Abstract
Background
Monoclonal gammopathy of undetermined significance (MGUS) is a common premalignant plasma cell disorder defined by evidence of immunoglobulin heavy chain (IgH expression). MGUS is the precursor lesion for most cases of multiple myeloma (MM), but up to 20% of MM is characterized by complete lack of IgH expression; the prevalence of a corresponding precursor entity, light chain MGUS (LC-MGUS) is unknown.
Methods
We studied 18,357 Olmsted County, Minnesota, residents age 50 years or older with the serum free light chain (FLC) assay. LC-MGUS was defined as an abnormal FLC-R with complete lack of IgH expression, plus elevation in the appropriate involved FLC.
Findings
An abnormal FLC-R was observed in 3.3% persons tested. Of these, 213 had IgH expression on immunofixation diagnostic of conventional MGUS, 57 of whom were previously undetected. Among the remaining 397 individuals, 146 had elevation of at least one FLC and met criteria for LC-MGUS. The prevalence of LC-MGUS was 0.8%, bringing the overall prevalence of MGUS+LC-MGUS to 4.2% among persons age 50-years and older. The risk of progression to MM among the LC-MGUS patients was 0.3% (95% confidence interval [CI], 0.1 to 0.8%)/year. Twenty-three percent of LC-MGUS patients had renal disease either at the time of or subsequent to acquisition of the test sample.
Interpretation
LC-MGUS is prevalent in approximately 1% of a predominantly Caucasian, general population and poses a risk of progression to light-chain MM and related conditions comparable to that of low-risk MGUS. LC-MGUS was also associated with a risk for renal disease.
INTRODUCTION
Monoclonal gammopathy of undetermined significance (MGUS) is a precursor lesion for multiple myeloma (MM), an incurable bone marrow malignancy with an annual incidence of approximately 4.6 per 100,000.1 Historically, MGUS has been characterized by evidence of immunoglobulin heavy chain (IgH) expression, but could therefore only account for 80% of MM cases since 20% of MM cases secrete no IgH (e.g., IgG, IgA IgD or IgE).2 The prevalence and risk of progression of the premalignant precursor entity for these light chain only MM (LC-MM) cases is unknown.
We hypothesized that an equivalent of MGUS in which IgH is not expressed, i.e. light chain MGUS (LC-MGUS), is the premalignant precursor of LC-MM. In our previous report on the prevalence of MGUS in Olmsted County, Minnesota, we used a serum agarose gel electrophoretic screening methodology and found that the prevalence of standard heavy chain associated MGUS to be 3.2% in the population of individuals age 50 years or older in Olmsted County MN (a predominantly Caucasian population), but identified only 2 patients among 21,463 tested to have a serum free light chain (FLC) without IgH expression.2 We postulated that the serum immunoglobulin FLC assay,3 which can detect imbalances of concentrations of unbound kappa (κ) and lambda (λ) light chains when the circulating levels are as low as 10–30 mg/L,4–7 might provide us with better sensitivity than the protein electrophoresis methodology to detect this putative entity of LC-MGUS. Clonality is defined as a ratio of a κ to λ ratio (FLC-R) outside of the reference range. This dependence on the ratio, rather than the absolute value of either independently, is because both renal disease and polyclonal activation of B-cells can result in a diffuse increase of both κ and λ plasma cells, yielding elevated absolute values of κ and λ FLC but maintaining a normal ratio. To estimate the prevalence of LC-MGUS in the Olmsted County population, we used this assay to test the large, well defined cohort of subjects previously assembled to estimate the prevalence and progression of plasma cell disorders.2 We also described progression of this novel disorder to myeloma or related malignancy.
METHODS
Study Subjects
We used a population-based cohort previously assembled by us to estimate the prevalence of MGUS.2 The original cohort comprised samples from 21,463 of the 28,038 enumerated Olmsted County residents aged 50-years or older as of January 1, 1995. Fifteen patients were found to have malignant plasma cell disorders, and 3.2% had MGUS.2 The original informed consent included permission for further tests and studies on the samples, but only 18,372 (86%) of the original 21,463 had sufficient remaining serum to perform the serum immunoglobulin FLC assay.
Laboratory Methodology
Following approval by the Mayo Clinic Institutional Review Board, the FLC assay (FREELITE™, The Binding Site Ltd., Birmingham, U.K.) was measured in the 18,372 stored serum samples on a Dade Behring BNII automated nephelometer (Siemens, Newark, Delaware). In addition to reporting the κ and λ FLC concentrations, the assay reports the FLC κ/λ ratio (FLC-R),8 and an abnormal result was defined as an abnormal FLC-R (normal diagnostic range: 0.26–1.65). The serum protein electrophoresis (PEL) assay and the serum immunofixation electrophoresis (IFE) were performed as previously reported.9 The limit of detection for these assays in our laboratory is 10 mg/L.
