Abstract
Background
Gold and carbon nanoparticles absorb non-ionizing radiofrequency (RF) energy and release heat. Solid gold nanoparticles are delivered to cancer cells via conjugation with targeting antibodies. Here, 20 nm gold particles were conjugated to cetuximab, an epidermal growth factor recpetor-1 (EGFR-1) antibody.
Methods
A pancreatic carcinoma cell line that highly expresses EGFR-1, Panc-1, and a breast carcinoma cell line that minimally expresses EGFR-1, Cama-1, were treated with 100 nM cetuximab-conjugated gold nanoparticles for 3 hours (n = 4). Thirty-six hours later, the dishes were placed in an RF field with a generator power of 200 W for 5 minutes. After another 36 hours, cell injury and death were evaluated with flow cytometry.
Results
The targeted cell line, Panc-1, had a viability of 45.5% ± 11.7% while Cama-1 cell had a viability of 91.7% ± 1.6% after RF field exposure (p < 0.008). Transmission electron microscopy showed gold nanoparticle uptake in Panc-1 cells, but negligible uptake by Cama-1 cells. Non-targeted cells do not internalize a sufficient amount of antibody-conjugated gold nanoparticles to induce injury in a noninvasive RF field.
Conclusion
This technique could be useful in cancer treatment provided a cancer-specific antibody is utilized to localize gold nanoparticles to malignant cells.
Introduction
Despite the growing use of radiofrequency ablation (RFA) in hepatic and other malignancies, standard invasive RFA of pancreatic cancers remain dangerous and ineffective for lasting cure.1, 2 Microwave ablation may offer benefits over RFA in some patients, but this has not been clearly demonstrated as superior, or even effective in prolonging survival.3 Unresectable pancreatic carcinoma, typically treated with systemic chemotherapy, carries a median survival of less than one year even when multiple sequential chemotherapeutic regimes are utilized.4, 5
Novel technologies are now developing into treatment modalities by investigations at the intersection of physics, chemistry, biology, and medicine. Nanoparticles are an extremely diverse group of materials with typical length scales of 10 nm to 1 μm. We have previously demonstrated the heating characteristics of gold nanoparticles and carbon nanotubes (hollow, single wall tubes of carbon) when placed in radiofrequency (RF) fields.6, 7 Specifically, RF fields heat solid gold nanoparticles in water at approximately ∼ 2°C/second in a concentration dependent fashion.7 The source of the RF energy is completely external to the sample and produces an electric field of approximately ∼ 10 kV/m at 600 W of power (Fig 1). Fortunately, shortwave RF fields are known to be safe for humans as they are used in multiple industries, military applications, and communication systems.8 Non-targeted, passively delivered gold nanoshells have been demonstrated in large animal models to be cytotoxic after being exposed to near-infrared radiation (NIR), but not without NIR exposure.9 However, unlike RF fields, NIR radiation is not transmitted more than a few centimeters through the body tissues, and as such, NIR therapy is greatly limited to treat superficial lesions.8, 10
Figure 1.

The 13.56 MHz RF field generator is seen with the Teflon sample holder in place. The air gap is approximately 10 cm between the transmitting and receiving heads.
We hypothesized that cells exposed to RF fields after internalization of antibody-conjugated gold nanoparticles (AuNP) will undergo thermally-induced cytotoxicity. The antibody, cetuximab (C225), is a well-known monoclonal antibody against the epidermal growth factor recepetor-1 (EGFR-1) surface receptor.11, 12 The cell lines of interest are a highly expressive EGFR-1 pancreatic carcinoma cell line, Panc-1, and a non-EGFR-1 expressing breast carcinoma cell line, Cama-1.
Materials & Methods
Cell culture
Panc-1 and Cama-1 cell lines were acquired from the American Type Culture Collection (Manassas, VA) and kept in standard conditions (37°C, 5% CO2). Cell line identities were confirmed by the Characterized Cell Line Core service (M. D. Anderson Cancer Center, Houston, TX, November 2009). Standard cell culture coated dishes were utilized for all experiments (Corning Inc., Corning, NY). All cells were maintained in Dulbecco's Modified Eagle's Medium (Mediatech, Inc., Manassas, VA) supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin/streptomycin.
