Abstract
Rationale
Oxidized low-density lipoprotein (LDL) is an important determinant of inflammation in atherosclerotic lesions. It has also been documented that certain chronic infectious diseases, such as periodontitis and chlamydial infection, exacerbate clinical manifestations of atherosclerosis. In addition, low-level but persistent metabolic endotoxemia is often found in diabetic and obese subjects and is induced in mice fed a high-fat diet.
Objective
In this study, we examined cooperative macrophage activation by low levels of bacterial LPS and by minimally oxidized LDL (mmLDL), as a model for subclinical endotoxemia-complicated atherosclerosis.
Methods and Results
We found that both in vitro and in vivo, mmLDL and LPS (Kdo2-LipidA) cooperatively activated macrophages to express pro-inflammatory cytokines Cxcl2 (MIP-2), Ccl3 (MIP-1alpha), and Ccl4 (MIP-1beta). Importantly, the mmLDL and LPS cooperative effects were evident at a threshold LPS concentration (1 ng/ml) at which LPS alone induced only a limited macrophage response. Analyzing microarray data with a de novo motif discovery algorithm, we found that genes transcribed by promoters containing an AP-1 binding site were significantly upregulated by co-stimulation with mmLDL and LPS. In a nuclear factor-DNA binding assay, the cooperative effect of mmLDL and LPS co-stimulation on c-Jun and c-Fos DNA binding, but not on p65 or p50, was dependent on mmLDL-induced activation of ERK1/2. In addition, mmLDL induced JNK-dependent derepression of AP-1 by removing the corepressor NCoR from the chemokine promoters.
Conclusions
The cooperative engagement of AP-1 and NF-kappaB by mmLDL and LPS may constitute a mechanism of increased transcription of inflammatory cytokines within atherosclerotic lesions.
Keywords: oxidized lipoprotein, endotoxin, inflammation, macrophage
INTRODUCTION
Lipoprotein oxidation plays an in important role in initiation and progression of atherosclerosis, a vascular inflammatory disease, which leads to complications that cause myocardial infarction and stroke 1. Oxidized low-density lipoprotein (LDL) accumulated in the vascular wall activates macrophages, endothelial and smooth muscle cells to produce pro-inflammatory cytokines and chemokines, thereby enhancing inflammation in the lesion. LDL oxidation is a gradual process, progressing from lipid hydroperoxides to degradation products, such as aldehydes and ketones, which can form covalent adducts with proteins and lipids. With the changing composition of lipid and protein oxidation products, different forms of oxidized LDL interact with different cellular and soluble receptors. Thus, we have demonstrated that hydroperoxide-rich minimally oxidized LDL (mmLDL) activates macrophages via toll-like receptor-4 (TLR4) 2–4. More extensively oxidized LDL (OxLDL), containing aldehydes and modified/degraded apoB-100, binds to CD36 and other scavenger receptors 5.
Certain chronic infectious diseases, such as periodontitis and chlamydial infection, accelerate atherosclerosis and exacerbate its clinical manifestations, leading to acute cardiovascular events 6–10. This is likely a consequence of immune responses to bacterial pathogens resulting in intensified vascular inflammation within atherosclerotic lesions. Moreover, recent studies have revealed that ingestion of high-fat meals leads to transient increases in plasma endotoxin levels 11, likely via chylomicron-mediated transport of endotoxin derived from intestinal microflora 12. Metabolic endotoxemia has been also observed in patients with type 2 diabetes mellitus 13 and in mice fed a high-fat diet 14. Thus, a combinatorial activation of vascular cells by oxidized LDL and by threshold levels of endotoxin is a likely in vivo event, particularly relevant to the development of atherosclerosis in overweight individuals consuming a Western-type diet.
Because we previously demonstrated that mmLDL activates macrophages via TLR4 2–4, which is also activated by bacterial lipopolysaccharide (LPS), a “classic” TLR4 ligand 15, we hypothesized that low levels of mmLDL and LPS would exert cooperative activation of macrophages. TLR4-mediated responses involve the recruitment of a number of adaptor proteins to the TLR4 cytoplasmic domain. At the plasma membrane, the LPS-induced recruitment of MyD88 to TLR4 results in rapid expression of NF-κB-dependent genes and activation of JNK and p38 kinases. In the endosomal compartment, the recruitment of TRIF to TLR4 induces IRF3-dependent gene expression. In contrast to the LPS-induced TLR4 responses, mmLDL induces moderate expression of proinflammatory genes, but profound cytoskeletal rearrangements and ROS generation in macrophages 2,3,16. These MyD88-independent effects of mmLDL are mediated by the recruitment of spleen tyrosine kinase (Syk) to the C-terminal domain of TLR4 and subsequent activation of ERK1/2 and PLCγ 4,16.
Because of the differences in the LPS- and mmLDL-induced activation of TLR4, we hypothesized that co-stimulation of macrophages by mmLDL and by low levels of bacterial LPS would result in cooperative effects that would result in greater activation than achieved by either stimulus alone. Herein, we demonstrate that mmLDL and low levels of Kdo2-LipidA (KLA), the active moiety of LPS, cooperatively upregulate expression of a number of proinflammatory genes, including chemokines Cxcl2 (MIP-2), Ccl3 (MIP-1α), and Ccl4 (MIP-1β), both in vivo and in vitro. A de novo motif analysis of promoters of cooperatively activated genes suggested involvement of AP-1 transcription factors. Indeed, ERK1/2-dependent activation of AP-1 was characteristic for mmLDL and LPS co-stimulation. In addition, mmLDL induced phosphorylation of c-Jun and the release of the corepressor NCoR from the promoter regions of Cxcl2, Ccl3 and Ccl4. These molecular mechanisms suggest that the combination of minimally oxidized LDL and low levels of LPS cooperatively activate inflammatory responses in macrophages.
