Abstract
Background.
Nuclear factor kappa B (NF-κB) is a critical signaling molecule of disuse-induced skeletal muscle atrophy. However, few studies have carefully investigated whether similar pathways are modulated with physical activity and age.
Methods.
The present study examined lean mass, maximal force production, and skeletal muscle NF-κB signaling in 41 men categorized as sedentary (OS, N = 13, 63.85 ± 6.59 year), physically active (OA, N = 14, 60.71 ± 5.54 year), or young and sedentary (YS, N = 14, 21.35 ± 3.84 year). Muscle tissue from the vastus lateralis was assayed for messenger RNA (mRNA) expression of the β subunit of IkB kinase (IKKβ), cytosolic protein content of phosphorylated inhibitor of kappa B alpha (pIKBα), and nuclear content of NF-κB subunits p50 and p65.
Results.
When compared with YS, OS demonstrated age-related muscle atrophy and reduced isokinetic knee extension torque. Physical activity in older individuals preserved maximal isokinetic knee extension torque. OS muscle contained 50% more pIKBα than OA and 61% more pIKBα than YS. Furthermore, nuclear p65 was significantly elevated in OS compared with YS. OS muscle did not differ from either of the other two groups for nuclear p50 or for mRNA expression of IKKβ.
Conclusions.
These results indicate that skeletal muscle content of nuclear-bound p65 is elevated by age in humans. The elevation in nuclear-bound p65 appears to be at least partially due to significant increases in pIKBα. A sedentary lifestyle appears to play some role in increased IKBα; however, further research is needed to identify downstream effects of this increase.
Keywords: Sarcopenia, Atrophy, Strength, Proteolysis
SKELETAL muscle comprises approximately 40% of the human body mass and is critical for a number of physiological functions including locomotion. Significant decreases in skeletal muscle mass and force production capacity are widespread problems that typically occur in the face of disuse (1), aging (2), and disease (3,4). Broadly, atrophy is the result of an imbalance between protein synthesis and protein degradation. Several intracellular and extracellular mechanisms are capable of degrading muscle proteins, including the ubiquitin–proteasome system, calcium-dependent proteases (calpains), lyosomal proteases, matrix metalloproteases, and apoptosis. Yet, interplay between these processes remains largely unknown. Understanding the precise signaling cascade involved in protein degradation is complicated by the large number of stimuli that can induce muscle atrophy.
One of the most prominent of these atrophy-related signaling cascades is one involving nuclear factor kappa B (NF-κB), an evolutionarily conserved transcription factor involved in stimulating more than 150 genes involved in inflammation and protein turnover (5). Genes involved in protein turnover that are targets of NF-κB include MuRF1, MyoD and cyclin D, ubiquitin-conjugating enzyme (E2), and proteasome C3 subunit (6). NF-κB is formed by the heterodimerization or homodimerization of proteins from the rel family—RelA (p65), RelB, cRel, p50, and p52—and subsequent binding to DNA.
Prior to DNA binding, NF-κB family members are retained in the cytoplasm bound to inhibitory proteins known as inhibitors of NF-κB (IkBs). Protein turnover is stimulated by the activation of kinases (IKKs), which pIKBα and thus allow translocation of NF-κB to the nucleus. Thus, skeletal muscle atrophy may be initiated by excessive NF-κB activation and subsequent protein turnover. To date, two different NF-κB pathways have been described which initiate muscle atrophy (7,8). These pathways differ in the formation of the DNA-binding dimer. The first discovered pathway, known as the classical pathway, involves DNA binding of a p50/p65 heterodimer (7). The second pathway, referred to as the alternative pathway, does not involve the p65 subunit but rather involves binding of a p50/p50 homodimer (8). Data have shown the classical pathway to be involved in disease-mediated atrophy (7) and the alternative pathway to be involved in disuse (8).
Meanwhile, the role of NF-κB in the progression of age-related atrophy remains largely unknown. Furthermore, no clinical data exist concerning the role of physical activity on NF-κB signaling in older adults. Regular exercise is well known to provide a host of physiological benefits to older skeletal muscle, including maintenance of mass (9,10) and force production capacity (11,12). Chronic training has also been shown to attenuate NF-κB activation within other cell types (13–15). Others have hypothesized that exercise may be capable of downregulating NF-κB activation in older individuals (16,17). Because of the paucity of evidence, there is a need to examine NF-κB signaling within aging skeletal muscle of individuals and the effect of physical activity. Therefore, the aim of the present study was to evaluate the effect of age and physical activity status on lean mass, muscle force production, and skeletal muscle NF-κB signaling.
