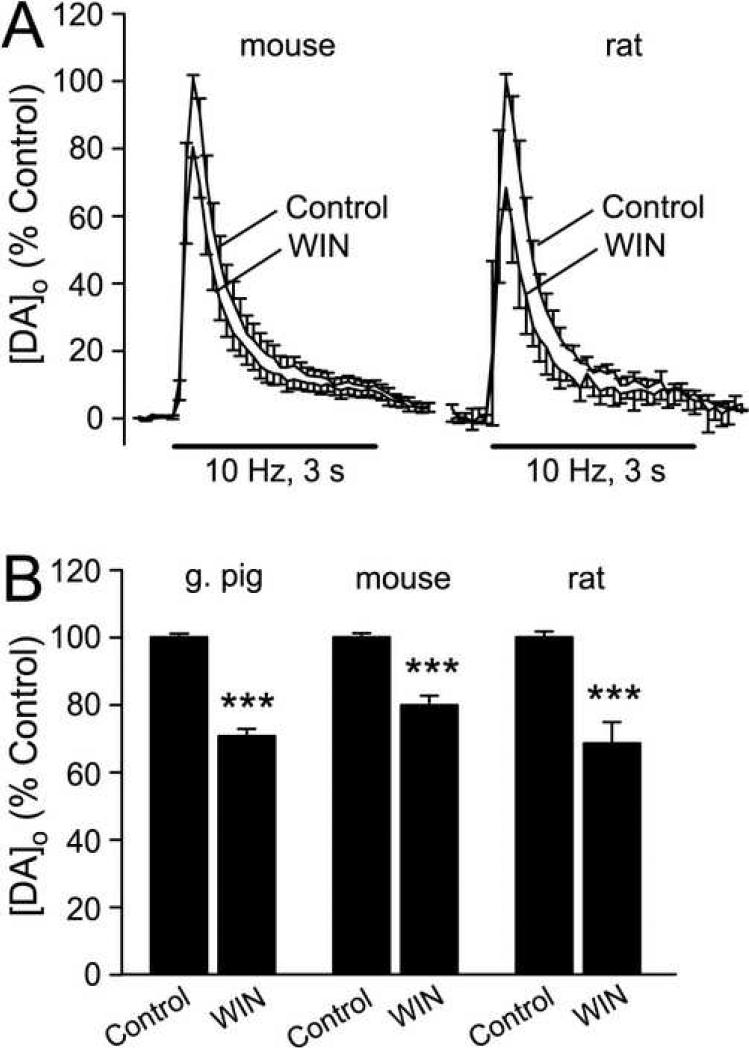
To ascertain that the effect of WIN55,212-2 was species-independent, we examined the effect of CB1 receptor activation in striatal slices from adult mouse (male, CD57/BL6) and adult rat (male, Wistar). WIN55,212-2 (5 μM) suppressed pulse-train evoked [DA]o in dorsal striatum in both species, with a decrease of ~20% in mouse striatum (n = 8, p < 0.001), and ~30% in rat striatum (n = 4, p < 0.001) (Fig. 2).
Figure 2. The effect of WIN55,212-2 on DA release is species independent.
A) Activation of CB1Rs by WIN55,212-2 (WIN; 5 μM) caused a similar decrease in pulse-train evoked [DA]o in the dorsolateral striatum of rats and mice to that seen in guinea pigs striatum (Fig. 1B). B) Comparison of the effect of WIN on average peak evoked [DA]o in guinea pig (***p < 0.001 WIN vs. same-site control; n = 13), rat (***p < 0.001; n = 4), and mouse (***p < 0.001; n = 8) striatum.
Effect of CB1R antagonists
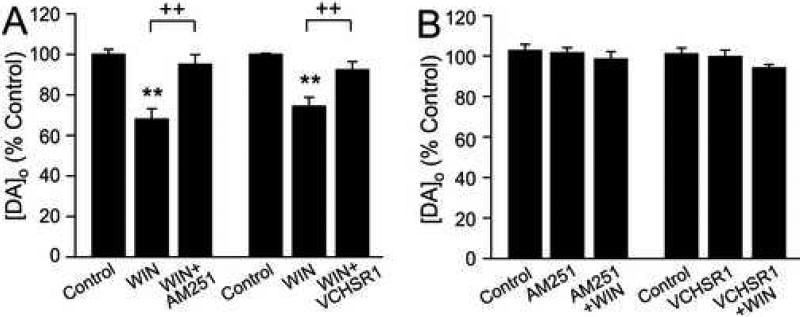
To confirm that the effect of WIN55,212-2 on DA release was CB1R-mediated, we examined whether release suppression could be reversed by CB1R antagonists. Although CB2 receptors have recently been identified in the CNS, these are found at much lower levels than CB1Rs (Van Sickle et al., 2005; Gong et al., 2006), so that CB2R involvement is unlikely. We examined the effect of a CB1R inverse agonist, AM251 (1-5 μM; Hájos and Freund, 2002; Kőfalvi et al., 2005; Brown et al., 2003; Ronesi and Lovinger 2005; Slanina et al., 2005; Kreitzer and Malenka, 2005; 2007) and a neutral antagonist, VCHSR1 (2 μM; Hurst et al., 2002). Consistent with action at CB1Rs, the effect of WIN55,212-2 (1-5 μM) was reversed by AM251 (1 μM) in the continued presence of WIN (p > 0.05 vs. control; n = 4) (Fig. 3A). The neutral antagonist VCHSR1 similarly reversed DA release suppression by WIN (p > 0.05; n = 3) (Fig. 3A), confirming that under the conditions tested, AM251 acted only as a CB1R antagonist.