METHODS
An expanded Methods section is available in the online-only Data Supplement.
Cells, LDL Preparations and Other Reagents
Murine macrophage-like cell lines RAW267.1 (abbreviated in the text as RAW) and J774A.1 (abbreviated as J774) were from ATCC. Mouse peritoneal macrophages were isolated as described below. LDL (density=1.019–1.063g/ml) was isolated from plasma of normolipidemic donors by sequential ultracentrifugation 17. Native and modified LDL preparations were tested for possible endotoxin contamination using a Limulus Amoebocyte Lysate kit (Cambrex, Walkersville, MD). LDL preparations with LPS content higher than 50 pg/mg protein, corresponding to 2.5 pg/ml in most cell culture experiments, were discarded. To produce mmLDL, we incubated 50 μg/ml of LDL in serum-free DMEM for 18 hours with murine fibroblast cells overexpressing human 15-lipoxygenase, as reported in detail 2,18,19. Kdo2-lipidA (KLA), obtained from Avanti Polar Lipids (Alabaster, AL), was used as a well-characterized active component of LPS and a highly specific TLR4 agonist 20.
Mice, intraperitoneal injections and macrophage isolation
All animal experiments were performed according toNIH guidelines and were approved by the Animal Subjects Committee of the UCSan Diego. C57BL/6J mice were purchased from the Jackson Laboratory. Mice were anesthetized by a short exposure to isoflurane and injected intraperitoneally with 1 ml of 50 μg/ml mmLDL, 5 ng/ml KLA or their combination. One hour later, mice were sacrificed, peritoneum was lavaged and peritoneal cells were isolated and prepared for real time qPCR as described below. In some experiments, CD11b+ cells (predominantly macrophages) were rapidly separated at 4oC using magnetic bead reagents from Miltenyi (Germany).
Real-time PCR–based quantitative gene expression analysis
Total RNA was isolated using RNeasy columns (Qiagen, Valencia, CA), treated with DNase and reverse transcribed using oligo-dT and a First Strand Synthesis kit (Invitrogen, Carlsbad, CA). Real-time qPCR analysis was performed using reagents from Applied Biosystems (Foster City, CA) and a Rotor Gene Q (Qiagen, Valencia, CA).
Microarray and motif discovery algorithm
RNA concentration and integrity were verified with a NanoDrop ND-1000 (Thermo Scientific, Waltham, MA) and an Agilent 2100 BioAnalyzer respectively (Agilent, Santa Clara, CA). RNA was amplified, labeled and hybridized to the Sentrix BeadChip array, MouseRef-8 (Illumina, San Diego, CA). The slides were then processed according to the Illumina’s protocol, stained with Cy3-streptavidin and scanned with a BeadStation scanner (Illumina, San Diego, CA). Intensities were normalized using the modified Loess procedure 21. Sorting of genes and statistical analysis of pathways and gene ontology terms were performed as described 21–23. De novo motif discovery was performed using HOMER (http://biowhat.ucsd.edu/homer/index.html), a comparative algorithm that searches for motifs that are specifically found in a set of regulated promoter sequences [−500, +100 bp] compared to an invariant background set of promoters.
Western blot analysis
SDS-PAGE and western blot were performed according to standard protocols as we previously described4.
Chemiluminescent assay for transcription factor-DNA binding
RAW cell nuclear extracts were isolated using a Nuclear Extraction kit (Active Motif, San Diego, CA). Transcription factor activation was measured using sensitive Active Motif’s TransAM NF-κB p65, NF-κB p50, c-Jun and c-Fos assays in an ELISA format.
Enzyme-linked immunosorbent assay
RAW cells or J774 cells were incubated for 5 hours with 50 μg/ml mmLDL, 1 ng/ml KLA or mmLDL + KLA in a serum-free DMEM. Supernatants were harvested and spun for 5 min at 10,000 rpm to remove floating cells. Levels of Cxcl2, Ccl3 and Ccl4 proteins were measured in ELISA assays using reagents from R&D Systems.
Chromatin Immunoprecipitation
The ChIP assay was performed as we previously reported 24. qPCR with SYBR Green (Invitrogen, Carlsbad, CA) was carried out with a Rotor Gene Q (Qiagen). Primer sequences for the promoter regions were: Cxcl2 forward, gggctctgtgcttcctgat; Cxcl2 reverse, cagtctggggctctgaggt 25; Ccl3 forward, cctcagtccctcactgtggt; Ccl3 reverse, catggaacggaaactctcgt; Ccl4 forward, ttgtggcaggtgtgaacatt; Ccl4 reverse, tgtcatggcatcgagaaaga. Results are presented as enrichment of the precipitated target sequence compared to input DNA.
Statistical analysis
Graphs represent means ± standard error. Significance of differences was calculated using one-way ANOVA, with Bonferroni correction, where indicated.