Western blot
EGFR-1 cell membrane expression was confirmed by Western blot analysis. Cell pellets were made by first lysing with cold radioimmunoprecipitation assay (RIPA) buffer with subsequent incubation for 30 minutes on ice. The lysates were centrifuged at 13,000 rpm for 30 minutes. Next, the protein extracts (50 μg/lane) were electrophoresed on 6% Bis-Tris protein gel and transferred to a PVDF membrane. The membranes were incubated for 1 hour in 5% dry milk and then incubated with an anti-EGFR primary antibody (BD Biosciences, Franklin Lakes, NJ) and an antibody against β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Next, the membranes were incubated with Peroxidase AffiniPure goat anti-rabbit IgG and goat anti-mouse IgG (H+L chains, Jackson ImmunoResearch West Grove, PA) secondary antibodies as appropriate. A peroxide solution (GenDEPOT, Inc., Barker, TX) detected the bands. Finally, images were acquired with a high resolution photo scanner (CanoScan 4400F, Canon, Inc., Lake Success, NY).
Antibody gold conjugation
Cetuximab (Bristol-Myers Squibb, New York, NY) was conjugated to 20 nm spherical gold nanoparticles (Ted Pella, Inc., Redding, CA) by modifying a previously described protocol.13 The pH of the gold nanoparticle solution was raised to approximately 8.5, the isoelectric point of C225. 450 μg of C225 was slowly added to 50 mL of the pH modified gold solution. It was placed on a continuous mixer and incubated at room temperature for 2 hours. Next, the conjugate was concentrated 15-fold in a 100,000 molecular weight centrifugation filter unit (Millipore Corp., Billerica, MA) at 3,500 × g. Finally, it was UV light sterilized.
Conjugation was confirmed by measuring the visible light absorbance of the solution (NS1, Applied NanoFluorescence, Houston, TX). A slight plasmon shift in the peak is consistent with conjugation, and indicative of a non-aggregated state. Dynamic light scattering (DLS, Horiba, Ltd., Irvine, CA) determined the average size of the conjugates.
Transmission electron microscopy (TEM) evaluated C225-conjugated nanoparticle internalization in the cells. TEM was performed with a JEM 1010 microscope (JEOL, Inc. Peabody, MA) and images were taken with an AMT Imaging System (Advanced Microscopy Techniques Crop., Danvers, MA).
Cell treatments
In quadruplicate, Panc-1, Cama-1, or both cells were plated in 60 mm cell culture dishes at 4 × 105 cells/dish. Twenty-four hours later, the cells were exposed to 100 nM C225-gold nanoparticle conjugates for 3 hours. The media was exchanged for fresh complete media without nanoparticles at that time. Thirty-six hours later, the cells were exposed to an RF field as described below.
RF field exposure
Panc-1 and Cama-1 cells were placed in a high voltage radiofrequency field (13.56 MHz) produced between 2 metal electrodes with an air gap of ∼ 10 cm (Fig 1, Therm Med, LLC, Inc., Erie, PA). A low RF field absorbing material, Teflon, holds the samples in place. RF field exposure was for 5 minutes at a generator power of 200W. An infrared camera continuously monitored the temperature (FLIR Systems, Inc., Boston, MA). All media temperatures in bulk remained less than 41.0°C.
Viability determination with flow cytometry
Thirty-six hours after RF field exposure, the cells were prepared for flow cytometry in order to investigate rates of apoptosis, necrosis, and cell death. First, the media was collected with any floating cells. Next, adherent cells were released with a trypsin-EDTA solution (Mediatech, Inc., Manassas, VA). The trypsin was neutralized with media and the cells were collected as well. The cells were sequentially washed with phosphate buffered saline (PBS) followed by Annexin Buffer 1 × (BD Biosciences, Franklin Lakes, NJ). They were suspended in 100 μL of buffer, stained with 5 μL propidium iodide (PI, BD Biosciences, Franklin Lakes, NJ) for necrosis/cell death, and stained with 5 μL Annexin-V conjugated to Horizon-V450 (BD Biosciences, Franklin Lakes, NJ) to measure external phosphatidylserine expression as a marker of apoptosis. Excess unconjugated fluorophore was removed by washing with buffer solution once and suspending in approximately 200 μL buffer. At least 50,000 single cell events were recorded per sample on a LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ), and often there were over 75,000 single cell events. Compensation was performed according to the manufacture's recommendation. Data were analyzed and plotted with FlowJo 8.8.6 (TreeStar, Inc., Ashland, OR).
Statistical Analysis
Results are means ± standard errors of the mean unless otherwise noted. Statistical significance, α, was set to p = 0.05. Two-tailed Student's t-test, unless otherwise noted, compared differences in means between groups. Data were analyzed and plotted with Microsoft Excel for Mac ver. 12 (Microsoft Corp., Redmond, WA) and FlowJo ver. 8.8.6 (Tree Star, Inc., Ashland, OR).
