Abstract
The impaired function of CD8+ T cells is characteristic of hepatitis C virus (HCV) persistent infection. HCV core protein has been reported to inhibit CD8+ T cell responses. To determine the mechanism of the HCV core in suppressing Ag-specific CD8+ T cell responses, we generated a transgenic mouse, core(+) mice, where the expression of core protein is directed to the liver using the albumin promoter. Using a recombinant adenovirus to deliver Ag, we demonstrated that core(+) mice failed to clear adenovirus-LacZ (Ad-LacZ) infection in the liver. The effector function of LacZ-specific CD8+ T cells was particularly impaired in the livers of core(+) mice, with suppression of IFN-γ, TNF-α, and granzyme B production by CD8+ T cells. In addition, the impaired CD8+ T cell responses in core(+) mice were accompanied by the enhanced expression of the inhibitory receptor programmed death-1 (PD-1) by LacZ-specific CD8+ T cells and its ligand B7-H1 on liver dendritic cells following Ad-LacZ infection. Importantly, blockade of the PD-1/B7-H1 inhibitory pathway (using a B7-H1 blocking antibody) in core(+) mice enhanced effector function of CD8+ T cells and cleared Ad-LacZ-infection as compared with that in mice treated with control Ab. This suggests that the regulation of the PD-1/B7-H1 inhibitory pathway is crucial for HCV core-mediated impaired T cell responses and viral persistence in the liver. This also suggests that manipulation of the PD-1/B7-H1 pathway may be a potential immunotherapy to enhance effector T cell responses during persistent HCV infection.
The hepatitis C virus (HCV)3 infection in humans is efficient in establishing persistent infection. Early CD4+ and CD8+ T cell responses are crucial for controlling HCV infection (1, 2). However, the magnitude of T cell responses is decreased in chronic HCV patients as compared with that in acute HCV patients (3, 4). Particularly, CTL effector functions (e.g., IFN-γ production and cytolytic activity) are significantly impaired in chronic HCV patients compared with other persistent viral infections, including hepatitis B virus (5, 6). This suggests that HCV has developed effective means to evade and/or subvert host immunity, leading to the high incidence of viral persistence.
HCV core protein has been reported to suppress T cell responses (7, 8). HCV core-mediated inhibition of T cell responses can occur via either modulation of proinflammatory cytokines by APCs (i.e., monocytes and dendritic cells (DCs)) or direct effect on T cells (9, 10). Because the liver is the major site of HCV infection, it is crucial to understand the regulation of host immunity by HCV core in the liver compartment and the impact of HCV core-induced immune dysregulation in facilitating HCV persistence. The lack of a small animal model has hampered studies attempting to elucidate the mechanism of HCV core-mediated suppression of antiviral CD8+ T cell activity. Thus, our laboratory has generated a core transgenic mouse, core(+), in which HCV core is expressed behind the albumin (Alb) promoter. We used this model to study HCV core-mediated dysregulation of intrahepatic T cell responses.
Recently, it has been reported that expression of the coinhibitory molecule programmed death-1 (PD-1) determines CD8+ antiviral T cell exhaustion. In addition, liver-infiltrating lymphocytes in chronic HCV patients display an exhausted phenotype with increased PD-1 expression (11–13). PD-1 is a negative signaling molecule inhibiting T cell responses, and the expression of PD-1 can be induced on T cells, B cells, NK T cells, and monocytes (14, 15). In vitro studies have shown that PD-1 signaling can inhibit proliferation and cytokine production by both resting and previously activated CD8+ T cells. The ligands for PD-1 have been identified as B7-H1 (PD-L1) and B7-DC (PD-L2). B7-H1 is expressed in various tissues including its constitutive expression by liver sinusoidal endothelial cells and Kupffer cells. In comparison, the expression of B7-DC appears to be limited to DCs and macrophages. Notably, B7-H1 and B7-DC were initially reported to exhibit a dual effect (stimulatory or inhibitory) on T cell responses; recent reports indicate that B7-H1 plays a role in inhibiting T cell responses while B7-DC has stimulatory functions (16–18). Furthermore, B7-H1 plays a pivotal role in the accumulation and deletion of intrahepatic CD8+ T cells (19). Importantly, the PD-1/B7-H1 inhibitory pathway has been shown to be involved in the regulation of intrahepatic T cell responses (20, 21). However, it is currently not known how the PD-1/B7-H1 pathway contributes to the HCV core-mediated immune dysregulation leading to viral persistence.
In this study, we demonstrate that HCV core causes failed clearance of adenovirus-LacZ (Ad-LacZ) from the liver. In these mice, core protein impairs the generation of β-galactosidase (βgal)-specific CD8+ T cells, effector cytokine production, and cytolytic potential in the liver following Ad-LacZ infection. Notably, the expression levels of PD-1 by intrahepatic βgal-specific CD8+ T cells and B7-H1 on liver DCs were increased in core(+) mice following Ad-LacZ infection as compared with that in core(−) mice. Importantly, blockade of the PD-1/B7-H1 inhibitory pathway restored effective CD8+ T cell responses in core(+) mice and enhanced viral clearance. Taken together, the PD-1/B7-H1 inhibitory pathway plays an important role in HCV core-mediated impairment of CD8+ T cell function. This suggests that HCV core may contribute to the impaired antiviral CD8+ T cell activity observed in chronic HCV patients via increased PD-1/B7-H1 expression.
Materials and Methods
Generation of transgenic core(+) mice
To generate HCV core transgenic mice, a fertilized oocyte at the single-cell stage, prepared from C57BL/6 (H-2b) × CBA (H-2k) mice, was microinjected with the transgene construct as shown in Fig. 1A. Candidate core(+) mice containing the core gene of the Hutchinson strain (genotype 1a) of HCV were analyzed by PCR of tail DNAs using core-specific primers, i.e., a sense primer (5′-AAGAAAAACCAAACGTAACACCA-3′) binding to nucleotides 24–46 and an antisense primer (5′-CCAGCTAGGC CGAGAGCCACG-3′) binding to nucleotides 301–321. The founder mice were backcrossed with C57BL/6 (H-2b) mice (Taconic Farms) for five generations. Transgenic mice expressing HCV core protein under the control of the Alb promoter were bred at the animal facilities of the University of Virginia (Charlottesville, VA). Progeny tail tissue samples were screened for the presence of HCV core DNA by PCR with HCV core-specific primers. The presence and absence of core expression in transgenic mice are designated as core(+) and core(−), respectively. All mice were bred in a pathogen-free facility and tested routinely for mouse hepatitis virus and other pathogens. All mice were handled according to protocols approved by the University of Virginia Institutional Animal Care and Use Committee.
FIGURE 1.