RESULTS
mmLDL and LPS cooperatively upregulate gene expression in RAW cells
To assess the effect of cooperative stimulation with mmLDL and LPS on a genome-wide scale, we conducted microarray analysis and focused on early (1 hour) gene expression responses. Throughout this study, we used Kdo2-LipidA (KLA; from Avanti Polar Lipids) as a well-characterized active component of bacterial LPS and a highly specific agonist of TLR4 20, at threshold levels of 1 ng/ml for in vitro experiments. We used 50μg/ml of mmLDL as previously reported 3,4,16. RNA isolated from RAW macrophages was analyzed using an Illumina microarray platform.
The analysis of macrophages stimulated with mmLDL alone and KLA alone demonstrated activation of both common and distinct signaling and metabolic pathways (Online Table I). While both mmLDL and KLA activated TLR, MAP kinase, NF-κB, and IL-6 proinflammatory signaling pathways, the mmLDL stimulation was uniquely characterized by activation of PI3K/Akt, ERK1/2 and signaling pathways involved in cytoskeletal rearrangements, which agrees with the mmLDL-induced effects we previously documented with other techniques 2–4,19,26. Downregulation of the sterol biosynthetic pathway by mmLDL was indicative of mmLDL uptake and intracellular cholesterol accumulation, as we have recently reported 4.
Although there was little overlap in expression of individual genes stimulated by mmLDL and KLA alone (Figure 1A), co-stimulation of macrophages with mmLDL and KLA resulted in additive and synergistic increases in expression of a number of genes, including transcription factors, kinases, phosphatases, and cytokines. In further studies, we focused on three proinflammatory chemokines important in the development of atherosclerosis, Cxcl2 (MIP-2), Ccl3 (MIP-1α), and Ccl4 (MIP-1β), which were additively/synergistically upregulated by mmLDL and KLA.
Figure 1. Gene expression changes in macrophages stimulated with mmLDL, KLA and their combination.
A, Microarray analysis. RAW macrophages were stimulated for 1 hour with 50 μg/ml mmLDL and/or 1 ng/ml KLA (Kdo2-LipidA, the active component of bacterial LPS). Gene expression in macrophages without any stimulation (media alone) was used as a reference.
B, Chemokine mRNA expression in RAW macrophages stimulated with mmLDL, KLA and their combination. RAW macrophages were incubated for 1 hour with 50 μg/ml mmLDL, 1 ng/ml KLA (Kdo2-LipidA), or 50 μg/ml mmLDL + 1 ng/ml KLA. The relative expression of Cxcl2, Ccl3 and Ccl4 mRNA was measured by qPCR and normalized to Gapdh, with subsequent normalization to the KLA sample in each experiment. Mean ± SEM from 4 independent experiments. *, p<0.05 vs. mmLDL; #, not statistically significant vs. mmLDL. ANOVA analysis with Bonferroni correction: p < 0.01 for all 3 chemokines.
To confirm the microarray data, we conducted independent experiments and measured the expression of Cxcl2, Ccl3, Ccl4 and Gapdh mRNA in RAW macrophages induced my mmLDL, KLA and their combination (Figure 1B). The co-stimulation with mmLDL and KLA resulted in 1.5–3 fold increases in chemokine expression (normalized to Gapdh), and ANOVA analysis demonstrated a significant trend toward the increase in gene expression in the mmLDL+KLA samples compared to mmLDL and KLA alone. These increases in RAW cells were moderately synergistic (Online Table II). The changes in the chemokine expression levels were not due to variations in the Gapdh mRNA levels (Online Figure I).
Cooperative stimulation of cytokine expression by mmLDL and LPS in vivo
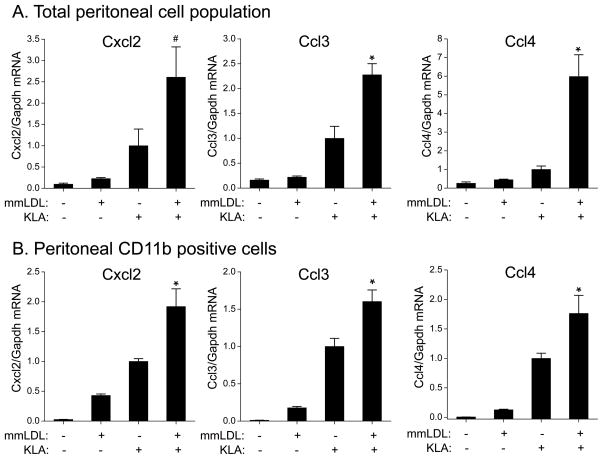
In order to test the in vivo relevance of our findings with RAW macrophages, we used a model of sterile peritonitis. Mice were injected intraperitoneally with vehicle, mmLDL, KLA, or mmLDL+KLA. One hour later, peritoneal cells were isolated and mRNA levels of Cxcl2, Ccl3 and Ccl4 were determined using qPCR. Similarly to the results with RAW cells, expression of Cxcl2, Ccl3, and Ccl4 was higher in peritoneal cells co-stimulated with mmLDL and KLA, but the synergistic effect was more dramatic, reaching as much as 2.5–5 fold increases compared to the cells stimulated with KLA alone (Figure 2A, Online Table II). Peritoneal macrophages constitute ~40% of all leukocytes in the peritoneum; other cells include lymphocytes, neutrophils, and dendritic cells. Using a rapid positive selection procedure with CD11b antibodies, we enriched the population of CD11b-positive cells (predominantly macrophages) from 40% to nearly 80% (Online Figure II) and confirmed that intraperitoneal stimulation with mmLDL and KLA resulted in cooperative stimulation of peritoneal macrophages (Figure 2B, Online Table II). These results, however, do not exclude the possibility that other peritoneal leukocytes can also respond to the mmLDL/KLA stimulation in an additive/synergistic manner.
