Abstract
Objective
Mitomycin C (MMC) is used clinically to treat corneal scarring in human patients. We investigated the safety and efficacy of MMC to treat corneal scarring in horses by examining its effects at the early and late stages of disease using an in-vitro model.
Procedure
An in-vitro model of equine corneal fibroblast (ECF) developed was used. The equine corneal fibroblast or myofibroblast cultures were produced by growing primary ECF in the presence or absence of transforming growth factor beta-1 (TGFβ1) under serum-free conditions. The MMC dose for the equine cornea was defined with dose-dependent trypan blue exclusion and MTT [(3-4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays after applying MMC to the cultures once for 2 minutes. The efficacy of MMC to control corneal scarring in horses was determined by measuring mRNA and protein expression of corneal scarring markers (α-smooth muscle actin and F-actin) with western blotting, immunocytochemistry and/or quantitative real-time polymerase chain reactions.
Results
A single 2 minutes treatment of 0.02% or less MMC did not alter ECF phenotype, viability, or cellular proliferation whereas 0.05% or higher MMC doses showed mild-to-moderate cellular toxicity. The TGFβ1 at 1ng/ml showed significant myofibroblast formation in ECF under serum-free conditions. A single 2 minute, 0.02% MMC treatment 24 hours (early) after TGFβ1 stimulation significantly reduced conversion of ECF to myofibroblasts, however, a single 0.02% MMC treatment 11 days after TGFβ1 stimulation showed moderate myofibroblast inhibition.
Conclusions
That MMC safely and effectively reduced scarring in ECF by reducing the degree of transdifferentiation of corneal fibroblasts to myofibroblasts in vitro. Further clinical in-vivo investigations are warranted using MMC in horses.
Keywords: equine, cornea, scarring, Mitomycin C, fibroblasts
INTRODUCTION
Scarring is a common ocular problem in horses, and may lead to significant visual deficits, and even blindness. Many factors contribute to the frequency of corneal ulceration including the size and anatomic location of the equine globe, as well as the environmental conditions to which they are exposed. Treatment of corneal ulceration can be costly, labor intensive, and prolonged.1 Conventional treatment of corneal ulcers in horses utilizes topical and systemic therapeutic agents such as antimicrobial/antifungal medications to control infection, anti-collagenases to decrease stromal degradation, mydriatics for ciliary spasm and stabilization of the blood-aqueous barrier, and non-steroidal anti-inflammatory drugs to alleviate ocular pain.1–3 To the best of our knowledge, no agent specifically used to inhibit equine corneal scarring has been investigated. The lack of a suitable experimental model has been a major limitation to these investigations. Our laboratory recently defined parameters for equine corneal keratocyte, fibroblast or myofibroblast cultures to study molecular mechanisms associated with equine corneal wound healing; our goal is to test the therapeutic potential of various agents effective for the treatment of equine corneal scarring. 4,5
Corneal wound healing is a complex physiological process and plays a critical role in the maintenance of corneal structural integrity and clarity.6–9 It affects corneal epithelial cell migration and proliferation, keratocyte apoptosis, extracellular matrix remodeling, and transdifferentiation of keratocyte to fibroblasts and myofibroblasts.6,9 Numerous factors, including growth factors, cytokines, and chemokines regulate this process.1,9 Transforming Growth Factor β (TGFβ1) has been shown to play a key role in the conversion of equine corneal fibroblasts (ECF) to myofibroblasts and thus the development of corneal scarring.1,10 While the production of myofibroblasts is a normal component of this complex process, the overproduction of myofibroblasts may be deleterious due to their adverse side-effect on corneal transparency. Corneal stromal keratocytes produce and maintain the extracellular matrix and actively participate in tissue repair. A change in the stromal environment alters keratocyte function.1,9 Corneal scar formation is influenced by numerous cytokines and results when myofibroblasts are overproduced.11–13 Myofibroblasts are contractile, metabolically active, opaque cells containing intracellular microfilament bundles of F-actin and alpha-smooth muscle actin (αSMA).14 Myofibroblasts are critical to normal corneal wound healing.7,8 Once wound repair processes are complete, myofibroblast numbers are believed to decrease via apoptosis, however the exact pathogenesis is still unknown.7,8 Multiple studies have shown that a decrease in myofibroblast production reduces corneal fibrosis or haze.9,14–16