Definition of LC-MGUS
LC-MGUS was defined as an abnormal FLC-R with complete lack of IgH expression, plus elevation in the appropriate involved FLC
Statistical Analysis
Although serum samples were collected over a period of several years,2 each subject was assessed only once. Age and gender-specific prevalence rates were calculated by using the number of persons with LC-MGUS in each age and sex stratum as the numerator, and the number of persons tested in the corresponding age and sex stratum as the denominator. Age-adjusted and overall age- and sex-adjusted prevalence rates were obtained by direct standardization to the U.S. population age 50 and older in 2000. Ninety-five percent confidence intervals (95%CI) for the prevalence rates were calculated assuming a Poisson distribution. The Poisson models were checked for overdispersion (a form of goodness of fit). The age-specific prevalence pattern was examined as a smoothed function of age, separately by sex, with the use of generalized additive model procedures for Poisson regression.10 The number of persons with abnormal FLC ratio, LC-MGUS or MGUS was entered as the dependent variable, age as the independent variable, and the number of people with samples tested as an offset. This method has been illustrated previously with regard to trends in the prevalence of hip fractures.11 In the calculation of progression 59 samples whose identifying data had been anonymized are necessarily omitted, leaving 133 LC-MGUS and 577 MGUS subjects. Progression rates were calculated using a person-years approach, where the number of observed progressions observed was divided by the total person-years of exposure. Rates are expressed as the number of progression events per 100 person-years. Risk of progression was also analyzed in the context of our previous MGUS risk model in which we found that patients with intact immunoglobulin MGUS and an abnormal FLC-R had a significantly higher risk of progression than with patients with a normal ratio (hazard ratio, 3.5; 95%CI, 2.3–5.5; P < 0.0001).12 Progression rates were depicted graphically using Kaplan-Meier curves. The Cox models were checked for goodness-of-fit with respect to outliers and proportional hazards using the methods of Therneau and Grambsch.13 We also compared the incidence of renal disorders in patients with LC-MGUS by cross referencing the FLC MGUS data with patients in our disease association study, which used the Hospital Adaptation of the International Classification of Diseases, Eighth Edition to assign diagnoses.14 All analysis was performed using SAS version 8.2 (SAS Institute Inc., Cary, NC) and Splus version 8.0 (TIBCO Corporation, Palo Alto, CA).
Role of the Funding Source
The Binding Site, UK, who provided the reagents for performing this study, had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication. A.R. Bradwell, director of The Binding Site, UK, participated in critical review of the manuscript. The time of the investigators was covered by the Mayo Clinic and NIH grants. Final responsibility for the decision to submit the manuscript was that of Dr. Dispenzieri and her Mayo Clinic colleagues. Neither the Binding Site, UK, nor Dr. A.R. Bradwell participated in this decision.
RESULTS
Prevalence
An abnormal FLC ratio was observed in 610 of the 18,357 Olmsted County residents (Table 1). Of these, serum immunofixation demonstrated IgH expression in 213 cases, indicating conventional MGUS. This included 57 cases previously undetected using serum protein electrophoresis as the screening test. As a result, the overall age-and sex-adjusted prevalence of MGUS in persons age 50 years or older in Olmsted County is 3.4% (95%CI, 3.2–3.7), an increase from the previously estimated MGUS prevalence of 3.2%.2
Table 1.
Distribution of free light chain ratio (FLC-R) abnormalities as they relate to intact immunoglobulin heavy chains (IgH) among Olmsted County, Minnesota, residents 50 years of age and older
| Normal FLC-R | Abnormal FLC-R | All | ||
|---|---|---|---|---|
| Neither FLC elevated | At least one FLC elevated | |||
| No IgH | 17337* | 251 | 146 | 17734 |
| Previously found IgH | 410 | 28 | 128 | 566 |
| Newly discovered IgH | ND* | 12 | 45 | 57 |
| Totals | 17747 | 291 | 319 | 18357 |
FLC, free light chain; FLC-R, free light chain ratio; IgH, monoclonal immunoglobulin heavy chain; ND, immunofixation electrophoresis not done
IgH not tested for in these patients since normal serum protein electrophoresis and FLC-R
Among the 397 individuals with an abnormal FLC-R and complete lack of IgH expression, the combinations of κ and λ concentrations relative to the normal range are delineated in Supplemental Table 1. Thus, 146 individuals (37%) had an elevation of the involved light chain that was the cause of the abnormal ratio and met criteria for LC-MGUS. The resulting age- and sex-specific prevalence of LC-MGUS in Olmsted County age 50 years or older is 0.8% (95%CI, 0.7–0.9), as shown in more detail in Figure 1 and Table 2. The age-standardized prevalence of LC-MGUS in men and women was 1.0 % (95%CI, 0.8–1.2) and 0.6% (95 %CI, 0.5–0.8), respectively. The overall prevalence of LC-MGUS combined with MGUS was 4.2% (95%CI, 3.9–4.5). The prevalence of an abnormal FLC-R without elevation of absolute values of either κ or λ is also shown in Figure 1.
