Abstract
Purpose
Preeclampsia is a leading cause of perinatal mortality and morbidity, and it increases maternal risk for future cardiovascular disease. The purpose of the study was to explore the relationships among stretching exercise, autonomic cardiac response, and the development of preeclampsia.
Design
Secondary data analysis.
Methods
Heart rate and pulse pressure were longitudinally examined in this secondary data analysis among women who engaged in stretching exercise daily from 18 weeks of gestation to the end of pregnancy compared with women who did walking exercise daily during the same time period. A total of 124 women were randomized to either stretching (n=60) or walking (n=64) in the parent study.
Findings
Heart rates in the stretching group were consistently lower than those in the walking group.
Conclusions
Based on the results of this secondary data analyses, a physiologic framework for possible beneficial effects of stretching exercise by enhancing autonomic responses on reducing risks for preeclampsia is proposed and discussed.
Clinical Relevance
If the protective effect is established, stretching exercise can be translated into nursing intervention for prenatal care.
Keywords: Pregnancy, heart rate, pulse pressure
Pregnancy is a healthy physiologic condition with pro-found cardiovascular system changes that expose women to risks for hypertension (Kaaja & Greer, 2005) and insulin resistance (Kaaja & Poyhonen-Alho, 2006). The autonomic nervous system (ANS) plays a central role in the adaptation of the cardiovascular system during pregnancy. Autonomic nervous activity shifts toward a lower sympathetic and higher vagal modulation in the first trimester, and then toward a higher sympathetic and lower vagal modulation in late pregnancy (Kuo, Chen, Yang, Lo, & Tsai, 2000). These conditions make pregnant women susceptible to various risks, including gestational hypertension, gestational diabetes, and preeclampsia. When pregnant women are overweight or obese, they are even more susceptible, because either condition adds risk for cardiovascular and metabolic diseases (Grassi et al., 1998; Kiel, Dodson, Artal, Boehmer, & Leet, 2007; Lombardi, Barton, O'Brien, Istwan, & Sibai, 2005).
Preeclampsia, a syndrome characterized by the sudden onset of hypertension and proteinuria in the latter half of pregnancy (National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy, 2000), accounts for 22% of maternal deaths (Panchal, Arria, & Labhsetwar, 2001) and 18% of all pre-mature births (Basso et al., 2006; McElrath et al., 2008; Redman & Sargent, 2005; Zhang, Meikle, & Trumble, 2003), and it increases maternal risk for future cardiovascular disease (Anderson, 2007; McDonald, Malinowski, Zhou, Yusuf, & Devereaux, 2008). Preceding the clinical manifestation of this disease is a period of largely asymptomatic gradual decline in health status, beginning in early pregnancy (Nishimoto et al., 2009), characterized by autonomic dysregulation (Fischer et al., 2004), labile blood pressure (Caritis, Sibai, Hauth, Lindheimer, VanDorsten, et al., 1998; Ohkuchi et al., 2006), insulin resistance (Kaaja et al., 2004; Moran et al., 2006; Parretti et al., 2006), and oxidative stress (Gupta, Agarwal, & Sharma, 2005; Hubel, 1999; Serdar, Gur, & Develioglu, 2006). Numerous studies have documented these pathologic changes in maternal systems, though the etiology of preeclampsia remains unknown—most likely multifactorial (Widmer et al., 2007)—and no preventive measures currently exist to eliminate the threat of developing this dangerous disease (Karumanchi & Lindheimer, 2008). Risk factors include certain genetic traits (Dekker, 1999; Nishimoto et al., 2009; Vural et al., 2009), obesity (Bodnar, Ness, Markovic, & Roberts, 2005; Sibai et al., 1995), nulliparous status (Dekker, 1999), history of preeclampsia (Caritis, et al., 1998), diabetes (Caritis, Sibai, Hauth, Lindheimer, VanDorsten, et al., 1998), hypertension (Caritis, Sibai, Hauth, Lindheimer, Klebanoff, et al., 1998; Caritis, Sibai, Hauth, Lindheimer, VanDorsten, et al., 1998; Ohkuchi et al., 2006), and sedentary lifestyle (Saftlas, Logsden-Sackett, Wang, Woolson, & Bracken, 2004; Sorensen et al., 2003). Unfortunately, many of these risk factors are not modifiable or are very difficult to modify, especially during pregnancy.
One potentially modifiable risk factor is physical activity, and large epidemiologic studies have shown that women who engaged in moderate to vigorous leisure time physical activities (LTPAs) before and during pregnancy experienced up to a 35% reduction in preeclampsia (Evenson, Savitz, & Huston, 2004; Ning et al., 2003; Rousham, Clarke, & Gross, 2006; Saftlas et al., 2004; Sorensen et al., 2003). However, few pregnant women (15.8%) engage in the recommended level of LTPAs, and the majority of pregnant women (84.2%; Evenson et al.) do not initiate (Ning et al.) or increase LTPAs during pregnancy, particularly in the latter half of pregnancy (Domingues & Barros, 2007; Hinton & Olson, 2001; Ning et al.; Yeo, 2009).