METHODS
Participants
Participant characteristics are shown in Table 1. Twenty-seven healthy men between the ages of 55 and 75 years (14 physically active [OA], 13 sedentary [OS]) and 14 men between the ages of 18 and 30 years (sedentary [YS]) matched for height participated in the study. Prior to the study, participants completed activity questionnaires and were considered physically active by participating in running or jogging, basketball, or resistance training, and so forth for at least 15 h/mo. Eleven of the 14 active participants reported resistance training at least 3 h/wk, whereas all participants participated in aerobic exercise at least 3 h/wk. Sedentary participants reported no physical activity beyond light walking required for daily activity for at least 1 year. OA reportedly exercised (mean ± SE) 6.15 ± 0.83 h/wk and with an average training history of 23.08 ± 3.37 years. Exclusionary criteria included smoking during previous year, use of nutritional supplements or supplemental androgens within the previous 6 months, current use of statins, recent history of cancer (within 2 years), neurological disease or cardiac arrhythmia interfering with physical function, peripheral vascular disease, congestive heart failure, previous stroke, complicated diabetes, chronic inflammatory disease, recent stroke (within 1 year), and renal and kidney disease. All eligible participants were asked to provide oral and written informed consent based on documents approved by the Institutional Review Board of Baylor University. Verbal explanation of the purpose of the research, the protocol to be followed, and the experimental procedures to be used were given to the participants. Participants were instructed to refrain from exercise for 48 hours and fast for 8 hours prior to testing. Furthermore, participants were instructed to refrain from the usage of anti-inflammatory medications for at least 7 days prior to the exercise-testing session.
Table 1.
Physical Characteristics of Study Participants
| YS (N = 14) | OS (N = 13) | OA (N = 14) | |
| Age (y) | 21.35 ± 3.84* | 63.85 ± 6.59* | 60.71 ± 5.54 |
| Height (cm) | 176.80 ± 5.79 | 173.99 ± 5.95 | 176.44 ± 4.54 |
| Body weight (kg) | 79.55 ± 17.09* | 93.69 ± 15.25* | 84.20 ± 7.24 |
| Resting heart rate (bpm) | 68.43 ± 9.39 | 68.54 ± 6.28 | 65.0 ± 8.44 |
| SBP (mm Hg) | 112.14 ± 9.72* | 136.62 ± 19.86*† | 122.0 ± 13.06† |
| DBP (mm Hg) | 76.00 ± 4.90 | 79.08 ± 7.05 | 76.71 ± 9.34 |
| Body fat (%) | 20.97 ± 7.83* | 31.71 ± 4.44*† | 24.38 ± 4.96† |
| VO2max | 43.42 ± 7.44* | 25.52 ± 5.15*† | 34.04 ± 3.5† |
Note: Values are means ± SD. bpm = beats per minute; DBP = diastolic blood pressure; OA = men aged between 55 and 75 years who exercise at least 15 h/mo; OS = physically inactive men aged between 55 and 75 years; SBP = systolic blood pressure; VO2max = maximal oxygen consumption; YS = physically inactive men aged between 18 and 30 years. Groups sharing a common symbol significantly different at p < .05.
* p < .05 between YS/OS.
† p < .05 between OA/OS.
Entry and Familiarization Session
Participants expressing interest in participating in this study were interviewed on the phone and/or via e-mail to determine whether they appeared to qualify to participate in this study. Participants believed to meet eligibility criteria were then invited to attend a familiarization session. Older participants were asked to bring signed physician approval of participation to the familiarization session. Participants were then familiarized to the study protocol, and the tests to be performed, via a verbal and written explanation outlining the study design.
Experimental Procedures
Upon reporting to the laboratory for the exercise-testing session, body mass, heart rate, and blood pressure were determined prior to analyzing body composition using dual x-ray absorptiometry (DEXA; Hologic, Waltham, MA; Table 1). Following the DEXA scan, muscle biopsy samples were obtained by standard and sterile procedures. Employing the Bergstrom technique, percutaneous muscle biopsies were obtained under local anesthesia of 1% Xylocaine with epinephrine from the middle portion of the vastus lateralis muscle of the dominant limb at the midpoint between the patella and the greater trochanter of the femur at a depth between 2 and 3 cm. The tissue sample was immediately frozen in liquid nitrogen and then stored at −80°C for future analyses.