Figure 1.
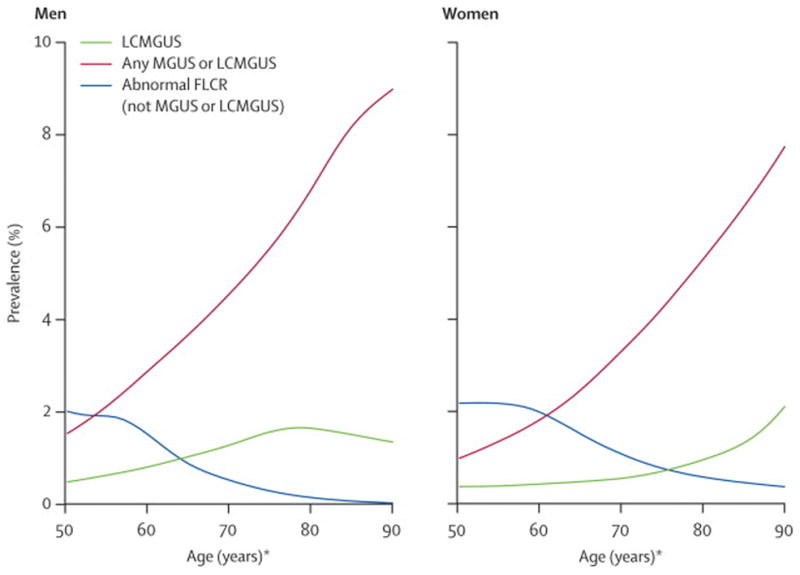
Prevalence of MGUS, light chain MGUS (LC-MGUS), and abnormal free light chain ratio (FLC-R) without elevated free light chain among Olmsted County, Minnesota residents 50 years of age and older
Table 2.
Age-specific and sex-specific prevalence of light chain MGUS (LC-MGUS) and Combined MGUS +LC-MGUS among Olmsted County, Minnesota residents 50 years of age and older.
| LC-MGUS*,† | |||
|---|---|---|---|
| Male, n (%) | Female, n (%) | Total, n (%) | |
| 50–59 years | 22/3450 (0.6) | 14/3717 (0.4) | 36/7167 (0.5) |
| 60–69 years | 25/2554 (1.0) | 15/2776 (0.5) | 40/5330 (0.8) |
| 70–79 years | 28/1608 (1.7) | 15/2242 (0.7) | 43/3850 (1.1) |
| 80–89 years | 6/577 (1.0) | 21/1433 (1.5) | 27/2010 (1.3) |
| Total | 81/8189 (1.0) | 65/10168 (0.6) | 146/18357 (0.8) |
|
‡ Age-standardized § Age- and sex-standardized |
1.0 (95%CI, 0.8–1.2)‡ -- |
0.6 (95 %CI, 0.5–0.8)‡ -- |
-- 0.8 (95%CI, 0.7–0.9)§ |
| Any MGUS or LC MGUS * | |||
| Male, n (%) | Female, n (%) | Total, n (%) | |
| 50–59 years | 95/3450 (2.8) | 64/3717 (1.7) | 159/7167 (2.2) |
| 60–69 years | 122/2554 (4.8) | 85/2776 (3.1) | 207/5330 (3.9) |
| 70–79 years | 116/1608 (7.2) | 112/2242 (5.0) | 228/3850 (5.9) |
| 80–89 years | 57/577 (9.9) | 118/1433 (8.2) | 175/2010 (8.7) |
| Total | 390/8189 (4.8) | 379/10168 (3.7) | 769/18357 (4.2) |
|
‡ Age- standardized § Age- and sex-standardized |
5.1 (95%CI, 4.6–5.6)‡ -- |
3.5 (95%CI, 3.2–3.9)‡ -- |
-- 4.2 (95%CI, 3.9–4.5)§ |
Abnormal FLC-R with at least one free light chain above the normal range
Excludes patients with intact heavy chain MGUS
Age-adjusted to the 2000 U.S. total population age 50 years and older
Age- and sex-adjusted to the 2000 U.S. total population age 50 years and older
Characterization of LC-MGUS
The demographics of residents with LC-MGUS, MGUS, and no MGUS are shown in Supplemental Table 2. The median age of those with LC-MGUS was 68 years as compared to 70 years for MGUS. Of those with LC-MGUS, 108 had κ and 38 had λ predominance (2.8:1). The monoclonal FLC was detectable on serum IFE (9 κ and 17 λ) for only 18% (26/146) of the individuals with LC-MGUS. Two of these subjects had been identified by the PEL screening method in the original survey2 but were not included as intact immunoglobulin MGUS cases. Two additional subjects with LC-MGUS had a discrepancy between IFE and FLC-R results, representing either assay error or biclonality. The median level of involved FLC in LC-MGUS cases who were IFE positive was significantly higher than that of LC-MGUS with a normal IFE (176 vs. 48 mg/L, P<0.001).