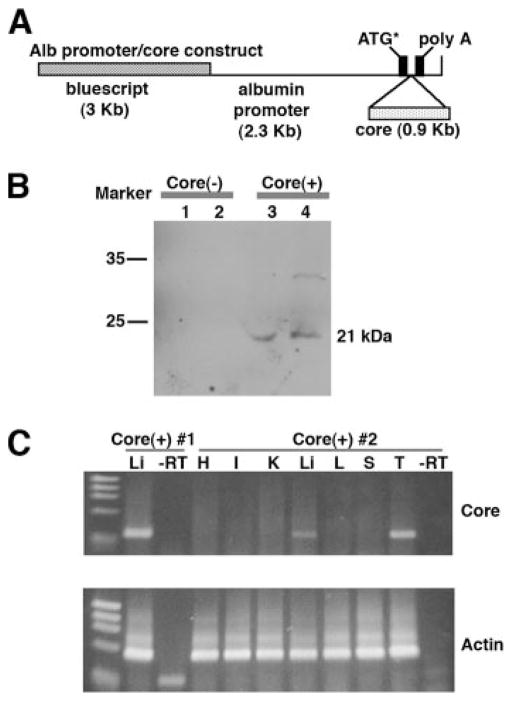
Intrahepatic expression of HCV core by transgenic mice. A, Diagram of the HCV core(+) transgene construct. The HCV core gene was inserted downstream of the Alb promoter. The dotted box indicates cDNA encoding the HCV core protein. B, Expression of core protein in core(+) mice. Liver samples from core(+) and core(−) mice were prepared for Western blot analysis using anti-core Ab. C, Liver-specific expression of core mRNA. Various tissues harvested from core(+) mice were used to determine the mRNA expression of the core gene by RT-PCR. As a control, reverse transcriptase was omitted from the indicated liver tissue samples (−RT). H, Heart; I, intestine; K, kidney; Li, liver; L, lung; S, spleen; T, thymus.
Ad-LacZ recombinant adenovirus
Recombinant adenovirus expressing the βgal protein under the control of the human CMV promoter (Ad-LacZ) and lacking E1 and E3 genes was generously provided by Dr. Greg Helm (University of Virginia). Ad-LacZ was propagated in 293A cells and purified by double cesium chloride gradient. Mice were injected i.v. with 5 × 108 PFU of Ad-LacZ.
Detection of HCV core mRNA
RNA was extracted from tissues (liver, heart, intestine, kidney, lung, spleen, and thymus) with TRIzol reagent as recommended by the manufacturer. After RNA was treated with RNase-free DNase I, reverse transcription of total RNAs was conducted. PCR for HCV core and β-actin was performed. As a control, reverse transcriptase was omitted from the indicated liver samples.
Western blot analysis for core protein
Liver samples from core(+) and core(−) mice were perfused with 1× PBS, flash frozen in liquid nitrogen, and then homogenized in 1 × x lysis buffer (Cell Signaling Technology) supplemented with Complete protease inhibitors (Roche). Liver lysates were boiled and then centrifuged at 10,000 × g at 4°C for 10 min. Equivalent amounts of lysates were subjected to SDS-PAGE separation and then transferred to an Immobilon-P polyvinylidene difluoride membrane (Millipore). Western blot analysis was performed using a polyclonal rabbit anti-core Ab that was generated by QED Bioscience. HRP-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories) and Super Signal West Pico chemiluminescent substrate (Pierce) were used for chemiluminescent detection.
Isolation of liver leukocytes and splenocytes
Intrahepatic lymphocytes were isolated from livers as described previously (22). Briefly, the liver was perfused with PBS via the portal vein and the median lobe was taken for histology. The rest of the liver was perfused with PBS plus 0.05% collagenase (Sigma-Aldrich) and then washed with IMDM supplemented with 10% newborn calf serum. The liver sections were finely minced and further digested with PBS plus 0.05% collagenase. Mononuclear cells were purified by Nycodenz gradient centrifugation. Splenocytes were prepared by mechanical disruption and isolation over a Ficoll gradient.
Ab staining and flow cytometric analysis
A PE-labeled H2-Kb βgal tetramer (ICPMYARV) was provided by the Tetramer Core Facility of the National Institutes of Health (Bethesda, MD). The following reagents were used for surface and intracellular staining: anti-CD8 allophycocyanin (clone 53-6.7), anti-PD-1 FITC (clone J43), anti-B7-H1 PE (clone MIH5), anti-CD8 FITC (clone 53-6.7), anti-IFN-γ PE (clone XMG1.2), and anti-TNF-α PE (clone MP6-XT22); all were purchased from eBioscience. The anti-granzyme B (clone GB12) Ab was purchased from Caltag Laboratories. For the cell surface labeling experiment, 2 × 106 cells were incubated with the corresponding Abs and tetramer for 30 min at 4°C in staining buffer (PBS with 1% FBS and 0.1% NaN3). After staining, cells were fixed in 1% formaldehyde for 10 min at room temperature. For granzyme B staining, freshly isolated liver lymphocytes were surface stained as described above with anti-CD8 and βgal tetramer. The cells were then stained for intracellular granzyme B using the Cytofix/Cytoperm kit (BD Bioscience) according to the manufacturer’s instructions. For intracellular cytokine staining, 2 × 106 cells were incubated for 5 h in IMDM supplemented with 10% FBS, 10 U/ml penicillin G, 2 mM L-glutamine, 0.05% 2-ME, 500 ng/ml ionomycin, 5 ng/ml PMA, and 1 μg/ml brefeldin-A (BD Biosciences). After incubation, the cells were surface labeled with anti-CD8 as described earlier, followed by intracellular staining for IFN-γ and TNF-α. Flow cytometry data for each of the experiments were acquired using BD FACSCaliber and BD FACSCanto devices (BD Immunocytometry Systems). Results were analyzed using FlowJo software (Tree Star).
LacZ staining
Liver cryosections were fixed with 0.2% glutaraldehyde for 10 min at room temperature and then incubated with a LacZ staining solution containing 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal; Roche), 35 mM potassium ferrocyanide (Sigma-Aldrich), 35 mM potassium ferricyanide (Sigma-Aldrich), 2 mM MgCl2 (Sigma-Aldrich), 0.02% Nonidet P-40 (Calbiochem-Novabiochem), and 0.01% sodium deoxycholate (Sigma-Aldrich) for 2 h at 37°C. Next, sections were counterstained with nuclear fast red. Quantification of LacZ staining was performed with ImageJ software. Briefly, five random ×40 images from the same tissue section were acquired and the percentage of the total area staining positive was calculated.
B7-H1 blocking Ab treatment
The blocking properties of functional grade-purified anti-mouse B7-H1 (clone MIH5) mAb have been reported recently (23). Anti-mouse B7-H1 (clone MIH5) mAb and control rat IgG2a (clone eBR2a) were purchased from eBioscience. Core(+) mice were infected with 5 × 108 PFU of Ad-LacZ i.v. On days 8 and 11 postinfection (p.i.) mice received either 200 μg of anti-mouse B7-H1 or control Ab i.p. On day 14 p.i. Ad-LacZ clearance was determined using LacZ staining and liver lymphocyte isolation was performed for flow cytometry analysis as mentioned earlier.
