Abstract
Dopamine increases in the nucleus accumbens after ethanol administration in rats, but the contributions of the core and shell subregions to this response are unclear. The goal of this study was to determine the effect of various doses of intravenous (i.v.) ethanol infusions on dopamine in these two subregions of the nucleus accumbens. Male Long-Evans rats were infused with either acute i.v. ethanol (0.5, 1.0, 1.5 g/kg), repeated i.v. ethanol (four 1.0 g/kg infusions resulting in a cumulative dose of 4.0 g/kg), or saline as a control for each condition. Dopamine and ethanol were measured in dialysate samples from each experiment. The in vivo extraction fraction for ethanol of probes was determined using i.v. 4-methylpyrazole, and was used to estimate peak brain ethanol concentrations after the infusions. The peak brain ethanol concentrations after the 0.5, 1.0, and 1.5 g/kg ethanol infusions were estimated to be 20, 49, and 57 mM, respectively. A significant dopamine increase was observed for the 0.5 g/kg ethanol group when collapsed across subregions. However, both the 1.0 g/kg and 1.5 g/kg ethanol infusions produced significant increases in dopamine levels in the shell that were significantly higher than those in the core. An ethanol dose-response effect on dopamine in the shell was observed when saline controls, 0.5, 1.0, and 1.5 g/kg groups were compared. For the cumulative-dosing study, the first, second, and fourth infusions resulted in significant increases in dopamine in the shell. However, these responses were not significantly different from one another. The results of this study show that the shell has a stronger response than the core to intravenous ethanol, and that dopamine in the shell increases in a dose-dependent manner between 0.5 -1.0 g/kg doses, but that the response to higher ethanol doses reaches a plateau.
Keywords: Alcohol, Microdialysis, 4-Methylpyrazole, Extraction Fraction, Long-Evans
Many brain regions and pathways may be involved in the reinforcing properties of drugs of abuse; however, the mesolimbic dopaminergic pathway is among the most highly studied. Dopaminergic neurons of this pathway originate in the ventral tegmental area and terminate in the nucleus accumbens and may be activated during the acquisition or expression of ethanol reinforcement (Spanagel and Weiss, 1999; Weiss and Porrino, 2002; Gonzales et al., 2004). It is well established in many animal models that extracellular dopamine levels increase in the nucleus accumbens after ethanol administration (Imperato and Di Chiara, 1986; Yoshimoto et al., 1991; Yoshimoto et al., 1992; Blomqvist et al., 1993; Weiss et al., 1993; Campbell and McBride, 1995; Kohl et al., 1998; Olausson et al., 1998; Yim et al., 2000; Tang et al., 2003). However, the potential contribution of environmental stimuli associated with ethanol administration, separate from a direct pharmacological effect, to the ethanol-evoked dopamine response is still not clear.
The nucleus accumbens has been described as having anatomically distinct core and shell subregions (Heimer et al., 1991; Zahm and Brog, 1992; Zahm, 1999), and these subregions may have different behavioral functions. The core of the nucleus accumbens has been implicated in associative conditioning and instrumental behaviors (Kelley et al., 1997; Sokolowski and Salamone, 1998; Bassareo and Di Chiara, 1999; Ito et al., 2000; Day et al., 2007; Gremel and Cunningham, 2008). The shell may be important during exposure to novel stimuli or the acquisition of place preference (Rebec et al., 1997; Bassareo and Di Chiara, 1999; Di Chiara et al., 2004; Fenu et al., 2006). However, the potential differential response of the core and shell to ethanol has not been well defined.
The ethanol-induced dopamine response in the core and shell of the nucleus accumbens may differ depending on the route of ethanol administration. Animals that self-administer ethanol orally encounter stimulus properties of the drinking solution, and animals that receive an intraperitoneal (i.p.) injection may experience handling stress. Environmental factors associated with ethanol self-administration, such as a cue lights, may also contribute to the stimulation of accumbal dopamine (Melendez et al., 2002). Intravenous (i.v.) ethanol administration may reduce or eliminate some confounding environmental factors that occur with i.p. or oral administration including handling stress and the stimulus properties of ethanol associated with its taste and smell. The i.v. administration of ethanol has been shown to enhance the firing rate of dopamine neurons in the ventral tegmental area, the origin of the mesolimbic pathway, in anesthestized or paralyzed naïve rats (Gessa et al., 1985; Foddai et al., 2004). However, it is not clear whether these increases in firing rate of dopamine neurons lead to dopamine release in the nucleus accumbens, particularly in freely-moving rats. Here, the i.v. method was used to compare extracellular dopamine in the core and shell in response to acute non-contingent administration of ethanol.
The major goal of this study was to determine whether there is a pharmacological effect of ethanol on dopamine in the core and shell of the nucleus accumbens over a wide range of ethanol doses, and whether this ethanol-evoked dopamine release differs in these subregions. We also estimated the tissue ethanol concentration in the area surrounding the microdialysis probe after the bolus injection of ethanol. This was done by determination of the in vivo extraction fraction for ethanol after inhibition of ethanol metabolism with an alcohol dehydrogenase inhibitor.
Experimental Procedures
Animals
A total of eighty-four male Long-Evans rats (Charles River Laboratories, Wilmington, MA), weighing 250-550 grams on dialysis day, were used for these experiments. Sixty-six were used for the acute ethanol studies, thirteen were used for the cumulative dosing study, and five were used to determine the extraction fraction for ethanol. The rats were housed individually in a temperature (25°C) and light (12 hour light/12 hour dark) controlled room and had access to food and water ad libitum. The rats were handled and weighed for at least four days prior to surgery. All procedures were carried out in compliance with the guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee of the University of Texas at Austin.
Surgical Procedures
A jugular catheter was inserted, and a guide cannula was placed over the nucleus accumbens in each rat using a modification of the procedure of Duvauchelle et al. (1998). The jugular catheter was fed subcutaneously to an incision on the head. Intravenous catheters were constructed from silastic tubing (0.30 mm ID, 0.64 mm OD, Fisher Scientific, Hampton, NH), a cannula (22 gauge, Plastics One, Roanoke, VA), and silicon adhesive (DAP Inc., Baltimore, MD). The rats were under isoflurane anesthesia (4.0 % during the induction period and 2.0 % during maintenance) during surgery. The guide cannula used for microdialysis (21 gauge, Plastics One, Roanoke, VA) was implanted above either the core (coordinates in mm relative to Bregma: AP +1.3, ML +1.6, DV -3.5 or -3.2) or shell (AP +2.2, ML +0.7, DV -4.0) of the nucleus accumbens while the animal was in a stereotaxic frame. The DV coordinate represents the bottom of the guide cannula, and the probe extends an additional 4.0 mm below the cannula when seated into the guide. We noted that some of the probes aimed at the core passed through the core and penetrated the ventrolateral shell. To minimize this, the core DV coordinate was changed to -3.2 midway through the study. An obturator was placed in the guide cannula to prevent blockage. Rats were allowed to recover from surgery for 2 (4-methylpyrazole experiments) or at least 4 days (acute and cumulative studies). A longer recovery period was allowed for the ethanol studies because we monitored their behavior following the injection. During the recovery period, the catheter was flushed daily with 0.1 ml of timentin (67 mg/ml; Henry Schein, Inc., Melville, KY) in heparinized saline (American Pharmaceutical Partners, Inc., Los Angeles, CA).