Currently, mitomycin C (MMC) is widely used clinically by physician ophthalmologists to prevent corneal haze formation that can be a secondary complication after photorefractive keratectomy and other refractive laser eye surgeries.10,17–20 Many physicians also advocate its use for treating pterygium and conjunctival and corneal neoplasia.10 MMC is a powerful alkylating agent with potent anti-tumor activity and has the ability to form a covalent linkage with DNA to inhibit DNA, RNA, and protein synthesis.8,21 MMC has been found to significantly inhibit proliferation of corneal epithelial, stromal and endothelial cells, conjunctival fibroblasts and retinal pigment epithelial cells.9,10,22–25 Furthermore, MMC demonstrated a significant decrease in corneal haze development by blocking corneal fibroblast proliferation, a known mechanism resulting in corneal haze/scar.10,26–30
The purpose of our study was to examine the toxicity and anti-fibrotic effects of MMC on cultured equine corneal fibroblast cells. Specifically, we investigated the safety and therapeutic efficiency of MMC at an early time point (24 hours) and late time point (11 days) of myofibroblast or scar production in equine cornea using an in-vitro model of equine corneal scarring. These two time points (24 hours and 11 days) were chosen to represent the early and late stages of corneal scarring, respectively. Corneal scarring is initiated immediately following corneal injury or infection, and cultures exposed to TGFβ1 for 24 hours represent this early stage of corneal scarring. In contrast, cultures exposed to TGFβ1 for 11 days mimic a later stage in corneal scarring.
MATERIALS AND METHODS
Equine corneal fibroblast, keratocyte and myofibroblast cultures
Five full-thickness 6-mm axial corneal buttons were aseptically harvested from healthy research horses undergoing humane euthanasia for reasons unrelated to this study. Slit-lamp biomicroscopy was performed by a board-certified veterinary ophthalmologist (EAG) prior to euthanasia to ensure that all samples were harvested from horses free of anterior segment disease. Corneal buttons were washed with minimum essential medium (MEM; Gibco, Invitrogen, Carlsbad, CA, USA) and the epithelium and endothelium was removed by gentle scraping using a #10 Bard Parker scalpel blade (BD, Franklin Lakes, NJ, USA). Corneal stroma was sub-sectioned and placed in tissue culture plates containing Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum to obtain ECF. ECF were then incubated in a humidified CO2 incubator (HERAcell, Thermo Scientific, USA) at 37°C. In approximately 3–10 days, fibroblasts began migrating from the stromal sub-sections. Once the primary culture reached 90% confluence, the stromal sub-sections were manually removed using rat-toothed forceps and discarded. The confluent cells were trypsinized, cellular concentrations calculated using a hemocytometer and re-plated on 60 mm tissue plates. Passages 1–3 of ECF were used for this experiment. The monocultures of corneal keratocytes, fibroblasts and myofibroblasts were selectively induced from the primary cultures generated from corneal explants by altering media or cell seeding conditions. Specifically, a concentration of 50,000 cells per 60 mm plate in serum-free MEM provided equine corneal keratocyte cultures. Equine fibroblast cultures were selectively produced by seeding 50,000 cells per 60 mm plate in MEM medium supplemented with 10% fetal bovine serum. Myofibroblast cultures were induced from seeding 10,000 fibroblasts per 60 mm plate in MEM medium supplemented with 10% fetal bovine serum or serum-free MEM medium containing 1ng/ml TGFβ1.
Cytotoxicity studies
The cytotoxicity of MMC to ECF was evaluated using phase-contrast microscopy and Trypan blue, MTT [(3-4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and TUNEL assays. Microscopy was used to evaluate cellular morphological changes, Trypan blue and MMT assays assessed cellular viability, and TUNEL assay detected apoptotic cells in cultures at 24hours and 11days. Microscopy investigations utilized a phase-contrast microscope (Leica DMIL) equipped with an imaging system (Leica DFC290). The ECF cultures treated with MMC were examined at different time intervals and cell morphology was recorded using digital photography.