Following the muscle biopsy, the participants performed a standard warm-up consisting of 5 minutes on a stationary bicycle at a comfortable speed. Then, participants performed muscle strength testing on the nondominant limb. Maximal isokinetic dynamic peak torque of the quadriceps extensors was assessed using a Biodex-System 3 (Biodex Medical Systems, Inc., Shirley, NY). This consisted of participants performing three submaximal trial repetitions at an estimated effort of 25%, 50%, 75% and two maximal (100% effort) repetitions, a rest period of 1 minute, followed by five maximal (100% effort) repetitions at 60 degrees/s. These contractions were performed over a 75 degree range of motion. Finally, participants completed a graded treadmill test to volitional exhaustion to determine maximal oxygen consumption with an automated open-circuit gas analyzer calibrated daily. The highest oxygen uptake per minute reached was defined as the maximal oxygen uptake (VO2max; Table 1).
Skeletal Muscle RNA Isolation
Muscle tissue was separated from adipose and/or connective tissue, and approximately 10 mg of muscle tissue was placed in a microcentrifuge tube and homogenized with a plastic pestle in a monophasic solution of phenol and guanidine isothiocyanate contained within the TRI-reagent (Sigma Chemical Co., St Louis, MO) as previously described (11,18). This procedure yielded intact RNA, free of DNA and proteins as indicated by prominent 28s and 18s ribosomal RNA bands on agarose gel and an OD260/OD280 ratio of approximately 2.0.
Reverse Transcription and Complementary DNA Synthesis
Two micrograms of total skeletal muscle RNA was then reverse transcribed to synthesize complementary DNA (cDNA) using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). A reverse transcription reaction mixture (2 μg of cellular RNA, 5× reverse transcription buffer [20 mM Tris–HCl, pH 8.3; 50 mM KCl; 2.5 mM MgCl2; 100 μg of bovine serum albumin per milliliter]; a dNTP mixture containing 0.2 mM each of dATP, dCTP, dGTP, and dTTP; 0.8 μM MgCl2; 0.5 μg/μL of oligo(dT) 15 primer; and 25 U/μg of MMLV RNAase H+ reverse transcriptase enzyme [Bio-Rad]) was incubated at 25°C for 5 minutes, 42°C for 30 minutes, heated to 85°C for 10 minutes, and then quick chilled on ice. The cDNA concentration was determined by using an OD260 equivalent to 50 μg/μL, and the starting cDNA template concentration was standardized by adjusting all samples to 200 ng prior to amplification.
Oligonucleotide Primers for Real-Time Polymerase Chain Reaction
The messenger RNA (mRNA) sequences of human skeletal muscle β-actin (NM_001101) and IKKB (NM_001556) published in the National Center for Biotechnology Information Entrez Nucleotide database (www.ncbi.nlm.nih.gov) were used to construct oligonucleotide polymerase chain reaction (PCR) primers using Beacon Designer software (Bio-Rad). The sense and antisense primers were then synthesized commercially (Integrated DNA Technologies, Coralville, IA). β-actin was used as an external control standard for each reaction due to its consideration as a constitutively expressed “housekeeping gene” and the fact that it has been shown to be an appropriate external reference standard in real-time PCR in human skeletal muscle following acute exercise (19).
Real-Time PCR Amplification and Quantitation
Two hundred nanograms of cDNA template were added to iQ SYBR Green Supermix (Bio-Rad), and each PCR reaction was amplified using RT quantitative PCR (iCycler IQ Real-Time PCR Detection System; Bio-Rad). The amplification profile was run for 40 cycles employing a denaturation step at 95°C for 30 seconds, primer annealing at 58°C for 30 seconds, and extension at 72°C for 30 seconds. Fluorescence was measured after each cycle resulting from the incorporation of SYBR green dye into each amplicon. The quantity of mRNA was determined relative to the expression of β-actin and the 2−ΔCT method used to compare gene expression (20). The specificity of the PCR was demonstrated with an absolute negative control reaction containing no cDNA template, and a single gene product was confirmed using DNA melt curve analysis. Positive amplification of the amplicons was assessed with agarose gel electrophoresis illuminated with UV transillumination (Chemi-Doc XRS; Bio-Rad).