Microdialysis
The evening before the dialysis experiment, a laboratory constructed probe (1.5 mm active membrane length, 270 μm OD, 18,000 molecular weight cut-off) was implanted through the guide cannula and perfused (CMA 100 microinjection pump, Acton, MA) with artificial cerebrospinal fluid (ACSF: 149 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 0.25 mM ascorbic acid, 5.4 mM D-glucose). The rats were placed in individual chambers with free access to water and food, and the flow rate was lowered to 0.2 μl/min overnight. After a stabilization period of 12-15 hours, the flow rate was increased to 2.0 μl/min, and two hours were allowed at this new flow rate before sample collection commenced using five-minute intervals.
For the ethanol dose-response experiments, four basal samples were taken before the infusion of ethanol (10%, w/v, in saline) or saline. For the 0.5 and 1.0 g/kg ethanol groups, post-infusion samples were taken for 30 minutes. For the 1.5 g/kg ethanol group post-infusion samples were collected for one hour. The i.v. infusions occurred over approximately 30 seconds, one minute, and one and a half minutes for the 0.5, 1.0, and 1.5 g/kg ethanol groups, respectively. For the cumulative dosing study, four basal samples were taken before the first infusion of 1.0 g/kg i.v. ethanol, and fifteen minutes elapsed between each of the remaining infusions. The rate of infusion was 15 seconds per milliliter of solution for all infusions. The volume of the infusions ranged from approximately 1.5 to 6.0 milliliters, depending on dose and body weight. For all groups, every dialysis sample was analyzed for dopamine, and ethanol was determined in the last two basal and the post-infusion samples in the ethanol groups. Upon completion of the experiment, the perfusate was switched to calcium-free ACSF. A five-minute sample was taken after one and a half hours to verify that dopamine recovered in the experimental samples was due to exocytotic release.
For each microdialysis experiment the behavior of the rat was observed, and sedative effects of ethanol were recorded. Behaviors that indicated sedation included loss of motor coordination during locomotion, or hypnosis.
In Vivo Extraction Fraction for Ethanol
Separate experiments in another group of rats were carried out to determine an in vivo extraction fraction for ethanol for our probes using the method of Robinson et al. (2000). This was done to enable us to estimate the brain tissue concentrations around the probe under our experimental conditions. This method allows us to estimate tissue concentrations after various ethanol doses with the use of a minimal number of animals, unlike that which would be necessary with ethanol analysis from tissue extractions. Briefly, five animals were given an alcohol dehydrogenase inhibitor, 4-methylpyrazole (2.0 mg/kg, i.v.) one hour before being given i.v. 10% ethanol infusions (0.5 g/kg). Alcohol dehydrogenase is the major pathway for ethanol metabolism (Matsumoto et al., 1994), and inhibiting this enzyme allowed blood ethanol concentrations to remain relatively constant. During this “pseudo-steady state”, blood was drawn from the jugular catheter one hour after the ethanol infusion. Then three five-minute dialysate samples were collected, which was followed by another blood draw. The second ethanol infusion (0.5 g/kg) was given after the second blood draw to bring the animal to a cumulative dose of 1.0 g/kg ethanol. The blood and dialysis sampling was repeated as described for the first ethanol infusion. Samples were analyzed for ethanol using gas chromatography.
The in vivo extraction fraction for ethanol was calculated for each dose by computing the ratio of the dialysate ethanol concentration to the blood ethanol concentration. The peak brain ethanol concentrations for each dose examined in the dose-response experiments was estimated by dividing the dialysate ethanol concentration in the first sample obtained after the infusion by the in vivo extraction fraction.
Histology
The day after dialysis, the animals were overdosed using an i.p. injection of sodium pentobarbital (150 mg/kg). After the animal was perfused intracardially with saline and then 10% formalin, the brain was extracted and placed in 10% formalin overnight. The brains were sectioned (100 μm thick) with a vibratome (Leica, Nussloch, Germany) and then stained with cresyl violet to confirm probe placement. The probe tracks were mapped using the atlas of Paxinos et al. (1999).
High Pressure Liquid Chromatography (HPLC)
Dialysate dopamine was analyzed using HPLC with electrochemical detection. The system used a Polaris 3 μm C18 column (50 × 2 mm, Varian, Lake Forest, CA). The mobile phase consisted of 0.50 g octanesulfonic acid, 0.05 g decanesulfonic acid, 0.13 g ethylenediaminetetraacetic acid, 11.1 g NaH2PO4, and 150 ml methanol in 1 liter of deionized water. The mobile phase had a pH equal to 5.6. Seven microliters of the dialysate sample were mixed with ascorbate oxidase at 4° C prior to injection. Dopamine was detected with an electrochemical detector (Model VT03, Antec Leyden, Netherlands) at a potential of + 450 mV (relative to an Ag/AgCl reference). A second system was used for some samples in which the reference was an in situ Ag/AgCl (ISAAC). KCl was added to the mobile phase in appropriate concentrations in this case. The limit of detection was approximately 0.3 nM. The peaks were recorded using EZChrom software, and the concentration of dopamine in each sample was determined using external standards. The signal to noise ratio was determined for an external standard (0.625 nM dopamine) and a basal sample for each animal. Only animals with a signal to noise ratio > 3 for the standard and > 6 for the basal sample were included in the analyses.
Ethanol Analysis by Gas Chromatography
Ethanol was analyzed in 2 μl aliquots that were transferred into 2 ml gas chromatography vials immediately after collection of the microdialysis sample. Blood alcohol concentration was determined in 10 μl of the blood sample that was immediately added to 90 μl of saturated sodium chloride solution in a gas chromatography vial. Dialysate and blood ethanol concentrations were determined following the method of Doyon et al. (2003). A Varian CP 3800 gas chromatograph with flame ionization detection and a Varian 8200 headspace autosampler was used to analyze the concentrations of ethanol in the samples. The stationary phase was an HP Innowax capillary column (30 m × 0.53 mm × 1.0 μm film thickness) and helium was the mobile phase. Resulting ethanol peaks were recorded using Varian Star Chromatography Workstation software, and calibration was achieved using external standards.
Statistical Analysis – Basal Dopamine Concentrations for Core vs. Shell
The basal dopamine concentrations for core and shell were collapsed across all experiments, and the values were compared using a t-test. Significance was assigned if p < 0.05.