Figure 3. CB1R antagonists prevent and reverse the effect of WIN55,212-2 on DA release, but have no effect alone.
A) The effect of WIN55,212-2 (WIN; 1-5 μM) on pulse-train evoked [DA]o was reversed by AM251 (1 μM) (**p < 0.01 WIN vs. control; ++p < 0.01 WIN vs. WIN + AM251; n = 4) and VCHSR1 (2 μM) (**p < 0.01 WIN vs. control; ++p < 0.01 WIN vs. WIN + VCHSR1; n = 3). B) Neither AM251 (1-5 μM) or VCHSR1 (2 μM) altered pulse-train evoked [DA]o, indicating the absence of endocannabinoid regulation of DA release under these conditions. AM251 and VCHSR1 also prevented the effect of WIN (1-5 μM) (*p > 0.05 for all comparisons; n = 3-4 per mean).
To test whether endogenous cannabinoid release contributed to DA-release regulation during local pulse-train stimulation, we examined the effect of CB1R blockade on pulse-train evoked [DA]o. Under the stimulation conditions examined (10 Hz for 3 s), neither AM251 (1-5 μM) (p > 0.05 vs. control; n = 4) nor VCHSR1 (2 μM) (p > 0.05; n = 3) altered peak evoked [DA]o (Fig. 3B), suggesting that modulatory endocannabinoids are not generated by this stimulation protocol. Lastly, WIN55,212-2 had no effect in the continued presence of these CB1R antagonists. Overall, therefore, these results show that the WIN55,212-2-induced suppression of evoked [DA]o is mediated by CB1Rs.
CB1R activation suppresses DA release by inhibiting striatal GABA release
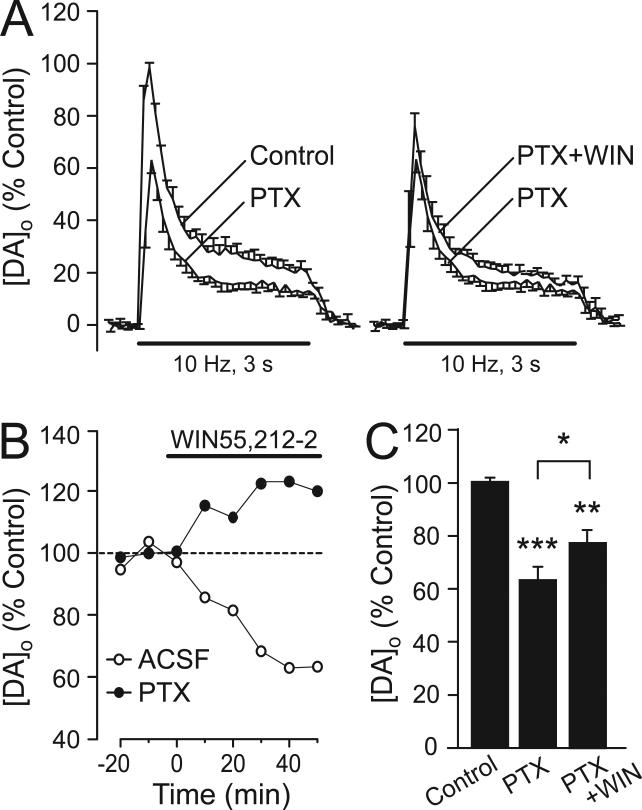
Local pulse-train stimulation elicits release not only of DA, but also of other transmitters, including GABA and glutamate. The effect of these concurrently released transmitters on evoked [DA]o can be revealed using receptor antagonists (Avshalumov et al., 2003). We noted that the suppression of pulse-train evoked [DA]o by WIN55,212-2 is similar to that seen when GABAA receptors (GABAARs) are blocked by picrotoxin (Avshalumov et al., 2003), which suggested that the effect on DA release might reflect inhibition of GABA release. To test this, we examined the effect of WIN55,212-2 (5 μM) when GABAARs were blocked by picrotoxin (100 μM; Avshalumov et al., 2003). As reported previously, picrotoxin alone decreased evoked [DA]o by 37 ± 5% (p < 0.001 vs. control; n = 5, Fig. 4A,C). In the continued presence of picrotoxin, the suppressing effect of WIN55,212-2 on evoked DA release was prevented, indicating a key role for GABA in mediating the effect of CB1R activation on DA release (Fig. 4). Interestingly, under these conditions, WIN caused a small but significant increase in evoked [DA]o, in contrast to the usual decrease (Fig. 4B,C) (p < 0.05, PTX vs. PTX + WIN; n = 5). This suggests that WIN55,212-2 also alters the release of at least one other modulatory transmitter, the influence of which was masked by the effect of GABA acting via GABAARs. Overall, these data confirm that the predominant effect of CB1R activation on striatal DA release is a consequence of GABA release inhibition.