Results
EGFR-1 expression
Variation in the cell surface expression of EGFR-1 was confirmed in the two cell lines (Fig 2). Panc-1 cells highly express EGFR-1 on their surface while Cama-1 cells have nearly no measureable expression. This was repeated twice to confirm.
Figure 2.
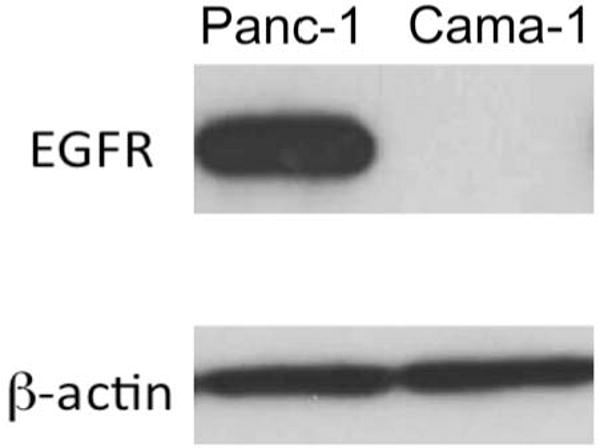
Cama-1 cells express negligible EGFR-1 on the cellular membrane (repeated for confirmation) while Panc-1 cells highly express the antigen.
Conjugation and delivery of C225 gold nanoparticles
After conjugation of cetuximab to 20 nm gold nanoparticles, the plasmon shift was approximately 2 nm (Fig 3). The conjugates were 35.9 nm ± 6.7 nm based on DLS measurements. Finally, delivery of gold nanoparticles into Panc-1 cells (Fig 3) and not Cama-1 cells (data not shown) was confirmed with TEM.
Figure 3.
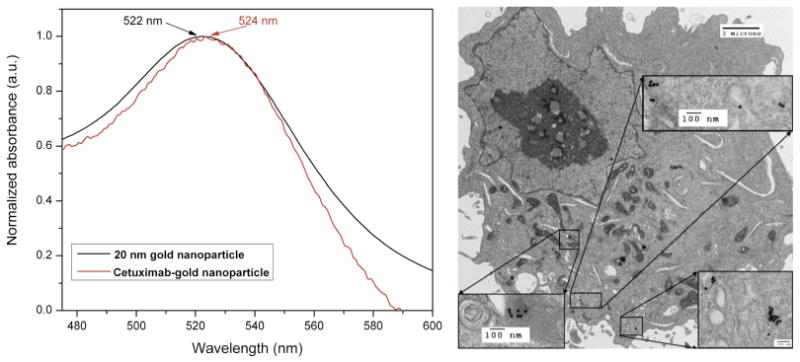
The slight shift of the plasmon peak (left) is indicative of successful conjugation of the antibody to the gold nanoparticles. Transmission electron microscopy (right) demonstrates cetuximab targeted gold nanoparticles in a Panc-1 cell.
Viability after C225-AuNP treatment and RF field exposure
All cells were analyzed after identification as single cell events on the scatter plot during flow cytometry (Fig 4). Thirty-six hours after RF field exposure with nanoparticle conjugate pre-treatment, the viability of Cama-1 cells was 91.7% ± 1.6% while Panc-1 cell viability was 45.5% ± 11.7% (Table 1, Fig 5, p ∼ 0.008). The viability of Cama-1 cells treated in the RF field with or without nanoparticle pretreatment was not significantly different, while Panc-1 cells treated with RF alone (no nanoparticles) were 92.7% ± 0.6% compared to 45.5% ± 11.7% in nanoparticles-treated cells (p ∼ 0.009). The decrease in Panc-1 viability was significantly associated with an increase in necrotic cells (Fig 6, p ∼ 0.006) and dead cells (Fig 7, p ∼ 0.016). Apoptosis was non-significantly increased in Panc-1 cells at this time point (Table 1, p > 0.10).
Figure 4.