Immunohistochemistry
For the detection of CD3 and Ki67, liver tissue samples were fixed in 10% buffered formalin solution overnight and then embedded in paraffin. To stain for CD3, tissue was permeabilized with 1 mM EDTA (pH 8.0) and then blocked in 10% donkey serum. The liver tissue was stained overnight at 4°C with goat anti-mouse CD3 (Santa Cruz Biotechnology). The number of CD3+ T cells in the periportal and lobular regions of the liver was determined by counting positive leukocytes in five random sections of each region at ×400 magnification. Ag retrieval was performed with 10 mM citrate buffer for the Ki67 staining. The tissue was incubated overnight at 4°C with rat anti-mouse Ki67 (DakoCytomation). The number of lymphocytes that express Ki67 was determined by counting Ki67 positive cells that morphologically resemble lymphocytes in five random sections of each region at ×400 magnification.
Statistical analysis
Student’s t tests were used to evaluate the significance of the differences. A value of p < 0.05 was regarded as statistically significant.
Results
Failure of CD8+ T cell-dependent clearance of Ad-LacZ infection in the liver of HCV core(+) transgenic mice
HCV core protein has been reported to exhibit an immunomodulatory function suppressing host immunity (24). To examine the underlying mechanism of HCV core protein in the alteration of host immune responses to viral infection in the liver compartment, we established a HCV core transgenic line, core(+) mice, where the expression of core protein is directed to the liver. To this end, a cDNA encoding core gene was inserted behind the Alb promoter (Fig. 1A) to localize HCV core expression to hepatocytes, the major sites of HCV infection and replication. After the presence of core gene was identified by PCR, these mice were then back-crossed onto C57BL/6 mice for at least five generations. To determine the expression of HCV core protein in the livers of core(+) mice, Western blot analysis was performed using anti-core polyclonal Ab. Cell lysates were prepared from core(+) mice and core(−) littermates. As shown in Fig. 1B, the core protein (21 kDa) was detected in core(+) mice but not in core(−) mice. Interestingly, the expression of HCV core protein in the livers of core(+) mice increases appreciably following Ad-LacZ infection (data not shown). The expression of HCV core mRNA in liver tissue was further confirmed by performing RT-PCR using core-specific primers. The expression of core-specific mRNA was detected in the liver and thymus but not in other tissues (kidney, lung, intestine, spleen, or heart) (Fig. 1C).
To determine whether the expression of HCV core protein by hepatocytes is capable of altering host immunity, core(+) mice and their core(−) littermate mice were infected with Ad-LacZ i.v. at an infectious dose of 5 × 108 PFU per mouse. The recombinant adenovirus (rAd) system (adenovirus 5, replication deficient) is advantageous to target the expression of Ag to the liver because i.v. injection of rAd predominantly delivers the expression of Ag to the liver (25). In addition, rAd expressing LacZ allows for monitoring the alteration of Ag-specific CD8+ T cell responses (i.e., βgal-specific CD8+ T cells) by HCV core. On days 7, 14, and 21 p.i., the clearance of Ad-LacZ infected cells was determined by performing LacZ staining of liver sections harvested from Ad-LacZ infected core(+) mice. Because LacZ encodes for βgal, which produces a blue product following conversion of X-gal substrate, the level of Ad-LacZ-infected cells was quantified by performing LacZ staining of liver tissues using X-gal as a substrate.
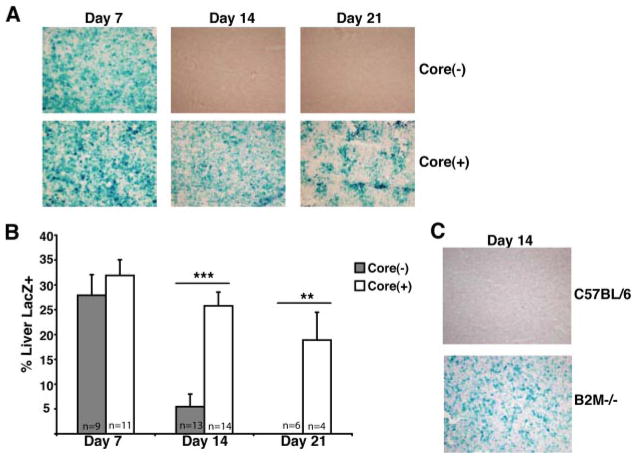
To this end, core(+) mice and their core(−) littermates were infected i.v. with 5 × 108 PFU of Ad-LacZ. Liver tissues were collected on days 7, 14, and 21 p.i. and LacZ staining on frozen liver sections was performed to evaluate HCV core-mediated modulation of Ag persistence. We did not observe a discernible difference in the clearance of Ad-LacZ infection between core(+) and core(−) mice at 7 days p.i. (Fig. 2A). However, core(−) mice were able to successfully clear the majority of Ag by day 14. In contrast, core(+) mice were impaired in their ability to clear LacZ Ag by day 14. Moreover, only modest clearance of Ad-LacZ infection between days 7 and 14 was observed in core(+) mice. This is in contrast to the robust clearance of LacZ Ag exhibited by core(−) mice. Interestingly, LacZ Ag persisted at significant levels in the livers of core(+) mice out to day 21 p.i., suggesting that the expression of core protein in the liver dysregulates effective intra-hepatic immune responses.
FIGURE 2.
HCV core-mediated dysregulation of Ad-LacZ clearance. A, Core(+) and core(−) mice were infected with 5 × 108 PFU of Ad-LacZ i.v. and mice were analyzed for clearance of βgal Ag on days 7, 14, and 21 p.i. Frozen liver sections were analyzed by LacZ staining (original magnification ×40). The displayed images are representative of at least three independent experiments. B, The percentage of liver area positive for LacZ staining was quantified using ImageJ software. The bars indicate the average percentage of liver area that scored positive for LacZ staining (±SEM). Data were pooled from separate experiments and the total number of mice analyzed for LacZ staining for each group is indicated in the bars of the graph (*, p < 0.05; **, p < 0.005;***, p < 0.0001). C, CD8+ T cell-dependent clearance of Ad-LacZ-infected hepatocytes. β2M−/− and control mice were infected with 5 × 108 PFU of Ad-LacZ i.v. The liver tissues were harvested on day 14 p.i. and used for LacZ staining.
These observations were further confirmed by examining the clearance of Ad-LacZ infection in large numbers of core(+) and core(−) mice following Ad-LacZ infection. LacZ-stained liver tissue slides were analyzed by ImageJ analysis and revealed an impaired clearance of Ad-LacZ infection in core(+) mice (Fig. 2B). These results suggest that the expression of HCV core in hepatocytes may be responsible for suppressing and/or delaying the host immune responses to viral infection.
Cell-mediated immune responses are critical to successful clearance of adenovirus from the liver (26, 27). To confirm the role of CD8+ T cell responses in clearing Ad-LacZ infection, we used Rag1−/− (deficient in T and B cells) and β2-microglobulin (β2M)−/− (deficient in CD8+ T cells) mice. To this end, Rag1−/− and β2M−/− mice were infected i.v. with 5 × 108 PFU of Ad-LacZ and the clearance of Ad-LacZ infection was examined. As expected, the expression of LacZ Ag was maintained in the livers of Rag1−/− mice on days 7 and 14 after Ad-LacZ infection (data not shown). Importantly, β2M−/− mice infected i.v. with Ad-LacZ also failed to clear Ad-LacZ infected cells by day 14 p.i. In comparison, C57BL/6 control mice infected with Ad-LacZ successfully cleared Ad-LacZ infection (Fig. 2C). This confirms that CD8+ T cells are crucial for the clearance of Ad-LacZ infection.