Statistical Analysis – Acute Intravenous Ethanol Experiments
A two-way ANOVA (mixed model with a randomized factor and a repeated measures factor) was used for the 0.5 g/kg ethanol experiments. The between-subject variable was subregion which had two levels (core and shell). Time was the within-subject variable. Three-way ANOVAs (mixed-models) were performed for the saline vs. 1.0 g/kg ethanol and the saline vs. 1.5 g/kg ethanol experiments (dose and subregion were between-subject variables). A two-way ANOVA (repeated measures, time; between-subject variable, dose) was performed for the shell data for the saline, 0.5, 1.0, and 1.5 g/kg ethanol groups to determine if a dose-response effect was present in this subregion. For all ANOVAs, if an interaction between the variables was observed, the simple effects were further analyzed to identify any sources of variation. Significance was assigned if p < 0.05. Because of a lack of homogeneity of variance in the between-subject variable for the 0.5 g/kg ethanol group, the 1.5 g/kg ethanol group, and the dose-response analysis, the analyses for these experiments were carried out on log-transformed data.
Statistical Analysis – Cumulative Intravenous Ethanol Experiments
For the cumulative dosing experiment, separate one-way repeated measures ANOVAs were used to analyze for the effect of time in the ethanol group and the saline group. Significance was assigned if p < 0.05. Because of a lack of homogeneity of variance the analysis for these experiments was carried out on log-transformed data.
Statistical Analysis – 4-Methylpyrazole Experiments
For the 4-methylpyrazole experiments, repeated measures ANOVA was used to compare the blood ethanol concentration before and after the dialysis sampling period at each of the two ethanol doses. Repeated measures ANOVA was also used to compare the extraction fraction values obtained after the 0.5 and 1.0 g/kg ethanol doses.
Statistical Analysis – 1.0 g/kg Ethanol-Evoked Dopamine in the Core and Shell
To compare the ability of ethanol to produce a dopamine response in the core and shell, the area under the dopamine response curve (AUC) and ethanol concentration curve was calculated. For the dopamine response AUC the basal values (nM) were subtracted to obtain a net response at each time point, and the sum of the post-infusion samples was obtained for successive samples in which two or more net responses were positive. The ethanol concentration AUC was computed by taking the sum of the post-infusion points. The ratio between the dopamine and ethanol AUC was calculated and a T-test was used to evaluate significance using p < 0.05 as the criterion.
Results
Basal Dialysate Dopamine Concentrations in the Core and Shell
In order to compare the basal values of extracellular dopamine in the core and shell, all experiments for each subregion were collapsed across ethanol doses. The overall concentrations were 1.5 ± 0.1 nM (n = 49) for the shell and 1.3 ± 0.1 nM (n = 30) for the core. These values were not significantly different from one another (T73 = -1.0, NS), and they agree with previously published results obtained with male Long-Evans rats (Blanchard et al., 1993; Benjamin et al., 1993; Hernandez et al., 2007)
Intravenous Infusion of 0.5 g/kg ethanol
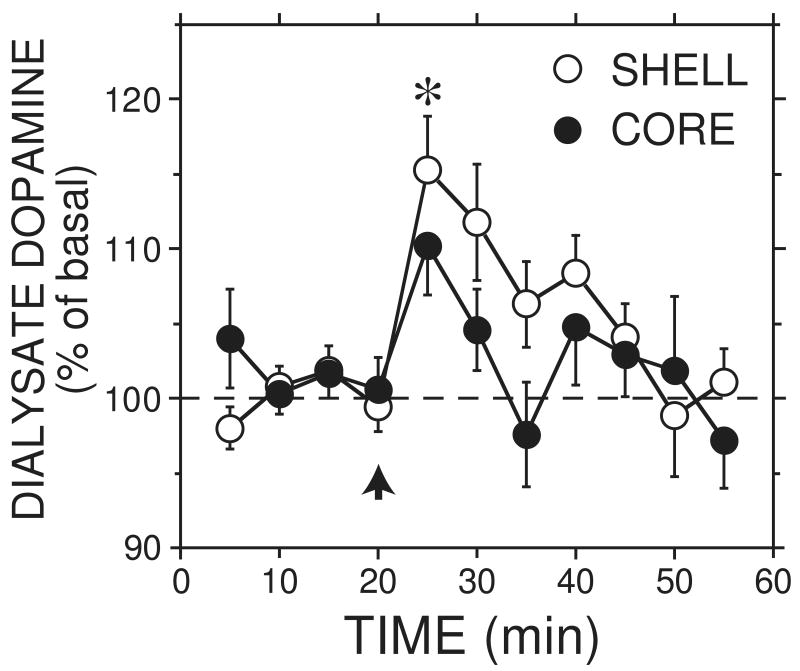
Infusion of 0.5 g/kg ethanol (i.v.) increased dopamine release in the core and shell (Figure 1; F10,150 = 4.5, p < 0.05 for effect of time), but there was no difference between core and shell (F10,150 = 1.4, NS for time × subregion interaction). One shell animal was given an equivalent volume of saline, and no increase in dopamine was observed (data not shown). A more complete saline control was not conducted because higher volumes of saline in the control experiments for the higher ethanol doses did not produce any significant increase in dopamine (see below). The peak dialysate ethanol concentration was obtained 5 minutes after the injection, and no difference was observed between core and shell time courses (Figure 2; F6,90 = 1.2, NS, for the interaction between time and subregion).
Figure 1.
Effect of 0.5 g/kg i.v. ethanol on accumbal core and shell dopamine concentrations. * p < 0.05 for core and shell when collapsed across dose and compared to basal. The average basal levels were 1.1 ± 0.2 nM for the core group and 1.5 ± 0.3 nM for the shell group. Arrow indicates bolus injection. Mean ± sem are shown (n=7-10).
Figure 2.
Core and shell ethanol concentrations after 0.5 g/kg i.v. ethanol infusion. Dialysate ethanol concentrations are indicated (mean ± sem, n=7-10). Arrow indicates bolus injection.
Intravenous Infusion of 1.0 g/kg ethanol
The i.v. infusion of 1.0 g/kg ethanol increased dialysate dopamine compared with saline (Figure 3). The ethanol-evoked dopamine response was significantly greater in the shell compared with the core (F10,230 = 1.9, p < 0.05, for subregion × time interaction). Furthermore, the dopamine response was significantly above basal in the shell but not the core (Figure 3; F10,230 = 4.5, p < 0.05 for the shell, and F10,230 = 0.6, NS for the core). The peak dialysate ethanol concentration was again obtained 5 minutes after the injection, and ANOVA revealed a significant difference in ethanol time courses between the core and shell (Figure 4; F6,54 = 3.5, p < 0.05, for the interaction between time and subregion). However, post hoc analyses did not show a difference between the subregions at any individual time point (F1,9 ≤ 6.2, NS). The overall difference in dialysate ethanol concentrations between the core and shell prompted us to examine whether the difference in dopamine response could be influenced by the concentration of ethanol reaching the sites from which dopamine is being sampled. Therefore, the ability of ethanol to produce a dopamine response in each subregion was calculated. First, the area under the curves (AUC) for both the dopamine concentration (nM) versus time and ethanol concentration (mM) versus time plots was computed for each rat. The ratio of the dopamine AUC to the ethanol AUC was 0.04 ± 0.01 for the core and 0.08 ± 0.02 for the shell (T5 = -2.1, p < 0.05), suggesting that the small difference in ethanol concentration between the subregions does not account for the differential dopamine response to ethanol.