Determination of pIKBα
To determine muscle content of pIKBα, approximately 25 mg of each muscle sample was placed in an autoclaved microcentrifuge tube. The skeletal muscle samples were homogenized with cell extraction buffer (1 mM EDTA, 6 M urea, 0.5% Triton-X 100, 0.0005% Tween 20 in phosphate buffered saline) supplemented with phenylmethanesulphonylfluoride and a protease inhibitor cocktail (Cat. No. P2714; Sigma Chemical Co.). Protein content of the samples was determined spectrophometrically using the DC Protein Assay (Bio-Rad) with bovine serum albumin utilized as the reference standard. Samples were then assayed in duplicate to determine muscle content of pIKBα (S32) relative to protein content of the sample using an ELISA kit from Invitrogen Corp. (Carlsbad, CA).
Determination of Nuclear p50 and p65
To determine nuclear p50 and p65, approximately 25 mg of each muscle sample was removed, weighed, and subsequently placed in an autoclaved microcentrifuge tube. Nuclear-localized NF-κB was quantified using a Transcription Factor ELISA kit (Panomics, Freemont, CA) to detect activated p50 and p65 subunits. Nuclear extracts were prepared using supplied reagents, and samples were then assayed for protein content utilizing the DC Protein Assay. Assays were then conducted in duplicate according to the manufacturer's instructions. Briefly, 5 g of nuclear extract was loaded in the wells of a 96-well plate coated with oligonucleotides containing the NF-κB consensus sequence (GGGACTTTCC). Then, the wells were incubated with an antibody against either the p50 or the p65 subunit of NF-κB, followed by incubation of a peroxidase-conjugated secondary antibody. The data were expressed as the optical density at 450 nm per milligram of protein.
Statistical Analyses
Data were initially analyzed for normality and homogeneity of variance. An analysis of variance was then conducted on each dependent variable, and Tukey's post hoc analysis was utilized to compare differences between OS/YS and OS/OA groups. All statistical procedures were performed using SPSS 16.0 software (Chicago, IL), and a probability level of less than .05 was adopted throughout.
RESULTS
Lower Limb Lean Mass and Muscular Performance
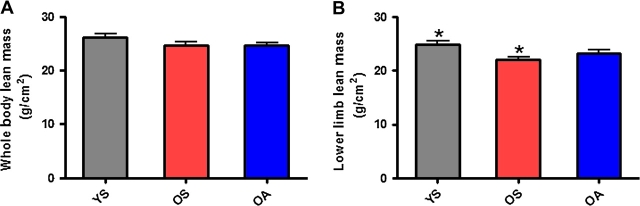
No significant differences were observed among groups for whole-body lean mass (Figure 1A). However, OS possessed significantly (p = .01) less lean mass (g/cm2) in the lower limb than did YS (Figure 1B). No significant differences existed between the two older groups. For isokinetic muscle testing, OS produced significantly less maximal torque compared with both YS (p = .03) and OA (p = .033; Figure 2A). When maximal peak torque was expressed relative to body weight, relative peak torque of OS was again significantly lower than the other two groups (YS [p < .001], OA [p = .004]; Figure 2B). Additionally, a significant difference was observed between OS and YS for isokinetic muscular power (p = .032; Figure 2C). No significant difference was observed between the two older groups for isokinetic muscular power (Figure 2C).
Figure 1.
Aging in the absence of physical activity leads to significant decreases in lower limb lean mass. (A) Whole-body lean mass (g/cm2) in young, physically inactive (gray), older, physically inactive (red), and older, physically active (blue) individuals. (B) Lower limb lean mass (g/cm2) of the nondominant leg. Bars depict means; error bars indicate standard error. Groups sharing a common symbol significantly different at *p < .05.
Figure 2.
