Abstract
Fat distribution changes with aging. Inherent changes in fat cell progenitors may contribute because fat cells turn over throughout life. To define mechanisms, gene expression was profiled in preadipocytes cultured from epididymal and perirenal depots of young and old rats. 8.4% of probe sets differed significantly between depots, particularly developmental genes. Only 0.02% differed with aging, despite using less stringent criteria than for comparing depots. Twenty-five genes selected based on fold change with aging were analyzed in preadipocytes from additional young, middle-aged, and old animals by polymerase chain reaction. Thirteen changed significantly with aging, 13 among depots, and 9 with both. Genes involved in inflammation, stress, and differentiation changed with aging, as occurs in fat tissue. Age-related changes were greater in perirenal than epididymal preadipocytes, consistent with larger declines in replication and adipogenesis in perirenal preadipocytes. Thus, age-related changes in preadipocyte gene expression differ among depots, potentially contributing to fat redistribution and dysfunction.
Keywords: Aging, Preadipocyte, Fat cell progenitor
AGE-RELATED changes in fat mass, distribution, and function are associated with elevated risk of common diseases, including atherosclerosis, diabetes, and hypertension (1–4). Together with age- and fat depot–related differences in innervation, hormonal exposure, or circulation, inherent properties of resident fat cell progenitors, preadipocytes, may contribute to variation in fat depot size and function with aging because fat tissue turns over throughout life (5). Indeed, fat cell numbers actually increase in certain fat depots between middle and old age (6–8). Fifteen to 50 percent of cells in fat are preadipocytes (9). Preadipocyte abundance increases or remains constant with aging in different fat depots. Preadipocyte replication and differentiation decline with aging at different rates among depots (10–14). Age-related and regional differences in preadipocyte function persist in colonies arising from single preadipocytes after many weeks in culture and multiple cell divisions (11,13,15–18). Differences in properties of preadipocytes during aging or among depots are reflected in the fat tissue that develops from them (12,14,18). Thus, inherent characteristics of preadipocytes may contribute to variation in fat tissue function with aging and among fat depots.
To test the hypothesis that age-dependent changes in gene expression can vary in progenitors from different regions of the same tissue, we compared expression profiles of preadipocytes that were isolated from epididymal and perirenal depots of young and old rats after being cultured under identical conditions. Cells from these two depots were studied because we previously found greater differences in capacities for replication and differentiation between perirenal (which are extraperitoneal) and epididymal preadipocytes than other fat depots with aging (13,14). We validated select differences in real-time polymerase chain reaction (PCR) and Western blot analyses.
METHODS
Preadipocyte Culture
Preadipocytes were isolated from epididymal and perirenal [caudal portion to exclude brown fat (14)] depots of 3- (young), 17- (middle-aged), and 30- (old) month male, barrier-reared, specific pathogen-free Brown Norway rats (median survival 32 months; maximum survival 43 months (19,20); National Institute on Aging colony maintained by Harlan Sprague Dawley, Indianapolis, IN). The protocol was approved by the Institutional Animal Care and Use Committee. Each N represents separate groups of animals, with animals in different age groups being studied in parallel. Animals were autopsied to exclude gross pathology. Fat depots were minced, digested in collagenase (1 mg collagenase/mL Hank’s balanced salt solution; 3 mL/g tissue), filtered, and centrifuged at 300 g for 10 minutes (14). The pellets were resuspended in α-minimal essential medium (α-MEM) containing 10% fetal bovine serum. Cells were plated for 20 hours, washed, trypsinized until 90% of the cells had lifted, and replated at a density of 4 × 104 cells/cm2. This results in more than 90% pure preadipocyte populations (determined by counting colonies derived from single cells that accumulate more lipid than similarly treated skin or lung fibroblasts), irrespective of age or depot of origin (13,18). Macrophage markers did not differ substantially with aging or in a consistent direction among depots (see Results section and Table 3). Preadipocyte recoveries, determined by adding various numbers of preadipocytes to fat tissue aliquots before processing, are similar across depots and age groups (9).
Table 3.
Macrophage Contamination Does Not Explain the Changes Observed With Aging in Preadipocyte Cultures
| Macrophage Marker | Young Epididymal | Old Epididymal | Young Perirenal | Old Perirenal | Min. Cycles | Age ANOVA p Value | Age × Depot ANOVA p Value |
| Cd 68 | 9.9 ± 2.2 | 15.9 ± 2.2 | 14.5 ± 3.7 | 15.6 ± 3.7 | 24 | 0.24 | 0.41 |
| Ccl3 | 14.5 ± 6.1 | 21.0 ± 4.5 | 11.3 ± 3.5 | 13.6 ± 4.0 | 27 | 0.35 | 0.65 |
| Cd 11b; Mip-1; α-M integrin | 9.2 ± 1.7 | 13.5 ± 1.8 | 13.9 ± 3.4 | 20.1 ± 3.5 | 25 | 0.06 | 0.72 |
| F4/80; Emr1 | 16.7 ± 4.2 | 20.3 ± 3.9 | 11.0 ± 3.9 | 10.1 ± 3.1 | 27 | 0.40 | 0.25 |
| Scya4; Mip-1β | 79.7 ± 7.0 | 90.6 ± 22.2 | 205.8 ± 22.1 | 152.8 ± 9.5 | From array | 0.09 | 0.24 |
Notes: Consistent differences in macrophage markers were not evident among preadipocyte cultures from different age groups. mRNA levels were assayed in primary preadipocyte cultures from perirenal and epididymal depots of young (3 mo) and old (30 mo) rats. Scya4 was the only transcript that met the criterion for being considered detectable on Affymetrix U230 arrays. The other transcripts were assayed by real-time polymerase chain reaction (PCR; N = 6 determinations, each from different sets of rats; means ± SEM are shown; Min. cycles = minimum number of PCR cycles required for detection). ANOVA = analysis of variance.
Expression Array processing and Data Analysis
Total RNA was isolated from preadipocytes with TRIzol (Invitrogen, Carlsbad, CA). Using a poly-dT primer incorporating a T7 promoter, double-stranded cDNA was synthesized from 10 μg total RNA using a Superscript cDNA synthesis kit (Invitrogen). Biotin-labeled cRNA was generated from the double-stranded cDNA template through in vitro transcription with T7 polymerase using an RNA transcript labeling kit (Enzo Diagnostics, Farmingdale, NY). Biotinylated cRNA was purified using RNeasy affinity columns (Qiagen, Valencia, CA) and fragmented in 40 mM Tris–acetate, pH 8.1, 100 mM KOAc, 30 mM MgOAc for 35 minutes at 94°C to 35–200 bases. cRNA (10 μg) and controls (Affymetrix, Santa Clara, CA) were hybridized to Affymetrix Rat Genome 230 2.0 GeneChip arrays containing 15,923 probe sets and washed and stained according to the Antibody Amplification for Eukaryotic Targets protocol (Affymetrix). The arrays were scanned at 488 nm using an Affymetrix GeneChip Scanner 3000 (Affymetrix). Expression estimates were derived using the rate monotonic analysis processing and normalizing algorithm (21). Data were deposited into the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/; accession number GSE6699).