Approximately 24% of pregnant women in the US are obese (Bodnar et al., 2005; Mumford, Siega-Riz, Herring, & Evenson, 2008). Obesity (Sibai et al., 1995; Wolf et al., 2001) and excessive weight gain during pregnancy (Kiel et al., 2007; Raatikainen, Heiskanen, & Heinonen, 2006) are both independent risk factors for preeclampsia. In a prospective cohort study, Bodnar et al. found that risk for preeclampsia was tripled for obese pregnant women compared with their nonobese counterparts. Dyslipidemia, inflammation, and oxidative stress are believed to be mechanisms shared by preeclampsia and obesity (Ray, Diamond, Singh, & Bell, 2006; Weissgerber, Wolfe, & Davies, 2004; Yeo & Davidge, 2001). Further, higher sympathetic nervous activity and reduced cardiac vagal tone are thought to contribute to these pathologic conditions (Moertl et al., 2008). Indeed, hypertensive disorders in pregnancy, including preeclampsia, have been characterized as a state of sympathetic overactivity (Fischer et al., 2004).
While previous studies have demonstrated that moderate to vigorous LTPAs reduce the risk for developing preeclampsia (Marcoux, Brisson, & Fabia, 1989; Saftlas et al., 2004; Sorensen et al., 2003), recent evidence suggests that the risk reduction may not apply to all pregnant women. In their prospective study, Magnus, Trogstad, Owe, Olsen, and Nystad (2008) found that un-like nonobese women, obese women did not receive any significant protective benefit of the vigorous physical activities with regard to preeclampsia. Participants in the study were asked the number of vigorous activities performed per month—from brisk walking to aerobic exercise—in their second trimester. Among those who reported 6 to 12 activities per month, obese women were at significantly higher risk for preeclampsia than women with normal weight. Thus, while obesity and sedentary lifestyle independently impose risks for preeclampsia, not all LTPAs reduce the risk for preeclampsia equally. For obese women, nonvigorous physical activities that are safe and effective in reducing risk for adverse outcomes need to be identified and examined.
Given the role of autonomic responses in both normal and pathologic processes of pregnancy, low-intensity exercise such as yoga, tai-chi, or prenatal stretching exercises (PSEs) may produce physiologic beneficial effects. Studies report some positive effects of yoga (Bowman et al., 1997) and tai-chi (Lu & Kuo, 2003) on autonomic responses as well as reducing stress (Jallo, Bourguignon, Taylor, & Utz, 2008). Yet, few studies to date examined similar effects of PSEs, which are commonly prescribed by nurses. Previously we conducted a randomized trial comparing two exercises, stretching and walking, on the incidence of preeclampsia among sedentary pregnant women, and found that women in the daily PSE program experienced a significantly lower incidence of preeclampsia than expected (Yeo et al., 2008). Unknown was the involvement of autonomic responses to daily exercises. Thus, the purpose of this study was to longitudinally compare autonomic responses—resting heart rates and blood pressures—between two exercise groups, stretching versus walking during pregnancy.
Methods
This was the secondary data analysis of a large randomized control trial. The primary purposes of the large study were to determine whether women who had led a sedentary lifestyle prior to pregnancy and were at risk for preeclampsia could exercise regularly during pregnancy and whether the exercise would reduce the incidence of preeclampsia (Yeo, 2009). Pregnant women were eligible for the study if they (a) were healthy but had been diagnosed with preeclampsia in a previous pregnancy; (b) had a lower than average cardiovascular fitness level (i.e., peak oxygen consumption equal to or less than the 50th percentile of age group women, as measured by fitness tests at 17 weeks of gestation; and (c) had a sedentary lifestyle (i.e., their estimated energy expenditure for daily physical activity during the index pregnancy was less than 840 kcal/week, as assessed by the Minnesota Leisure Time Physical Activity Questionnaire). They were excluded if they had (a) a diagnosis of chronic hypertension or pregestational diabetes, (b) any medical or physical condition prohibiting daily regular exercise, (c) a recommendation from the primary care provider not to participate, or (d) inability to reasonably communicate with research staff because of language or mental status. The details of the design and methods have been published (Yeo, 2006).
Sample
From November 2001 through July 2006, 386 pregnant women from nine clinics in two health systems in a Midwest urban area were contacted. A total of 124 eligible women were randomized to either walking (n=64) or stretching (n=60) at 18 weeks of gestation. Power analysis indicated 30 participants in each group would have had 80% of power to detect 10 beats/min in heart rate difference in two groups. The majority of the sample (85%) identified themselves as non-Hispanic White. They were on average 31±5 years of age; had 15±2 years of education; were relatively affluent (52% reported household incomes above $75,000); were employed (80%); and worked an average of 30±13 hr/week. The majority (82%) had one child at home. At baseline, there were no significant differences between the stretching and walking groups on any variables.