Trypan blue assay was performed following manufacturer’s instructions. Briefly, at selected time points, cells were trypsinised and mixed with equal amounts of 0.4% trypan blue solution (Invitrogen, Carlsbad, CA, USA). Dead cells with ruptured membranes stained blue and live cells white were counted with Neubauer’s chamber. Cellular viability was calculated as a percent.
The MTT assay is colorimetric assay which utilizes a tetrazolium compound, MTT (Sigma-Aldrich, St Louis, MO). The MTT is bio-reduced by viable cells to a purple color formazan product which is detected at an absorbance of 570 nm by spectrophotometry. Ten μl of MTT reagent was added the each well of 96-well plate containing 100 μl DMEM and 70% confluent ECF cultures treated with or without MMC. Wells containing media alone without cells served as negative controls. Plates were incubated at 37°C in a humidified 5% CO2 incubator and absorbance was recorded at 570 nm (BioTEk FLx 800, Winooski, VT, USA).
A fluorescence-based TUNEL assay was used to detect DNA fragmentation and apoptosis in ECF. The MMC treated and untreated ECF cultures were fixed in acetone at −20°C for 2 minutes, dried at room temperature for 5 minutes, and placed in PBS balanced salt solution. Apoptosis was detected with ApopTag apoptosis detection kit (Millipore, Billerica, MA, USA) following manufacturer’s instructions. Photographs were obtained using a fluorescent microscope (Leica, Wetzlar, Germany) equipped with a digital camera (SpotCam RT KE, Diagnostic Instruments Inc., Sterling Heights, MI, USA).
Immunocytochemistry and quantification of anti-fibrotic effect of MMC
Immunofluorescent staining for αSMA, a marker for myofibroblasts responsible for corneal fibrosis, was performed using mouse monoclonal antibody for αSMA. The myofibroblast formation in ECF was stimulated with TGFβ1.4 To evaluate anti-fibrotic effect of MMC, 0.02% MMC was added to cultures for 2 minutes once at 24 hours (early) after TGFβ1 stimulation or at 11 days (late) after TGFβ1 stimulation. A single 0.02% MMC treatment for 2 minutes demonstrated least toxicity to ECF. At study end-point (11days), cultures were washed twice with PBS and incubated at room temperature with mouse monoclonal antibody for F-actin (Invitrogen, Carlsbad, CA, USA) αSMA (DAKO, Carpinteria, CA, USA) at a 1:200 dilution in 1X PBS for 90 minutes and with secondary antibody Alexa 488 or 594 goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) at a dilution of 1:500 for 1 hour. Cells were mounted with Vectashield containing DAPI (Vector Laboratories, Inc., Burlingame, CA, USA) to allow visualization of nuclei. Irrelevant isotype-matched primary antibody, secondary antibody alone, and tissue sections from naïve eyes were used as negative controls. Tissue culture plates were examined and photographed with Leica fluorescent microscope (Leica, Wetzlar, Germany) equipped with a digital camera (SpotCam RT KE, Diagnostic Instruments Inc., Sterling Heights, MI, USA) at the conclusion of the study. The αSMA and DAPI-stained cells in ten randomly selected areas were counted per 400X microscope field.
Immunoblotting
ECF were washed with ice-cold PBS and lysed in RIPA lysis buffer containing protease inhibitor cocktail (Roche Applied Sciences, Indianpolis, IN, USA). The samples were suspended in Laemmli’s denaturing sample buffer (30 μl) containing β-mercaptoethanol, vortexed for 1 minute, centrifuged for 5 minutes at 10,000× g, and boiled at 70°C for 10 minutes. Protein samples were resolved by 4%–10%SDS–PAGE and transferred to a 0.45-μm pore size PVDF membrane (Invitrogen, San Diego, CA). The membrane was incubated with αSMA (DAKO, Carpinteria, CA, USA), fibronectin or GAPDH primary antibodies (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) followed by secondary anti-mouse-or goat antibodies (Santa Cruz, Biotechnology Inc, Santa Cruz, CA, USA).