Real-Time PCR Analysis
Total RNA was prepared as earlier. First strand cDNA was prepared from total RNA using a SuperScript II reverse transcriptase kit (Invitrogen). Real-time PCR was carried out using TaqMan Fast Universal PCR Master Mix 2× in a 7500 Fast Real Time PCR System (Applied Biosystems, Foster City, CA). In brief, 10 μl of Fast PCR Master Mix were combined with 5 μl of cDNA, 1 μl of the appropriate TaqMan primer, and 4 μl of water. Following an initial 95°C incubation for 20 seconds, PCR was carried out for 40 cycles at 95°C for 3 seconds and 60°C for 30 seconds. RNA was analyzed by relative quantification using 18S rRNA as an internal control.
Western Blot Analysis
Matrix metalloproteinase (MMP) 3 and 12 proteins were assayed by Western blot analysis (15). Briefly, protein was extracted using radioimmunoprecipitation assay buffer, and concentrations were determined by Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). Thirty micrograms of protein in 5× loading buffer were heated at 95°C for 10 minutes, placed on ice, and then briefly centrifuged. Y79 (MMP3) and J774 (MMP12) cell lysates (10 μg; Santa Cruz Biotechnology, Santa Cruz, CA) were used as positive controls. Protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10%) and transferred to poly-vinylidene fluoride membranes (Amersham Biosciences, Piscataway, NJ). Equal amounts of protein from undifferentiated preadipocytes from young or old rats were loaded in parallel on the same gels. Protein transfer was confirmed by Ponceau dye staining. Membranes were blocked with 5% milk fat with or without 0.5% bovine serum albumin, then incubated with primary antibody overnight at 4°C (goat anti-MMP3 IgG [1:200] or goat anti-MMP12 IgG [1:200]). Antibodies were from Santa Cruz Biotechnology. Satisfactory antibodies for rat Stmn-2 were not available. Membranes were incubated with secondary antibody: donkey anti-goat horse radish peroxidase (1:2000) for 1 hour at room temperature. Secondary antibody binding was visualized by chemiluminescense. Scanning densitometry was performed using a Hewlett Packard 3970 scanner (Hewlett Packard, Palo Alto, CA) and Quantiscan software (Biosoft, Ferguson, MT). Densitometric results were expressed as a percentage of total optical density within each gel and normalized to reflect differences in cellular protein content (total protein contents were 305 ± 32, 325 ± 36, and 335 ± 39 pg/cell [± SEM] in undifferentiated perirenal preadipocytes from 3-, 17-, and 30-month old rats, respectively, and 336 ± 32, 346 ± 35, and 360 ± 39 pg/cell in epididymal cells, respectively [N = 16 in each group]).
Data Analysis
Only those probe sets that exhibited sequence-specific hybridization intensity in at least one of the samples were included in the analysis (10,983 of 15,923 probe sets). Fold change was calculated using the average normalized signal from the samples in each of the groups. The significance of observed expression differences was determined using two-way analysis of variance (ANOVA) in which age and depot were the main effects. The limma package within R was used in part for this analysis (22). To correct probabilities of differential expression for multiple hypothesis testing, we used the false discovery rate (FDR) method (23) that estimates the proportion of type I errors within a group of probe sets meeting a significance cutoff. To assess similarities and differences among gene expression profiles, we used hierarchical clustering (using R; http://www.cran.r-project.org). Genes were annotated using NetAffx (Affymetrix) and Resourcerer (The Institute for Genomic Research, Rockville, MD). For analyses of real-time PCR and Western blot studies, t tests or ANOVA with appropriate post hoc comparisons were used (24,25). Unless otherwise noted, two-tailed hypothesis tests with p less than .05 were considered significant.
RESULTS
Preadipocytes From Different Fat Depots Have Distinct Expression Profiles
Consistent with the contention that preadipocytes from different fat depots are distinct cell subtypes, we found that expression of many genes differs considerably between epididymal and perirenal preadipocytes (Figure 1A). Eight percent of probe sets detected in undifferentiated preadipocytes were significantly differentially expressed between depots (FDR < 0.01 by ANOVA) and varied at least twofold. Developmental genes were prominent among the gene categories that varied between depots (Table 1), as we previously reported in human preadipocytes (17) and as has been found in mice (26). Indeed, rat homologs of human developmental genes that vary inherently among human abdominal subcutaneous, mesenteric, and omental preadipocytes (17) also differed between rat epididymal and perirenal preadipocytes (Table 2). Other genes that varied among depots were those involved in cell dynamic processes (replication, differentiation, apoptosis) and metabolism (Table 1). Fat tissue, particularly from visceral depots, can contain macrophages. The differences we found are not likely to be due to contamination of our primary cultures with macrophages as (a) differential plating was used to remove macrophages, (b) preadipocytes were plated at subconfluent density and replicated until confluence, which dilutes any macrophages as they do not divide, (c) fewer than two per 106 cells had the distinct morphology of macrophages by microscopic examination, and (d) macrophage markers were not consistently higher in cultures from either the perirenal or epididymal depot or from old compared with young animals (Table 3).
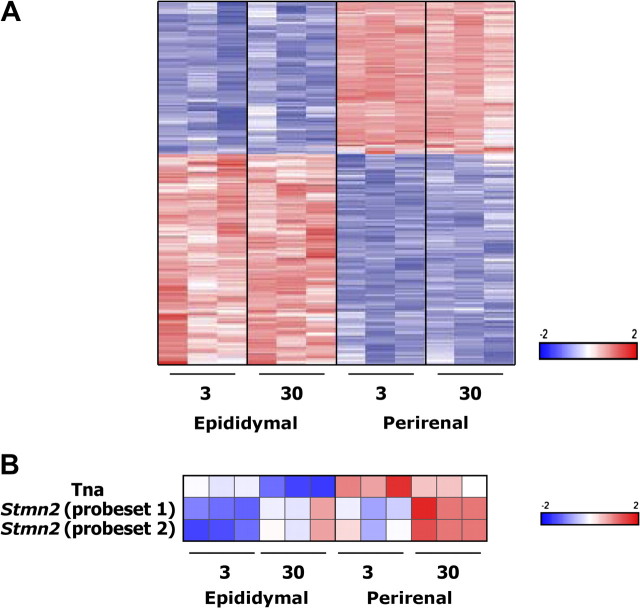
Figure 1.