Walking or Stretching Interventions
Both groups were asked to perform the assigned exercise five times a week throughout the pregnancy. Walkers were trained to walk at moderate intensity. They were consistently monitored by a Polar S810 Heart Rate Monitor (Warminster, PA) and the Rating Perceived Exertion Scale (rating of 12 or 13, moderate level of intensity) and were instructed to look for warning signs to stop exercising and call their care providers (e.g., vaginal bleeding, uterine contractions, etc.). Stretchers were instructed to follow videotaped movements developed for the study, consisting of slow muscle movements that had neither aerobic nor muscle resistance components. The stretching exercise consists of a sequence of large skeletal muscles from the neck to the upper limbs, to the torso, and to the lower limbs, accompanied by deep breathing. We selected these movements from various PSEs and used in our preliminary studies. Upper body movements included slouch stretch with overcorrection, side-bending neck stretch, neck rotation, neck flexion-extension, chin tuck, arm-across-chest stretch, forearm and hands stretch, wrist extension stretch, and seated side bend stretch. Lower body movements included child's pose, cat-camel stretch, hamstring stretch, seated cross-legged hip rotator stretch, seated hamstring stretch, V-sit stretch, and groin stretch.
Participants visited the research laboratory once a week and exercised under the supervision of the research staff exercise specialist. In the walking group, the average adherence was 3.77 times per week at 18 weeks, 3.14 times per week at 28 weeks, and 2.71 times per week at 35 weeks. In the stretching group, the average adherence was 4.20 times per week at 18 weeks, 3.81 times per week at 28 weeks, and 3.53 times per week at 35 weeks (Yeo, 2009).
The main outcomes of the original study have been published elsewhere (Yeo, 2009; Yeo et al., 2008). Briefly, of the 124 participants, 13 (10%) developed preeclampsia. The walking group had a higher incidence of preeclampsia than the stretching group (10 cases [16%] verses 3 cases [5%]; exact conditional analyses, p=.054). Given the population estimate of 18% for preeclampsia in women with a history of the disease (Caritis, Sibai, Hauth, Lindheimer, VanDorsten, et al., 1998) the finding of 10 preeclampsia cases in the walking group was within the expected range of incidence, but the finding of 3 cases in the stretching group was significantly lower than expected (p<.005).
No statistically significant group differences were observed in birth outcomes. The preterm birth rate was twice as high in the walking group (22%) compared with that in the stretching group (11%), but the difference was not statistically significant (Yeo et al., 2008).
Measures
Heart rate and blood pressure were measured at weekly laboratory visits prior to exercise sessions. After having participants sit quietly for 10 minutes, heart rate and blood pressure were taken. Pulse pressures were calculated by subtracting diastolic pressure from systolic pressure. All measures were taken by trained research assistants following the American College of Sports Medicine (ACSM) guidelines (ACSM, 2000). Briefly, resting heart rates were manually recorded by placing the index and middle fingers on the thumb side of the lower part of the forearm, followed by manual measurement of blood pressure.
Data Analysis
Two sample t tests were used to examine differences between the walking and stretching groups. Repeated measure analysis of variance was used to compare continuous variables over time. Restricted maximum likelihood (REML) regression was used to compare group differences, gestation weeks, and their interaction with adherence, resting heart rate, and blood pressure changes during statistical analyses. REML is a method for fitting linear mixed models and can produce unbiased estimates of variance and covariance parameters (West, Welch, & Galecki, 2007).
Results
Resting Heart Rate
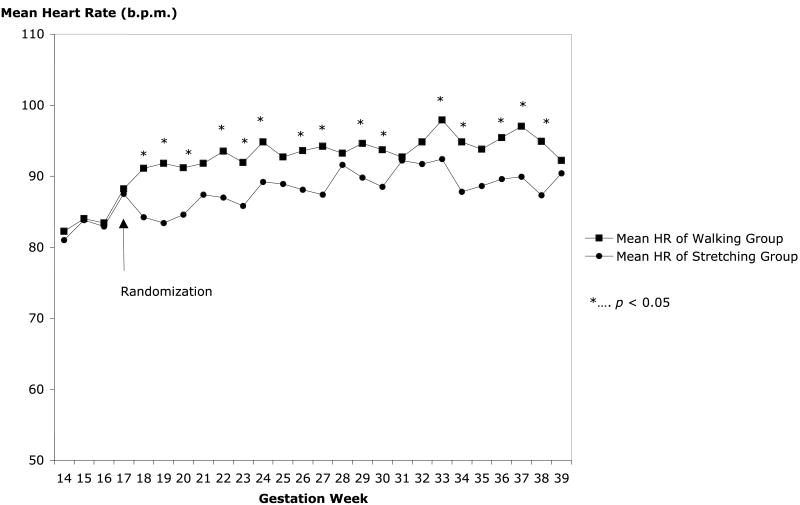
Resting heart rate at baseline (14–17 weeks) was 84±11 beats/min for the sample as a whole (mean±SD; 95% confidence interval [CI]: 82, 86), and there were no group differences (p=.51). Figure 1 shows weekly mean resting heart rate changes before and after randomization. For walkers, resting heart rate increased an average of 14±16 beats/min (95% CI: 9.1, 17.9) during the period between 18 and 28 weeks of gestation (p<.001). For stretchers, resting heart rate increased an average of 8.0±11 beats/min (95% CI: 5.1, 11.2) during that period (p<.001). With linear mixed-model analysis, group effect (walking vs. stretching), gestation effect (18–28 weeks), its quadratic term (gestation square), the group-gestation interaction effect, and the group-gestation quadratic effect were significant: group (p<.001), gestation (p<.001), gestation square (p<.001), interaction between group and gestation (p=.002), and interaction between group and gestation square (p=.002).