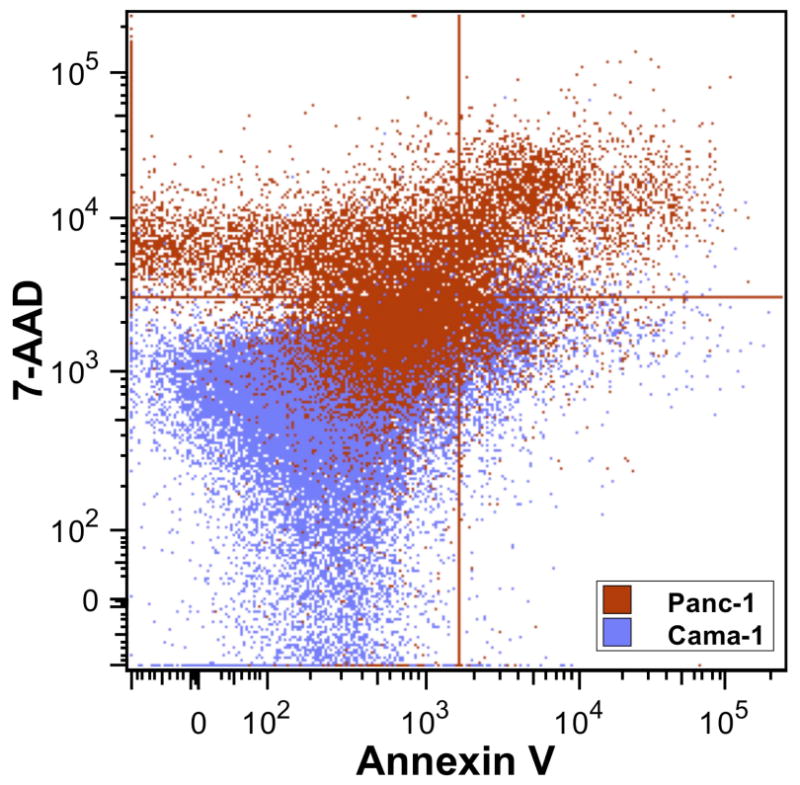
The viability dot plot demonstrates the shift from viable cells (lower left) to necrotic cells (upper left) and dead cells (upper right). Neither cell line demonstrates a significant difference in the apoptotic fraction (lower right). Cama-1 cells (blue) are predominately viable while Panc-1 cells (red) are predominately necrotic and dead.
Table 1.
Panc-1 cells were killed significantly more than Cama-1 cells after exposure to RF fields with prior treatment of C225-AuNP treatment. Surprisingly, the degree of apoptosis at this time point (36 hours) was not significantly different.
| Viability | Cama-1 | Panc-1 | p value |
|---|---|---|---|
| Viable | 91.7% ± 1.6% | 45.5% ± 11.7% | 0.0078 |
| Apoptotic | 6.0% ± 1.5% | 8.8% ± 3.1% | > 0.10 |
| Necrotic | 1.3% ± 0.3% | 29.6% ± 6.8% | 0.0058 |
| Dead | 1.1% ± 0.3% | 16.0% ± 4.5% | 0.016 |
Figure 5.
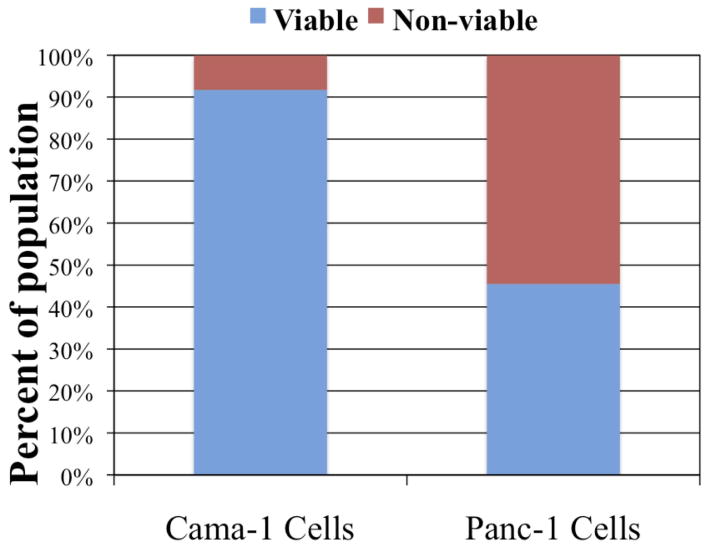
The viability of Panc-1 cells was approximately half that of Cama-1 cells after treatment with C225-AuNP and RF field exposure based on the flow cytometric single cell population (p = 0.0078).
Figure 6.