Preadipocyte expression profiles differ extensively among fat depots and less prominently with aging. A). Regional variation in preadipocyte expression profiles. Nine hundred and twenty-one transcripts (of 10,983 probe sets detected) demonstrated both a twofold or greater difference in expression between undifferentiated perirenal compared with epididymal preadipocytes from 3- and 30-month-old rats and a depot-effect false discovery rate (FDR) less than 0.01 by analysis of variance (ANOVA). Data shown were z-score normalized and organized by hierarchical clustering, with each column representing a single animal. Full names, accession numbers, and hybridization intensities are in Supplementary Table 1. B). Stathmin-like 2 (Stmn-2) and tetranectin (Tna) increase with aging in preadipocytes. Only 3 out of 10,983 probe sets demonstrated significant age-dependent differences in expression in comparisons of preadipocytes isolated from 3-month compared with 30-month-old rats, despite use of less stringent criteria than those used to detect differences among fat depots (FDR < 0.08 by ANOVA and no fold-change criterion).
Table 1.
Developmental Genes Are Overrepresented Within Transcripts Differentially Expressed Between Depots
| Key Word | Key Word Searches of the 921 Genes
Differing by Depot |
Key Word Searches of the 10,986 Present
Probe Sets |
Adjusted p Value | ||
| No. of Genes | % of Total | No. of Genes | % of Total | ||
| Development | 67 | 7.27 | 128 | 1.16 | 0.000* |
| Stress | 12 | 1.30 | 63 | 0.57 | 0.236 |
| Immune | 7 | 0.76 | 45 | 0.41 | 0.684 |
| Cytokine | 4 | 0.43 | 70 | 0.64 | 0.836 |
| Metabolism | 52 | 5.64 | 306 | 2.79 | 0.011* |
| Biosynthesis | 32 | 3.47 | 254 | 2.31 | 0.305 |
| Growth | 24 | 2.60 | 260 | 2.37 | 1.000 |
| Apoptosis | 28 | 3.04 | 118 | 1.07 | 0.011* |
| Differentiation | 32 | 3.47 | 80 | 0.73 | 0.000* |
| Proliferation | 40 | 4.34 | 76 | 0.69 | 0.000* |
Notes: Proportions of transcripts differentially expressed between depots in different gene ontology functional categories are shown (921 transcripts varied significantly between depots out of 10,983 probe sets detected by array analysis). Fisher’s exact tests were performed to measure overrepresentaiton or underrepresentation of each functional category; p values were adjusted by using false discovery rate procedure to control the number of false-positive results.
*p < .05.
Table 2.
Rat Homologues of Developmental Genes That Vary Among Depots in Human Preadipocytes
| Gene Symbol | Perirenal/Epididymal Fold Difference | Adjusted p Value (Perirenal vs Epididymal) |
| Krt1-18 (Krt-18) | 3.39 | 0.0332 |
| Hoxa4 | 2.49 | 0.0056 |
| Hoxa5 | 2.53 | 0.0060 |
| Hoxa2 | 1.03 | 0.7610 |
| Pitx2 | 4.17 | 0.0212 |
| Twist1 | 7.72 | 0.0106 |
| Prrx1 (Pmx-1) | 9.78 | 0.0003 |
| Prrx2 (Prx-2) | 1.48 | 0.0003 |
| Kitl (Kitlg) | 2.80 | 0.0007 |
| Hoxa10 | 9.36 | 0.0012 |
Notes: We previously confirmed inherent variation of 20 developmental genes among human preadipocytes isolated from different fat depots [figure 4 in (17)]. Of these, 11 have homologs on Affymetrix rat 230 array sets, 10 of which met the same scaling criteria for expression in rat preadipocytes as in our human studies. Of the 10 genes present, 9 exhibited differences in expression between perirenal and epididymal preadipocytes (false discovery rate < 0.05), despite species differences and differences in depots being compared in the human and rat studies.
Fewer Genes Varied With Aging Than Among Fat Depots
Age-related changes in expression profiles of undifferentiated preadipocytes were modest compared with regional differences. Despite using less stringent criteria than those used to detect variation among fat depots, only three probe sets representing two genes exhibited a statistically significant difference between young (3 months) and old (30 months) rats in the array analyses. These probe sets were selected from a two-way ANOVA that examined the effect of age and depot on gene expression. Age effect p values were adjusted by the FDR method, and those with FDR less than 0.08 were selected, with no constraints on the fold change between age groups (Figure 1B). These genes were stathmin-like 2 (Stmn-2) and tetranectin (Tna). Notably, substantial changes in developmental gene expression were not found with aging.