Figure 4. CB1R-dependent suppression of DA release is mediated by GABA release inhibition.
A) Averaged evoked [DA]o records showing a significant decrease in pulse-train evoked [DA]o when GABAARs are blocked by pictrotoxin (PTX, 100 μM, n = 5) (left panel). The usual effect of WIN55,212-2 was prevented in the continued presence of PTX (right panel) (n = 5). B) Representative peak evoked [DA]o versus time profiles showing the effect of WIN55,212-2 (WIN; 5 μM) alone (open circles) and WIN applied in the presence of PTX (100 μM) (filled circles) unmasking an increase in evoked [DA]o with WIN when GABAARs were blocked. Baseline data were normalized to respective control conditions (ACSF or PTX; see Results). C) Comparison of mean peak evoked [DA]o in the presence of PTX, and PTX +WIN confirmed a slight, but significant increase in evoked [DA]o with WIN application in the presence PTX (***p < 0.001 control vs. PTX; *p < 0.05 PTX vs. PTX+WIN; n = 5); all means are normalized to control (ACSF alone).
H2O2 and ATP-sensitive K+ channels mediate CB1R-dependent suppression of DA release
Previous studies have shown that GABAA-receptor blockade increases activity-dependent production of the diffusible second messenger H2O2, which suppresses axonal DA release in striatum via opening of ATP-sensitive K+ (KATP) channels (Avshalumov et al., 2003; Avshalumov and Rice, 2003). To evaluate whether H2O2 is required for the suppression of evoked [DA]o by CB1R activation, we applied WIN55,212-2 (2 μM) in the presence of the H2O2 metabolizing enzyme catalase (500 U/mL). Catalase prevented the effect of WIN55,212-2 (p > 0.05; n = 4) (Fig. 5), indicating that the diffusible messenger H2O2 is essential for the effects of CB1R activation on DA release. In the presence of heat-inactivated catalase, WIN had its usual effect (p < 0.001; n = 3) (Fig. 5). We then tested whether KATP channels play a role in the mediation of CB1R-dependent suppression of DA release. When KATP channels were blocked either with tolbutamide (200 μM, n = 4) or glybenclamide (10 μM, n = 5), the effect of WIN55,212,2 (up to 5 μM) on evoked [DA]o was prevented (Fig. 6).
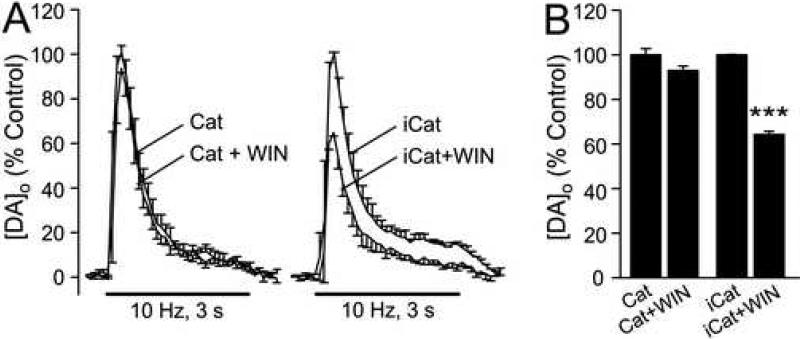
Figure 5. The effect of CB1R activation on evoked [DA]o is prevented by the H2O2-metabolizing enzyme catalase.