Figure 3.
Effect of 1.0 g/kg ethanol and saline infusion (i.v.) on dopamine concentrations in the core (left) and shell (right). * p < 0.05 for ethanol when compared to basal. The average basal levels were 1.7 ± 0.4 nM for the core ethanol group, 1.4 ± 0.2 nM for the core saline group, 1.7 ± 0.3 nM for the shell ethanol group, and 1.4 ± 0.2 nM for the shell saline group. Arrow indicates bolus injection (n=5-9).
Figure 4.
Core and shell ethanol concentrations after 1.0 g/kg i.v. ethanol infusion. Dialysate ethanol concentrations are indicated (mean ± sem, n=5-6). Arrow indicates bolus injection.
Intravenous Infusion of 1.5 g/kg ethanol
The i.v. infusion of 1.5 g/kg ethanol increased dialysate dopamine differentially in the core and the shell when compared to saline for each subregion (Figure 5; F17,306 = 1.87, p < 0.05, for interaction between dose, subregion, and time). Again, the response was significantly larger in the shell relative to the core (F17,306 = 2.2, p < 0.05, for interaction between subregion and time). The peak dialysate ethanol concentration was also obtained 5 minutes after the injection, and the overall ethanol time course did not differ between core and shell (Figure 6; F13,130 = 1.4, NS).
Figure 5.
Effect of 1.5 g/kg ethanol and saline infusion (i.v.) on dopamine concentrations in the core (left) and shell (right). * p < 0.05 for ethanol when compared to basal. The average basal levels were 1.4 ± 0.2 nM for the core ethanol group, 1.1 ± 0.3 nM for the core saline group, 2.2 ± 0.4 nM for the shell ethanol group, and 1.5 ± 0.3 nM for the shell saline group. Arrow indicates bolus injection (n=4-6).
Figure 6.
Core and shell ethanol concentrations after 1.5 g/kg i.v. ethanol infusion. Dialysate ethanol concentrations are indicated (mean ± sem, n=6 for each group). Arrow indicates bolus injection.
Dose Response Effect in the Shell of the Nucleus Accumbens
A significant difference in dialysate dopamine from the shell after infusion of saline, 0.5, 1.0, or 1.5 g/kg ethanol was observed (F30,320 = 4.1, p < 0.05, for the dose × time interaction). Post-hoc analyses revealed that the saline and 0.5 g/kg ethanol groups individually differed from the 1.0 and 1.5 g/kg groups (F10,320 ≥ 3.3, p < 0.05), but that the saline and 0.5 g/kg groups, and the 1.0 and 1.5 g/kg groups did not differ from each other (F10,320 = ≤ 2.0, NS). Also, a significant effect of dose was observed for the ethanol concentrations across groups (F12,108 = 13.0, p < 0.05). Post-hoc analyses showed that the ethanol concentrations resulting from 0.5 g/kg intravenous infusions significantly differed from the 1.0 and 1.5 g/kg infusions (F6,108 = ≥12.9, p < 0.05); however, the 1.0 and 1.5 g/kg infusions did not result in concentrations that significantly differed from one another (F6,108 = 1.6, NS)
Cumulative Intravenous Ethanol Experiments
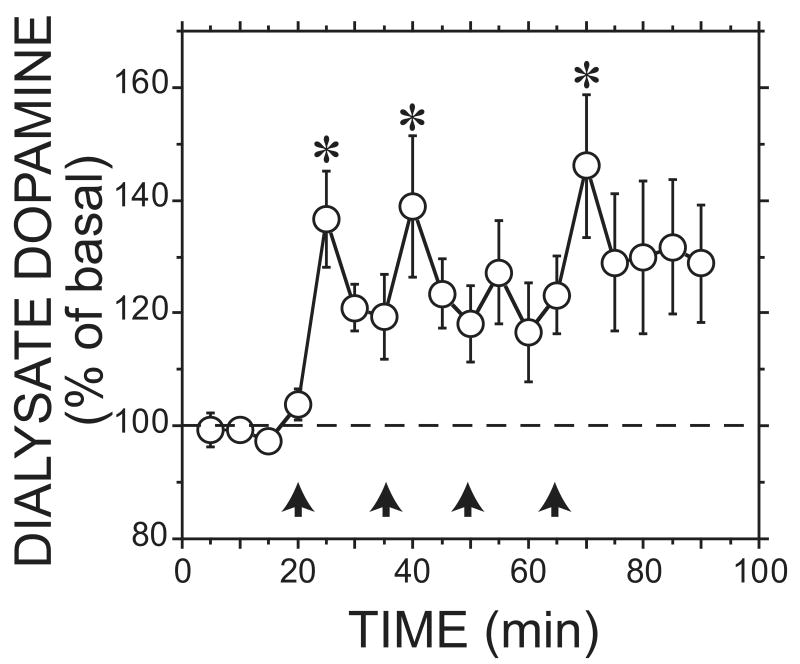
In the ethanol group, a significant increase in dialysate dopamine in the shell of the nucleus accumbens was observed (Figure 7; F17,136 = 3.9, p < 0.05, for effect of time). In contrast, no effect was observed in the group receiving repeated saline infusions (data not shown. F17,51 = 1.1, NS). The first, second, and fourth i.v. ethanol infusions of 1.0 g/kg significantly increased dialysate dopamine concentrations compared with basal values (F4,135 = ≥6.1, p < 0.05). No significant difference was observed between the dopamine increases following the four 1.0 g/kg ethanol infusions (F3,135 = 0.9, NS). Each peak dialysate ethanol concentration was observed 5 minutes after each infusion, and were all significantly different from baseline and from one another (Figure 8; F15,120 = 628.7, p < 0.05, for effect of time; F3,120 = 485.1, p < 0.05, for comparison of the four injection time points).
Figure 7.
Effect of cumulative 1.0 g/kg ethanol infusions on dopamine in the nucleus accumbens shell. * p < 0.05 for ethanol when compared to basal. The average basal level was 1.3 ± 0.2 nM. Arrows indicate bolus injections (n=9).
Figure 8.