Figure 2. Chemokine mRNA expression in murine peritoneal cells stimulated in vivo with mmLDL, KLA and their combination.
One milliliter of either vehicle (media), 50 μg/ml mmLDL, 5 ng/ml KLA (Kdo2-LipidA) or 50 μg/ml mmLDL + 5 ng/ml KLA were injected intraperitoneally into C57BL6/J mice. Peritoneal cells were isolated 1 hour post injection, and mRNA expression of Cxcl2, Ccl3 and Ccl4 was measured by qPCR. All values were normalized to the KLA response.
A, Total peritoneal cell population. qPCR measurement from individual mouse samples. Four mice per group. Mean ± SEM.
B, In a separate experiment, CD11b-positive cells were isolated from the peritoneal lavages (pooled from 3 mice) by positive selection as described in Methods. Representative results from two independent experiments are shown. Mean ± SD from technical triplicates.
*, p<0.05 vs. mmLDL and vs. KLA; #, non-significant vs. KLA; ANOVA with Bonferroni correction: p < 0.01 for all 3 chemokines.
Co-stimulation with mmLDL and LPS upregulates AP-1 transcriptional activity
Further analysis of the microarray data, using a de novo motif discovery algorithm developed in our group 27, suggested that promoter regions of many genes upregulated in macrophages co-stimulated with mmLDL and LPS, including Cxcl2, Ccl3 and Ccl4, contained the common motif presented in Figure 3. This motif matched with the known c-Jun/c-Fos consensus sequence, suggesting the involvement of AP-1 transcription factor regulation in macrophages co-stimulated with mmLDL and LPS. The most common AP-1 components, transcription factors c-Jun and c-Fos, are regulated by MAP kinases JNK and ERK1/2, respectively. Indeed, microarray analysis demonstrated highly significant activation of MAP kinase related pathways (Figure 3). These data agree with our earlier findings of strong ERK1/2 phosphorylation and Ccl5 (RANTES) expression in macrophages stimulated with mmLDL alone 3,4,16.
Figure 3.
Promoter motif discovery and pathway analysis in macrophages co-stimulated with mmLDL and KLA.
Next, we compared time-dependent ERK1/2 activation in macrophages stimulated with either mmLDL or KLA. Interestingly, mmLDL induced ERK1/2 phosphorylation in RAW cells as early as at 5 minutes post stimulation, but the levels of phosphorylated ERK1/2 rapidly declined and by 60 min were back to the levels in unstimulated cells (Figure 4). In contrast, ERK1/2 phosphorylation by a low dose of KLA (1 ng/ml) was delayed by nearly 30 minutes, but was sustained for over 1 hour. Co-stimulation with mmLDL and KLA resulted in a pattern of ERK1/2 phosphorylation similar to that of mmLDL, except that the length of activation was more sustained, for the entire hour of the experiment. Thus, we hypothesized that early and sustained phosphorylation of ERK1/2 increases activation of the AP-1 transcription complex during combined mmLDL/KLA stimulation.
Figure 4. ERK1/2 phosphorylation in RAW macrophages stimulated with mmLDL, KLA and their combination.
RAW macrophages were incubated for indicated times with 50 μg/ml mmLDL, 1 ng/ml KLA (Kdo2-LipidA), or 50 μg/ml mmLDL + 1 ng/ml KLA. Cell lysates were separated on SDS-PAGE and probed with antibodies against phosphorylated ERK1/2 and GAPDH and pan-Actin. Representative blots from 4 independent experiments.
To test this hypothesis, we measured binding of c-Jun and c-Fos to consensus AP-1 DNA binding sites. mmLDL alone and KLA alone induced only moderate c-Jun and c-Fos DNA binding, which was synergistically increased in RAW macrophages co-stimulated with mmLDL and KLA (Figure 5). Importantly, inhibition of ERK1/2 phosphorylation completely abolished the synergistic effect of mmLDL and KLA. NF-κB transcription factors p50 and p65 showed the same DNA binding pattern as the AP-1 transcription factors, but, in contrast, inhibition of ERK1/2/did not have a significant effect on p50 and p65 DNA binding. These data agree with the role of ERK1/2 in regulation of AP-1, but not the NF-κB transcription program.
Figure 5. AP-1 and NF-κB DNA binding in RAW macrophages stimulated with mmLDL, KLA and their combination.
RAW macrophages were incubated for 1 hour, in the presence or absence of 5 μM U0126, with 50 μg/ml mmLDL, 1 ng/ml KLA (Kdo2-LipidA), or 50 μg/ml mmLDL + 1 ng/ml KLA; “no inhibitor” samples were supplemented with an equal volume of DMSO. Nuclear extracts were analyzed for transcription factor DNA binding using TransAM plate-based assays. Representative results of 3 independent experiments.