The impaired Ag-specific CD8+ T cell responses are restricted to the liver, but not to the spleen, in core(+) mice
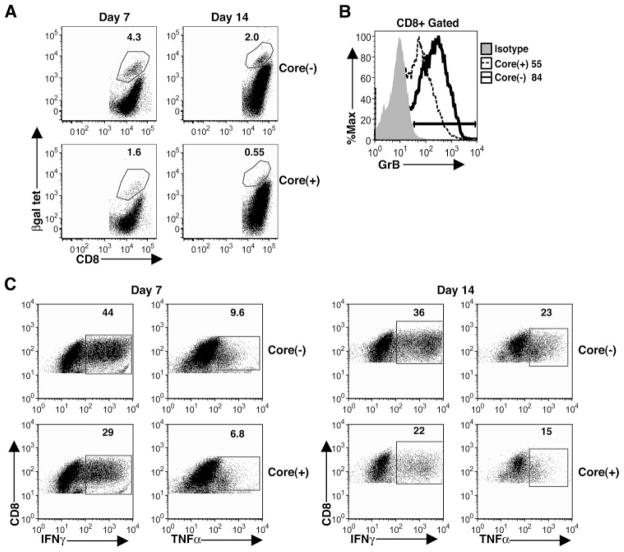
To determine whether impaired CD8+ T cell responses could account for the failure to clear Ad-lacZ infection in core(+) mice, we examined the magnitude of βgal-specific CD8+ T cell responses and their effector function in the livers of core(+) mice. Core(+) and core(−) mice were infected i.v. with 5 × 108 PFU of Ad-LacZ, and liver lymphocytes were isolated on days 7 and 14 p.i. to examine the impact of the core on CD8+ T cell responses, which have been shown to be critical for the control of Ad-LacZ infection. As shown in Fig. 3A, the frequency of βgal-specific CD8+ T cells staining positive for class I MHC H2-Kb restricted βgal tetramer (ICPMYARV) in core(+) mice was 2-fold lower at day 7 and 3-fold lower at day 14 than that in core(−) mice. In addition, the percentage of CD8+ T cells expressing the cytolytic protease granzyme B was significantly diminished in core(+) mice at day 14 (Fig. 3B). IFN-γ and TNF-α production by intrahepatic CD8+ T cells in core(+) mice on days 7 and 14 p.i. was also suppressed as compared with core(−) mice following PMA/ionomycin stimulation (Fig. 3C). Interestingly, we have also observed impaired effector cytokine production by intrahepatic CD8+ T cells in core(+) mice during vaccinia virus infection (data not shown). This suggests that HCV core-expressing hepatocytes significantly impair antiviral effector CD8+ T cell responses in the liver.
FIGURE 3.
Impaired CD8+ T cell responses in the livers of core(+) mice. Core(+) and core(−) mice were infected with 5 × 108 PFU of Ad-LacZ i.v. A, βgal-specific CD8+ T cells were analyzed from the livers of Ad-LacZ-infected core(+) and core(−) mice on days 7 and 14 p.i. using MHC class I H2-Kb βgal tetramer (tet). The percentages of CD8+ T cells that are βgal tetramer+ are indicated. Data show one representative mouse per group (n = 3). Results are representative of three independent experiments for the day 7 data and four independent experiments for the day 14 data. B, Granzyme B (GrB) expression by intrahepatic CD8+ T cells 14 days p.i. The numbers adjacent to the histogram legends represent the percentage of liver CD8+ T cells expressing granzyme B. The results are representative of four separate experiments with at least three mice per experimental group. C, Effector cytokine production (IFN-γ and TNF-α) by intrahepatic CD8+ T cells on day 7 and 14 p.i. with Ad-LacZ. Liver lymphocytes were restimulated with PMA/ionomycin for 5 h and intracellular IFN-γ and TNF-α production was determined for the CD8+ T cell population. The numbers in the dot plots represent the percentage of CD8+ T cells that are positive for the corresponding cytokine. Data show one representative mouse per group (n = 3). The results are representative of two independent experiments.
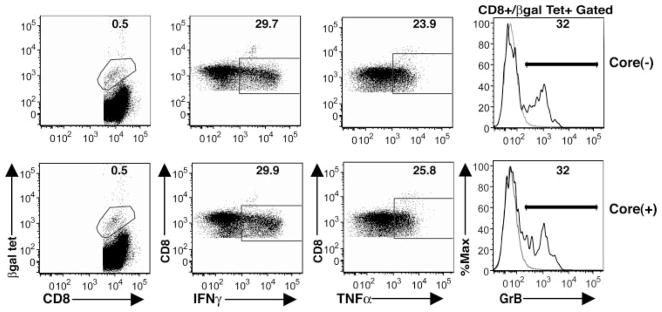
To determine whether core-mediated impairment of CD8+ T cell responses is restricted to the liver compartment (where core protein is expressed), splenic CD8+ T cell responses were analyzed in core(+) and core(−) mice at day 14 following Ad-LacZ infection. Core expression in the liver did not alter the magnitude of the splenic βgal-specific CD8+ T cell population (Fig. 4). The percentage of βgal tetramer+ CD8+ T cells in the spleen was comparable between core(+) and core(−) mice at day 14 p.i. In addition, the spleen compartment of core(+) mice did not exhibit decreased levels of effector cytokine production (IFN-γ and TNF-β) or granzyme B production by CD8+ T cells. This suggests that CD8+ T cell dysregulation in core(+) mice is restricted to the liver compartment where HCV core is expressed. These findings also suggest that core-mediated dysregulation of CD8+ T cell responses occurs at the effector phase and not during the initiation of the immune response or T cell induction phase.
FIGURE 4.
No difference in splenic βgal-specific CD8+ T cell responses between core(+) and core(−) mice. Core(+) mice and core(−) littermates were injected i.v. with 5 × 108 PFU of Ad-LacZ. On day 14 p.i., lymphocytes from spleen tissues were analyzed for the magnitude of βgal-specific CD8+ T cells and their effector function as described in the legend of Fig. 3. Data show one representative mouse per group (n = 3). The results are representative of two independent experiments. Tet, Tetramer.