Figure 1.
Heart rate changes in the two groups.
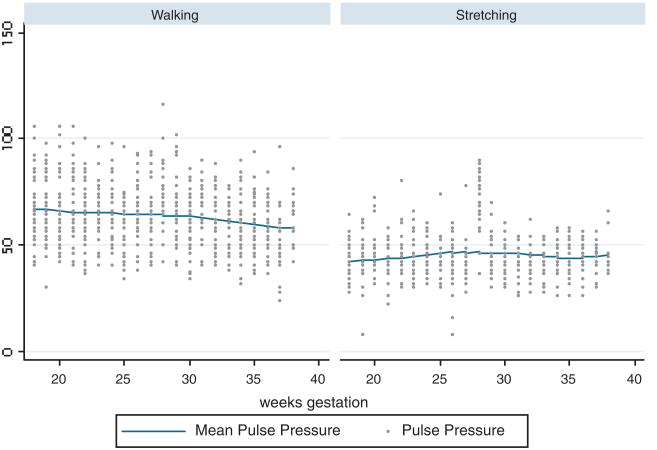
Pulse Pressure
Figure 2 shows pulse pressures over the study period by exercise groups. At the baseline, mean group difference of pulse pressure was 25.0 mmHg (standard error of estimate [SE]: 2.59) and at the end of the study period, the mean difference was 14.1 mmHg (SE: 3.15). They were statistically significant (p<.001). Group differences over the study period did not reach statistically significant level.
Figure 2.
Pulse pressures during the study period in the two exercise groups.
Discussion
PSE is a low-intensity exercise (i.e., structured LTPA) consisting of a series of skeletal muscle stretches accompanied by deep breathing. Deep breathing (abdominal breathing) naturally accompanies stretching but is also repeated between stretching sequences. In PSE, a single muscle or a series of muscles are deliberately elongated to their fullest length (Nelson & Kokkonen, 2007; Weerapong, Hume, & Kolt, 2004). PSE is recommended for releasing stress (Babycenter, 2009) and minor low back pain (Lowdermilk & Perry, 2007). While it is widely accepted as stress reduction in clinical practice, no specific theory has been examined to link PSE and the intended benefits such as reducing gestational diabetes or preeclampsia. The results of the current study indicate a possible role of autonomic nervous systems in stretching exercises.
The evidence indicates that signals from skeletal muscles produced by stretching exercise ascend to the central nervous system, influencing the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) independently and interactively (Martens, Greenberg, & Allen, 2008). The use of PSE as a preventive intervention may be supported on a basis that the stimulation of mechanoreceptors on skeletal muscles influences muscle parasympathetic activities with minimum perturbation of sympathetic activities. For example, Cui, Blaha, Moradkhan, Gray, and Sinoway (2006) tested whether stretching of the calf muscle for 5 s followed by 15 to 25 seconds of rest, repeated 25 times, induced responses in muscle sympathetic nerve activity (MSNA) with young healthy adults (6 men and 6 women). They found that MSNA and heart rate increased transiently from baseline, followed by a transient increase in blood pressure. These investigators hypothesized that the reason for the transient nature of the response was the activation of vagal responses, noting that increased MSNA-induced vasoconstriction, coupled with a rise in cardiac output due to the rise in heart rate, activated the vagal reflex to suppress the MSNA and heart rate—the vagal preponderance. This finding suggests that isolated passive muscle stretching in humans activates sympathetic responses; it also suggests that vagal engagement immediately follows.
Murata and Matsukawa used an animal model to test the hypothesis that when skeletal muscles are stretched, parasympathetic and sympathetic discharges show opposing reflex responses in magnitude and time course (Murata & Matsukawa, 2001). Using the hindlimb muscles of unanesthetized decerebrate cats, they measured cardiac sympathetic efferent nerve activity (SNA) and cardiac vagal efferent nerve activity (VNA). They found that SNA changed at the outset of the passive stretching, followed by gradual VNA changes during the stretching of the hindlimb, and this change (vagal tone) was sustained throughout the stretching. These investigators concluded that the muscle mechanoreflex contributed to the regulation of cardiac sympathetic and parasympathetic efferent discharges during the stretching of the skeletal muscle. This study provided the basis for the model to explain the possible beneficial effects of PSE. The model posits that muscle stretching directly elicits a short SNA followed by a sustained VNA, that is, a small sympathetic response followed by a sustained and robust vagal tone.
Similarly, deep breathing (i.e., voluntarily controlling breathing pattern) might influences ANS function, including heart rate variability and cardiac vagal tone, chemoreflex sensitivity, baroreflex, and central nervous system excitation (Brown & Gerbarg, 2005). The PSE program combines muscle stretching and deep breathing.
Effects of Prenatal Stretching Exercise on the Autonomic Nervous Systems
Based on previous studies and the current preliminary study results, it is proposed that PSE may stimulate sympathetic activity and parasympathetic activity, leading to the state of vagal preponderance. The term vagal preponderance reflects the fact that under normal circumstances, when the SNS is stimulated, the PNS exerts inhibitory effects on SNS excitatory influences (Martens et al., 2008). Thus, daily PSE may influence vagal tone enhancement.