RNA extraction, cDNA synthesis and quantitative real-time PCR
The total RNA from ECF cultures (24hours and 11days) was extracted using RNeasy kit (Qiagen Inc., Valencia, CA, USA) and reverse-transcribed to cDNA following manufacturer’s instructions (Promega, Madison, WI, USA). Real-time PCR was performed using iQ5 real-time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). A 50 μL’s reaction mixture containing 2 μL cDNA (250ng), 2 μL forward (200nM), 2 μL reverse primer (200nM), and 25 μL 2X iQ SYBR green super mix (Bio-Rad Laboratories) was run at universal cycle (95°C 3 min, 40 cycles of 95°C 30 sec followed by 60°C 60 sec) following manufacturer’s instructions. For αSMA, forward primer sequence TGGGTGACGAAGCACAGAGC and reverse primer sequence CTTCAGGGGCAACACGAAGC were used. The house keeping gene beta actin forward primer sequence was CGGCTACAGCTTCACCACCA and reverse primer sequence was CGGGCAGCTCGTAGCTCTTC. The threshold cycle (CT) was used to detect the increase in the signal associated with an exponential growth of PCR product during the log-linear phase. The relative gene expression was calculated using the following formula: 2−ΔΔCT. The CT is a PCR cycle number at which the fluorescence meets the threshold in the amplification plot and ΔCT is a subtraction product of target and housekeeping genes CT values. The 2−ΔΔCT is a method for determining relative target mRNA quantity in samples. The amplification efficiency for all used templates was validated by insuring that the difference between linear slopes for all templates <0.1. Three independent reactions were performed and the average (± SEM) results were calculated.
Image and statistical analysis
The results were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using two-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test for cell toxicity assay. The real-time PCR data results were analyzed using one-way ANOVA followed by Tukey’s multiple comparison tests. A p-value less than 0.05 was considered significant. The immunoblotting data was analyzed using image J 1.38 X image analysis software (NIH, USA).
RESULTS
Cellular Viability
The experimental data collected from dose-dependent trypan blue and MTT assays presented in Figures 1 and 2. A single 2 minute, 0.02% MMC treatment dose was the most effective dose at reducing myofibroblast formation in ECF. One 0.02% MMC treatment to ECF on day-1 (early) or day-11 (late) did not alter ECF cellular viability (Figure 1) or phenotype (Figure 2). No statistically significant differences in the MMC treated or untreated control groups were noted.
Figure 1.
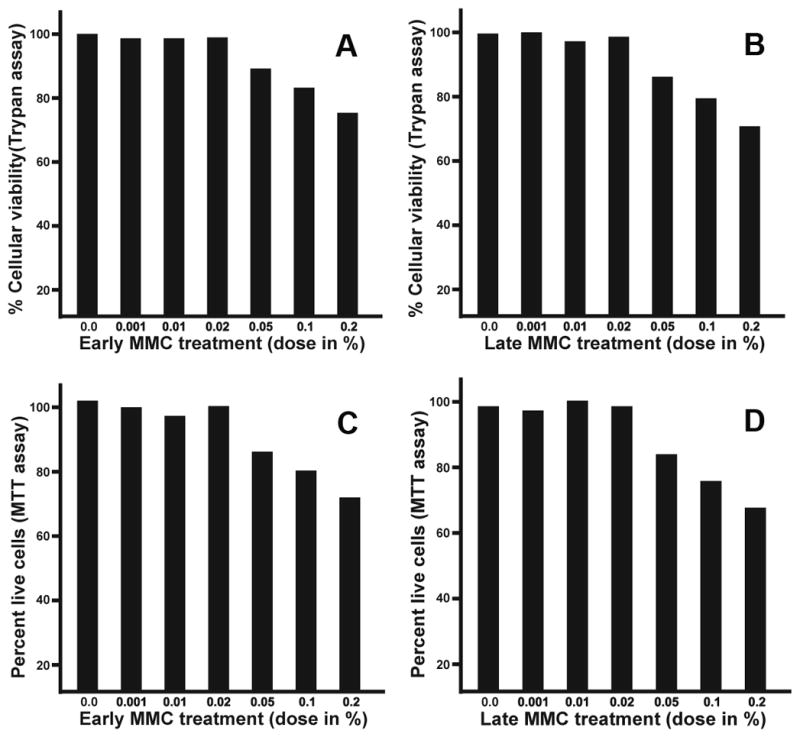
Dose-dependent effect of early and late MMC treatment to ECF. Panels A and B show data collected with trypan blue assay and panels C and D show MTT assay data. The doses of MMC (0.02% or less) did not alter ECF viability whereas MMC doses higher than 0.02% showed mild-to-moderate toxicity.