Progression to myeloma and related disorders
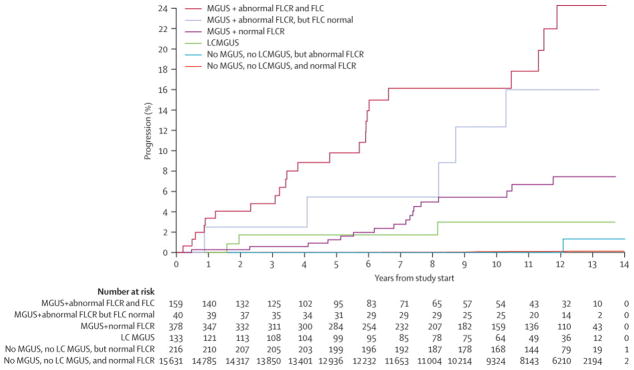
With follow-up of 155,542 patient-years for the entire cohort thus far, progression to MM or other disease has occurred in 62 patients (Figure 2 and Table 3). Only 3 of the 133 unblinded LC-MGUS have progressed in 1100 patient-years of follow-up (Table 4). These 3 LC-MGUS progressions, which occurred at 19, 23, and 98 months, were all to LC-MM. The 0.3%/year (95%CI 0.1–0.8) rate of progression to any lymphoproliferative disorder is higher than the normals’ rate of 0.01%/year (95%CI 0.01–0.02), indicating that LC-MGUS is a premalignant plasma cell disorder analogous to MGUS.
Figure 2.
Rates of progression to multiple myeloma and related disorders.
Table 3.
Risk of progression to multiple myeloma and related disorders among Olmsted County, Minnesota residents with light chain-monoclonal gammopathy of undetermined significance (LC-MGUS), conventional MGUS, and no MGUS
| Number of progressors/Person-years [Progression rate per 100-years (95%CI)] | ||||
|---|---|---|---|---|
| Abnormal FLC with elevated light chain | FLC ratio abnormal but no high component | FLC ratio normal | Total | |
| No conventional MGUS | 3/1,110* [0.27 (0.06, 0.79)*] | 1/2,290 [0.04 (0, 0.24)] | 13/147,620 [0.01 (0.01, 0.02)] | 17/151,020 [0.01 (0.01, 0.02)] |
| MGUS (detected in original study) 2 | 20/850† [2.36 (1.44, 3.65)†] | 4/250† [1.59 (0.43, 4.07) †] | 17/3,080‡ [0.55 (0.32, 0.89)‡] | 41/4,170 [0.98 (0.71, 1.33)] |
| MGUS (newly detected in current study) | 3/240† [1.27 (0.26, 3.7) †] | 1/120† [0.87 (0.02, 4.84)†] | NA‡ [NA‡] | 4/350 [1.14 (0.31, 2.91)] |
| Total | 26/2,190 [1.19 (0.77, 1.74)] | 6/2,660 [0.23 (0.08, 0.49)] | 30/150,690 [0.02 (0.01, 0.03)] | 62/155,540 [0.04 (0.03, 0.05)] |
Light chain MGUS population
Conventional MGUS, increased risk as defined as abnormal FLC-R
Conventional MGUS, low risk as defined as normal FLC-R
NA, not applicable
Table 4.
Characteristics of four LC-MGUS patients who progressed to multiple myeloma.