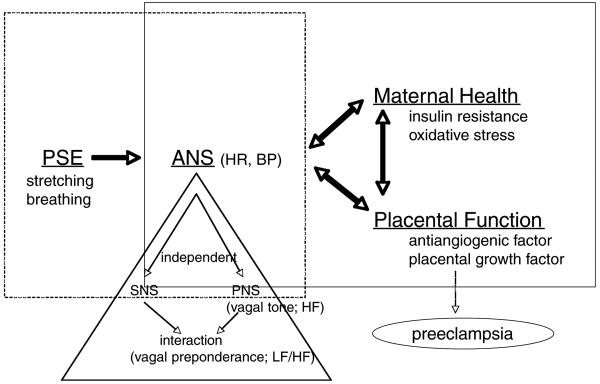
To demonstrate this beneficial effect, one must consider that the progress of pregnancy lowers vagal tone over time. The effect of PSE on levels of SNS and PNS interactions can be examined, and indexed by assessing heart rate variability. Overall ANS balance can be assessed by measuring heart rate and blood pressure. A physiologic model is depicted in Figure 3.
Figure 3.
A physiological model. PSE=prenatal stretching exercise; ANS=autonomic nervous systems; HR=heart rate; BP=blood pressure; SNS=sympathetic nervous system; PNS=parasympathetic nervous system; HF=high-frequency spectrum of heart rate variability; LF/HF=ratio of low- versus high-frequency spectrum of heart rate variability.
Effects of the Autonomic Nervous Systems on Maternal Health
Elevated sympathetic outflow contributes to an imbalance between the SNS and PNS. Hemodynamically, preeclampsia is characterized by peripheral vasoconstriction (Fischer et al., 2004). Stimulation of the SNS might be a component, though probably a secondary component, of a multifactorial etiology leading to preeclampsia (Brown, 1997). Endothelial dysfunction, leading to oxidative stress (Redman & Sargent, 2005; Weissgerber et al., 2004) with an imbalance of vasoconstrictor and vasodilating activity, may contribute to the peripheral vasoconstriction hypertension seen in preeclampsia (Fischer et al.). In a prospective study (n=22), Fischer et al. looked at whether increased sympathetic activity preceded preeclampsia or preeclampsia preceded the increase in sympathetic activity. They prospectively measured MSNA at 22 and 33 weeks of gestation and at post-partum and found that MSNA levels were significantly augmented during all of pregnancy. Though all participants showed pregnancy-induced sympathetic overactivity, only a subset of participants developed preeclampsia. Based on these results, the researchers postulated that preeclampsia developed when the physiologic vasodilating mechanism failed. PSE may prevent or slow down the physiologic failure of the vasodilating mechanism through enhancing vagal tone.
Autonomic dysregulation is also closely involved in the metabolic complications associated with hyperglycemia (Carnethon, Jacobs, Sidney, & Liu, 2003). Heart rate variability changes in people with insulin resistance, and one group of investigators (Laitinen et al., 1999) found that a state of hyperinsulinemia caused a significant decrease in high-frequency power and an increase in the low-frequency or high-frequency ratio; both are indexes derived from heart rate variability. Heart rate also increased significantly during hyperinsulinemia. Further, insulin resistance in early pregnancy is a strong predictor of preeclampsia (Parretti et al., 2006). Therefore, it is possible that PSE helps to prevent the development of insulin resistance through an enhanced cardiac autonomic control.
Conclusions
Based on the results of this secondary data analyses, a physiologic framework for possible beneficial effects of stretching exercise by enhancing autonomic responses on reducing risks for preeclampsia is proposed.
Clinical Implications
The preliminary study results and theoretical model in this article connecting PSE and autonomic nervous systems may provide nurses with a rationale to recommend PSE. The model supports encouraging PSE for lowering the risk for preeclampsia, as well as for the promotion of health. PSE also may help manage symptoms such as nausea or bloating because the ANS controls associated reflexes. While pregnant women should enjoy other forms of exercise, stretching exercises may be recommended when women cannot adhere to other forms of exercise, since pregnant women adhere to PSE regimens better than to other forms of exercise in the second and third trimesters (Yeo, 2009). For overweight and obese pregnant women, clinicians may wish to recommend stretching exercises accompanied by sensible interventions to monitor weight gain.
Clinical Resources
Prenatal Exercise http://www.babies.sutterhealth.org/during/preg_exercises.html
Healthy Pregnancy http://www.webmd.com/baby/guide/pregnancy-safe-exercises
ACOG Patient Education Pamphlets http://www.acog.org/publications/patient_education/bp119.cfm
Preeclampsia Foundation http://www.preeclampsia.org/
Acknowledgments
The author was supported by NINR(R01-NR05002) and a Faculty Research Opportunity Grant at the School of Nursing, University of North Carolina at Chapel Hill.
References
- American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. Vol. 6. Philadelphia: Lippincott, Williams, & Wilkins; 2000. [Google Scholar]
- Anderson CM. Preeclampsia: Exposing future cardiovascular risk in mothers and their children. Journal of Obstetrics Gynecology &Neonatal Nursing. 2007;36:3–8. doi: 10.1111/j.1552-6909.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- Babycenter. Great pregnancy exercise: Stretching. 2009 Retrieved July 23, 2009, from http://www.babycenter.com/0_great-pregnancy-exercise-stretching_588.bc.
- Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. Trends in fetal and infant survival following preeclampsia. JAMA. 2006;296:1357–1362. doi: 10.1001/jama.296.11.1357. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Ness RB, Markovic N, Roberts JM. The risk of preeclampsia rises with increasing prepregnancy body mass index. Annals of Epidemiology. 2005;15:475–482. doi: 10.1016/j.annepidem.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Bowman AJ, Clayton RH, Murray A, Reed JW, Subhan MM, Ford GA. Effects of aerobic exercise training and yoga on the baroreflex in healthy elderly persons. European Journal of Clinical Investigation. 1997;27:443–449. doi: 10.1046/j.1365-2362.1997.1340681.x. [DOI] [PubMed] [Google Scholar]
- Brown MA. Pre-eclampsia: A case of nerves? Lancet. 1997;349(9048):297–298. doi: 10.1016/S0140-6736(05)62819-X. [DOI] [PubMed] [Google Scholar]
- Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression: Part I—Neurophysiologic model. Journal of Alternative & Complemental Medicine. 2005;11:189–201. doi: 10.1089/acm.2005.11.189. [DOI] [PubMed] [Google Scholar]
- Caritis S, Sibai B, Hauth J, Lindheimer M, VanDorsten P, Klebanoff M, et al. Predictors of pre-eclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. American Journal of Obstetrics & Gynecology. 1998;179:946–951. doi: 10.1016/s0002-9378(98)70194-2. [DOI] [PubMed] [Google Scholar]
- Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units [see comment] New England Journal of Medicine. 1998;338:701–705. doi: 10.1056/NEJM199803123381101. [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Jacobs DR, Jr, Sidney S, Liu K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: The CARDIA study. Diabetes Care. 2003;26:3035–3041. doi: 10.2337/diacare.26.11.3035. [DOI] [PubMed] [Google Scholar]
- Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. Journal of Physiology. 2006;576(Pt. 2):625–634. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker GA. Risk factors for preeclampsia. Clinical Obstetrics & Gynecology. 1999;42:422–435. doi: 10.1097/00003081-199909000-00002. [DOI] [PubMed] [Google Scholar]
- Domingues MR, Barros AJ. Leisure-time physical activity during pregnancy in the 2004 Pelotas Birth Cohort Study. Revista de Saúde Pública. 2007;41:173–180. doi: 10.1590/s0034-89102007000200002. [DOI] [PubMed] [Google Scholar]
- Evenson KR, Savitz DA, Huston SL. Leisure-time physical activity among pregnant women in the US. Paediatric and Perinatal Epidemiology. 2004;18:400–407. doi: 10.1111/j.1365-3016.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- Fischer T, Schobel HP, Frank H, Andreae M, Schneider KT, Heusser K. Pregnancy-induced sympathetic overactivity: A precursor of preeclampsia. European Journal of Clinical Investigation. 2004;34:443–448. doi: 10.1111/j.1365-2362.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, et al. Endothelial dysfunction: A link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;49:90–95. doi: 10.1161/01.HYP.0000251522.18094.d4. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, et al. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97:2037–2042. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- Gupta S, Agarwal A, Sharma RK. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstetrical & Gynecological Survey. 2005;60:807–816. doi: 10.1097/01.ogx.0000193879.79268.59. [DOI] [PubMed] [Google Scholar]
- Hinton PS, Olson CM. Predictors of pregnancy-associated change in physical activity in a rural white population. Maternal and Child Health Journal. 2001;5:7–14. doi: 10.1023/a:1011315616694. [DOI] [PubMed] [Google Scholar]
- Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proceedings of the Society for Experimental Biology & Medicine. 1999;222:222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- Jallo N, Bourguignon C, Taylor AG, Utz SW. Stress management during pregnancy: Designing and evaluating a mind-body intervention. Family & Community Health. 2008;31:190–203. doi: 10.1097/01.FCH.0000324476.48083.41. [DOI] [PubMed] [Google Scholar]
- Kaaja R, Laivuori H, Pulkki P, Tikkanen MJ, Hiilesmaa V, Ylikorkala O. Is there any link between insulin resistance and inflammation in established preeclampsia? Metabolism: Clinical & Experimental. 2004;53:1433–1435. doi: 10.1016/j.metabol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. Journal of the American Medical Association. 2005;294:2751–2757. doi: 10.1001/jama.294.21.2751. [DOI] [PubMed] [Google Scholar]
- Kaaja RJ, Poyhonen-Alho MK. Insulin resistance and sympathetic overactivity in women. Journal of Hypertension. 2006;24:131–141. doi: 10.1097/01.hjh.0000194121.19851.e5. [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Lindheimer MD. Preeclampsia pathogenesis: “triple a rating”—Autoantibodies and antiangiogenic factors. Hypertension. 2008;51:991–992. doi: 10.1161/HYPERTENSIONAHA.107.100735. [DOI] [PubMed] [Google Scholar]
- Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: How much is enough? Obstetrics & Gynecology. 2007;110:752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- Kuo CD, Chen GY, Yang MJ, Lo HM, Tsai YS. Biphasic changes in autonomic nervous activity during pregnancy. British Journal of Anaesthesiology. 2000;84:323–329. doi: 10.1093/oxfordjournals.bja.a013433. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Vauhkonen IK, Niskanen LK, Hartikainen JE, Lansimies EA, Uusitupa MI, et al. Power spectral analysis of heart rate variability during hyperinsulinemia in nondiabetic offspring of type 2 diabetic patients: Evidence for possible early autonomic dysfunction in insulin-resistant subjects. Diabetes. 1999;48:1295–1299. doi: 10.2337/diabetes.48.6.1295. [DOI] [PubMed] [Google Scholar]
- Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clinical Obstetrics and Gynecology. 2005;48:372–386. doi: 10.1097/01.grf.0000160313.82606.d7. [DOI] [PubMed] [Google Scholar]
- Lombardi DG, Barton JR, O'Brien JM, Istwan NK, Sibai BM. Does an obese prepregnancy body mass index influence outcome in pregnancies complicated by mild gestational hypertension remote from term? American Journal of Obstetrics & Gynecology. 2005;192:1472–1474. doi: 10.1016/j.ajog.2004.12.072. [DOI] [PubMed] [Google Scholar]
- Lowdermilk DL, Perry S. Maternity nursing. 7th. St. Louis, MO: Mosby; 2007. [Google Scholar]
- Lu WA, Kuo CD. The effect of Tai Chi Chuan on the autonomic nervous modulation in older persons. Medicine & Science in Sports & Exercise. 2003;35:1972–1976. doi: 10.1249/01.MSS.0000099242.10669.F7. [DOI] [PubMed] [Google Scholar]
- Magnus P, Trogstad L, Owe KM, Olsen SF, Nystad W. Recreational physical activity and the risk of preeclampsia: A prospective cohort of Norwegian women. American Journal of Epidemiology. 2008;168:952–957. doi: 10.1093/aje/kwn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoux S, Brisson J, Fabia J. The effect of leisure time physical activity on the risk of pre-eclampsia and gestational hypertension. Journal of Epidemiology and Community Health. 1989;43:147–152. doi: 10.1136/jech.43.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens A, Greenberg J, Allen JJ. Self-esteem and autonomic physiology: Parallels between self-esteem and cardiac vagal tone as buffers of threat. Personality and Social Psychology Review. 2008;12:370–389. doi: 10.1177/1088868308323224. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. American Heart Journal. 2008;156:918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: An epidemiologic approach to classification. American Journal of Epidemiology. 2008;168:980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moertl MG, Ulrich D, Pickel KI, Klaritsch P, Schaffer M, Flotzinger D, et al. Changes in haemodynamic and autonomous nervous system parameters measured non-invasively throughout normal pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2008;144(Suppl. 1):S179–S183. doi: 10.1016/j.ejogrb.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Moran C, Sandoval T, Duque X, Gonzalez S, Moran S, Bermudez JA. Increased insulin levels independent of gestational overweight in women with preeclampsia. Archives of Medical Research. 2006;37:749–754. doi: 10.1016/j.arcmed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Mumford SL, Siega-Riz AM, Herring A, Evenson KR. Dietary restraint and gestational weight gain. Journal of American Dietary Association. 2008;108:1646–1653. doi: 10.1016/j.jada.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata J, Matsukawa K. Cardiac vagal and sympathetic efferent discharges are differentially modified by stretch of skeletal muscle. American Journal of Physiology—Heart and Circulatory Physiology. 2001;280:H237–H245. doi: 10.1152/ajpheart.2001.280.1.H237. [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American Journal of Obstetrics & Gynecology. 2000;183:S1–S22. [PubMed] [Google Scholar]
- Nelson AG, Kokkonen J. Stretching anatomy. Champaign, IL: Human Kinetics; 2007. [Google Scholar]
- Ning Y, Williams MA, Dempsey JC, Sorensen TK, Frederick IO, Luthy DA. Correlates of recreational physical activity in early pregnancy. Journal of Maternal Fetal & Neonatal Medicine. 2003;13:385–393. doi: 10.1080/jmf.13.6.385.393. [DOI] [PubMed] [Google Scholar]
- Nishimoto F, Sakata M, Minekawa R, Okamoto Y, Miyake A, Isobe A, et al. Metal transcription factor-1 is involved in hypoxia-dependent regulation of placenta growth factor in trophoblast-derived cells. Endocrinology. 2009;150:1801–1808. doi: 10.1210/en.2008-0949. [DOI] [PubMed] [Google Scholar]
- Ohkuchi A, Iwasaki R, Suzuki H, Hirashima C, Takahashi K, Usui R, et al. Normal and high-normal blood pressures, but not body mass index, are risk factors for the subsequent occurrence of both preeclampsia and gestational hypertension: A retrospective cohort study. Hypertension Research—Clinical & Experimental. 2006;29:161–167. doi: 10.1291/hypres.29.161. [DOI] [PubMed] [Google Scholar]
- Panchal S, Arria AM, Labhsetwar SA. Maternal mortality during hospital admission for delivery: A retrospective analysis using a state-maintained database. Anesthesia & Analgesia. 2001;93:134–141. doi: 10.1097/00000539-200107000-00028. [DOI] [PubMed] [Google Scholar]