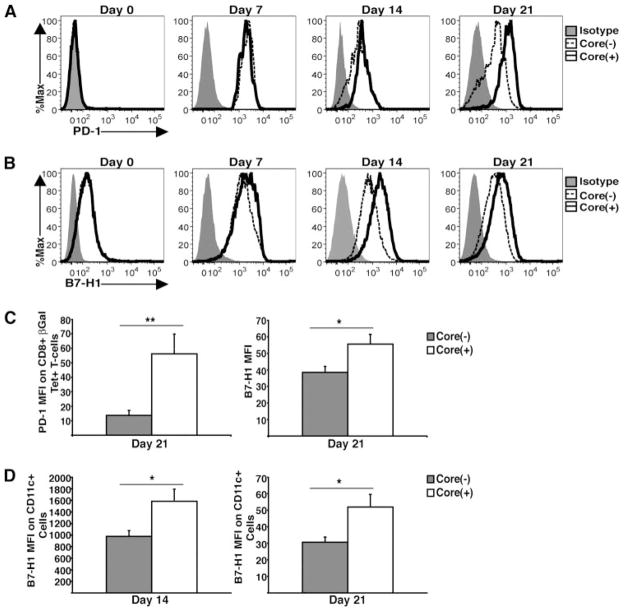
PD-1 and B7-H1 expression remains up-regulated during Ad-LacZ infection in core(+) mice
The PD-1/B7-H1 inhibitory pathway has been reported to play an important role in the dysregulation of effector T cell responses during viral infection (28). For instance, the expression of PD-1 is increased on functionally exhausted CD8+ T cells during chronic viral infection. In addition, B7-H1 regulates the accumulation and deletion of activated CD8+ T cells in the liver. To assess the role of the PD-1/B7-H1 inhibitory pathway in core-mediated dysregulation of antiviral CD8+ T cell responses, we examined the kinetics of expression for both PD-1 and B7-H1 in the livers of core(+) and core(−) mice following Ad-LacZ infection (Fig. 5). In naive mice, PD-1 is not expressed by CD8+ T cells (Fig. 5A, Day 0) while B7-H1 is constitutively expressed by liver DCs. Interestingly, there was not a basal difference in the expression of B7-H1 by bulk liver cells (data not shown) or liver DCs observed between core(+) and core(−) mice (Fig. 5B, Day 0). During early time points after Ad-LacZ infection (day 7), the level of PD-1 expression by βgal-specific CD8+ T cells was similar between core(+) and core(−) mice. At day 14 p.i., PD-1 expression by βgal-specific CD8+ T cells in core(−) mice begins to decrease. Notably, the down-regulation of PD-1 expression seen in core(−) mice corresponds with successful clearance of Ad-LacZ Ag. In contrast, the expression of PD-1 by βgal-specific T cells remained elevated out to day 21 p.i. in core(+) mice.
FIGURE 5.
Kinetics of PD-1 and B7-H1 expression in the livers of core(+) and core(−) mice. Core(+) and core(−) mice were infected with 5 × 108 PFU of Ad-LacZ i.v. A, Kinetics of PD-1 expression by βgal-specific CD8+ T cells on intrahepatic lymphocytes from core(+) and core(−) mice infected with Ad-LacZ. Representative data of one mouse from a litter of core(+) and core(−) mice are shown. Data are representative of at least two independent experiments. For day 0 the expression of PD-1 was assessed on the bulk CD8+ T cell population. B, Kinetics of B7-H1 expression by liver DCs from core(+) and core(−) mice infected with Ad-LacZ. Representative data of one mouse from a litter of core(+) and core(−) mice are shown. C, The MFI values of PD-1 expressed on βgal-specific CD8+ T cells and B7-H1 expressed by bulk liver cells from core(+) and core(−) mice on day 21 following Ad-LacZ infection were pooled from two independent experiments (±SEM) (*, p < 0.05; **, p < 0.005). The bar graph on the left represents the MFI of PD-1 expressed on βgal-specific CD8+ T cells from core(+) and core(−) mice on day 21 following Ad-LacZ infection. The bar graph on the right represents the MFI of B7-H1 expressed by bulk liver cells on day 21 p.i. D, The MFI expression of B7-H1 (±SEM) by intrahepatic CD11c+ leukocytes from core(+) and core(−) mice on days 14 and 21 p.i. (n = 3). Data are representative of four independent experiments for the day 14 data and two independent experiments for the day 21 data (*, p < 0.05).
Interestingly, higher levels of B7-H1 expression by DCs were observed in the livers of core(+) mice at various time points (days 7, 14, and 21) following Ad-LacZ infection (Fig. 5B). The elevated expression of PD-1 by βgal-specific CD8+ T cells and B7-H1 by bulk liver cells seen in the core(+) mice at day 21 p.i. was statistically significant when mean fluorescence intensity (MFI) values from two independent experiments were pooled (Fig. 5C). DCs have been shown to play an important role in shaping CD8+ T cell responses at the site of the infection. To determine whether B7-H1 expression by DCs is altered in core(+) mice, the intensity of B7-H1 expressed by CD11c+ cells was analyzed at days 14 and 21 p.i. We observed elevated expression of B7-H1 by DCs in core(+) mice at both time points (Fig. 5D). Taken together, enhanced expression of PD-1 by virus-specific CD8+ T cells and B7-H1 in the livers of core(+) mice during Ad-LacZ infection suggests a potential role for the PD-1/B7-H1 inhibitory pathway in the core-mediated dysregulation of effector CD8+ T cell responses and the Ag persistence observed in core(+) mice.
Blockade of the PD-1/B7-H1 inhibitory pathway enhances viral clearance and CD8+ T cell responses in core(+) mice
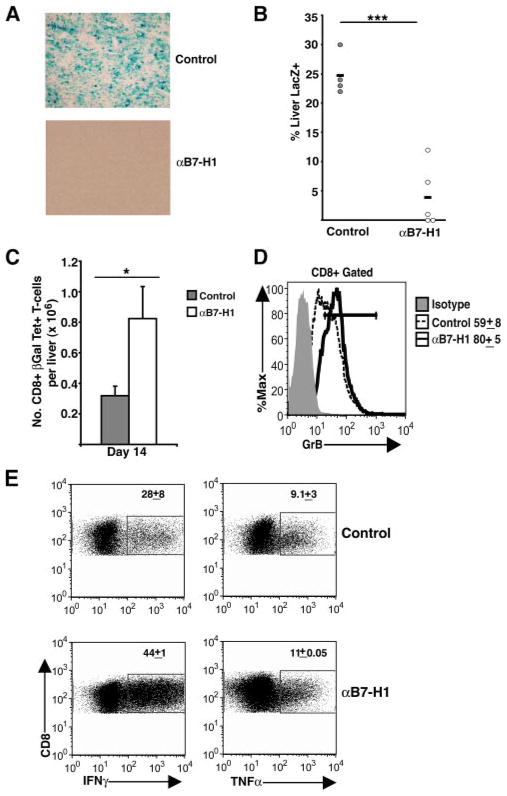
To assess the role of the PD-1/B7-H1 inhibitory pathway in core-mediated dysregulation of effector T cell responses and Ag persistence, we treated Ad-LacZ infected core(+) mice with either B7-H1 blocking Ab or control rat IgG2a Ab on days 8 and 11 p.i. (Fig. 6). Viral Ag clearance was monitored by performing LacZ staining on frozen liver sections isolated on day 14 p.i. (Fig. 6A). Treatment of core(+) mice with B7-H1 blocking Ab reduced viral Ag load by >5-fold in core(+) mice when compared with core(+) mice treated with control Ab (Fig. 6B). Strikingly, B7-H1 blockade completely cleared LacZ Ag in two of the five core(+) mice. Furthermore, treatment of core(+) mice with B7-H1 blockade significantly increased the numbers of intrahepatic βgal-specific T cells and enhanced their effector function (Fig. 6, C–E). Intrahepatic CD8+ T cells showed significantly up-regulated expression of granzyme B (Fig. 6D) and enhanced production of IFN-γ with a slight increase in TNF-α production (Fig. 6E) following anti-B7-H1 treatment of core(+) mice. In contrast, only a slight increase in CD8+ T cell effector response was detected in core(−) mice treated with anti-B7-H1 Ab (data not shown).