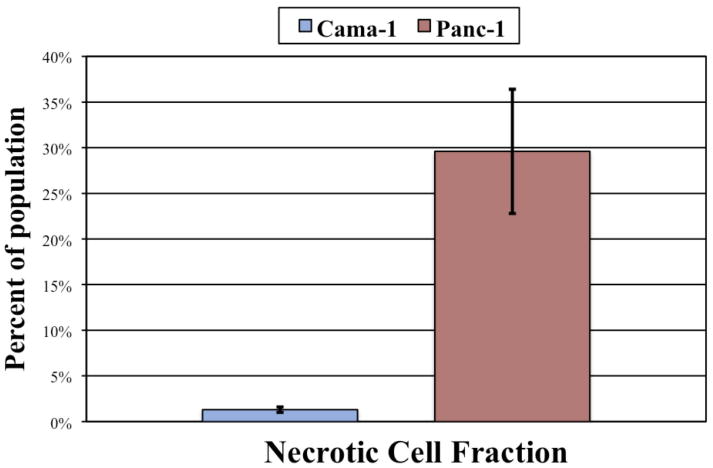
Panc-1 cells demonstrated an over 20-fold increase in the necrotic fraction (PI positive only) compared to Cama-1 cells based on flow cytometric analysis (p = 0.0058).
Figure 7.
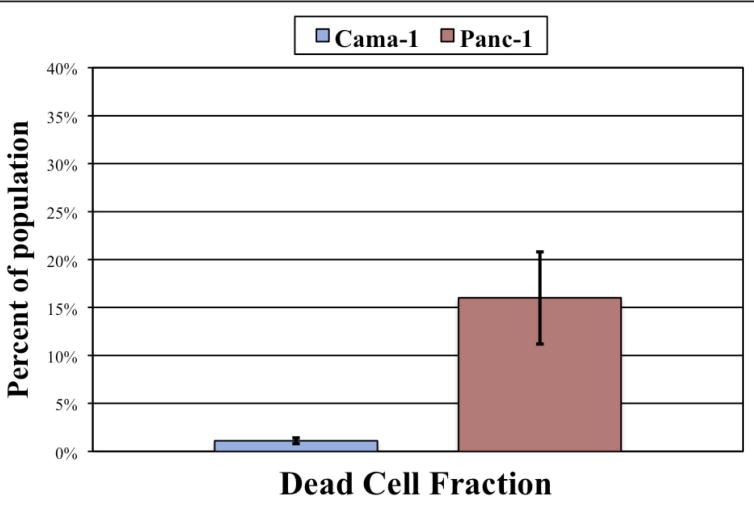
In concordance with the necrotic fraction, Panc-1 cells were 15 time more likely to be dead (Annexin V and PI positive) despite being part of the single cell population (p = 0.016).
Discussion
Targeted delivery of therapeutic gold nanoparticles is a developing treatment modality that often yields significant cytotoxicity without major bystander injury.11, 14, 15 This, obviously, is the goal of any cancer treatment. Unlike NIR-mediated cytotoxicity, the shortwave RF field penetrates deep into tissue, and thus it may develop into a treatment for deep-seated or circulating cancer cells in the future. The specificity of the targeting agent- the antibody- in these experiments dictates where the gold nanoparticles will be delivered. In this case, we utilized the antibody cetuximab as a proof of principle for this targeted nanoparticle approach. In addition, one can theoretically tailor an RF field to include only certain areas of the body in order to prevent non-cancerous cell injury if the pattern of antigen expression is not completely cancer specific. New antibody targets, specifically identification of antigens expressed on metastatic lesions not expressed in the normal cells surrounding the metastases, will permit the selective use of highly specific antibody- or drug-conjugated nanoparticles with tailored RF fields in order to induce hyperthermic cytotoxicity selectively.
Here, we demonstrate a necrotic pathway of cellular injury. Other studies have demonstrated apoptosis at later time points.11 This describes a pattern of injury similar to invasive RF ablation used clinically. A region of nearly immediate necrosis is created close to the needle electrode while an apoptotic zone develops circumferentially around the treatment site at later time points.16
Interestingly, there is an opportunity for multimodality treatments by utilizing a cytotoxic antibody (i.e., EGFR-1 antagonists) in combination with gold nanoparticle delivery. Again, the hyperthermic component is introduced by noninvasive RF field exposure. It is even possible to conjugate a chemotherapeutic agent to the same nanoparticle, and in so doing, truly offer the patient a nearly simultaneous multimodality-based therapy.
It may be possible to offer treatments for circulating tumor cells, micrometastatic disease, or even dormant tumor cells.17 For example, in colorectal cancer, a specific cell adhesion molecule, L1, has been identified as a marker for worse prognosis and early metastatic spread.18 Functional targets, such as those involved in adhesion and metastatic spread, are especially ideal targets for this novel RF treatment.