Age-Related Differences in Expression Profiles Are Fat Depot Dependent
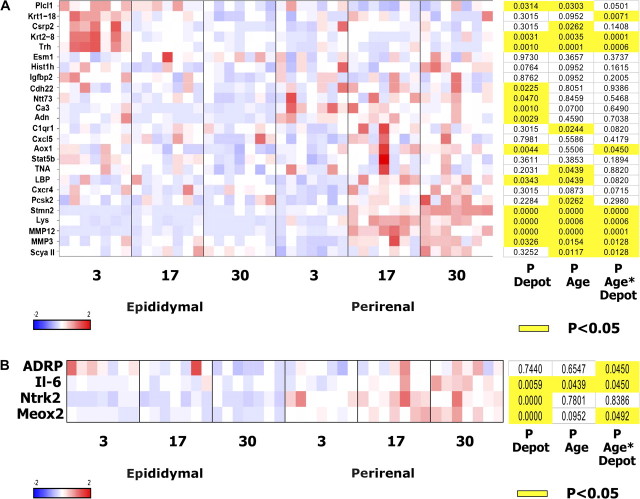
From the array data, 30 probe sets were selected for further analysis by PCR based on fold changes across age groups only, without focusing on depot-related expression changes or known function. These probe sets, which encoded 25 known genes, were chosen through this approach to avoid potential bias involved in selection based on known function. Undifferentiated perirenal and epididymal preadipocytes were cultured from middle-aged as well as additional young and old animals to distinguish changes during the maturational phase of the life span from those during the senescent phase and to increase statistical power. Changes in expression of the 25 genes across age groups were generally more extensive in perirenal than epididymal preadipocytes, consistent with the hypothesis that extent of age-related changes in progenitor properties varies among regions, as well as with our previous observation that age-related declines in replicative potential and adipogenesis are greater in perirenal than epididymal preadipocytes [Figure 2A (13)]. Twelve of the 25 genes are involved in inflammation, tissue repair, or stress, and five are involved in regulating differentiation (Table 4). Interestingly, the nine genes (C1qr1, Tna, lipopolysaccharide-binding protein, Pcsk-2, Stmn-2, lysozyme, small inducible cytokine A2, Mmp-3, and Mmp-12) that significantly increased in expression with aging tended to do so to a greater extent in perirenal than epididymal preadipocytes, whereas the four genes that decreased with aging tended to do so to a greater extent in epididymal cells (phospholipase C–like1, Csrp2, Krt2-8, and Trh). Of the nine genes exhibiting a significant change in expression with respect to the Depot × Age interaction, three differed significantly with aging in epididymal cells and six differed significantly with aging in the perirenal cells (Tukey’s test). Thus, changes in gene expression occur in undifferentiated preadipocytes with aging, some of these changes are fat depot dependent, and genes involved in cellular stress and regulation of differentiation are among those that change. Adiponectin decreased in epididymal preadipocytes with aging but changed less in perirenal cells, so although preadipocyte adiponectin was similar between depots in young animals, it was higher in perirenal than epididymal preadipocytes of old animals (p < .05; Student’s t test). Four additional genes relevant to fat tissue function or with potentially distinctive patterns suggested by the gene array studies were also analyzed by real-time PCR (Figure 2B). IL-6 increased with aging in a fat depot–dependent manner. Depot-dependent changes in Stmn-2, Mmp-3, and Mmp-12 with aging indicated in Figure 2A are plotted in Figure 3A–C, respectively.
Figure 2.
Changes in gene expression with aging tend to be fat depot dependent. A). Genes most upregulated or downregulated between age groups were further tested by real-time polymerase chain reaction (PCR) in additional animals. RNA was isolated from perirenal and epididymal undifferentiated preadipocytes from young (3 months), middle-aged (17 months), and old (30 months) rats. Data shown are z-score-normalized and organized by hierarchical clustering. Each column represents data from a single animal (N = 7 animals in each age group). p values were computed by two-way fixed effects analysis of variance and adjusted using the false discovery rate method. Gene descriptions are in Table 4. B). Four additional genes relevant to fat tissue function or with distinctive profiles suggested from the array analyses were assayed by real-time PCR.
Table 4.
Function of Genes Examined by Real-Time Polymerase Chain Reaction for Changes With Aging
| Name | Symbol | Aliases | Function |
| Phospholipase C–like 1 | Plcl1 | PLC-L (PLC-ϵ), PLDL | Catalyzes hydrolysis of phosphatidylinositol 4,5 biphosphate to diacylglycerol and inositol 1,4,5 triphosphate |
| Keratin, type I cytoskeletal 18 | Krt1-18 | Keratin 18 | Structural constituent of cytoskeleton that dimerizes with KRT8 |
| Cysteine and glycine-rich protein 2 | Csrp2 | Cysteine-rich protein, smooth muscle cell LIM protein | Downregulated in response to platelet-derived growth factor or cell injury, promotes smooth muscle cell proliferation and dedifferentiation |
| Keratin, type II cytoskeletal 8 | Krt2-8 | Keratin 8, cardiac autoantigen 2, 120 kDa | Keratin 8, type II, early embryonic, dimerizes with KRT1 |
| Thyrotropin-releasing hormone | Trh | Thyroliberin precursor | Regulates thyroid stimulating hormone biosynthesis in the anterior pituitary, neurotransmitter/neuromodulator in the central nervous system and peripheral nervous system |
| Endothelial cell–specific molecule 1 | Esm1 | ESM-1 secretory protein | Implications in lung endothelial cell–leukocyte interactions, induction by tumor necrosis factor (TNF)-α and IL-1β |
| Testis-specific histone H2B | Hist1h | Histone 1, H2ba, histone H2B, testis | Histone H2B family protein, nucleosome assembly activity, chromatin organization, and remodeling |
| Insulinlike growth factor–binding protein 2 | Igfbp2 | IBP-2 | Alters interaction of IGF with cell surface receptors, inhibits or stimulates growth promoting effects of IGF |
| Cadherin-22 | Cdh22 | Calcium-dependent cell adhesion proteins, sorting of heterogeneous cell types | |
| Solute carrier family 6 member 15 | Ntt73 | Solute carrier family 6 (neurotransmitter transporter), member 15, orphan transporter v7-3 | Orphan transporter |
| Carbonic anhydrase 3 | Ca3 | Reversible hydration of carbon dioxide | |
| Adiponectin | Adn | Adipocyte, C1q and collagen domain–containing protein, adipose most abundant gene transcript 1, adipocyte complement related protein of 30 kDa | Negative regulator of endothelial nuclear factor-κB and TNF-α signaling, control of fat metabolism and insulin sensitivity |
| Complement component 1, q subcomponent, receptor 1 | C1qr1 | Lymphocyte antigen 68, C1q/MBL/SPA receptor, CD93 antigen | Receptor (or element of a larger receptor complex) for C1q, mannose-binding lectin (MBL2), and pulmonary surfactant protein A (SPA) |
| Chemokine (C-X-C motif) ligand 5 | Cxcl5 | SCYB5, Neutrophil-activating peptide ENA-78 | Neutrophil activation, inflammation/injury |
| Aldehyde oxidase | Aox1 | Enzyme | |
| Signal transducer and activator of transcription 5B | Stat5b | Signal transduction and activation of transcription, activated by interleukin 2 and growth hormone | |
| Tetranectin | TNA | C-type lectin domain family 3, member B, plasminogen kringle 4–binding protein | Binds to plasminogen and isolated kringle 4, may be involved in packaging of molecules destined for exocytosis |
| Lipopolysaccharide-binding protein | LBP | Binds to lipid A moiety of bacterial lipopolysaccharides, LBP/LPS complexes interact with CD14 receptors, inflammation | |
| Chemokine (C-X-C motif) receptor 4 | Cxcr4 | CD184 antigen, leukocyte-derived seven transmembrane domain receptor, neuropeptide Y receptor Y3 | Receptor for the C-X-C chemokine CXCL12/SDF-1, involved in hematopoiesis and vascularization of the gastrointestinal tract, inflammation |
| Proprotein convertase subtilisin/kexin type 2 | Pcsk2 | NEC 2, KEX2-like endoprotease 2, prohormone convertase 2 | Release of protein hormones and neuropeptides from their precursors, generally by hydrolysis of -Lys-Arg- bonds |
| Stathmin-like 2 | Stmn2 | SCG10, superior cervical ganglion-10 protein | Role in neuronal differentiation and modulating membrane interaction with the cytoskeleton during neurite outgrowth |
| Lysozyme | Lys | 1,4-beta-N-acetylmuramidase C, lysozyme C precursor | Bacteriolytic, associated with the monocyte–macrophage system, inflammation |
| Matrix metalloproteinase 12 | MMP12 | Macrophage elastase, macrophage metalloelastase precursor | Involved in tissue injury and remodeling, elastolytic activity |
| Matrix metalloproteinase 3 | MMP3 | Matrix metallopeptidase 3, stromelysin 1, progelatinase | Degrades fibronectin; laminin; gelatins; collagen III, IV, X, and IX; and cartilage proteoglycans; activates procollagenase, inflammation/injury |
| Small inducible cytokine A2 | Scya II | CCL2, monocyte chemotactic protein, monocyte secretory protein | Attracts monocytes and basophils, augments monocyte antitumor activity, inflammation |
Note: Full gene names, abbreviations, and function of transcripts depicted in Figure 2A are shown.