To confirm that ERK1/2 activation mediates cytokine expression in macrophages co-stimulated with mmLDL and LPS, we measured CXCL2, CCL3 and CCL4 protein secretion by RAW and by J774 cells. We found that co-stimulation of both macrophage cell lines with mmLDL and KLA led to additive-to-synergistic upregulation of cytokine secretion, which, importantly, was blocked with the inhibition of ERK1/2 signaling (Figure 6).
Figure 6. Chemokine secretion by RAW and J774 macrophages stimulated with mmLDL, KLA and their combination.
RAW (top) or J774 (bottom) macrophages were incubated for 5 hours, in the presence or absence of 5 μM U0126, with 50 μg/ml mmLDL, 1 ng/ml KLA (Kdo2-LipidA), or 50 μg/ml mmLDL + 1 ng/ml KLA; “no inhibitor” samples were supplemented with an equal volume of DMSO. Cell culture media were collected and CXCL2, CCL3 and CCL4 protein levels were measured in an ELISA assay. Results shown are technical duplicates of biological duplicates and are representative of 2 independent experiments.
mmLDL-induced NCoR derepression
Nuclear receptor co-repressor (NCoR) is an important check point in the regulation of inflammatory responses to LPS via TLR4 signaling 25. LPS induces IKKε-dependent phosphorylation of c-Jun, which results in detachment of NCoR from c-Jun and recruitment of c-Fos to complete the AP-1 assembly on a promoter binding site 25. This ultimately leads to the transcription of inflammatory genes. Since mmLDL also signals via TLR4 and phosphorylates c-Jun 3,4, we tested whether mmLDL has the ability to detach NCoR from the promoters of Cxcl2, Ccl3 and Ccl4, the mmLDL-induced proinflammatory genes. First, we measured time-dependent phosphorylation of c-Jun in macrophages stimulated with mmLDL, KLA and their combination. We found the same pattern as with phosphorylation of ERK1/2, characterized by rapid and transient c-Jun phosphorylation with mmLDL and delayed c-Jun phosphorylation with KLA (Figure 7). As with ERK1/2, the mmLDL and KLA combination induced early and sustained c-Jun phosphorylation.
Figure 7. c-Jun phosphorylation in RAW macrophages stimulated with mmLDL, KLA and their combination.
RAW macrophages were incubated for indicated times with 50 μg/ml mmLDL, 1 ng/ml KLA (Kdo2-LipidA), or 50 μg/ml mmLDL + 1 ng/ml KLA. Cell lysates were separated on SDS-PAGE and probed with antibodies against phosphorylated c-Jun and pan-Actin (pan-Actin loading control in this figure is identical to that in Fig. 4 as the same cell lysates were probed for p-ERK1/2 and p-c-Jun). Representative blots from 4 independent experiments.
In addition to ERK1/2, mmLDL activation of macrophages also results in JNK phosphorylation 3. Thus, we hypothesized that in contrast to LPS-induced c-Jun phosphorylation, which was dependent on IKKε25, mmLDL stimulates c-Jun phosphorylation via JNK. Indeed, a specific JNK inhibitor SP600125 effectively inhibited mmLDL-induced c-Jun phosphorylation (Figure 8A). The specificity of SP600125 was validated by the lack of inhibition of ERK1/2 phosphorylation in the same macrophage lysates.
Figure 8. mmLDL-induced c-Jun phosphorylation and NCoR detachment from the Cxcl2 and Ccl3 promoters.
A, RAW macrophages were incubated for indicated times with 50 μg/ml mmLDL, in the presence or absence of 25 μM SP600125. Cell lysates were separated on SDS-PAGE and probed with antibodies against phosphorylated c-Jun, phosphorylated ERK1/2 and GAPDH.
Representative blots from three independent experiments.
B, RAW macrophages were incubated for 0, 15 or 30 minutes with 50 μg/ml mmLDL. NCoR ChIP and promoter qPCR were performed as described in Methods. Representative data from two independent experiments. Mean ± SEM of technical triplicates.
C, The NCoR ChiP assay was repeated for macrophages incubated with mmLDL for 15 minutes in the presence or absence of 25 μM SP600125; “no inhibitor” samples were supplemented with an equal volume of DMSO. The dashed line at 100% represents the NCoR occupancy levels measured in non-stimulated cells. Representative results of three independent experiments. Mean ± SEM of technical triplicates.
Next, in a chromatin immunoprecipitation experiment, we measured NCoR occupancy on the Cxcl2, Ccl3 and Ccl4 promoters. We found that as early as 15 minutes post stimulation mmLDL induced release of approximately 40–60% of NCoR molecules from the promoter regions of Cxcl2 and Ccl3 (Figure 8B), but the results with the Ccl4 promoter were inconsistent. Importantly, inhibition of JNK with SP600125 abrogated mmLDL-induced NCoR release from the promoter regions of Cxcl2 and Ccl3 (Figure 8C).