| RESEARCH SAMPLE | AT CLINICAL PROGRESSION PRIOR TO INSTITUING CHEMOTHERAPY | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Date | FLC, mg/L | S-IFE | Date | Symptoms | Hb, g/dL Ca, mg/dL Cr, mg/dL |
FLC* | S-IFE | UIFE/UPEP, mg/day | ISS | BM BX | Bone disease |
| 1 | 5/30/96 | κ 68.8 λ 15.6 Ratio 4.4 |
Neg | 3/98 | 84 y.o female with h/o osteoporosis, hypertension, sick sinus syndrome, primary hyperparathyroidism, goiter presented with 3 months left hip pain and new onset anemia | 9.2 10.9 1.1 |
κ 85.7 λ 1.58 Ratio 54.2 |
Neg | κ/713 | II | 40% | Yes |
| 2 | 1/17/97 | κ 602 λ 16.7 Ratio 36.0 |
Neg | 8/98 | 65 y.o male patient with no medical history presented with 3 months of back pain | 11.9 14.5 1.6 |
κ 7.83 λ 1.24 Ratio 6.29 |
Neg | κ/NM | II | 25% | Yes |
| 3 | 5/17/96 | κ 66.2 λ 10.7 Ratio 6.19 |
Neg | 7/04 | 79 y.o male with h/o pseudogout, PVC’s, BPH, hyperlipidemia, IBS presented with 2–3 months of fatigue and renal insufficiency. Renal biopsy showed cast nephropathy. | 10.9 10.8 2.3 |
κ 1360 λ 0.54 Ratio 2500 |
κ/NM | κ/1980 | III | 60% | Yes |
Done retrospectively on stored serum sample
BM BX, bone marrow biopsy percent myelomatous involvement; BPH, benign prostatic hypertrophy; Ca, serum calcium; Cr, serum creatinine; FLC, free light chain; Hb, hemoglobin; h/o, history of; IBS, irritable bowel syndrome; ISS, International Staging System; Neg, negative; NM, not measurable; PVC’s, premature ventricular complexes; S-IFE, serum immunofixation electrophoresis; UIFE, urine immunofixation electrophoresis; UPEP, urine protein electrophoresis; y.o, year old
We next compared the risk of progression for LC-MGUS to that of conventional MGUS, which is known to have an overall annual progression rate of 1.0%/year.2 A coexistent abnormal FLC-R increased the risk of progression within the conventional MGUS cohort, but the risk of progression for the low-risk conventional MGUS patients (i.e., no coexistent abnormal FLC-R) and for the LC-MGUS patients was not significantly different (0.6%/year [95%CI 0.4–1.0] versus 0.3%/year [95%CI 0.1–0.8]), meaning either that the rates were similar or that the sample size limited the power to detect a difference.
Finally, the overall survival of all groups was evaluated in an attempt to correct for missed progression events (e.g. undiagnosed amyloidosis) that may have contributed to early mortality. Overall survival mirrored progression events (data not shown).
LC-MGUS and renal disease
It is known that renal disease raises serum levels of both κ and λ free light chains and it has recently been proposed that a “renal reference range” be used to interpret the FLC-R among patients with renal insufficiency given altered catabolism of light chains in the setting of renal failure.15 When the renal reference range of 0.37–3.1 (as opposed to 0.26–1.65) was applied to the entire cohort, 69 individuals with apparent κ restriction were no longer deemed LC-MGUS, but 57 additional individuals with apparent λ restriction were added (Figure 3), including one individual who developed IgG λ multiple myeloma 90 months after her research specimen was acquired. Although this was an interesting exercise, because all of the medical information is not available for all patients, the renal reference range was not used to calculate the prevalence figures. However, we found an association between LC-MGUS and renal diagnoses. By merging the present data set with that of our MGUS disease association paper,14 we found that among the 16,255 patients in both data sets, there was an increased risk for a renal diagnosis among patients with MGUS or LC-MGUS (Table 5). Twenty-three percent of LC-MGUS patients had some form of renal diagnosis that was not recognized as being related to a plasma cell dyscrasia either at the time of acquisition of the test sample (8.5%) or subsequent to it (14.7%). Sixty percent of patients who had renal disease, had λ restricted LC-MGUS.
Figure 3.

Definition of light chain MGUS (LC-MGUS) depends on free light chain ratio (FLC-R) cut-offs: normal reference range versus the renal reference range.
Table 5.
Relationship between light chain MGUS (LC-MGUS) and renal disease.
| N | Renal disease, N (%) | |||
|---|---|---|---|---|
| Pre-FLC measurement*,† | Post-FLC measurement‡,§,¶ | Pre or Post FLC measurement | ||
| 1. LC-MGUS | 129 | 11 (8.5) | 19 (14.7) | 30 (23.3) |
| 2. MGUS | 566 | 35 (6.2) | 133 (23.5) | 168 (29.7) |
| 3. No MGUS, LC-MGUS, but abnormal FLC-R | 211 | 2 (0.9) | 11 (5.2) | 13 (6.2) |
| 4. No MGUS, LC-MGUS, and normal FLC-R | 15,349 | 531 (3.5) | 1,965 (12.8) | 2,496 (16) |
| All patients with diagnostic data | 16,255 | 579 (3.6) | 2,128 (13.1) | 2,707 (16.7) |
1-and-2 versus 3-and-4, p<0.0001 by chi-square analysis
1 versus 2 versus 3-and-4, p<0.0001 by chi-square analysis
1 versus 3-and-4, hazard ratio is 1.3, p=0.2, using Cox model that excludes patients with previously found renal diagnosis.