Shell ethanol concentrations after cumulative 1.0 g/kg ethanol infusions. * Indicates the ethanol dose differs from all other doses. Dialysate ethanol concentrations are indicated (mean ± sem, n=9). Arrows indicate bolus injections.
Behavioral Analysis
Behavioral observations were recorded for the first 5 minutes after each ethanol infusion. We focused on this period because ethanol concentrations peaked during this time in all experiments. Peak dopamine responses generally occurred during this period as well. For the 0.5 g/kg i.v ethanol group, 0 of 5 core animals and 2 of 7 shell animals showed signs of sedation during the first 5 min post-infusion, corresponding to the peak ethanol concentrations. For the 1.0 g/kg acute i.v. ethanol group, 5 of 8 core and 5 of 7 shell animals showed signs of sedation. For the 1.5 acute i.v. ethanol group, 4 of 6 core and 6 of 7 shell animals showed signs of sedation. For the cumulative ethanol dosing group (all shell animals), 6 of 9 animals after the first 1.0 g/kg ethanol infusion and 9 of 9 animals after each of the additional three infusions showed signs of sedation in the 5-min period post-infusion.
4-Methylpyrazole Experiment: In vivo Extraction Fraction for Ethanol
A separate group of rats was used to determine the in vivo extraction fraction for ethanol for the probes used in this study. The major metabolic pathway of ethanol was blocked by inhibiting alcohol dehydrogenase, and this produced “pseudo-steady state” blood ethanol levels, and presumably, the brain ethanol concentrations were also relatively stable during this period (Gonzales et al., 2002; Robinson et al., 2000). Table 1 shows the blood ethanol concentration, dialysate ethanol concentration, decrease in blood ethanol concentration during the sampling period, and the extraction fraction after 0.5 and 1.0 g/kg ethanol (i.v.) doses. The slight decrease in blood ethanol concentrations during the dialysis sampling period was significant during each sampling period (F1,4 = 12.9, p < 0.05 for 0.5 g/kg; F1,4 = 20.9, p < 0.05 for 1.0 g/kg). This small drop in blood ethanol concentrations reflects the contribution of catalase and cytochrome P450 to the metabolism (Zimatkin and Buben, 2007; Zimatkin et al., 2006). The extraction fractions obtained for the 0.5 and 1.0 g/kg ethanol doses were not significantly different (F1,4 = 2.2, NS), and therefore, a mean extraction fraction was calculated (0.14). The peak brain ethanol concentrations were estimated for each of the ethanol doses used previously by dividing the peak dialysate concentration by the mean extraction fraction. This was also done for the 1.5 g/kg dose also because the in vivo extraction fraction for ethanol is independent of the concentration. These estimates yielded peak concentrations of 20, 49, and 57 mM for the 0.5, 1.0, and 1.5 g/kg acute i.v. infusion experiments, respectively. For the cumulative dosing study, we estimate that the peak tissue concentrations after each infusion to be 35, 62, 79, and 103 mM, respectively.
Table 1.
Blood and dialysate ethanol concentrations, change in blood concentration, and derived in vivo extraction fraction values for ethanol at two doses after i.v. administration.
| i.v. Ethanol Dose | Blood Ethanol (mM)a | Dialysate Ethanol (mM) b | Decrease in Blood Ethanol during Sampling Period (mM)c | Extraction Fractiond |
|---|---|---|---|---|
| 0.5 g/kg | 11.2 ± 0.7 | 1.5 ± 0.1 | 1.1 ± 0.3 | 0.14 ± 0.01 |
| 1.0 g/kg | 18.6 ± 1.1 | 2.7 ± 0.2 | 2.4 ± 0.5 | 0.15 ± 0.02 |
The mean ethanol concentration (± sem) from the blood taken before and after the dialysis sampling period
The mean dialysate ethanol concentration (± sem) in three samples taken at five minute intervals
Calculated as the concentration difference between the pre- and post- dialysis sample blood draws
The extraction fraction was derived from the ratio of the dialysate ethanol concentration to the blood ethanol concentration
Probe Placement Verification
Figure 9 shows the representation of the probe placements for the single dose ethanol experiments. None of the probes aimed at the shell overlapped with the core, although some of the core probes sampled from the ventral shell to a limited degree. For the core analysis we only included animals with probes that penetrated the shell by no more than 30% of the active dialysis membrane length. For the cumulative experiments, all probes were placed in the shell (Figure 10), and for 4-methylpyrazole experiments, all probes were placed on the medial border of the shell (Figure 11).
Figure 9.
Coronal sections indicating microdialysis probe placements in the core and shell of the nucleus accumbens for the 0.5 g/kg (n = 7 for the core, n = 10 for the shell), 1.0 g/kg and saline control (n = 13 for the core, n = 14 for the shell), and 1.5 g/kg and saline control (n = 10 for the core, n = 12 for the shell) groups. This figure was adapted from Paxinos and Watson (1998) and is representative of all probes collapsed onto one slice for either the core or shell.
Figure 10.
Coronal sections indicating microdialysis probe placements in the shell of the nucleus accumbens for the cumulative dosing experiments using intravenous saline (right) or ethanol (left). This figure was adapted from Paxinos and Watson (1998) and is representative of all probes collapsed onto one slice.
Figure 11.
Coronal sections indicating microdialysis probe placements in the shell of the nucleus accumbens for the 4-methylpyrazole experiments. This figure was adapted from Paxinos and Watson (1998) and is representative of all probes collapsed onto one slice.
Discussion
Two major findings are reported in the present study. First, the results show a larger dopamine response to ethanol in the shell subregion of the nucleus accumbens compared with the core in naïve Long-Evans rats. Increases in dialysate dopamine were seen after infusions of 0.5 g/kg ethanol when compared to saline; however, there was no significant difference between the effect in the core (7% above baseline) and the effect in the shell (15% above baseline). In contrast, the 16-38% increase observed after an infusion of 1.0 g/kg ethanol was statistically different between the two subregions. The 25-51% increase in dialysate dopamine observed following the 1.5 g/kg ethanol infusion was also significantly different between core and shell. The second major finding is the concentration-dependence of the accumbal dopamine response after i.v. ethanol administration. The 0.5 g/kg infusion of ethanol produced a peak accumbal ethanol concentration of 20 mM, and a modest dopamine response was observed. The 20 mM concentration produces moderate intoxication in non-tolerant humans (equivalent to a blood alcohol concentration of 0.9 mg/ml) and would be achieved after consumption of approximately 4 standard drinks within 60 min (Duarte et al., 2008; Schweizer et al., 2006; Brasser et al., 2004; Erickson, 2007). In contrast, the 1.0 g/kg and 1.5 g/kg ethanol infusions produced a robust dopamine response in the shell at estimated peak brain concentrations of 49 mM and 57 mM. These concentrations would produce severe intoxication in humans accompanied by motor incoordination and hypnosis (blood alcohol concentration of 2.3-2.7 mg/ml; Erickson, 2007. The concentration-dependence of the ethanol-stimulated dopamine response reported in the present study is not consistent with the idea that concentrations of ethanol that are associated with low to moderate intoxication strongly activate the mesolimbic dopamine system.