Figure 3.
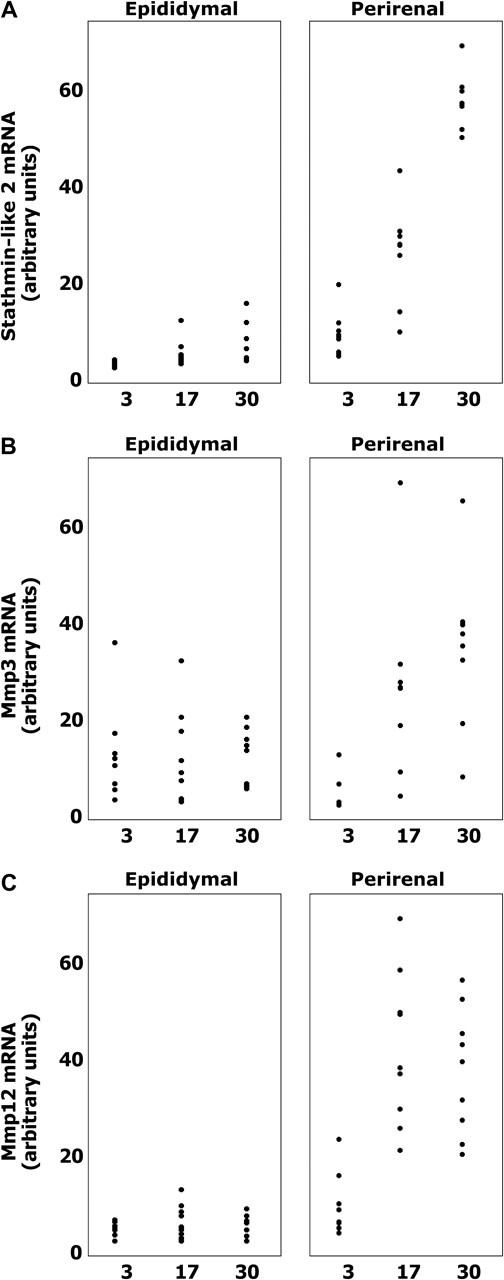
Stmn-2, Mmp-3, and Mmp-12 increase more extensively with aging in perirenal than epididymal preadipocytes. Stathmin-like 2 (A), matrix metalloproteinase 3 (Mmp-3); (B), and Mmp-12 (C) mRNA levels in undifferentiated perirenal and epididymal preadipocytes cultured from young (3 months), middle-aged (17 months), and old (30 months) rats were assayed by real-time polymerase chain reaction (N = 7 animals in each group; *p < .05; Duncan’s multiple range test; data plotted from Figure 2A).
Age-Related Changes in MMP3 and MMP12 Protein Are Depot Dependent
Mmp-3 and Mmp-12 protein levels increased in perirenal preadipocytes with aging, unlike epididymal cells (Figure 4), as was the case with Mmp-3 and Mmp-12 mRNA (Figure 3). Unlike Mmp-3 and Mmp-12 mRNA, protein levels in epididymal preadipocytes were not substantially lower than in perirenal cells, suggesting differences in translational or posttranslational processing of these proteins between depots.
Figure 4.
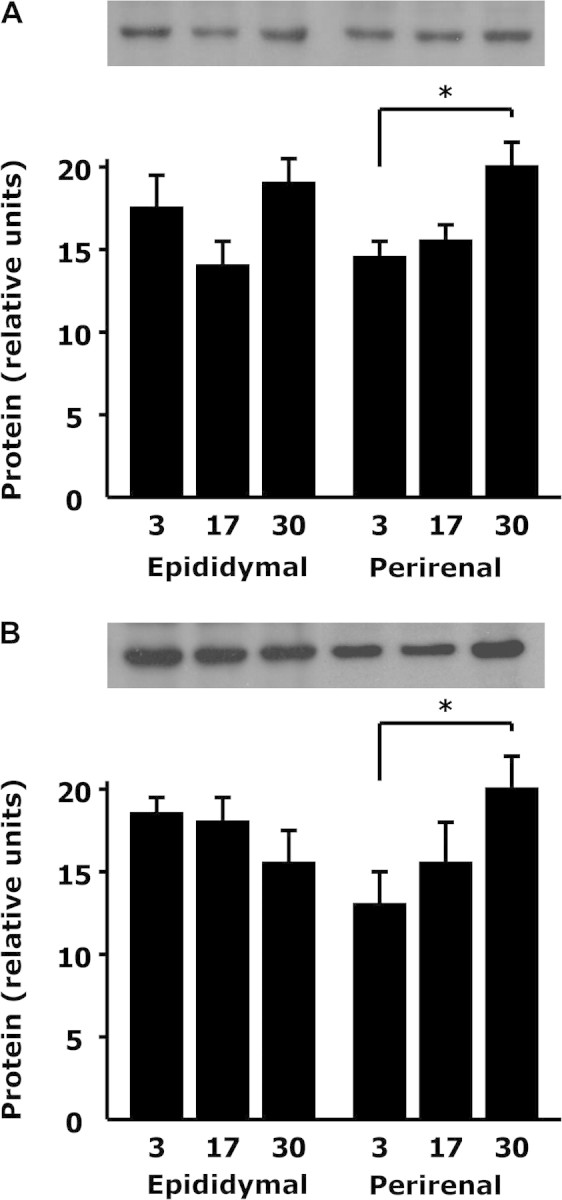
Matrix metalloproteinase (Mmp) 3 and Mmp-12 proteins increase with aging in a depot-dependent manner. Lysates of undifferentiated perirenal and epididymal preadipocytes cultured from young (3 months), middle-aged (17 months), and old (30 months) rats were analyzed by Western blotting for Mmp3 (A) and Mmp12 (B). *p < .05 by analysis of variance; N = 7.