1-and-2 versus 3-and-4, hazard ratio is 2.1, p<0.0001, using Cox model that excludes patients with previously found renal diagnosis.
1 versus 2, HR: 0.60, p= 0.03, using Cox model that excludes patients with previously found renal diagnosis.
DISCUSSION
The term, MGUS, was coined at the Mayo Clinic over 30 years ago in reference to asymptomatic individuals with an intact serum monoclonal protein (both immunoglobulin heavy and light chain detectable) less than 30 g/L and with fewer than 10% bone marrow plasma cells.2, 16, 17 MGUS was observed to be the precursor lesion for intact immunoglobulin MM.18 Approximately 80% of MM cases have IgH expression (i.e., IgG, IgA, IgM, IgD, and IgE); in the remaining 20%, there is no IgH expression. Prior to our original description of LC-MGUS,19 a premalignant lesion for LC-MM corresponding to MGUS had not been systematically described. However, a condition called idiopathic Bence Jones proteinuria was defined in 7 patients who had at least one gram of urinary monoclonal protein without evidence of MM or related disorders.20 In the parlance of the current manuscript, these 7 patients could be considered the first cases of LC-MGUS, but prevalence was not addressed. Herein, we have shown that LC-MGUS comprises 19% of the total cases of MGUS and LC-MGUS, consistent with the proportion of LC-MM cases among newly diagnosed MM patients, 21 and that an estimated that 0.8% of the general population age 50 years and older has LC-MGUS.
Recognition and description of this entity is important for two reasons. First, it provides insight into an additional precursor lesion for MM, a devastating malignancy. Second, the natural history of LC-MGUS, which affects 0.8% of the Caucasian population age 50 years and older—likely more than 750,000 Americans—needs to be understood to promote appropriate testing on these patients who have a relatively benign condition. Patients with LC MGUS had a progression rate of only 0.3%/year, which is lower than their conventional MGUS counterparts, who have an overall annual risk of 1.0%/year, but 23% of them have or will develop renal disease. This low progression rate was perhaps unexpected in the context of our prior observation that patients with intact immunoglobulin MGUS, but who also have an abnormal FLC-R, are at higher risk of progression than are their counterparts with a normal FLC-R.12 We demonstrate, however, that the progression rates of the low-risk conventional MGUS patients (i.e. those without coexistent abnormal FLC-R) and the LC-MGUS patients were similar.
Whether differential risks of progression among patients with conventional MGUS or LC-MGUS are a function of tumor burden, biology, or a combination of the two is unknown. It is conceivable that the transforming events that produce a LC-MGUS and a conventional IgH MGUS (with a normal FLC-R) are the same, whereas conventional IgH MGUS combined with an abnormal FLC-R is a manifestation of another “hit” and therefore one step closer to full transformation to active MM.22 It is also plausible that the cases of LC-MGUS are being detected at an earlier time point (i.e., at a point of earlier tumor burden) given the relatively higher sensitivity of the serum FLC ratio determination (which can detect involved FLC of less than 10–30 mg/L) compared to the serum protein electrophoresis screening (1–2 g/L).3,8 In this study, we detected an additional 57 unrecognized MGUS patients by FLC assay, increasing the prevalence of IgH MGUS from 3.2%2 to 3.4% and defining the prevalence of LC-MGUS plus MGUS as 4.2%. A final explanation could be that a small fraction of our LC-MGUS cohort, i.e. those with the least abnormal ratios, does not have a true clonal disorder, but merely renal dysfunction or polyclonal activation.
Our description of the LC-MGUS entity is supported by three recent observations. Landgren et al., in collaboration with our group showed that the either MGUS or LC-MGUS is present in over 96% of MM patients more than 6 years prior to the diagnosis of their malignancy.23 If LC-MGUS were excluded, only 93% of MM patients had a premalignant MGUS preceding the diagnosis. This shows that there is a small subset of MM patients in whom the precursor lesion is not MGUS, but rather LC-MGUS. A second similar study showed that LC-MGUS was the preceding premalignant lesion in LC-MM.24 These results support our previous report that IgH MGUS progresses only to MM with IgH expression. Taken together, they lend strong support to the findings of the present study. Finally, in a slightly different vein, Tsai et al found that among a cohort of 109 CLL patients, the prevalence of an abnormal FLC-ratio prior to CLL diagnosis was 38% (95%CI 29–47%);25 none of the LC-MGUS cases in our cohort has yet evolved to CLL.