A) Averaged pulse-train evoked [DA]o records in the presence of the H2O2 metabolizing enzyme, catalase (Cat; 500 U/mL) and in catalase + WIN55,212-2 (WIN; 2 μM).an in heat-inactivated catalase (iCat) and iCat + WIN. B) Catalase prevented the effect of WIN (p > 0.05 Cat vs. Cat + WIN; n = 4), whereas the usual WIN-induced suppression of evoked [DA]o was seen in iCat (***p < 0.001 vs. same-site control; n = 3).
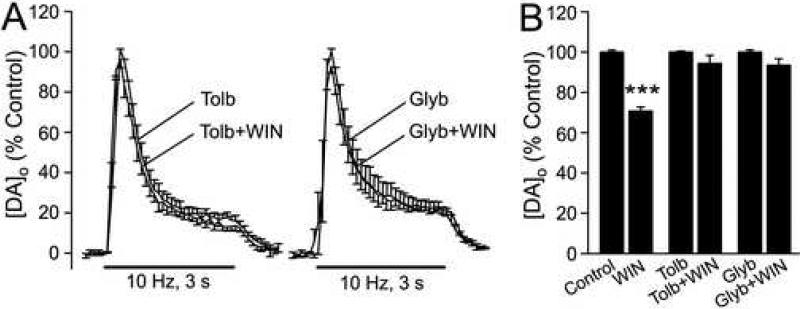
Figure 6. KATP-channel activation is required for CB1R-dependent suppression of DA release.
A) Averaged pulse-train evoked [DA]o records in the presence of a KATP channel blocker tolbutamide (Tolb, 200 μM, n = 4), or glybenclamide (Glyb, 10 μM, n = 5) and in Tolb or Glyb + WIN55,212-2 (up to 5 μM); KATP channel blockade prevented the effect of WIN on evoked [DA]o. B) Comparison of the effect of WIN on average peak evoked [DA]o under control conditions (***p < 0.001; n = 13), in tolbutamide (p > 0.05, n = 4), and in glybenclamide (p > 0.05, n = 5).
Discussion
The data presented here demonstrate that activation of CB1Rs within the dorsolateral striatum leads to inhibition of axonal DA release, independent of any effects in DA cell body regions. The lack of effect of WIN55,212-2 on single-pulse evoked DA release is consistent with the absence of CB1Rs on DA axons. This further implies that suppression of [DA]o evoked by pulse-train stimulation must be indirect. Indeed, the data indicate that modulation of striatal DA release is mediated predominantly by inhibition of GABA release, which leads to enhanced generation of the diffusible messenger H2O2, and consequent activation of KATP channels. This study therefore reveals that inhibition of DA release by CB1R activation in striatum involves a nonsynaptic mechanism, in which direct CB1-agonist-mediated inhibition of one transmitter, GABA, regulates release of another, DA, via generation of a diffusible messenger, H2O2. This is analogous to the mechanism of DA release regulation involving glutamate receptor activation and consequent increase in [DA]o via diffusible nitric oxide (NO), described previously (for review see Kiss and Vizi, 2001; Kiss et al., 2004).
CB1R activation inhibits axonal DA release in the striatum
Increasing evidence indicates that CB1R activation can inhibit transmitter release, including GABA release in the striatum (Szabo et al., 1998; Kőfalvi et al., 2005; Freiman et al., 2006). Inhibition of GABA release is mediated directly by presynaptic CB1Rs, with localization of CB1R protein on GABAergic terminals in rodent striatum (Julian et al., 2003; Fusco et al., 2004; Kőfalvi et al., 2005; Mátyás et al., 2006; Uchigashima et al., 2007) and coexpression of mRNAs for CB1Rs and the GABAergic markers parvalbumin (Marsicano and Lutz, 1999) and GAD67 (Hohmann and Herkenham, 2000). By contrast, CB1R localization on DA axons remains controversial. Although there is some evidence for a low level of CB1 expression on DA axons from double immunohistochemical labeling for CB1Rs and tyrosine hydroxylase (TH), the rate-limiting enzyme in DA synthesis (Wenger et al., 2003; Kőfalvi et al., 2005), other studies found no colocalization of CB1Rs and DAT protein (Uchigashima et al., 2007) or CB1Rs and TH mRNAs (Julian et al., 2003). Voltammetric studies, showing a lack of effect of WIN55,212-2 on single-pulse evoked [DA]o in striatal slices including data reported here, further support the absence of CB1Rs on DA axons in dorsal striatum (Szabo et al., 1999 and Fig. 1A).