DISCUSSION
Preadipocytes, which give rise to new fat cells throughout life, are among the most abundant type of progenitor in the body. Their numbers can exceed those of other cell types in fat tissue, including fat cells (9). The fat tissue that develops from them is, in turn, at the nexus of processes involved in longevity and the genesis of age-related metabolic disease. Preadipocyte gene expression profiles are distinct from those of fat cells, as is their function (17). For example, preadipocytes are a greater source of inflammatory cytokines than fat cells and produce chemokines that attract lymphocytes and macrophages (27–29). They also process and release paracrine factors and hormones in a fashion distinct from fat cells. Thus, given their numbers and distinct function, they are an important cell type to study in their own right (30). Unlike many other progenitor types, fat cell progenitors are generally resident in fat tissue (17,26), with circulating progenitors making a minor contribution to new fat cell development under certain conditions (31,32). Preadipocytes from different fat depots vary in capacities for replication and differentiation. Distinct characteristics of preadipocytes from different depots affect the fat tissue that develops from them, as we showed previously (18,33), with different fat depots being effectively distinct mini-organs. Thus, trajectories of age-related changes in preadipocyte function with aging could vary among fat depots, potentially contributing to the fat redistribution and metabolic dysfunction that are common in old age (1).
To our surprise, we found that although changes in preadipocyte gene expression patterns do occur with aging, they are considerably less prominent than differences among depots. This finding is consistent with parabiosis experiments, which suggested that tissue microenvironmental changes with aging might have a bigger effect on progenitor function than inherent effects of aging on progenitors themselves (34). Transcriptional measures of 921 probe sets showed statistically significant differential expression across depots, with developmental genes being prominent among those that vary, consistent with studies in mice and humans (17,26). Although more than 8% of genes differed among depots in our array analyses using stringent criteria, only two genes varied with aging using less stringent criteria. Unlike differences among fat depots, aging did not involve substantial changes in developmental gene expression.
Because (a) overall expression measures of preadipocytes from different depots were distinct in the array analyses with respect to 921 probe sets, (b) regional variation in extent of age-related changes in preadipocyte replication and capacity for adipogenesis have been reported (11–13), and (c) trajectories of age-related changes in fat depot size, preadipocyte number, and fat cell size and number are depot dependent (6,7,9), we tested the hypothesis that age-related changes in preadipocyte gene expression are depot dependent. We conducted further studies by real-time PCR of genes that appeared to have age-dependent expression changes in the microarray studies, but did not meet our criteria for identification as age-dependent genes in the microarray study. The PCR analyses were done in cells from additional animals and included cells from middle-aged in addition to young and old animals. With the increase in numbers of animals and ages and the greatly reduced multiple comparison correction, the PCR studies uncovered further genes that changed significantly with aging.
The genes whose expression changed with aging in the PCR analyses included cellular stress response–, injury-, and differentiation-related genes. These age-related changes in preadipocyte gene expression were evident, despite culturing cells from the same animals under identical conditions in parallel for a week. This suggests that these changes in gene expression are at least partly inherent, consistent with our previous finding that age-related declines in preadipocyte replicative potential and adipogenesis remain evident in colonies derived from single preadipocytes isolated from rats of different ages, even after a month in culture (13). Furthermore, the larger changes in perirenal than epididymal preadipocyte gene expression with aging in the current study are consistent with the larger declines in adipogenesis and replicative potential with aging in the perirenal than epididymal clones. The increases in preadipocyte stress response– and injury-related genes and declines in differentiation-related gene expression changes with aging in our study are similar to age-related changes in these gene categories reported in whole fat tissue from old compared with younger mice (35,36). Thus, the potentially inherent age-related changes in expression of these gene categories that occur in preadipocytes appear to carry through to the fat tissue that develops from them.
In the cases of genes that changed with aging, expression in middle-aged animals appeared to be intermediate between that of young and old animals, consistent with changes in fat cell progenitor function occurring steadily with chronological aging. Expression of these genes did not correlate with changes in body weight and fat, which increase during maturation (between 3 and 17 months of age) and decline in old age. The progressive nature of the changes in gene expression is consistent with the contention chronological processes, such as progressive accumulation of senescent progenitors or cellular damage, make a major contribution to age-related changes in progenitor function. Indeed, the gene categories involved (increased inflammation decreased differentiation genes with aging) and individual genes (eg, IL-6, Mmp-3) are related to cellular senescence and stress responses.
Importantly, the age-related changes in gene expression were fat depot specific. For example, expression of Stmn-2, Lbp, and Mmp-3 increased markedly in perirenal preadipocytes with aging, but underwent little if any change in epididymal cells. Genes upregulated with aging tended to change to a greater extent in perirenal preadipocytes, whereas those downregulated did so more in epididymal cells. These findings support the contentions that (a) cell-autonomous properties of preadipocytes may contribute to age- and fat depot–dependent changes in adipose tissue growth and function and (b) progenitors from different regions of the same tissue can undergo age-related changes that are distinct.
There were greater increases in stress response, proinflammatory, and matrix-remodeling genes in perirenal than epididymal preadipocytes with aging, as indicated in our PCR studies. This could be related to regional variation in extent of preadipocyte turnover. Larger increases in perirenal than epididymal fat cell numbers occur during maturation (18). These are associated with greater capacity of perirenal than epididymal preadipocytes for replication and differentiation in young animals (11,13,18). Over a lifetime, more extensive utilization of the perirenal than epididymal preadipocyte pools may lead to greater activation of stress, proinflammatory, and matrix-remodeling responses in older individuals. Alternatively, regional differences in the local microenvironment or abundance of other cell types, such as macrophages, could contribute. In support of this, the already high numbers of macrophages in rat epididymal depots of young animals do not increase further with aging, whereas the lower numbers of macrophages in the inguinal subcutaneous fat depots of young animals do increase (37). As in subcutaneous fat, macrophage markers increase with aging in perirenal fat tissue fragments (38). Despite lack of exposure to macrophages for a week in our culture system, possibly macrophages in the vicinity of preadipocytes in vivo impart a persisting effect. Thus, cell autonomous mechanisms and persistent microenvironmental influences could be responsible for depot-dependent age-related changes in preadipocyte function.