For the purposes of disease definition, we excluded from the LC-MGUS category those individuals with an abnormal FLC-R who had no elevation in the involved light chain. This group, constituting 63% of all individuals with an abnormal FLC-R and absent IgH expression, may include a subset of patients with true LC-MGUS, but were felt to be false positives based on two arguments. First, since an abnormal FLC-R in the context of B-cell disorders is used as a surrogate for clonality, abnormality in the FLC-R due merely to relative suppression of the other immunoglobulin FLC seems insufficient evidence for a clone. Second, when we explored the prevalence of abnormal FLC-R with neither elevated κ or λ concentrations, the age distribution was the inverse of what is seen in MGUS.
A more specific definition of LC-MGUS could include only that subset that were immunofixation positive (26 of the 146 LC-MGUS cases), but such a definition would have missed all 3 cases of LC-MGUS that progressed to MM in this series. Since the FLC-R is more sensitive at detecting free light chains than serum immunofixation;4–7, 9 relying on immunofixation alone would also greatly underestimate the true prevalence of LC-MGUS. Our trust in the FLC-R to detect clonal disorders in the setting of a negative immunofixation to is supported by the report of Weiss et al.24 In their series, among the 30 patients with MM who had pre-diagnostic serum available for retrospective testing for monoclonal gammopathy from 2.2 to 15.3 years prior to MM diagnosis, the first evidence of a plasma cell clone was the FLC-R alone in 6 patients, two of whom subsequently had an IgH detected by immunofixation prior to their diagnosis of non-LC MM.
Another “more specific” definition of LC-MGUS might employ the renal reference range.15 This exercise increased the number of λ cases by 57 and decreased the number of κ cases by 69, which did not significantly change the prevalence rate, but did dramatically alter the population at risk. Because the current definition of renal range does not take into account degrees of renal dysfunction,15 it was not possible to select those patients to whom the renal reference range should have been applied. Employing the normal reference range rather than the renal range, albeit imperfect, allowed for the greatest consistency in the setting of this large population study. Another confounder of our prevalence estimates is that the recognition of MGUS—be it conventional MGUS or LC-MGUS—is dependent on the screening methods used. As the sensitivity of the screening technology increases, the prevalence of an entity also “increases.” In the case of conventional MGUS, our detection rates have increased with increasing sensitivity of the screening method as we have evolved from paper electrophoresis,16 to agarose gel electrophoresis2 to FLC measurements.
In summary, we define a new clinical entity representing the light chain equivalent of MGUS, namely LC-MGUS. LC-MGUS is prevalent in almost 1% of the general Caucasian population age 50 and older, is associated with increased progression to MM or related malignancy. An important limitation of this study is the absence of an African American presence with the Olmsted County cohort. Conventional MGUS is 2–3 times as common in African Americans as it is in Caucasians, and our population study does not provide information about the prevalence of LC-MGUS in African Americans.26 We also found an association with renal disease that needs further study. We do not recommend screening for this disorder; however, we expect patients to be identified incidentally when a FLC assay is ordered as part of a diagnostic evaluation for a variety of symptoms and laboratory abnormalities such as anemia, hypercalcemia, or renal failure. If LC-MGUS is identified in the context of one of these abnormalities, as with MGUS we recommend excluding a diagnosis of MM, amyloidosis, and related conditions by performing a bone marrow examination with clonality testing, imaging studies, renal biopsy, and/or Congo red staining of fat or other tissue as clinically appropriate. If no malignant condition is recognized, the free light chain assay should be repeated in 6 months and yearly thereafter; no therapy is indicated as long as there is no progression. Our data would suggest that monitoring renal function periodically is prudent given the fact that 23% of these patients either have or will develop renal disease. LC-MGUS patients are at risk for LC-MM, amyloidosis, and renal disorders and hence will need further evaluation if symptoms suggestive of these disorders are encountered during follow up. We hypothesize that LC-MGUS occurs as a consequence of translocations involving the immunoglobulin heavy chain region at chromosome 14q32 which results in total suppression of heavy chain production.27
Supplementary Material
Acknowledgments
Investigators listed herein are supported in part by grants CA 125614, CA062242, CA107476, CA150831, CA93842, CA83724, CA100080, and CA100707 from the National Cancer Institute. Support was also provided by the Robert A. Kyle Hematologic Malignancies Fund. We thank The Binding Site, UK, for providing the reagents for performing this study and Ms. Tara Phelps and Ms. Carol Shipman for their work maintaining the samples and the Dysproteinemia database, respectively.
Footnotes
CONTRIBUTORS
A.D., J.A.K., R.A.K., S.K.K., and S.V.R. designed the study. A.D., J.A.K., R.A.K., S.K.K., D.R.L., L.J.M., C.L.C., T.M.T., R.C., and S.V.R. gathered and analyzed the data. The manuscript was written by A.D., J.A.K., R.A.K., S.K.K., D.R.L., L.J.M., T.T., A.B., R.F., D.F.J., and S.V.R. A.D. and S.V.R. vouch for the data and the analyses. All authors agreed upon publishing the paper.