As noted above, a change in evoked [DA]o can reflect a change in either DA release or in DAT-mediated DA uptake. Indeed, previous studies from Kiss et al. (1999) showed that NO increases basal [DA]o in striatum monitored using in vivo microdialysis. Importantly, this effect was prevented by a DAT inhibitor, showing that NO acted via DA uptake, rather than release. We used a similar rationale here to test whether CB1R activation inhibited DA release or enhanced DA uptake, by applying the WIN55,212-2 in the presence of the DAT inhibitor GBR 12909. DAT blockade did not alter the inhibitory effect of WIN (Fig. 1C,D), which is consistent with the lack of effect on DA uptake observed in vivo (Cheer et al., 2004) and in vitro (Kőfalvi et al., 2005). Although a recent chronoamperometric study suggested that WIN might decrease striatal DAT activity in vivo, this was not altered by CB1R blockade, indicating a non-receptor mediated effect (Price et al., 2007). We found no evidence for such an effect in the present studies, since the time course of single-pulse evoked [DA]o was unaltered by WIN55,212-2 (Fig. 1A). Overall, therefore, our data confirm that CB1R activation in the present studies indeed modulates DA release, not uptake.
CB1R-dependent inhibition of DA release requires GABA, H2O2 and KATP channels
How then does CB1R activation lead to DA release inhibition? We have reported previously that locally released glutamate and GABA in dorsolateral striatum can modulate DA release by modulating levels of the diffusible messenger H2O2 (Avshalumov et al., 2003; 2007). Based on the apparent absence of ionotropic glutamate or GABA receptors on DA axons, but abundant expression of these receptors on striatal medium spiny neurons (MSNs) (Bernard and Bolam, 1998; Chen et al., 1998; Fujiyama et al., 2000), we have proposed that modulatory H2O2 is generated in MSNs. Our working model involves a triad of DA, glutamate, and GABA synapses converging on MSN dendritic spines (Fig. 7, left panel) (Avshalumov et al., 2007). In this model, glutamatergic corticostriatal input activates MSNs via AMPA receptors, which leads to increased production of mitochondrial H2O2; H2O2 then diffuses to adjacent DA axons and activates KATP channels that inhibit DA release. Importantly, GABA acting at GABAARs opposes glutamate-dependent excitation of MSNs and consequent H2O2 generation (Fig. 7, left panel). When GABAARs are blocked by picrotoxin, therefore, H2O2 generation is enhanced, which causes a further inhibition of DA release that is prevented by catalase (Avshalumov et al. 2003).
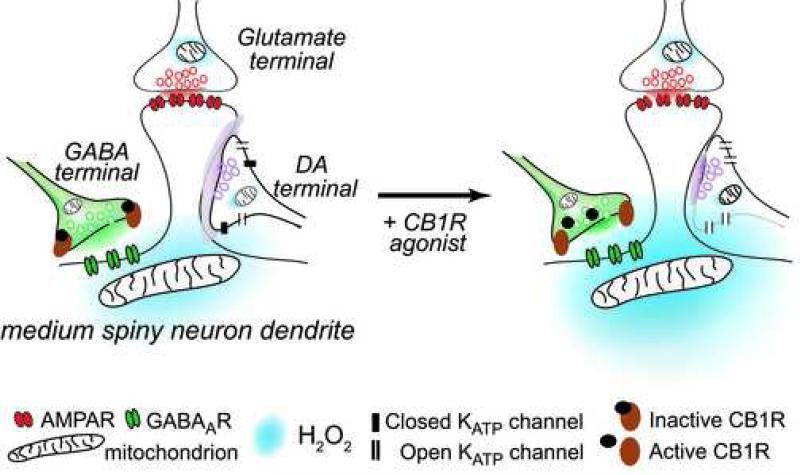
Figure 7. Schematic diagram showing the proposed mechanism for the effect of CB1R-dependent suppression of evoked DA release.
Glutamate, GABA, and DA synapses converging on a medium spiny neuron (MSN) dendrite. Left panel: AMPA receptor activation by glutamate on a dendritic spine results in increased mitochondrial H2O2 production, which diffuses to dopaminergic terminals to open KATP channels and thereby decrease DA release. This process is attenuated by activation of GABAARs. The amount of H2O2 produced is therefore determined by the net effect of glutamatergic excitation and GABAergic inhibition. Right panel: Activation of CB1Rs on GABAergic terminals by the agonist WIN55,212-2 results in the suppression of GABA release, and a consequent facilitation of H2O2 production. As a result, more KATP channels will be opened on dopaminergic terminals, causing suppression of evoked DA release.
Based on this model, one would predict that if activation of presynaptic CB1Rs on GABA terminals inhibits GABA release, this would mimic the effects of GABAAR blockade and lead to decreased DA release (Fig. 7, right panel). A decrease in local pulse-train evoked DA release was in fact seen when CB1Rs were activated by WIN55,212-2 (Fig. 1B,D). In further consonance with this model, the effect of WIN55,212-2 was prevented by catalase and by the KATP-channel blockers tolbutamide and glybenclamide, confirming a key role for H2O2 and KATP channel activation in DA release regulation by CB1Rs.