DISCUSSION
Inflammation plays a major role in the pathogenesis of atherosclerosis, and chronic infections are known to exacerbate its complications. In this study, we demonstrated that two likely pro-inflammatory agonists involved in endotoxemia-complicated atherogenesis, minimally oxidized LDL and bacterial LPS, induced cooperative upregulation of proinflammatory genes in macrophages when applied together. Specifically, we found that mmLDL and threshold levels of LPS (1 ng/ml KLA) cooperatively activated RAW and J774 macrophages to express pro-inflammatory genes, including chemoattractant cytokines Cxcl2, Ccl3, and Ccl4 (Figures 1 and 6, and Online Table II). This cooperative effect was even more pronounced in vivo, achieving synergistic levels in peritoneal macrophages and possibly in other peritoneal cells (Figure 2). We further found that AP-1 response elements were enriched in the promoter regions of the genes responsive to co-stimulation with mmLDL and KLA (Figure 3) and that c-Jun and c-Fos binding to the response element DNA sequence was higher in the nuclear lysates of macrophages co-stimulated with mmLDL and KLA compared to mmLDL or KLA alone (Figures 4 and 5). In addition, mmLDL rapidly induced AP-1 derepression by phosphorylating c-Jun and releasing corepressor NCoR from the chemokine promoters (Figures 7–8).
The premise for our study was that low concentrations of LPS and mmLDL, although having one common signaling receptor, TLR4, nevertheless, activate separate as well as common signaling pathways. mmLDL stimulates TLR-dependent, but MyD88-independent generation of ROS and cytoskeletal rearrangements in macrophages, the latter leading to macropinocytosis and lipoprotein uptake. These mmLDL effects are mediated by the recruitment of Syk to the cytoplasmic domain of TLR4 and subsequent Syk-dependent activation of ERK1/2 4,16. In this study, we compared the time courses of ERK1/2 phosphorylation and found that mmLDL activates ERK1/2 as early as in 5 min, but LPS (KLA) at a concentration of 1 ng/ml showed no significant ERK1/2 activation before 30 min. Interestingly, co-stimulation with mmLDL and KLA maintained the early ERK1/2 phosphorylation, characteristic for mmLDL only, which was sustained for a longer period of time due to the KLA component (Figure 4). It has been suggested that sustained ERK1/2-dependent phosphorylation of c-Fos leads to the AP-1 complex assembly and its DNA binding 28.
In addition to the ERK1/2-mediated mechanism, mmLDL also activates a JNK pathway leading to c-Jun phosphorylation and AP-1 derepression via the detachment of corepressor NCoR (Figures 7–8). The dependence of NCoR detachment on c-Jun phosphorylation has been demonstrated in experiments with overexpressed phospho-mimic c-Jun mutant 25. However, as we suggested earlier, the mechanism of LPS-induced c-Jun phosphorylation is via IKKε and is independent of JNK activity 25. In contrast, experiments of this study demonstrate that mmLDL-induced c-Jun phosphorylation and NCoR detachment do depend on JNK activity (Figure 8). These findings of separate pathways by which mmLDL and LPS regulate NCoR derepression further explain cooperative upregulation of proinflammatory cytokines induced by these two stimuli. Another difference in the mmLDL- and LPS- induced mechanisms is that mmLDL induces translocation of p65 to the nucleus but not its phosphorylation nor binding to DNA, whereas LPS stimulates all these steps of NF-κB activation 3. Thus, taken together, mmLDL and LPS complement each other by strengthening AP-1 and NF-κB promoter activity of target proinflammatory genes.
Importantly, the mmLDL and LPS cooperative effects were evident at a threshold LPS concentration (1 ng/ml) at which LPS alone induced a very limited macrophage response. Such levels of bacterial endotoxin can be found in human plasma during low-grade but sustained chronic infections, like periodontitis or infections with Chlamydia, which have been shown to accelerate the progression of atherosclerosis 6,7,9. In fact, the American Journal of Cardiology and the Journal of Periodontology jointly published an Editors’ Consensus, recommending the early treatment of periodontitis to slow the progression of atherosclerosis in affected subjects 29.
Recent clinical and experimental evidence suggests that low-level endotoxemia may be considerably more prevalent than previously realized 11, underscoring the importance of our findings. The gastrointestinal tract constitutes an enormous reservoir of biologically active bacterial products, including LPS, and a small proportion of gut-derived LPS can translocate into the circulation even in relatively healthy subjects 11. In the Bruneck (Italy) Study, plasma levels of endotoxin in the general population (516 men and women aged 50 to 79 years old) ranged from 6 to 209 pg/ml, with a 3-fold higher risk of carotid atherosclerosis in individuals with endotoxin levels 50 pg/ml and higher (90th percentile) 30. A more recent study of a smaller population in England reported endotoxin levels of 3.1 EU/ml in non-diabetic and 5.5 EU/ml in patients with type 2 diabetes, which correspond to 310 pg/ml and 550 pg/ml LPS, respectively (according to the FDA’s EC-6 standard of 10 EU/ng) 13. Miller et al. analyzed ethnic differences and found a graded increase in endotoxin levels from black Africans to whites to South Asians, reporting significantly higher values than in other studies, as much as 14.4 EU/ml, or 1.44 ng/ml in South Asian men, and a strong correlation between plasma endotoxin and triglyceride concentrations 31. In addition, emerging evidence suggests that dietary habits alone may alter systemic exposure to endotoxin. For example, Erridge et al. have demonstrated that a single high-fat meal markedly increases endotoxin concentrations postprandially in healthy human subjects, sufficient to activate vascular cells in vitro 11. These findings were supported by studies by Amar et al., correlating energy intake with endotoxemia in healthy subjects 32. In mice, a 4-week high-fat (72%) diet increased plasma endotoxin concentrations 2.7-fold to 4.9 EU/ml, or 490 pg/ml 14. Although absolute values vary considerably in different studies, the presence of sub-nanogram to nanogram per milliliter levels of endotoxin in plasma of seemingly normal subjects is well documented. To put the above numbers in perspective, the fatality rates for meningococcal patients with endotoxin plasma levels of 10–50 EU/ml, 50–100 EU/ml, 100–150 EU/ml, and > 150 EU/ml were 30%, 100%, 89% and 100%, respectively 33. Because a large portion of LPS is carried in plasma by lipoproteins 34,35, it is also plausible that LPS is present in atherosclerotic lesions, but this is yet to be determined.