During the cumulative dosing study, 1.0 g/kg i.v. ethanol infusions were administered every fifteen minutes to reach a dose of 4.0 g/kg. While the first infusion resulted in an increase in dopamine in the shell (37%) similar to our 1.0 g/kg ethanol shell group in the acute study (38%), the additional three infusions did not increase dopamine release in a dose-dependent manner. Therefore, this experiment indicates that the effect of ethanol on dopamine release in the shell is relatively constant over a three-fold concentration range starting with an initial dose of 1.0 g/kg. This is in marked contrast to the steep dose-dependence of dopamine release in the shell observed between 0.5 and 1.0 g/kg, which produces peak ethanol concentrations in the range of 20-49 mM.
The core and shell subregions of the nucleus accumbens may be differentially activated by ethanol exposure depending on the environmental context. For example, Bassareo et al. (2003) showed that administration of ethanol through an intraoral catheter produced a transient dopamine response in the shell in naïve rats, but not in rats that had been exposed to ethanol the previous day. This is consistent with the proposed role of the shell in the recognition of novel stimuli (Rebec et al., 1997; Di Chiara et al., 2004). On the other hand, the core may show a more robust dopaminergic response after the formation of associations between ethanol and conditioned stimuli during behaviors that require instrumental responses or in operant conditioning (Kelley et al., 1997; Sokolowski and Salamone, 1998; Bassareo and Di Chiara, 1999; Ito et al., 2000). In the present study, the ethanol was administered to naïve rats, so the larger dopamine response we observed in the shell is consistent with the idea that the dopamine signaling in the shell may code for a novel experience. However, we found that the dopamine response in the core was also significantly enhanced in the 1.5 g/kg ethanol group, and it may be possible that the core also plays this role, although not to the same extent as the shell.
Our conclusion that the shell has a higher dopamine response to ethanol than the core is tempered by two issues. First, we found that the peak ethanol concentration is slightly higher in the shell than the core after the 1.0 g/kg dose, a finding that was not replicated at a lower or higher dose. To account for the difference in ethanol concentrations, we compared the ratio of the dopamine AUC to the ethanol AUC in both subregions. This analysis showed that a significantly greater dopamine response was produced in the shell compared with the core after the 1.0 g/kg dose, in agreement with the 1.5 g/kg dose. A second issue that should be considered is the large volume of fluid that we infused, particularly for the 1.0 and 1.5 g/kg doses. It is likely that physiological changes in cardiovascular parameters occur after these infusions. However, volume-matched saline infusions did not alter accumbal dopamine release demonstrating that the accumbal dopamine response is specific to ethanol. However, we can not rule out the possibility that the physiological changes in cardiovascular function may interact with the pharmacological effects of ethanol to contribute to the overall responses we measured. In any case, this issue does not invalidate our conclusion that the shell responds to ethanol with a greater magnitude than the core.
To our knowledge, the present study is the first to specifically compare dialysate dopamine in core versus shell after ethanol administration in rats. However, the present results contradict a previous study of ethanol-evoked dopamine release in the core and shell in which no difference was reported (Zocchi et al., 2003). This discrepancy could be due, in part, to the use of mice by Zocchi et al. (2003). Mice have smaller core and shell subregions compared with rats, and it may be more difficult to place probes exclusively in the core or shell subregions in the mouse model. Alternatively, the core-shell difference in ethanol response we report may be found in rats but not mice.
The measurement of the in vivo extraction fraction for ethanol allowed us to clearly define the concentration-dependence of the ethanol-evoked accumbal dopamine response. Relatively high concentrations of ethanol (∼20 mM) yield modest dopamine release (7-15%) in the nucleus accumbens after a bolus i.v. infusion of 0.5 g/kg. This effect is lower in magnitude to that previously reported after a higher dose (1.0 g/kg) given i.p. Previous work has consistently shown that i.p. administration of 1.0 g/kg ethanol elicits a more robust accumbal dopamine response (40-200% above baseline) (Carboni et al., 1989; Yoshimoto et al., 1991; Acquas et al., 1993; Heidbreder and De Witte, 1993; Kiianmaa et al., 1995; Yim et al., 2000). Although these doses and routes of administration differ (0.5 g/kg, i.v. vs. 1.0 g/kg, i.p.), both produce similar peak brain concentrations (Nurmi et al., 1994). Based on Nurmi's work using one minute microdialysis samples, a 1.0 g/kg ethanol injection i.p. should produce a peak ethanol brain concentration around 35 mM. Although the estimated peak brain concentration for the 0.5 g/kg ethanol group was 20 mM, this value was determined from the average brain concentration during the first five minutes, whereas the peak is actually much higher within 1-2 minutes after the injection and is more likely to be in the range of 35 mM (Robinson et al., 2002). Preliminary data in our lab also indicate that a 0.5 g/kg i.v. infusion and 1.0 g/kg i.p. infusion produce similar peak concentrations of ethanol in the brain (dialysate concentration of 3.0 mM) and yet yield very different increases in dopamine (16% increase in dopamine above baseline after i.v. infusion and 44% increase above baseline after the i.p. injection). These considerations lead us to suggest that the accumbal dopamine response previously reported after i.p. injection of low to moderate doses of ethanol is due, in part, to physiological mechanisms, in addition to, a direct pharmacological action on the mesolimbic system.
The reasons for this discrepancy between the results of this study and previous studies are not clear, but several potential explanations can be offered. The differing routes of administration could contribute because the i.p. route involves handling of the animal, whereas in the present study the i.v. route eliminated handling-induced stress. The stress associated with handling and saline injection has been demonstrated to increase accumbal dopamine in some (Barrot et al., 2000; Tang et al., 2003), but not all previous studies (Yoshimoto et al., 1991; Acquas et al., 1993; Heidbreder and De Witte, 1993; Kiianmaa et al., 1995; Yan, 1999). Intraperitoneal injection-induced stress may interact with ethanol to enhance the effect of ethanol on the accumbal dopamine response reported in previous studies (Imperato and Di Chiara, 1986; Carboni et al., 1989; Yoshimoto et al., 1991; Acquas et al., 1993; Blanchard et al., 1993; Heidbreder and De Witte, 1993; Kiianmaa et al., 1995; Samson et al., 1997; Tanda and Di Chiara, 1998; Yan, 1999). In addition, it is possible that i.p. administration of ethanol produces a unique sensation in the peritoneum of the animal after injection. For example, many previous studies used 15-20% ethanol, and this concentration may dehydrate the peritoneal lining to produce a visceral sensation. This novel sensation may contribute to an increase in dopamine in the shell of the accumbens (Rebec et al., 1997; Di Chiara et al., 2004).