In addition to the predominant role of GABA and GABAARs in mediating effects of CB1R activation on striatal DA release, the increase in evoked [DA]o seen with WIN55,212-2 in the presence of the GABAAR antagonist picrotoxin (Fig. 4A) revealed the involvement of at least one other neurotransmitter system. The most likely candidates are ACh and glutamate, both of which are known to modulate striatal DA release. For example, tonically released ACh strongly regulates striatal DA release, such that a decrease in cholinergic tone markedly suppresses single-pulse evoked [DA]o (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004). Arguing against a significant influence of CB1R activation on ACh release in the present studies, however, is the lack of effect of WIN55,212-2 on single-pulse evoked [DA]o, which would be predicted to cause a marked decrease in evoked [DA]o. A lack of ACh involvement is further supported by previous studies showing no effect of WIN on evoked [3H]ACh release from striatal slices (Cadogan et al., 1997). Moreover, anatomical data suggest that localization of CB1Rs on striatal cholinergic interneurons is minimal. Although Fusco et al. (2004) reported low levels of colocalization of CB1Rs and choline acetyl-transferase (ChAT), Uchigashima et al. (2007) found no CB1Rs on terminals labeled with vesicular acetylcholine transporter (VAChT) and Hohmann and Herkenham (2000) found no colocalization of mRNAs for CB1R and the cholinergic markers VAChT or ChAT in striatum.
Glutamate also modulates striatal DA release: voltammetric studies show that blockade of glutamatergic AMPA receptors causes an increase in pulse-train evoked [DA]o in striatal slices via diffusible H2O2 (Avshalumov et al., 2003) (see Fig. 7, left panel). Importantly, anatomical studies have confirmed the presence of CB1Rs on glutamatergic terminals in striatum (Kőfalvi et al., 2005; Uchigashima et al., 2007). Moreover, WIN55,212-2 has been shown to suppress evoked [3H]glutamate from striatal slices (Kőfalvi et al., 2005) and to inhibit glutamate-dependent EPSCs in MSNs (e.g., Gerdeman and Lovinger, 2001; Huang et al., 2001; Kreitzer and Malenka, 2005; 2007). Regardless of whether glutamate or yet another transmitter might also influence striatal DA release during CB1R activation, the present studies show that the primary effect of CB1R activation on DA release is mediated by GABA.
Implications
The data reported here demonstrate that CB1R activation can indeed suppress DA release within striatum, independent of long feedback pathways. Dose-dependent variation in motor responses after administration of THC or synthetic CB1R agonists that range from increased motor activity to catalepsy (Gough and Olley, 1978; Navarro et al., 1993; Onaivi et al., 1996; Sañudo-Peña et al., 2000; Shi et al., 2005) might reflect in part the competing consequences of DA release suppression on the efficacy of the direct and indirect motor pathways, as well as CB1R-mediated effects in other basal ganglia structures (Albin et al., 1989). Furthermore, the present findings suggest a physiological substrate for the clinically relevant observations that CB1R agonists can attenuate motor tics in Tourette's syndrome (Muller-Wahl et al., 2003) and DOPA-induced dyskinesia a Parkinson's disease model (Sieradzan et al., 2001; Ferrer et al., 2003; Segovia et al., 2003; Venderova et al., 2004).
More generally, our findings reveal a mechanism by which CB1R activation can inhibit transmitter release from sites that lack presynaptic CB1Rs, thereby mediating nonsynaptic communication (see Fuxe and Agnati, 1991; Vizi, 2000 for reviews). Consequences of CB1R activation extend beyond synaptic boundaries by regulating GABA release, which in turn enhances production of the diffusible messenger H2O2, to inhibit DA release from adjacent axonal release sites. It is likely that endogenous cannabinoids can also act through these same pathways to inhibit DA release via suppression of GABA release and subsequent activation of H2O2-sensitive KATP channels. Although we found no evidence for endocannabinoid release with the stimulation protocol used in the present studies, previous work has shown that higher frequency stimulation paradigms do generate endocannabinoids, which induce synaptic plasticity in MSNs (Gerdeman et al., 2002; Ronesi et al., 2004; Kreitzer and Malenka, 2005, 2007). Appreciation of the indirect consequences of CB1R activation via GABA release inhibition and diffusible H2O2 may be important for interpretation of data from such studies.
Overall, our findings from motor striatum should help clarify the emerging roles of cannabinoid agents in the regulation of movement by the basal ganglia. Additionally, given the dorsoventral continuum of striatal circuitry (Voorn et al., 2004), the findings may also have implications for understanding regulation of DA release by CB1R activation in the nucleus accumbens, which receives mesolimbic DA input from the ventral tegmental area (van der Stelt and Di Marzo, 2005).