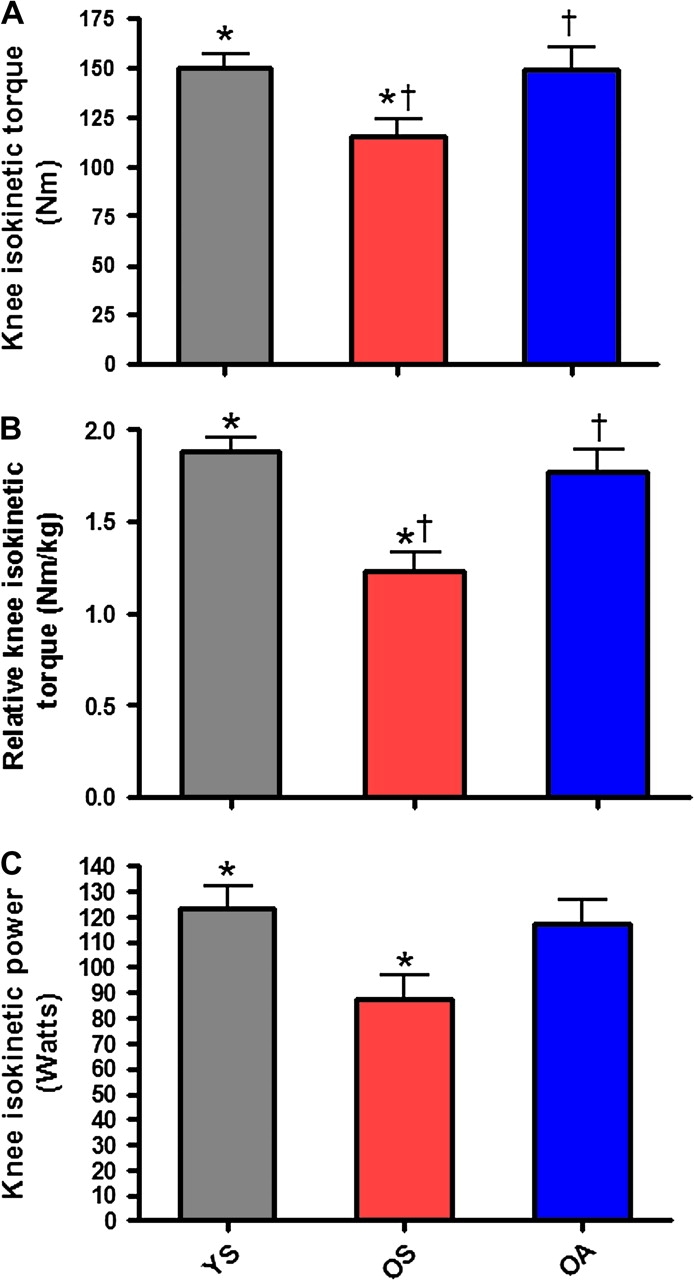
Muscle force production declines significantly as an effect of age and/or inactivity. (A) Maximal isokinetic peak torque (Nm) production of the quadriceps extensors of the nondominant limb in young, physically inactive (gray), older, physically inactive (red), and older, physically active (blue) individuals. (B) Maximal isokinetic peak torque relative to body mass (Nm/kg) of the quadriceps extensors of the nondominant limb. (C) Maximal isokinetic power (W) production of the quadriceps extensors of the nondominant limb. Bars depict means; error bars indicate standard error. Groups sharing a common symbol significantly different at p < .05
* p < .05 between YS/OS.
† p < .05 between OA/OS.
NF-κB Signaling
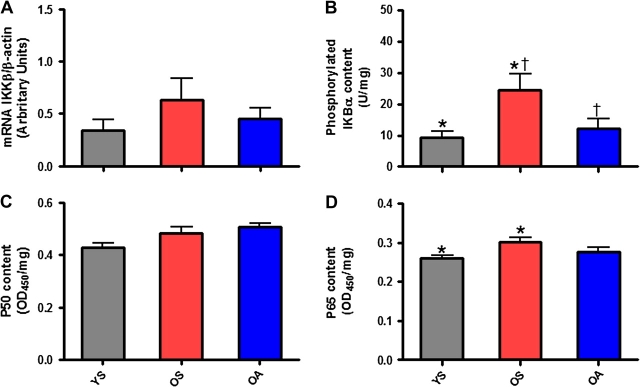
pIKBα was 50% and 61% lower, respectively, in OA (p = .05) and YS (p = .013) compared with OS (Figure 3A). Surprisingly, mRNA expression of skeletal muscle IKKβ relative to expression of β-actin was not significantly different among groups (Figure 3B).
Figure 3.