An important component of this system is the ability to maintain temperatures in bulk in the range of those typically tolerated by patients (i.e., 37°C - 41°C). Although patients may feel some warmth, it is reassuring that here, we demonstrate clinically and statistically significant cell death without bulk hyperthermia. This suggests that intracellular, targeted hyperthermia mediates cell death in this in vitro model. The next step is clear- to move forward with small and medium sized animals to demonstrate not only further proof of principle, but also demonstrate safety associated with both gold nanoparticle and RF field treatments.
Acknowledgments
The authors would like to acknowledge the assistance of Kristine Ash and Yolanda Taylor-Brittan for administrative assistance.
This work was funded by NCI #1U54CA143837-01 and an unrestricted research grant from the Kanzius Foundation (SAC, Erie, PA). ESG is an NIH T32 research fellow (5 T32 CA09599). The Cell Line Characterization Core and High Resolution Transmission Electron Microscopy Core are funded by NCI # CA16672.
Footnotes
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hadjicostas P, Malakounides N, Varianos C, Kitiris E, Lerni F, Symeonides P. Radiofrequency ablation in pancreatic cancer. HPB (Oxford) 2006;8:61–4. doi: 10.1080/13651820500466673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, et al. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392–5. doi: 10.1002/jso.20580. [DOI] [PubMed] [Google Scholar]
- 3.Lygidakis NJ, Sharma SK, Papastratis P, Zivanovic V, Kefalourous H, Koshariya M, et al. Microwave ablation in locally advanced pancreatic carcinoma--a new look. Hepatogastroenterology. 2007;54:1305–10. [PubMed] [Google Scholar]
- 4.Van Cutsem E, Aerts R, Haustermans K, Topal B, Van Steenbergen W, Verslype C. Systemic treatment of pancreatic cancer. Eur J Gastroenterol Hepatol. 2004;16:265–74. doi: 10.1097/00042737-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Custodio A, Puente J, Sastre J, Diaz-Rubio E. Second-line therapy for advanced pancreatic cancer: a review of the literature and future directions. Cancer Treat Rev. 2009;35:676–84. doi: 10.1016/j.ctrv.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J Nanobiotechnology. 2008;6:2. doi: 10.1186/1477-3155-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran CH, Wainderdi SM, Cherukuri TK, Kittrell C, Wiley BJ, Nicholas NW, et al. Size-Dependent Joule Heating of Gold Nanoparticles Using Capacitively Coupled Radiofrequency Fields. Nano Res. 2009;2:400–5. [Google Scholar]
- 8.Tell RA, Harlen F. A review of selected biological effects and dosimetric data useful for development of radiofrequency safety standards for human exposure. J Microw Power. 1979;14:405–24. doi: 10.1080/16070658.1979.11689176. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, et al. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res. 2009;69:1659–67. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- 10.Garn H, Gabriel C. Present knowledge about specific absorption rates inside a human body exposed to radiofrequency electromagnetic fields. Health Phys. 1995;68:147–56. doi: 10.1097/00004032-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Curley SA, Cherukuri P, Briggs K, Patra CR, Upton M, Dolson E, et al. Noninvasive radiofrequency field-induced hyperthermic cytotoxicity in human cancer cells using cetuximab-targeted gold nanoparticles. J Exp Ther Oncol. 2008;7:313–26. [PubMed] [Google Scholar]
- 12.Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008;68:1970–8. doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
- 13.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901–6. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654–65. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- 15.Lal S, Clare SE, Halas NJ. Nanoshell-Enabled Photothermal Cancer Therapy: Impending Clinical Impact. Accounts of Chemical Research. 2008;41:1842–51. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 16.Curley SA. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol. 2008;15:11–3. doi: 10.1245/s10434-007-9668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGowan PM, Kirstein JM, Chambers AF. Micrometastatic disease and metastatic outgrowth: clinical issues and experimental approaches. Future Oncol. 2009;5:1083–98. doi: 10.2217/fon.09.73. [DOI] [PubMed] [Google Scholar]
- 18.Kaifi JT, Reichelt U, Quaas A, Schurr PG, Wachowiak R, Yekebas EF, et al. L1 is associated with micrometastatic spread and poor outcome in colorectal cancer. Mod Pathol. 2007;20:1183–90. doi: 10.1038/modpathol.3800955. [DOI] [PubMed] [Google Scholar]