Acknowledgements
Supported by National Institutes of Health grants R01 NS036362 (MER) and F31 DA021065 (ZS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Rice ME. Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proceedings of National Academy of Sciences (USA) 2003;100:11729–11734. doi: 10.1073/pnas.1834314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Marshall SP, Pena DM, Rice ME. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. The Journal of Neuroscience. 2003;23:2744–2750. doi: 10.1523/JNEUROSCI.23-07-02744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Bao L, Patel JC, Rice ME. H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers. Antioxidants and Redox Signaling. 2007;9:219–231. doi: 10.1089/ars.2007.9.219. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. The Journal of Neuroscience. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Bolam JP. Subcellular and subsynaptic distribution of the NR1 subunit of the NMDA receptor in the neostriatum and globus pallidus of the rat: co-localization at synapses with the GluR2/3 subunit of the AMPA receptor. European Journal of Neuroscience. 1998;10:3721–3736. doi: 10.1046/j.1460-9568.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- Brown TM, Brotchie JM, Fitzjohn SM. Cannabinoids decrease corticostriatal synaptic transmission via an effect on glutamate uptake. The Journal of Neuroscience. 2003;23:11073–11077. doi: 10.1523/JNEUROSCI.23-35-11073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadogan AK, Alexander SP, Boyd EA, Kendall DA. Influence of cannabinoids on electrically evoked dopamine release and cyclic AMP generation in the rat striatum. Journal of Neurochemistry. 1997;69:1131–1137. doi: 10.1046/j.1471-4159.1997.69031131.x. [DOI] [PubMed] [Google Scholar]
- Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C, Parkin SG, Fox P, Wright D, Hobart J, Zajicek JP. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004;63:1245–1250. doi: 10.1212/01.wnl.0000140288.48796.8e. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PEM, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. The Journal of Neuroscience. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. The Journal of Neuroscience. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Veenman L, Knopp K, Yan Z, Medina L, Song WJ, Surmeier DJ, Reiner A. Evidence for the preferential localization of glutamate receptor-1 subunits of AMPA receptors to the dendritic spines of medium spiny neurons in rat striatum. Neuroscience. 1998;83:749–761. doi: 10.1016/s0306-4522(97)00452-1. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. European Journal of Neuroscience. 2003;18:1607–1614. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. The Journal of Physiology. 2006;575:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Fritschy JM, Stephenson FA, Bolam JP. Synaptic localization of GABAA receptor subunits in the striatum of the rat. Journal of Comparative Neurology. 2000;416:158–172. [PubMed] [Google Scholar]
- Fusco FR, Martonara A, Giampa C, de March Z, Farini D, D'Angelo V, Cancesario G, Bernardi G. Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse. 2004;53:159–167. doi: 10.1002/syn.20047. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, editors. Volume Transmission in the Brain. Raven Press; New York: 1991. [Google Scholar]
- Gerdeman GL, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. Journal of Neurophysiology. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nature Neuroscience. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Research. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Gough AL, Olley JE. Catalepsy induced by intrastriatal injections of Δ9-THC and 11-OH-Δ9-THC in the rat. Neuropharmacology. 1978;17:137–144. doi: 10.1016/0028-3908(78)90126-0. [DOI] [PubMed] [Google Scholar]
- Hájos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proceedings of National Academy of Sciences USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. The Journal of Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Research. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Hohman AG, Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. Journal of Physiology. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst DP, Lynch DL, Barnett-Norris J, Hyatt SM, Seltzman HH, Zhong M, Song ZH, Nie J, Lewis D, Reggio PH. N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Molecular Pharmacology. 2002;62:1274–1287. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–318. doi: 10.1016/s0306-4522(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Hennings EC, Zsilla G, Vizi ES. A possible role of nitric oxide in the regulation of dopamine transporter function in the striatum. Neurochemistry International. 1999;34:345–350. doi: 10.1016/s0197-0186(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends in Neurosciences. 2001;24:211–215. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Zsilla G, Vizi ES. Inhibitory effect of nitric oxide on dopamine transporters: interneuronal communication without receptors. Neurochemistry International. 2004;45:485–489. doi: 10.1016/j.neuint.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Kőfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlágh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunocytochemical and pharmacological analysis. The Journal of Neuroscience. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. The Journal of Neuroscience. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Localization of cannabinoid receptor in the human developing and adult basal ganglia. Higher levels in the striatonigral neurons. Neuroscience Letters. 1992;148:173–176. doi: 10.1016/0304-3940(92)90832-r. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. European Journal of Neuroscience. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Mátyás F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Millar J, Pelling CW. Improved methods for construction of carbon fibre electrodes for extracellular spike recording. Journal of Neuroscience Methods. 2001;110:1–8. doi: 10.1016/s0165-0270(01)00411-3. [DOI] [PubMed] [Google Scholar]