Nuclear factor kappa B (NF-κB) activity is elevated by advanced age, and p65 is the critical subunit for regulating NF-κB signaling in age-related muscle atrophy. Skeletal muscle messenger RNA (mRNA) expression of IKKβ (IKKβ/β-actin) from biopsies taken from the vastus lateralis of the dominant limb in (A) young, physically inactive (gray), older, physically inactive (red), and older, physically active (blue) individuals. (B) Skeletal muscle content of IKBα phosphorylated at serine 32 (U/mg protein) from vastus lateralis biopsies. (C) Nuclear p50 (OD450/mg protein) from skeletal muscle biopsies of the vastus lateralis. (D) Nuclear p65 (OD450/mg protein) from skeletal muscle biopsies of the vastus lateralis. Bars depict means; error bars indicate standard error. Groups sharing a common symbol significantly different at p < .05
* p < .05 between YS/OS.
† p < .05 between OA/OS.
In conjunction with elevations in pIKBα, OS muscle contained significantly (p = .022) more nuclear p65 (+14%) compared with YS (Figure 3C). Finally, no group differences were observed among groups for nuclear p50 (Figure 3D).
DISCUSSION
Previous data indicate that individuals lose 20%–40% of skeletal muscle mass between the third and ninth decades of life (2,21,22). As our population ages, the loss of skeletal muscle function is affecting large numbers of people and their ability to carry out daily tasks, such as climbing stairs, rising from the toilet, or carrying groceries. Estimates have indicated that approximately 45% of older Americans are sarcopenic (23), whereas approximately 20% are functionally disabled (24). In addition to the individual loss of functional capabilities, the economic impact of sarcopenia is also dramatic as it has been estimated that the direct health care costs of sarcopenia in the United States were approximately $18 billion at the turn of the century (25).
Subsequently, researchers are actively looking for promising molecular biomarkers that may serve as targets for therapeutic interventions for sarcopenia. Although NF-κB is involved in numerous physiological processes, including immunity and inflammation (5), tumorigenesis (26), and tissue development and differentiation (27), it has also been shown to be a crucial signaling molecule in the pathogenesis of skeletal muscle atrophy (28). Previous data showed that NF-κB signaling is upregulated within aged skeletal muscle (16,29,30). However, clinical studies investigating the role of NF-κB in skeletal muscle atrophy are limited.
The present study examined NF-κB signaling in human skeletal muscle biopsy samples from sedentary young and older men. In addition, the study investigated whether NF-κB activation was decreased in older men performing regular physical exercise compared with their sedentary counterparts. Our results indicated that age increases nuclear-bound content of p65 as the protein was significantly higher in OS than YS. These results agree with previous data from animal models, which indicated that aging results in greater p65 content with skeletal muscle (29,30).
Not surprisingly, elevated nuclear p65 in OS was associated with significantly increased pIKBα. It was surprising, however, that nuclear content of neither p50 nor p65 differed between OS and OA in spite of a twofold difference in pIKBα. Equally as surprising is the relatively small difference in nuclear p65 between groups when pIKBα differed quite robustly. We believe each of these findings may be explained by changes in ubiquitin–proteasome activity. IKB phosphorylation and subsequent degradation by the proteasome are thought to immediately precede NF-κB translocation to the nucleus. We speculate that when IKBα phosphorylation is increased, proteasome degradation of pIKBα is slowed as compensatory mechanism. This proposed mechanism may serve to slow NF-κB nuclear translocation in attempt to slow protein degradation. Future data are needed to support or refute this speculation.
The authors acknowledge that the present study has several limitations. First, the results are only applicable to men as women were not included in the study due to resource restraints. Second, if additional resources were available, a group consisting of trained younger men would have added significant value in determining the precise role that physical activity and NF-κB signaling plays across the life span. Finally, the study provides utility as a cross-sectional study, but future longitudinal studies should include untrained individuals who either remain sedentary or adhere to a training program to further elucidate the effects of exercise on NF-κB signaling within skeletal muscle.
Nevertheless, we demonstrated that skeletal muscle content of pIKBα is altered by both aging and physical activity status in older men. Furthermore, p65 appears to be upregulated in older skeletal muscle. Future investigations are needed to examine the apparent incongruence between changes in pIKBα and nuclear-bound NF-κB family members. In addition, future longitudinal studies should examine specific training modalities and their effects on NF-κB signaling in older adults.