FIGURE 6.
Blockade of the PD-1/B7H1 inhibitory pathway increases Ad-LacZ clearance and CD8+ T cell function in core(+) mice. Core(+) mice were infected with 5 × 108 PFU of Ad-LacZ i.v. and then received either anti-B7-H1 (αB7-H1; 200 μg i.p.) or control (rat IgG2a) treatment on days 8 and 11 p.i. Liver tissues were harvested on day 14 p.i. A, LacZ staining was performed on the liver sections isolated 14 days p.i. The displayed images are representative of two independent experiments. B, The percentage of liver area positive for LacZ staining was quantified using ImageJ software (***, p < 0.0001). Results are pooled from two independent experiments. C, βgal-specific CD8+ T cell numbers in the liver were analyzed on day 14 (±SEM). Data pooled from two independent experiments are represented (*, p < 0.05). D, Granzyme B (GrB) expression by intrahepatic CD8+ T cells. The numbers adjacent to the histogram represent the percentage of liver CD8+ T cells expressing granzyme B (±SEM). Represented data was pooled from two independent experiments. E, Effector cytokine production (IFN-γ and TNF-α) by intrahepatic CD8+ T cells 14 days p.i. with Ad-LacZ. Following PMA/ionomycin stimulation, intracellular IFN-γ and TNF-α production was determined for the CD8+ T cell population as described in the legend of Fig. 3C. The numbers in the dot plots represent the percentage of CD8+ T cells that are positive for the corresponding cytokine (±SEM). Data are representative of two independent experiments.
Blockade of the PD-1/B7-H1 inhibitory pathway in core(+) mice enhances T cell proliferation and infiltration to the lobular region of the liver
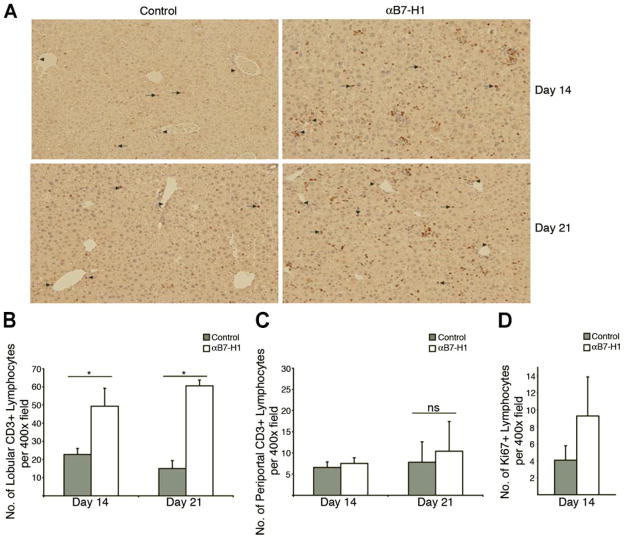
A crucial factor involved in the successful control of intrahepatic infections is the effective extravasation and infiltration of virus-specific CD8+ T cells into the lobular region of the liver (29–32). The slow flow of the liver compartment allows for deletion of virus-specific CD8+ T cells by liver sinusoidal endothelial cells as they migrate into the liver (33–36). To examine the effect of anti-B7-H1 treatment on the infiltration of T cells into the liver, we analyzed the localization of CD3+ cells by performing immunohistochemistry on liver tissues harvested from core(+) mice treated with either B7-H1 blocking Ab or control Ab (Fig. 7A). Importantly, we observed a significant increase in the total number of T cells that are able to infiltrate into the lobular areas of the liver of B7-H1-treated core(+) mice (Fig. 7B). However, there was only a negligible difference in the number of T cells in the periportal regions between the B7-H1 blockade treatment group and the control group (Fig. 7C). Interestingly, anti-B7-H1 blockade treatment was still able to enhance T cell infiltration into the lobular regions of infected livers when it was administered to the mice at later time points (days 14 and 17) (data not shown).
FIGURE 7.
Blockade of the PD-1/B7-H1 inhibitory pathway increases the number intrahepatic T cells infiltrating into lobular regions of core(+) mice. Core(+) mice were infected with 5 × 108 PFU of Ad-LacZ i.v. and then received either anti-B7-H1 (αB7-H1; 200 μg i.p.) or control (rat IgG2a) treatment on days 8 and 11 p.i. and liver tissue was harvested on day 14 p.i. The day 21 mice received either anti-B7-H1 blocking treatment or control Ab as mentioned above on days 14 and 17 p.i. Liver tissue was harvested either on day 14 or day 21 p.i. A, Harvested liver tissue was analyzed by immunostaining with anti-CD3 Abs (original magnification ×100). Arrows indicate the infiltration of T cells into lobular regions. T cells residing in the periportal region of the liver are designated with arrowheads. Sections are representative of two independent experiments. B and C, Quantification of lobular (B) and periportal T cells (C) in the liver of the core(+) mice. The number of CD3+ T cells in the periportal and lobular regions of the liver was determined by counting positive leukocytes in five random sections of each region at ×400 magnificaiton. Mean numbers of T cells in each compartment are indicated (±SEM). Data are representative of two independent experiments (*, p < 0.05; ns, not statistically significant). D, Liver tissue was harvested from Ad-LacZ infected core(+) mice that received either anti-B7-H1 or control Ab treatment as mentioned above and sections were stained for Ki67 to analyze leukocyte proliferation in the liver. The number of Ki67+ leukocytes was quantified by counting positive leukocytes in five random sections of each region at ×400 magnification. Mean numbers of Ki67+ lymphocytes in the livers of infected mice are indicated (±SEM). Data are representative of three independent experiments.
To assess the ability of anti-B7-H1 blockade treatment to enhance T cell proliferation at the site of Ad-LacZ infection in core(+) mice, we determined the expression of Ki67 by intrahepatic lymphocytes by immunohistochemistry. As shown in Fig. 7D, blockade of the PD-1/B7-H1 inhibitory pathway in core(+) mice was able to increase the proliferation of liver lymphocytes. Taken together, these findings suggest that blockade of the PD-1/B7-H1 pathway can improve T cell infiltration into infected tissue where antiviral T cells can carry out their cytotoxic T cell responses on virally infected cells. In addition, we have also found that anti-B7-H1 blockade treatment enhances T cell proliferation and expansion at the site of Ad-LacZ infection.
Discussion
In this report, we demonstrate that intrahepatic expression of HCV core impairs βgal-specific CD8+ T cells, resulting in failed clearance of Ad-LacZ infection. The functional analysis of βgal-specific CD8+ T cells revealed that the expression of HCV core by hepatocytes alters the magnitude of the βgal-specific CD8+ T cell response and suppresses both CD8+ T cell effector cytokine production (i.e., IFN-γ and TNF-α) and cytolytic potential. In addition, high levels of PD-1 expression by intrahepatic βgal-specific CD8+ T cells and B7-H1 expression by liver DCs were sustained in core(+) mice as compared with that in core(−) mice. Suppression of CD8+ T cell responses was limited to the liver compartment where core is expressed and was not observed in the spleen. Importantly, blockade of the PD-1/B7-H1 inhibitory pathway rescued exhausted CD8+ T cells in core(+) mice and led to successful clearance of Ad-LacZ infection.