- Parretti E, Lapolla A, Dalfra M, Pacini G, Mari A, Cioni R, et al. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension. 2006;47:449–453. doi: 10.1161/01.HYP.0000205122.47333.7f. [DOI] [PubMed] [Google Scholar]
- Perkins AV. Endogenous anti-oxidants in pregnancy and preeclampsia. Australian & New Zealand Journal of Obstetrics & Gynaecology. 2006;46(2):77–83. doi: 10.1111/j.1479-828X.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- Raatikainen K, Heiskanen N, Heinonen S. Transition from overweight to obesity worsens pregnancy outcome in a BMI-dependent manner. Obesity. 2006;14:165–171. doi: 10.1038/oby.2006.20. [DOI] [PubMed] [Google Scholar]
- Ray JG, Diamond P, Singh G, Bell CM. Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. BJOG: An International Journal of Obstetrics & Gynaecology. 2006;113:379–386. doi: 10.1111/j.1471-0528.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Rousham EK, Clarke PE, Gross H. Significant changes in physical activity among pregnant women in the UK as assessed by accelerometry and self-reported activity. European Journal of Clinical Nutrition. 2006;60:393–400. doi: 10.1038/sj.ejcn.1602329. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Logsden-Sackett N, Wang W, Woolson R, Bracken MB. Work, leisure-time physical activity, and risk of preeclampsia and gestational hypertension. American Journal of Epidemiology. 2004;160:758–765. doi: 10.1093/aje/kwh277. [DOI] [PubMed] [Google Scholar]
- Serdar Z, Gur E, Develioglu O. Serum iron and copper status and oxidative stress in severe and mild preeclampsia. Cell Biochemistry & Function. 2006;24:209–215. doi: 10.1002/cbf.1235. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, et al. Risk factors for preeclampsia in healthy nulliparous women: A prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. American Journal of Obstetrics & Gynecology. 1995;172(2 Pt. 1):642–648. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension. 2003;41:1273–1280. doi: 10.1161/01.HYP.0000072270.82815.91. [DOI] [PubMed] [Google Scholar]
- Thadhani R, Ecker JL, Mutter WP, Wolf M, Smirnakis KV, Sukhatme VP, et al. Insulin resistance and alterations in angiogenesis: Additive insults that may lead to preeclampsia. Hypertension. 2004;43:988–992. doi: 10.1161/01.HYP.0000124460.67539.1d. [DOI] [PubMed] [Google Scholar]
- Vural P, Degirmencioglu S, Dogru-Abbasoglu S, Saral NY, Akgul C, Uysal M. Genetic polymorphisms in DNA repair gene APE1, XRCC1 and XPD and the risk of pre-eclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2009;146:160–164. doi: 10.1016/j.ejogrb.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Weerapong P, Hume PA, Kolt GS. Stretching: Mechanisms and benefits for sports performance and injury prevention. Physical Therapy Review. 2004;9(4):189–206. [Google Scholar]
- Weissgerber TL, Wolfe LA, Davies GAL. The role of regular physical activity in preeclampsia prevention. Medicine & Science in Sports & Exercise. 2004;36:2024–2031. doi: 10.1249/01.mss.0000147627.35139.dc. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear mixed models: A practical guide using statistical software. New York: Taylor & Francis Group; 2007. [Google Scholar]
- Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M. Mapping the theories of preeclampsia and the role of angiogenic factors: A systematic review. Obstetrics & Gynecology. 2007;109:168–180. doi: 10.1097/01.AOG.0000249609.04831.7c. [DOI] [PubMed] [Google Scholar]
- Wolf M, Hubel CA, Lam C, Sampson M, Ecker JL, Ness RB, et al. Preeclampsia and future cardiovascular disease: Potential role of altered angiogenesis and insulin resistance. Journal of Clinical Endocrinology & Metabolism. 2004;89:6239–6243. doi: 10.1210/jc.2004-0548. [DOI] [PubMed] [Google Scholar]
- Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: The potential role of inflammation. Obstetrics and Gynecology. 2001;98(5 Pt. 1):757–762. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- Yeo S. A randomized comparative trial of the efficacy and safety of exercise during pregnancy: Design and methods. Contemporary Clinical Trials. 2006;27:531–540. doi: 10.1016/j.cct.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Yeo S. Adherence to walking or stretching, and risk of preeclampsia in sedentary pregnant women. Research in Nursing & Health. 2009;32:379–390. doi: 10.1002/nur.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo S, Davidge ST. Possible beneficial effect of exercise, by reducing oxidative stress, on the incidence of preeclampsia. Journal of Women's Health & Gender-Based Medicine. 2001;10:983–989. doi: 10.1089/152460901317193558. [DOI] [PubMed] [Google Scholar]
- Yeo S, Davidge ST, Ronis DL, Antonakos CL, Hayashi R, O'Leary S. A comparison of walking versus stretching exercise to reduce the incidence of preeclampsia: A randomized clinical trial. Hypertension in Pregnancy. 2008;27(2):113–130. doi: 10.1080/10641950701826778. [DOI] [PubMed] [Google Scholar]
- Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertension in Pregnancy. 2003;22(2):203–212. doi: 10.1081/PRG-120021066. [DOI] [PubMed] [Google Scholar]