Little is known about preadipocyte or fat cell expression of stathmin-like 2 (Stmn-2; also called Scg-11). Of all 10,983 transcripts detected in the array study, Stmn-2 increased most with aging. This pattern was also found in the real-time PCR analysis. Stmn-2 is a stress-responsive microtubule-destabilizing protein that regulates neurite outgrowth and differentiation of oligodendrocytes (39–41). It is upregulated in response to neural injury, but this becomes attenuated with aging (42). Given its regionally specific role in regulation of nerve cell differentiation, a role of Stmn-2 in development of different fat depots is possible. Tetranectin, a 68-kDa cell surface protease that regulates mesenchymal development and cell migration, proliferation, and differentiation (43–45), also varied with aging in both the array and PCR studies. It might also have a role in fat tissue development, particularly because preadipocytes arise from mesenchymal progenitors.
Both Mmp-3 and Mmp-12 mRNA and protein increased in perirenal preadipocytes with aging, but much less so (or not at all) in epididymal cells. Mmp-3 and Mmp-12 are involved in inflammation and tissue remodeling. Consistent with these increases in Mmp-3 with aging in perirenal preadipocytes, Mmp-3 increases in human skin fibroblasts and mouse subcutaneous fat cells with aging (46,47). Mmp-3 is lower in preadipocytes from obese than lean subjects (48). To date, little information about regional differences in fat tissue Mmp-3 has been available. Fat tissue Mmp-12 expression and activity are increased in obesity and after high fat feeding (49,50), as well as in unstable atherosclerotic plaques and invasive cancers (51,52), conditions associated with inflammation and tissue remodeling, consistent with the upregulation of preadipocyte proinflammatory genes we found with aging [Figure 2 (27)].
Genes involved in early developmental segmentation and patterning were prominent among those that varied among fat depots. These genes, which regulate such fundamental cell dynamic processes as replication, differentiation, and susceptibility to apoptosis, may affect progenitor pool utilization and set the stage for regional variation in trajectories of age-related changes in gene expression. Differing nature and rates of age-related changes in progenitor gene expression profiles could themselves contribute to age-related changes in fat distribution and depot function. Altered fat distribution is a prominent feature of the aging phenotype in humans. Subcutaneous fat begins to decrease in the 70s, whereas intra-abdominal fat decreases later (53,54), leading to an effective shift of fat from subcutaneous to intra-abdominal depots, with eventual deposition of fat ectopically in muscle, bone marrow, liver, and elsewhere. This is associated with increased prevalence of metabolic syndrome, particularly after age 70 (4). It will be interesting to test if the depot-related differences in trajectories of age-related changes in preadipocyte gene expression in rats occur in humans and predispose to fat redistribution and metabolic disease.
FUNDING
This work was supported by the National Institutes of Health (AG13925 to J.L.K.), the Noaber Foundation, and the Robert and Arlene Kogod Center on Aging.
Supplementary material
Array data and primary analyses have been deposited in the GEO database (http://www.ncbi.nlm.nih.gov/geo/; accession number GSE6699). Supplementary Table 1 gives names for genes shown in Figure 1. Supplementary material can be found at: http://biomed.gerontologyjournals.org/
Acknowledgments
The authors are grateful for the advice of J. L. Armstrong and technical assistance of Maria M. A. Lopez.
References
- 1.Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol. 2007;42:463–471. doi: 10.1016/j.exger.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkland JL, Corkey BE, Tchkonia T. Current views of the fat cell as an endocrine cell: lipotoxicity. Endocr Updat. 2006;26:105–118. [Google Scholar]
- 3.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism. 2005;54:542–547. doi: 10.1016/j.metabol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand HA, Lynd FT, Masoro EJ, Yu BP. Changes in adipose mass and cellularity through the adult life of rats fed ad libitum or a life-prolonging restricted diet. J Gerontol. 1980;35:827–835. doi: 10.1093/geronj/35.6.827. [DOI] [PubMed] [Google Scholar]
- 7.Bertrand HA, Masoro EJ, Yu BP. Increasing adipocyte number as the basis for perirenal depot growth in adult rats. Science. 1978;201:1234–1235. doi: 10.1126/science.151328. [DOI] [PubMed] [Google Scholar]
- 8.Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Lifespan study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth lean body mass and disease. J Gerontol. 1982;37:130–141. doi: 10.1093/geronj/37.2.130. [DOI] [PubMed] [Google Scholar]
- 9.Kirkland JL, Hollenberg CH, Kindler S, Gillon WS. Effects of age and anatomic site on preadipocyte number in rat fat depots. J Gerontol. 1994;49:B31–B35. doi: 10.1093/geronj/49.1.b31. [DOI] [PubMed] [Google Scholar]
- 10.Björntorp P, Karlsson M, Pettersson P. Expansion of adipose tissue storage capacity at different ages in rats. Metabolism. 1982;31(4):366–373. doi: 10.1016/0026-0495(82)90112-3. [DOI] [PubMed] [Google Scholar]
- 11.Djian P, Roncari DAK, Hollenberg CH. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J Clin Invest. 1983;72:1200–1208. doi: 10.1172/JCI111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karagiannides I, Tchkonia T, Dobson DE, et al. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol. 2001;280:R1772–R1780. doi: 10.1152/ajpregu.2001.280.6.R1772. [DOI] [PubMed] [Google Scholar]
- 13.Kirkland JL, Hollenberg CH, Gillon WS. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol. 1990;258:C206–C210. doi: 10.1152/ajpcell.1990.258.2.C206. [DOI] [PubMed] [Google Scholar]
- 14.Kirkland JL, Hollenberg CH, Gillon WS. Effects of fat depot site on differentiation-dependent gene expression in rat preadipocytes. Int J Obesity. 1996;20:S102–S107. [PubMed] [Google Scholar]
- 15.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 16.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 17.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Kirkland JL, Hollenberg CH. Varying capacities for replication of rat adipocyte precursor clones and adipose tissue growth. J Clin Invest. 1989;83:1741–1746. doi: 10.1172/JCI114075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazzard DG, Soban J. Studies of aging using genetically defined rodents: a bibliography. Exp Aging Res. 1988;14:59–81. doi: 10.1080/03610738808259727. [DOI] [PubMed] [Google Scholar]