FUNDING
This work was supported by the Baylor University Young Investigator Development Program (Award No. 030153134 [M.B.C.]), the Doctoral Student Research Program Award by the National Strength and Conditioning Association (T.W.B.), and the Exercise and Biochemical Nutrition Laboratory at Baylor University. T.W.B. is currently supported by the University of Florida Institute on Aging and Claude D. Pepper Older Americans Independence Center (1 P30 AG028740).
Acknowledgments
The authors would like to thank all 41 study participants for their willingness to participate in the study. In addition, we thank Geoffrey Hudson, Brian Shelmadine, and Liz Redd for assistance with performance testing.
References
- 1.Berg HE, Dudley GA, Haggmark T, Ohlsen H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol. 1991;70:1882–1885. doi: 10.1152/jappl.1991.70.4.1882. [DOI] [PubMed] [Google Scholar]
- 2.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 3.Cesari M, Pahor M. Target population for clinical trials on sarcopenia. J Nutr Health Aging. 2008;12:470–478. doi: 10.1007/BF02982708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Cancer cachexia. Langenbecks Arch Surg. 2004;389:299–305. doi: 10.1007/s00423-004-0486-7. [DOI] [PubMed] [Google Scholar]
- 5.Cai D, Frantz JD, Tawa NE, Jr,, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 7.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 8.Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 9.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 11.Willoughby DS, Stout JR, Wilborn CD. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids. 2007;32:467–477. doi: 10.1007/s00726-006-0398-7. [DOI] [PubMed] [Google Scholar]
- 12.Porter MM, Nelson ME, Fiatarone Singh MA, et al. Effects of long-term resistance training and detraining on strength and physical activity in older women. J Aging Phys Act. 2002;10:260–270. [Google Scholar]
- 13.Radak Z, Chung HY, Naito H, et al. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18:749–750. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Lopez D, Cuevas MJ, Almar M, Lima E, De Paz JA, Gonzalez-Gallego J. Effects of eccentric exercise on NF-kappaB activation in blood mononuclear cells. Med Sci Sports Exerc. 2007;39:653–664. doi: 10.1249/mss.0b013e31802f04f6. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez-Jimenez R, Cuevas MJ, Almar M, et al. Eccentric training impairs NF-kappaB activation and over-expression of inflammation-related genes induced by acute eccentric exercise in the elderly. Mech Ageing Dev. 2008;129:313–321. doi: 10.1016/j.mad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Shai M, Carmeli E, Reznick AZ. The role of NF-kappaB in protein breakdown in immobilization, aging, and exercise: from basic processes to promotion of health. Ann N Y Acad Sci. 2005;1057:431–447. doi: 10.1196/annals.1356.034. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Shai M, Carmeli E, Ljubuncic P, Reznick AZ. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-kappaB activation. Free Radic Biol Med. 2008;44:202–214. doi: 10.1016/j.freeradbiomed.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Willoughby DS. Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc. 2004;36:574–582. doi: 10.1249/01.mss.0000121952.71533.ea. [DOI] [PubMed] [Google Scholar]
- 19.Mahoney DJ, Carey K, Fu MH, et al. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol Genomics. 2004;18:226–231. doi: 10.1152/physiolgenomics.00067.2004. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 22.Short KR, Nair KS. Muscle protein metabolism and the sarcopenia of aging. Int J Sport Nutr Exerc Metab. 2001;11(suppl):S119–S127. doi: 10.1123/ijsnem.11.s1.s119. [DOI] [PubMed] [Google Scholar]
- 23.Melton LJ, 3rd, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- 24.Manton KG, Gu X. Changes in the prevalence of chronic disability in the united states black and nonblack population above age 65 from 1982 to 1999. Proc Natl Acad Sci U S A. 2001;98:6354–6359. doi: 10.1073/pnas.111152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 26.Perkins ND. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. 2004;14:64–69. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Ventura JJ, Tenbaum S, Perdiguero E, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med. 2008;86:1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar-Shai M, Carmeli E, Coleman R, et al. The effect of hindlimb immobilization on acid phosphatase, metalloproteinases and nuclear factor-kappaB in muscles of young and old rats. Mech Ageing Dev. 2005;126:289–297. doi: 10.1016/j.mad.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]