Another issue to consider is the strain of rat used in this experiment (Long-Evans). Blanchard et al. (1993) reported that a low dose of i.p. ethanol (0.25 g/kg) stimulates accumbal dopamine release in male Long-Evans rats, but that higher doses (0.5 and 1.0 g/kg, i.p.) were ineffective, and the highest dose used (2.0 g/kg, i.p.) inhibited dopamine release. However, Samson et al. (1997) observed that 1.0 g/kg ethanol (i.p.) stimulated accumbal dopamine release in this strain. Thus, there is no clear consensus in the literature regarding the dose-dependence of ethanol-stimulated accumbal dopamine release in male Long-Evans rats when given by the i.p. route. Our results using the i.v. route of administration match those of Samson et al. (1997), although caution should be maintained in comparing results from an i.v. study with an i.p. study. Many other investigators have reported that 1-2 g/kg doses of ethanol given i.p. stimulate accumbal dopamine release in Sprague-Dawley and Wistar rats (Imperato and Di Chiara, 1986; Acquas et al., 1993; Yim et al., 1998; Yim et al., 2000; Campbell and McBride, 1995; Kohl et al., 1998). Because the present study is the first to report the effect of i.v. ethanol administration on dialysate accumbal dopamine concentrations, it is not clear whether results obtained in Long-Evans rats will generalize to other rat strains.
The results of this study can also be compared to previous behavioral studies such as ethanol self-administration or ethanol-induced conditioned place preference using peak tissue concentration estimates. The present data show that the concentrations of ethanol that produce a robust accumbal dopamine response (20-50%) are considerably higher than those in rats that orally self-administer ethanol (Weiss et al., 1993; Weiss et al., 1996; Gonzales and Weiss, 1998; Melendez et al., 2002; Doyon et al., 2003; Doyon et al., 2005). Although tissue concentrations weren't measured in these previous studies, based on the amounts of ethanol consumed (0.5 -1.4 g/kg), and the dialysate ethanol levels reported when dopamine peaks, it is unlikely that concentrations higher than those of the 0.5 g/kg i.v. ethanol group in this study would have been reached. The 0.5 g/kg i.v. ethanol group only showed dopamine responses of 7% and 15% above basal in the core and shell, respectively. Therefore, this adds further support to the suggestion that accumbal dopamine is not regulated by ethanol through only a pharmacological mechanism during self-administration (Doyon et al., 2005), but rather also through physiological processes associated with ethanol consumption. For example, during self-administration animals are exposed to stimulus cues from the drinking solution, and over time the animal may associate these cues with ethanol's rewarding effects. Eventually, these cues may be responsible for producing the change in dopamine (Schultz et al., 1997).
Another method of assessing the rewarding properties of ethanol is conditioned place preference (Cunningham et al., 2003). Most studies with rats have used the i.p. route of administration, although one report showed that 0.6 g/kg (i.v.) ethanol did not produce place conditioning after four conditioning trials (van der Kooy et al., 1983). In contrast, ethanol-induced conditioned place preference was demonstrated by several groups after prolonged place conditioning (at least 14 pairing trials). Unfortunately, the dose-dependence of this effect has not been firmly established with one group showing that 0.5 g/kg (i.p.) ethanol did not produce place conditioning (Biala and Kotlinska, 1999), whereas others have reported success using this dose and route of administration (Bozarth, 1990; Zhu et al., 2007). The peak brain concentrations after 0.5 g/kg ethanol (i.p.) should be approximately 17 mM (Nurmi et al., 1994), and our present data suggest that this concentration will produce a modest, threshold effect on dopamine release. Taken together, it is tempting to speculate that ethanol-induced conditioned place preference in rats is largely independent of an accumbal dopamine response. However, a major caveat with this speculation is that the present data are from naïve rats, and conditioned place preference requires repeated exposure to ethanol, similar to what is required to establish ethanol self-administration. It can be noted that that repeated exposure to ethanol may induce sensitization of the dopamine response (Szumlinski et al., 2005), although this finding has not been reported in all studies (Zapata et al., 2006). Further research is needed to define the mechanisms that are responsible for the stimulation of accumbal dopamine activity in behavioral contexts in which the rewarding properties of ethanol are apparent.
In conclusion, the results of this study indicate that dopamine release in the shell is higher than the core in response to acute i.v. ethanol administration. Also, tissue concentrations near 20 mM only produce modest dopamine increases in these subregions. The results also indicate that dopamine in the shell increases in a dose-dependent manner between 0.5 -1.0 g/kg doses, but higher doses result in a plateau of the response. These findings imply that the robust accumbal dopamine response observed in previous studies using i.p. administration or oral self-administration in which similar concentrations have been achieved may be due to stimulus cues associated with ethanol or an interaction between ethanol and handling stress.
Acknowledgments
This work was supported by a grant from NIH/NIAAA (AA11852). ECH was supported by training grants from NIDA (DA018926), NIAAA (AA007471) and a Ruth L. Kirchstein National Service Research Award (AA016874). The authors would like to thank Dr. Donita Robinson for helpful comments on the manuscript and Laura West for assistance with experiment preparation.