CONFLICT OF INTEREST
No conflict of interest for J.A.K., D.R.L, D.R.L., C.L.C., L.J.M., T.M.T., R.C., S.K.K., R.F., D.F.J., or S.V.R. A.D. and R.A.K. have received honoraria from the Binding Site. A.B. is a director of The Binding Site, UK, which manufactures and sells the free light chain immunoassay kits.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ., 3rd Incidence of multiple myeloma in Olmsted County, Minnesota: Trend over 6 decades. Cancer. 2004;101(11):2667–74. doi: 10.1002/cncr.20652. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. The New England journal of medicine. 2006;354(13):1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 3.Bradwell AR, Carr-Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4):673–80. [PubMed] [Google Scholar]
- 4.Mead GP, Carr-Smith HD, Drayson MT, Bradwell AR. Detection of Bence Jones myeloma and monitoring of myeloma chemotherapy using immunoassays specific for free immunoglobulin light chains. Clin Lab. 2003;49(1–2):25–7. [PubMed] [Google Scholar]
- 5.Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361(9356):489–91. doi: 10.1016/S0140-6736(03)12457-9. [DOI] [PubMed] [Google Scholar]
- 6.Katzmann JA, Dispenzieri A, Kyle RA, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc. 2006;81(12):1575–8. doi: 10.4065/81.12.1575. [DOI] [PubMed] [Google Scholar]
- 7.Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001;97(9):2900–2. doi: 10.1182/blood.v97.9.2900. [DOI] [PubMed] [Google Scholar]
- 8.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437–44. [PubMed] [Google Scholar]
- 9.Katzmann JA, Kyle RA, Benson J, et al. Screening Panels for Detection of Monoclonal Gammopathies. Clin Chem. 2009;55(8):1517–22. doi: 10.1373/clinchem.2009.126664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hastie TJ, Tibshirani RJ. Generalized additive models. London: Chapman & Hall; 1990. [Google Scholar]
- 11.Melton LJ, 3rd, Therneau TM, Larson DR. Long-term trends in hip fracture prevalence: the influence of hip fracture incidence and survival. Osteoporos Int. 1998;8(1):68–74. doi: 10.1007/s001980050050. [DOI] [PubMed] [Google Scholar]
- 12.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106(3):812–7. doi: 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Therneau TM, Gronbach PM. Modeling survival data. Extending the Cox model. New York: Springer-Verlag; 2000. [Google Scholar]
- 14.Bida JP, Kyle RA, Therneau TM, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc. 2009;84(8):685–93. doi: 10.4065/84.8.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(6):1684–90. doi: 10.2215/CJN.02290508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyle RA. Monoclonal gammopathy of undetermined significance. Natural history in 241 cases. Am J Med. 1978;64(5):814–26. doi: 10.1016/0002-9343(78)90522-3. [DOI] [PubMed] [Google Scholar]
- 17.Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–57. [PubMed] [Google Scholar]
- 18.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. The New England journal of medicine. 2002;346(8):564–9. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Kyle R, Plevak M, et al. Prevalence of Light-Chain Monoclonal Gammopathy of Undetermined Significance (LC-MGUS) among Olmsted County, Minnesota Residents Aged 50 Years or Greater. ASH Annual Meeting Abstracts. 2006;108(11):5060. [Google Scholar]
- 20.Kyle RA, Greipp PR. “Idiopathic” Bence Jones proteinuria: long-term follow-up in seven patients. New England Journal of Medicine. 1982;306(10):564–7. doi: 10.1056/NEJM198203113061002. [DOI] [PubMed] [Google Scholar]
- 21.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Ayliffe MJ, Davies FE, de Castro D, Morgan GJ. Demonstration of changes in plasma cell subsets in multiple myeloma. Haematologica. 2007;92(8):1135–8. doi: 10.3324/haematol.11133. [DOI] [PubMed] [Google Scholar]
- 23.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) precedes multiple myeloma: a prospective study. Blood. 2009 doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113(22):5418–22. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai H-T, Caporaso NE, Kyle RA, et al. Evidence of serum immunoglobulin abnormalities up to 9.8 years prior to diagnosis of chronic lymphocytic leukemia: a prospective study. Blood. 2009 doi: 10.1182/blood-2009-08-237651. blood-2009-08-237651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107(3):904–6. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magrangeas F, Cormier ML, Descamps G, et al. Light-chain only multiple myeloma is due to the absence of functional (productive) rearrangement of the IgH gene at the DNA level. Blood. 2004;103(10):3869–75. doi: 10.1182/blood-2003-07-2501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.