- Muller-Vahl KR, Schneider U, Prevedel H, Theloe K, Kolbe H, Daldrup T, Emrich HM. Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. Journal of Clinical Psychiatry. 2003;64:459–465. doi: 10.4088/jcp.v64n0417. [DOI] [PubMed] [Google Scholar]
- Navarro M, Fernandez-Ruiz JJ, de Miguel R, Hernandez ML, Cebeira M, Ramos JA. An acute dose of delta 9-tetrahydrocannabinol affects behavioral and neurochemical indices of mesolimbic dopaminergic activity. Behavioural Brain Research. 1993;57:37–46. doi: 10.1016/0166-4328(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annual Review of Neuroscience. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Chakrabarti A, Gwebu ET, Chaudhuri G. Neurobehavioral effects of Δ9-THC and cannabinoid (CB1) receptor gene expression in mice. Behavioural Brain Research. 1996;72:115–125. doi: 10.1016/0166-4328(96)00139-8. [DOI] [PubMed] [Google Scholar]
- Palij P, Bull DR, Sheehan MJ, Millar J, Stamford J, Kruk ZL, Humprey PPA. Presynaptic regulation of dopamine release in corpus striatum monitored in vitro in real time by fast cyclic voltammetry. Brain Research. 1990;509:172–174. doi: 10.1016/0006-8993(90)90329-a. [DOI] [PubMed] [Google Scholar]
- Price DA, Owens WA, Gould GG, Frazer A, Roberts JL, Daws LC, Giuffrida A. CB(1)-independent inhibition of dopamine transporter activity by cannabinoids in mouse dorsal striatum. Journal of Neurochemistry. 2007;101:389–396. doi: 10.1111/j.1471-4159.2006.04383.x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward related signals in striatum. Nature Neuroscience. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. Journal of Neurophysiology. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. The Journal of Neuroscience. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Lovinger DM. Induction of striatal long-term synaptic depression by moderate frequency activation of cortical afferents in rat. The Journal of Physiology. 2005;562:245–256. doi: 10.1113/jphysiol.2004.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker J,M. Activational role of cannabinoids on movement. European Journal of Pharmacology. 2000;391:269–274. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Segovia G, Mora F, Crossman AR, Brotchie JM. Effects of CB1 cannabinoid receptor modulating compounds on the hyperkinesias induced by high-dose levodopa in the reserpine-treated rat model of Parkinson's disease. Movement Disorders. 2003;18:138–149. doi: 10.1002/mds.10312. [DOI] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, Chang JY. Dose and behavioral context dependent inhibition of movement and basal ganglia neural activity by Δ9-tetrahydrocannabinol during spontaneous and treadmill locomotion tasks in rats. Synapse. 2005;55:1–16. doi: 10.1002/syn.20088. [DOI] [PubMed] [Google Scholar]
- Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson's disease: a pilot study. Neurology. 2001;57:2108–2111. doi: 10.1212/wnl.57.11.2108. [DOI] [PubMed] [Google Scholar]
- Slanina KA, Roberto M, Schweitzer P. Endocannabinoids restrict hippocampal long-term potentiation via CB1 receptors. Neuropharmacology. 2005;49:660–668. doi: 10.1016/j.neuropharm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Sulcova E, Mechoulam R, Fride E. Biphasic effects of anandamide. Pharmacology Biochemistry and Behavior. 1998;59:347–352. doi: 10.1016/s0091-3057(97)00422-x. [DOI] [PubMed] [Google Scholar]
- Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Szabo B, Muller T, Koch H. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. Journal of Neurochemistry. 1999;73:1084–1089. doi: 10.1046/j.1471-4159.1999.0731084.x. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. The Journal of Neuroscience. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. European Journal of Pharmacology. 2003;480:133–150. doi: 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Venderova K, Ruzicka E, Vorisek V, Visnovsky P. Survey on cannabis use in Parkinson's disease: subjective improvement of motor symptoms. Movement Disorders. 2004;19:1102–1106. doi: 10.1002/mds.20111. [DOI] [PubMed] [Google Scholar]
- Vizi E,S. Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacological Reviews. 2000;52:63–89. [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends in Neurosciences. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wenger T, Moldrich G, Fürst S. Neuromorphological background of cannabis addiction. Brain Research Bulletin. 2003;61:125–128. doi: 10.1016/s0361-9230(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature Neuroscience. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nature Neuroscience. 2001;4:1224–1249. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]