List of Abbreviations
- AUC
area under curve
- i.p
intraperitoneal
- i.v
intravenous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acquas E, Meloni M, Di Chiara G. Blockade of delta-opioid receptors in the nucleus accumbens prevents ethanol-induced stimulation of dopamine release. Eur J Pharmacol. 1993;230:239–241. doi: 10.1016/0014-2999(93)90809-v. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Aresu M, Aste A, Ariu T, Di Chiara G. Differential adaptive properties of accumbens shell dopamine responses to ethanol as a drug and a motivational stimulus. Eur J Neurosci. 2003;17:1465–1472. doi: 10.1046/j.1460-9568.2003.02556.x. [DOI] [PubMed] [Google Scholar]
- Benjamin D, Grant ER, Pohorecky LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Research. 1993;621:137–140. doi: 10.1016/0006-8993(93)90309-b. [DOI] [PubMed] [Google Scholar]
- Biala G, Kotlinska J. Blockade of the acquisition of ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol. 1999;34:175–182. doi: 10.1093/alcalc/34.2.175. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacol Biochem Behav. 1990;35:485–487. doi: 10.1016/0091-3057(90)90191-j. [DOI] [PubMed] [Google Scholar]
- Brasser SM, McCaul ME, Houtsmuller EJ. Alcohol effects during acamprosate treatment: a dose-response study in humans. Alcohol Clin Exp Res. 2004;28:1074–1083. doi: 10.1097/01.alc.0000130802.07692.29. [DOI] [PubMed] [Google Scholar]
- Campbell AD, McBride WJ. Serotonin-3 receptor and ethanol-stimulated dopamine release in the nucleus accumbens. Pharmacol Biochem Behav. 1995;51:835–842. doi: 10.1016/0091-3057(95)00050-7. [DOI] [PubMed] [Google Scholar]
- Carboni E, Acquas E, Frau R, Di Chiara G. Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur J Pharmacol. 1989;164:515–519. doi: 10.1016/0014-2999(89)90259-8. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93:1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Duarte R, McNeill A, Drummond G, Tiplady B. Comparison of the sedative, cognitive, and analgesic effects of nitrous oxide, sevoflurane, and ethanol. Br J Anaesth. 2008;100:203–210. doi: 10.1093/bja/aem369. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Sapoznik T, Kornetsky C. The synergistic effects of combining cocaine and heroin (“speedball”) using a progressive-ratio schedule of drug reinforcement. Pharmacol Biochem Behav. 1998;61:297–302. doi: 10.1016/s0091-3057(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Erickson CK. The Science of Addiction, From Neurobiology to Treatment. W. W. Norton & Company, Inc.; New York, NY: 2007. [Google Scholar]
- Fenu S, Spina L, Rivas E, Longoni R, Di Chiara G. Morphine-conditioned single-trail place preference: role of nucleus accumbens shell dopamine receptors in acquisition, but not expression. Psychopharmacology (Berl) 2006;187:143–153. doi: 10.1007/s00213-006-0415-2. [DOI] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Tang A, Robinson DL. Quantitative microdialysis for in vivo studies of pharmacodynamics. In: Liu Y, Lovinger D, editors. Methods in Alcohol Related Neuroscience Research. Boca Raton, FL: CRC Press; 2002. pp. 287–315. [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Roles of the nucleus accumbens and amygdale in the acquisition and expression of ethanol-conditioned behavior in mice. J Neurosci. 2008;28:1076–1084. doi: 10.1523/JNEUROSCI.4520-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder C, De Witte P. Ethanol differentially affects extracellular monoamines and GABA in the nucleus accumbens. Pharmacol Biochem Behav. 1993;46:477–481. doi: 10.1016/0091-3057(93)90383-5. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wholtmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Hernandez G, Haines E, Rajabi H, Stewart J, Arvanitogiannis A, Shizgal P. Predictable and unpredictable rewards produce similar changes in dopamine tone. Behav Neurosci. 2007;121:887–895. doi: 10.1037/0735-7044.121.5.887. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate recap tor activation in the nucleus accumbens core. Proc Natl Acad Sci. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiianmaa K, Nurmi M, Nykanen I, Sinclair JD. Effect of ethanol on extracellular dopamine in the nucleus accumbens of alcohol-preferring AA and alcohol-avoiding ANA rats. Pharmacol Biochem Behav. 1995;52:29–34. doi: 10.1016/0091-3057(95)00097-g. [DOI] [PubMed] [Google Scholar]
- Kohl RR, Katner JS, Chernet E, McBride WJ. Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology (Berl) 1998;139:79–85. doi: 10.1007/s002130050692. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Fujimiya T, Fukui Y. Role of alcohol dehydrogenase in rat ethanol elimination kinetics. Alcohol Alcohol Suppl. 1994;29:15–20. [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26:318–325. [PubMed] [Google Scholar]
- Nurmi M, Kiianmaa K, Sinclair JD. Brain ethanol in AA, ANA, and Wistar rats monitored with one-minute microdialysis. Alcohol. 1994;11:315–321. doi: 10.1016/0741-8329(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Olausson P, Ericson M, Petersson A, Kosowski A, Soderpalm B, Engel JA. Nefazodone attenuates the behavioral and neurochemical effects of ethanol. Alcohol. 1998;15:77–86. doi: 10.1016/s0741-8329(97)00101-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Paxinos G, Kus L, Ashwell KWS, Watson C. Chemoarchitectonic atlas of the rat forebrain. Academic Press; San Diego, CA: 1999. [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Lara JA, Brunner LJ, Gonzales RA. Quantification of ethanol concentrations in the extracellular fluid of the rat brain: in vivo calibration of the microdialysis probes. J Neurochem. 2000;75:1685–1693. doi: 10.1046/j.1471-4159.2000.0751685.x. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Brunner LJ, Gonzales RA. Effect of gender and estrous cycle on the pharmacokinetics of ethanol in the rat brain. Alcohol Clin Exp Res. 2002;26:165–172. [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Erickson HL, Niehus JS, Gerhardt GA, Kalivas PW, Floyd EA. The effects of local application of ethanol in the n. accumbens on dopamine overflow and clearance. Alcohol. 1997;14:485–492. doi: 10.1016/s0741-8329(96)00216-9. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31:1301–1309. doi: 10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. The role of accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav. 1998;59:557–566. doi: 10.1016/s0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Di Chiara G. A dopamine-mu1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10:1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Tang A, George MA, Randall JA, Gonzales RA. Ethanol increases extracellular dopamine concentration in the ventral striatum in C57BL/6 mice. Alcohol Clin Exp Res. 2003;27:1083–1089. doi: 10.1097/01.ALC.0000075825.14331.65. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, O'Shaughnessy M, Mucha RF, Kalant H. Motivational properties of ethanol in naive rats as studied by place conditioning. Pharmacol Biochem Behav. 1983;19:441–445. doi: 10.1016/0091-3057(83)90117-x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q. Extracellular dopamine and serotonin after ethanol monitored with 5-minute microdialysis. Alcohol. 1999;19:1–7. doi: 10.1016/s0741-8329(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22:367–374. [PubMed] [Google Scholar]
- Yim HJ, Robinson DL, White ML, Jaworski JN, Randall PK, Lancaster FE, Gonzales RA. Dissociation between the time course of ethanol and extracellular dopamine concentrations in the nucleus accumbens after a single intraperitoneal injection. Alcohol Clin Exp Res. 2000;24:781–788. [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1991;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Ethanol enhances the release of dopamine and serotonin in the nucleus accumbens of HAD and LAD lines of rats. Alcohol Clin Exp Res. 1992;16:781–785. doi: 10.1111/j.1530-0277.1992.tb00678.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann NY Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Bie B, Pan ZZ. Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci. 2007;27:289–298. doi: 10.1523/JNEUROSCI.3912-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimatkin SM, Buben AL. Ethanol oxidation in the living brain. Alcohol Alcohol. 2007;42:529–532. doi: 10.1093/alcalc/agm059. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Pronko SP, Vasiliou V, Gonzales FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res. 2006;30:1500–1505. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Girlanda E, Varnier G, Satori I, Zanetti L, Wildish GA, Lennon M, Mugnaini M, Heidbreder CA. Dopamine responsiveness to drugs of abuse: a shell-core investigation in the nucleus accumbens of the mouse. Synapse. 2003;50:293–302. doi: 10.1002/syn.10271. [DOI] [PubMed] [Google Scholar]