In summary, our data demonstrate that cooperative engagement of AP-1 and NF-κB transcription factors by mmLDL and LPS results in additive/synergistic upregulation of pro-inflammatory genes in macrophages. This may constitute a mechanism of enhanced inflammatory activation within atherosclerotic lesions leading to the disease progression and increased risk of acute cardiovascular events, as suggested by human epidemiologic studies of atherosclerosis complicated by chronic infections and other conditions associated with subclinical endotoxemia in apparently healthy subjects.
Novelty and Significance.
What is known?
Oxidation of low-density lipoprotein (LDL) significantly contributes to the progression of atherosclerosis, and oxidized LDL induces inflammatory responses in vascular cells
Certain chronic infectious diseases, such as periodontitis and chlamydial infection, exacerbate clinical manifestations of atherosclerosis
Low-level but persistent metabolic endotoxemia is often found in diabetic and obese subjects, who are at risk of cardiovascular disease, and is induced in mice fed a high-fat diet
What new information does this article contribute?
Minimally oxidized LDL (mmLDL) and low doses of bacterial lipopolysaccharide (LPS) cooperatively activate macrophages to express higher levels of pro-inflammatory cytokines Cxcl2, Ccl3, and Ccl4
The mechanism of this synergistic stimulation involves early and sustained activation of ERK1/2 and JNK, release of corepressor NCoR from the gene promoter regions, and derepression of the AP-1 transcription program
Chronic inflammation in atherosclerotic lesions in coronary and cerebral arteries leads to formation of plaques vulnerable to rupture. Ruptured plaques cause intravascular thrombosis, which often results in a myocardial infarction or a stroke. In this study, we tested the hypothesis that two factors involved in the pathogenesis and complications of atherosclerosis – mmLDL and LPS – may synergize in inducing pro-inflammatory responses in macrophages. We demonstrated that mmLDL and LPS have different modes of activation of intracellular signaling pathways and that combined mmLDL and LPS stimulation of macrophages leads to enhanced transcriptional activity and elevated expression of proinflammatory cytokines. These mechanistic findings may explain why chronic infectious diseases, such as periodontitis and chlamydial infection, characterized by low-level but systemic elevations in LPS levels, accelerate atherosclerosis and exacerbate its clinical manifestations, leading to acute cardiovascular events. Our results are also relevant to the development of atherosclerosis in diabetic and overweight individuals consuming a Western-type diet, since recent studies have demonstrated that high-fat diets result in subclinical metabolic endotoxemia. Because lipoprotein oxidation is a well-documented event in the development of atherosclerosis, our data showing cooperative activation of macrophages with mmLDL and LPS, suggest a possible mechanism of accelerated atherosclerosis in patients with subclinical endotoxemia.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This study was supported by the NIH grants HL081862 (Y.I.M.), GM069338 (C.K.G., J.L.W. and Y.I.M.), HL088093 (J.L.W. and Y.I.M.), the American Recovery and Reconstruction Act’s administrative supplement HL081862-S1 (P.W. and Y.I.M.), as well as a grant from the Leducq Fondation (P.W., C.K.G., J.L.W. and Y.I.M.).
Non-standard Abbreviations and Acronyms
- LDL
low-density lipoprotein
- mmLDL
minimally oxidized LDL
- OxLDL
extensively oxidized LDL
- LPS
lipopolysaccharide
- KLA
Kdo2-lipidA
- TLR
toll-like receptor
- NCoR
nuclear receptor co-repressor
Footnotes
DISCLOSURES
No conflicts of interest to disclose.