- 20.Shimokawa I, Higami Y, Hubbard GB, McMahan CA, Masoro EJ, Yu BP. Diet and the suitability of the male Fischer 344 rat as a model for aging research. J Gerontol. 1993;48:B27–B32. doi: 10.1093/geronj/48.1.b27. [DOI] [PubMed] [Google Scholar]
- 21.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3(1):1–26. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57:289–300. [Google Scholar]
- 24.Kachigan SK. Statistical Analysis. New York, NY: Radius Press; 1986. [Google Scholar]
- 25.Keppel G. Design and Analysis: A Researcher’s Handbook. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- 26.Gesta S, Bluher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchkonia T, Cartwright M, Wise B, et al. Increased TNFa and CCAAT/enhancer binding protein homologous protein (CHOP) with aging predispose preadipocytes to resist adipogenesis. Am J Physiol. 2007;293:E1810–E1819. doi: 10.1152/ajpendo.00295.2007. [DOI] [PubMed] [Google Scholar]
- 28.Charriere G, Cousin B, Arnaud E, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 29.Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol. 2009;297:E999–E1003. doi: 10.1152/ajpendo.00377.2009. [DOI] [PubMed] [Google Scholar]
- 30.Kirkland JL, Dobson DE. Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat cell function. J Am Geriatr Soc. 1997;45(8):959–967. doi: 10.1111/j.1532-5415.1997.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 31.Crossno JT, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomiyama K, Murase N, Stolz DB, et al. Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem Cells. 2008;26:330–338. doi: 10.1634/stemcells.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caserta F, Tchkonia T, Civelek V, et al. Fat depot origin affects fatty acid handling in cultured rat and human preadipocytes. Am J Physiol. 2001;280:E238–E247. doi: 10.1152/ajpendo.2001.280.2.E238. [DOI] [PubMed] [Google Scholar]
- 34.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 35.Higami Y, Barger JL, Page GP, et al. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006;136(2):343–352. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- 36.Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J. 2004;18(2):415–417. doi: 10.1096/fj.03-0678fje. [DOI] [PubMed] [Google Scholar]
- 37.Jerschow E, Anwar S, Barzilai N, Rosenstreich D. Macrophages accumulation in visceral and subcutaneous adipose tissue correlates with age. J Allergy Clin Immunol. 2007;119(suppl 1):S179. [Google Scholar]
- 38.Linford NJ, Beyer RP, Gollahon K, et al. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell. 2007;6:673–688. doi: 10.1111/j.1474-9726.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 39.Bondallaz P, Barbier A, Soehrman S, Grenningloh G, Riederer BM. The control of microtubule stability in vitro and in transfected cells by MAP1B and SCG10. Cell Motil Cytoskeleton. 2006;63:681–695. doi: 10.1002/cm.20154. [DOI] [PubMed] [Google Scholar]
- 40.Hossain-Ibrahim MK, Rezajooi K, MacNally JK, Mason MR, Lieberman AR, Anderson PN. Effects of lipopolysaccharide-induced inflammation on expression of growth-associated genes by corticospinal neurons. BMC Neurosci. 2006;7:8. doi: 10.1186/1471-2202-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang PL, Izrael M, Ainbinder E, Ben-Simchon L, Chebath J, Revel M. Increased myelinating capacity of embryonic stem cell derived oligodendrocyte precursors after treatment by interleukin-6/soluble interleukin-6 receptor fusion protein. Mol Cell Neurosci. 2006;31:387–398. doi: 10.1016/j.mcn.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Mori N, Morii H. SCG10-related neuronal growth-associated proteins in neural development, plasticity, degeneration, and aging. J Neurosci Res. 2002;70:264–273. doi: 10.1002/jnr.10353. [DOI] [PubMed] [Google Scholar]
- 43.Graversen JH, Lorensten RH, Jacobsen C, et al. The plasminogen binding site of the C-type lectin tetranectin is located in the carbohydrate recognition domain, and binding is sensitive to both calcium and lysine. J Biol Chem. 1998;273:29241–29246. doi: 10.1074/jbc.273.44.29241. [DOI] [PubMed] [Google Scholar]
- 44.Wewer UM, Iba K, Durkin ME, et al. Tetranectin is a novel marker for myogenesis during embryonic development, muscle regeneration, and muscle cell differentiation in vitro. Dev Biol. 1998;200:247–259. doi: 10.1006/dbio.1998.8962. [DOI] [PubMed] [Google Scholar]
- 45.Wewer UM, Ibaraki K, Schjorring P, Durkin ME, Young MF, Albrechtsen R. A potential role for teranectin in mineralization during osteogenesis. J Cell Biol. 1994;127:1767–1775. doi: 10.1083/jcb.127.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hazane F, Valenti K, Sauvaigo S, et al. Ageing effects on the expression of cell defense genes after UVA irradiation in human male cutaneous fibroblasts using cDNA arrays. J Photochem Photobiol B. 2005;79:171–190. doi: 10.1016/j.jphotobiol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Gasparrini M, Rivas D, Elbaz A, Duque G. Differential expression of cytokines in subcutaneous and marrow fat of aging C57BL/6J mice. Exp Gerontol. 2009;44:613–618. doi: 10.1016/j.exger.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Traurig MT, Permana PA, Nair S, Kobes S, Bogardus C, Baier LJ. Differential expression of matrix metalloproteinase 3 (MMP3) in preadipocytes/stromal vascular cells from nonobese nondiabetic versus obese nondiabetic Pima Indians. Diabetes. 2006;55:3160–3165. doi: 10.2337/db06-0373. [DOI] [PubMed] [Google Scholar]
- 49.Huber J, Loffler M, Bilban M, et al. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes. 2007;31(6):1004–1013. doi: 10.1038/sj.ijo.0803511. [DOI] [PubMed] [Google Scholar]
- 50.Chavey C, Mari B, Monthouel MN, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278:11888–11896. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- 51.Hofmann HS, Hansen G, Richter G, et al. Matrix metalloproteinase-12 expression correlates with local recurrence and metastatic disease in non-small cell lung cancer patients. Clin Cancer Res. 2005;11:1086–1092. [PubMed] [Google Scholar]
- 52.Morgan AR, Rerkasem K, Gallagher PJ, et al. Differences in matrix metalloproteinase-1 and matrix metalloproteinase-12 transcript levels among carotid atherosclerotic plaques with different histopathological characteristics. Stroke. 2004;35:1310–1315. doi: 10.1161/01.STR.0000126822.01756.99. [DOI] [PubMed] [Google Scholar]
- 53.Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev. 2004;5:13–19. doi: 10.1111/j.1467-789x.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 54.Silver AJ, Guillen CP, Kahl MJ, Morley JE. Effect of aging on body fat. J Am Geriatr Soc. 1993;41:211–213. doi: 10.1111/j.1532-5415.1993.tb06693.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.