Chronic HCV infection in humans is characterized by CD8+ T cell exhaustion and dysfunction (37). Recently, it has been reported that increased expression of PD-1 is associated with the impaired HCV-specific CD8+ T lymphocytes observed in chronic HCV patients (11–13). However, the underlying mechanism(s) for HCV-mediated impaired CD8+ T cell responses has yet to be determined. Based on our finding of impaired intrahepatic CD8+ T cell responses in core(+) mice, it is possible that HCV core-induced T cell dysfunction may contribute one of the viral factors that causes impaired CD8+ T cell responses as seen in chronic HCV patients. From our study we found that splenic CD8+ T cell responses are not altered in core(+) mice. One possible explanation is that HCV core-expressing hepatocytes may not inhibit the CD8+ T cell response at the induction phase. Instead, the inhibition may rather occur at the point where CD8+ T cells enter the livers of core(+) mice and either undergo apoptosis or become defective in further proliferation/effector activity. That is, it is likely that HCV core-expressing hepatocytes alter the liver inflammatory conditions, thereby inhibiting CD8+ T cell responses either directly or indirectly by DC dysregulation. Furthermore, we have observed increased caspase-3 staining by intrahepatic CD8+ T cells in core(+) mice during Ad-LacZ infection, indicating enhanced apoptosis of T cells as a potential mechanism for the observed dysregulation of T cell responses in the livers of core(+) mice (data not shown).
Based on our findings that impaired CD8+ T cell responses correlate with sustained PD-1 expression in core(+) mice, it is likely that the PD-1/B7-H1 pathway plays a major inhibitory role in our HCV core transgenic model. The role of PD-1/B7-H1 as a mechanism for liver tolerance has been well established. Indeed, PD-1 expression by T cells has been shown to inhibit intrahepatic antiviral immune responses at the effector phase (20, 21, 38). The initial induction of PD-1 by CD8+ T cells in both core(+) and core(−) mice at the early stage of viral infection provides further evidence that PD-1 plays an important role in controlling intrahepatic T cell responses. However, at later time points the expression of PD-1 by βgal-specific intrahepatic CD8+ T cells remains significantly elevated in core(+) mice whereas it decreases appreciably in core(−) mice. Our studies suggest that enhanced expression of PD-1 and B7-H1 in core(+) mice following Ad-LacZ infection modulates both antiviral immunity and effector CD8+ T cell deletion. The loss of PD-1 expression by CD8+ T cells in core(−) mice at later time points during Ad-LacZ infection is likely due to successful viral control, leading to clearance of Ag from the liver. The sustained PD-1 expression in core(+) mice is due to higher levels of B7-H1 expression by liver DCs in core(+) mice and an inability to rapidly clear viral Ag. More so, we believe that enhanced expression of PD-1 on βgal-specific CD8+ T cells is due to a partially exhausted phenotype exhibited by the T cells in an environment characterized by Ag persistence.
Currently, it remains unclear how intrahepatic expression of HCV core suppresses CD8+ T cell responses during Ad-LacZ infection. It is possible that HCV core is secreted from hepatocytes in core(+) mice and that extracellular core protein exerts an immunomodulatory function. However, we were unable to detect secreted HCV core by using ELISA on our core(+)-infected mice (data not shown). This could be due to a detection limit with our core ELISA. However, we would rather believe that the expression of HCV core alone (not as polyproteins including the E1 and E2 glycoproteins) might not allow for an appreciable secretion of HCV core in core(+) mice. Thus, it is likely that intracellular core protein plays a role in the impaired antiviral activity of CD8+ T cells as observed in core(+) mice.
Based on the importance of innate immunity linking to adaptive immunity, it is tempting to speculate on the potential mechanism of HCV core-mediated suppression of antiviral CD8+ T cell responses. First, HCV core has been shown to alter type 1 IFN responses in vitro (39–41). Type 1 IFNs induce DC maturation and NK cell activation. Furthermore, the production of type 1 IFNs is believed to be critical to overcome baseline tolerance in the liver and thus HCV core-mediated alteration of type 1 IFNs in our murine model may explain the observed dysregulation of CD8+ T cell responses. Second, HCV core expression by hepatocytes has been shown to impair NK-mediated activation of DC cells in vitro (42, 43). Indeed, a human cell line expressing HCV core has been reported to up-regulate HLA-E, a ligand for NK inhibitory receptors. Recently, the immunoregulatory role for NK cells has emerged such that NK cells can influence adaptive immune responses by their ability to secrete immunomodulatory cytokines and chemokines. It is possible that altered expression of NK inhibitory ligands in core(+) mice impairs the induction of effector immune responses. Third, IL-10 has been shown to be a key mediator in the suppression of T cell responses during persistent viral infection (44, 45). Interestingly, anti-IL-10R treatment during chronic lymphocytic choriomeningitis virus infection was shown to enhance antiviral T cell responses and reduce expression of PD-1 by T cells (46). It is possible that HCV core-expressing hepatocytes alter DC activation and function and lead to enhanced IL-10 production. As a result of DC dysfunction, core(+) mice might not generate effective CD8+ T cell responses.
Nevertheless, our studies demonstrate that in vivo treatment with anti-B7-H1 blocking Ab can restore effective antiviral intra-hepatic T cell responses in core(+) mice. Importantly, blockade of this pathway enhances viral clearance by releasing HCV core-mediated immune suppression. At this point, it is not clear if the increased numbers of virus-specific T cells observed in the B7-H1 blockade group is due to decreased deletion of activated T cells in the liver. We have shown thus far that the proliferation marker Ki67 is expressed at higher levels in core(+) mice that received anti-B7-H1 blocking Ab. Importantly, we have also observed a decreased TUNEL-positive signal in the livers of core(+) mice treated with B7-H1 blockade (J. R. Lukens, unpublished observations). These results suggest that the observed increase in the frequency of βgal-specific T cells in the anti-B7-H1 treatment group is due to both decreased deletion of CD8+ T cells and enhanced proliferation of virus-specific CD8+ T cells. Interestingly, blockade of the PD-1 pathway might affect the ability of activated CD8+ T cells to infiltrate into the lobular region where virus-infected hepatocytes reside. Furthermore, anti-B7-H1 blockade treatment may be a potential therapeutic treatment strategy to treat chronic HCV patients. The HCV core transgenic model described in this report will be useful for testing the efficacy of therapeutic agents to restore antiviral CD8+ T cell activity as well as their effector function on viral clearance.
In summary, we report that the expression of HCV core in the liver compartment of mice impairs adenovirus clearance and CD8+ T cell responses. The suppressed CD8+ effector T cell responses observed in core(+) mice coincides with an elevated expression of PD-1 by virus-specific CD8+ T cells and B7-H1 by liver DCs. Importantly, blockade of the PD-1/B7-H1 inhibitory pathway rescues viral clearance, CD8+ T cell effector cytokine production, and granzyme B expression in core(+) mice. These findings implicate a potential therapeutic strategy to manipulate the PD-1/B7-H1-negative signaling pathway to restore effector T cell responses in chronic HCV patients.