References
- 1.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally Modified LDL Binds to CD14, Induces Macrophage Spreading via TLR4/MD-2, and Inhibits Phagocytosis of Apoptotic Cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 3.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 4.Choi S-H, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boullier A, Gillotte KL, Hörkkö S, Green SR, Friedman P, Dennis EA, Witztum JL, Steinberg D, Quehenberger O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J Biol Chem. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 6.Lehr HA, Sagban TA, Ihling C, Zahringer U, Hungerer KD, Blumrich M, Reifenberg K, Bhakdi S. Immunopathogenesis of atherosclerosis: endotoxin accelerates atherosclerosis in rabbits on hypercholesterolemic diet. Circulation. 2001;104:914–920. doi: 10.1161/hc3401.093153. [DOI] [PubMed] [Google Scholar]
- 7.Kalayoglu MV, Libby P, Byrne GI. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA. 2002;288:2724–2731. doi: 10.1001/jama.288.21.2724. [DOI] [PubMed] [Google Scholar]
- 8.Takaoka N, Campbell LA, Lee A, Rosenfeld ME, Kuo CC. Chlamydia pneumoniae Infection Increases Adherence of Mouse Macrophages to Mouse Endothelial Cells In Vitro and to Aortas Ex Vivo. Infect Immun. 2008;76:510–514. doi: 10.1128/IAI.01267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8:38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- 10.Pussinen PJ, Tuomisto K, Jousilahti P, Havulinna AS, Sundvall J, Salomaa V. Endotoxemia, Immune Response to Periodontal Pathogens, and Systemic Inflammation Associate With Incident Cardiovascular Disease Events. Arterioscler Thromb Vasc Biol. 2007;27:1433–1439. doi: 10.1161/ATVBAHA.106.138743. [DOI] [PubMed] [Google Scholar]
- 11.Erridge C. The Roles of Pathogen-Associated Molecular Patterns in Atherosclerosis. Trends Cardiovasc Med. 2008;18:52–56. doi: 10.1016/j.tcm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Creely SJ, McTernan PG, Kusminski CM, Fisher F, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 14.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R. Toll-like receptors and innate immunity. Nature Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 16.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages Generate Reactive Oxygen Species in Response to Minimally Oxidized Low-Density Lipoprotein: Toll-Like Receptor 4- and Spleen Tyrosine Kinase-Dependent Activation of NADPH Oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havel RJ, Bragdon JH, Eder HA. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benz DJ, Mol M, Ezaki M, Mori-Ito N, Zelaan I, Miyanohara A, Friedmann T, Parthasarathy S, Steinberg D, Witztum JL. Enhanced levels of lipoperoxides in low density lipoprotein incubated with murine fibroblast expressing high levels of human 15-lipoxygenase. J Biol Chem. 1995;270:5191–5197. doi: 10.1074/jbc.270.10.5191. [DOI] [PubMed] [Google Scholar]
- 19.Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized LDL. J Biol Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raetz CRH, Garrett TA, Reynolds CM, Shaw WA, Moore JD, Smith DC, Jr, Ribeiro AA, Murphy RC, Ulevitch RJ, Fearns C, Reichart D, Glass CK, Benner C, Subramaniam S, Harkewicz R, Bowers-Gentry RC, Buczynski MW, Cooper JA, Deems RA, Dennis EA. Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J Lipid Res. 2006;47:1097–1111. doi: 10.1194/jlr.M600027-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Sasik R, Woelk CH, Corbeil J. Microarray truths and consequences. J Mol Endocrinol. 2004;33:1–9. doi: 10.1677/jme.0.0330001. [DOI] [PubMed] [Google Scholar]
- 22.Sasik R, Calvo E, Corbeil J. Statistical analysis of high-density oligonucleotide arrays: a multiplicative noise model. Bioinformatics. 2002;18:1633–1640. doi: 10.1093/bioinformatics/18.12.1633. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa S, Lozach J, Jepsen K, Sawka-Verhelle D, Perissi V, Sasik R, Rose DW, Johnson RS, Rosenfeld MG, Glass CK. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. PNAS. 2004;101:14461–14466. doi: 10.1073/pnas.0405786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghisletti S, Huang W, Jepsen K, Benner C, Hardiman G, Rosenfeld MG, Glass CK. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang W, Ghisletti S, Perissi V, Rosenfeld MG, Glass CK. Transcriptional Integration of TLR2 and TLR4 Signaling at the NCoR Derepression Checkpoint. Mol Cell. 2009;35:48–57. doi: 10.1016/j.molcel.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller YI, Worrall DS, Funk CD, Feramisco JR, Witztum JL. Actin polymerization in macrophages in response to oxidized LDL and apoptotic cells: role of 12/15-lipoxygenase and phosphoinositide 3-kinase. Mol Biol Cell. 2003;14:4196–4206. doi: 10.1091/mbc.E03-02-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hevener AL, Olefsky JM, Reichart D, Nguyen MTA, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M. Macrophage PPAR+| is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalmers CJ, Gilley R, March HN, Balmanno K, Cook SJ. The duration of ERK1/2 activity determines the activation of c-Fos and Fra-1 and the composition and quantitative transcriptional output of AP-1. Cell Sign. 2007;19:695–704. doi: 10.1016/j.cellsig.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Friedewald VE, Kornman KS, Beck JD, Genco R, Goldfine A, Libby P, Offenbacher S, Ridker PM, Van Dyke TE, Roberts WC. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: Periodontitis and Atherosclerotic Cardiovascular Disease. Amer J Cardiol. 2009;104:59–68. doi: 10.1016/j.amjcard.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: Prospective results from the bruneck study. J Amer Coll Cardiol. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 31.Miller MA, McTernan PG, Harte AL, Silva NF, Strazzullo P, Alberti KG, Kumar S, Cappuccio FP. Ethnic and sex differences in circulating endotoxin levels: A novel marker of atherosclerotic and cardiovascular risk in a British multi-ethnic population. Atherosclerosis. 2009;203:494–502. doi: 10.1016/j.atherosclerosis.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 33.Brandtzaeg P, Bjerre A, Ovstebo R, Brusletto B, Joo GB, Kierulf P. Invited review: Neisseria meningitidis lipopolysaccharides in human pathology. J Endotoxin Res. 2001;7:401–420. [PubMed] [Google Scholar]
- 34.Kallio KAE, Buhlin K, Jauhiainen M, Keva R, Tuomainen AM, Klinge B, Gustafsson A, Pussinen PJ. Lipopolysaccharide associates with pro-atherogenic lipoproteins in periodontitis patients. Innate Immunity. 2008;14:247–253. doi: 10.1177/1753425908095130. [DOI] [PubMed] [Google Scholar]
- 35.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.