Acknowledgments
We thank Susan Landes and Travis Lillard for technical assistance. We also thank Shawn Gill for critical review of this manuscript.
Footnotes
This work was supported by National Institutes of Health Grants (DK063222 to Y.S.H.) and Training Fellowship Grant 5T32AI10749608.
Abbreviations used in this paper: HCV, hepatitis C virus; Ad-LacZ, adenovirus-LacZ; Alb, albumin; βgal, β-galactosidase; β2M, β2-microglobulin; DC, dendritic cell; MFI, mean fluorescence intensity; p.i., post infection; PD-1, programmed death-1; rAD, recombinant adenovirus; X-gal, 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, Eichenlaub D, Pape GR. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 2.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 4.Chisari FV. Cytotoxic T cells and viral hepatitis. J Clin Invest. 1997;99:1472–1477. doi: 10.1172/JCI119308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruner NH, Gerlach TJ, Jung MC, Diepolder HM, Schirren CA, Schraut WW, Hoffmann R, Zachoval R, Santantonio T, Cucchiarini M, et al. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:1528–1536. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 6.Rehermann B, Chang KM, McHutchison JG, Kokka R, Houghton M, Chisari FV. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J Clin Invest. 1996;98:1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Large MK, Kittlesen DJ, Hahn YS. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J Immunol. 1999;162:931–938. [PubMed] [Google Scholar]
- 8.Sarobe P, Lasarte JJ, Casares N, Lopez-Diaz de Cerio A, Baixeras E, Labarga P, Garcia N, Borras-Cuesta F, Prieto J. Abnormal priming of CD4+ T cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J Virol. 2002;76:5062–5070. doi: 10.1128/JVI.76.10.5062-5070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao ZQ, Nguyen DT, Hiotellis AI, Hahn YS. Hepatitis C virus core protein inhibits human T lymphocyte responses by a complement-dependent regulatory pathway. J Immunol. 2001;167:5264–5272. doi: 10.4049/jimmunol.167.9.5264. [DOI] [PubMed] [Google Scholar]
- 10.Eisen-Vandervelde AL, Waggoner SN, Yao ZQ, Cale EM, Hahn CS, Hahn YS. Hepatitis C virus core selectively suppresses interleukin-12 synthesis in human macrophages by interfering with AP-1 activation. J Biol Chem. 2004;279:43479–43486. doi: 10.1074/jbc.M407640200. [DOI] [PubMed] [Google Scholar]
- 11.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 13.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, et al. Role of PD-1 and its ligand, B7–H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radhakrishnan S, Celis E, Pease LR. B7-DC cross-linking restores antigen uptake and augments antigen-presenting cell function by matured dendritic cells. Proc Natl Acad Sci USA. 2005;102:11438–11443. doi: 10.1073/pnas.0501420102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Gajewski TF, Fu YX, Zheng P, Liu Y. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med. 2003;197:1721–1730. doi: 10.1084/jem.20022089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7–H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 20.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007;178:2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 22.Cruise MW, Melief HM, Lukens J, Soguero C, Hahn YS. Increased Fas ligand expression of CD4+ T cells by HCV core induces T cell-dependent hepatic inflammation. J Leukocyte Biol. 2005 doi: 10.1189/jlb.0105005. [DOI] [PubMed] [Google Scholar]
- 23.Aramaki O, Shirasugi N, Takayama T, Shimazu M, Kitajima M, Ikeda Y, Azuma M, Okumura K, Yagita H, Niimi M. Programmed death-1-programmed death-L1 interaction is essential for induction of regulatory cells by intratracheal delivery of alloantigen. Transplantation. 2004;77:6–12. doi: 10.1097/01.TP.0000108637.65091.4B. [DOI] [PubMed] [Google Scholar]
- 24.Hahn YS. Subversion of immune responses by hepatitis C virus: immunomodulatory strategies beyond evasion? Curr Opin Immunol. 2003;15:443–449. doi: 10.1016/s0952-7915(03)00076-1. [DOI] [PubMed] [Google Scholar]
- 25.Krebs P, Scandella E, Odermatt B, Ludewig B. Rapid functional exhaustion and deletion of CTL following immunization with recombinant adenovirus. J Immunol. 2005;174:4559–4566. doi: 10.4049/jimmunol.174.8.4559. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Ertl HC, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Xiang Z, Ertl HC, Wilson JM. Upregulation of class I major histocompatibility complex antigens by interferon γ is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 29.Ando K, Guidotti LG, Cerny A, Ishikawa T, Chisari FV. CTL access to tissue antigen is restricted in vivo. J Immunol. 1994;153:482–488. [PubMed] [Google Scholar]
- 30.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 31.Freeman AJ, Pan Y, Harvey CE, Post JJ, Law MG, White PA, Rawlinson WD, Lloyd AR, Marinos G, Ffrench RA. The presence of an intrahepatic cytotoxic T lymphocyte response is associated with low viral load in patients with chronic hepatitis C virus infection. J Hepatol. 2003;38:349–356. doi: 10.1016/s0168-8278(02)00424-5. [DOI] [PubMed] [Google Scholar]
- 32.Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, et al. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 34.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 35.Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL, Kubes P. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782–2790. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, Hegenbarth S, Gerken G, Lohse AW. Induction of cytokine production in naive CD4+ T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116:1428–1440. doi: 10.1016/s0016-5085(99)70508-1. [DOI] [PubMed] [Google Scholar]
- 37.Spangenberg HC, Viazov S, Kersting N, Neumann-Haefelin C, McKinney D, Roggendorf M, von Weizsacker F, Blum HE, Thimme R. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42:828–837. doi: 10.1002/hep.20856. [DOI] [PubMed] [Google Scholar]
- 38.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8+ T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 39.de Lucas S, Bartolome J, Carreno V. Hepatitis C virus core protein down-regulates transcription of interferon-induced antiviral genes. J Infect Dis. 2005;191:93–99. doi: 10.1086/426509. [DOI] [PubMed] [Google Scholar]
- 40.Bode JG, Ludwig S, Ehrhardt C, Albrecht U, Erhardt A, Schaper F, Heinrich PC, Haussinger D. IFN-α antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003;17:488–490. doi: 10.1096/fj.02-0664fje. [DOI] [PubMed] [Google Scholar]
- 41.Lin W, Kim SS, Yeung E, Kamegaya Y, Blackard JT, Kim KA, Holtzman MJ, Chung RT. Hepatitis C virus core protein blocks interferon signaling by interaction with the STAT1 SH2 domain. J Virol. 2006;80:9226–9235. doi: 10.1128/JVI.00459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jinushi M, Takehara T, Tatsumi T, Yamaguchi S, Sakamori R, Hiramatsu N, Kanto T, Ohkawa K, Hayashi N. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, Hayashi N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 44.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. Hepatic expansion of a virus-specific regulatory CD8+ T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–972. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]