Figure 2.
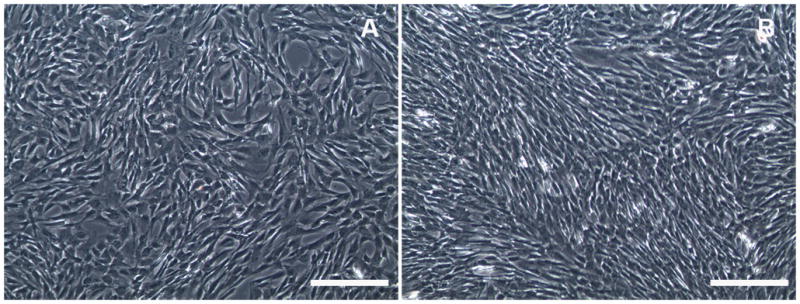
Phase-contrast light microscopy images of ECF. No morphological changes to ECF were observed when 0.02% of MMC early (A) or late (B) treatment was performed, thus establishing MMC safety to ECF in-vitro. Bar = 100μm
Apoptosis
The TUNEL assay detected increased apoptotic cell death in ECF cultures treated with 0.02% MMC early (Figure 3B) and late (Figure 3D) compared to untreated controls (Figure 3A and 3C).
Figure 3.
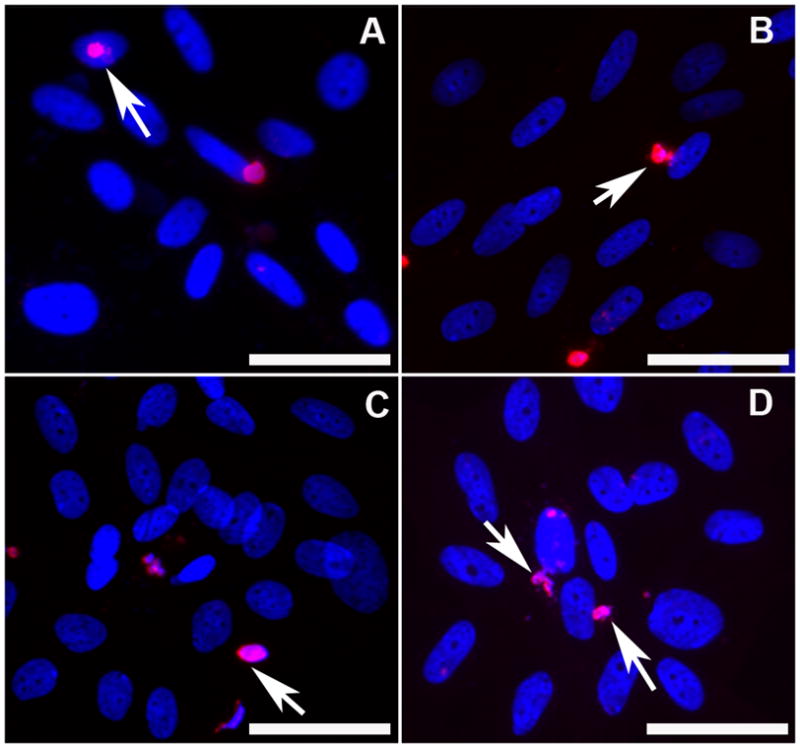
TUNEL assay detecting apoptosis induced by 0.02% MMC in ECF. Panel A demonstrates the early control of no MMC treatment, panel B shows effects of early MMC treatment, panel C is late control (no MMC) and panel D shows effects of late MMC treatment on ECF. TUNEL-positive cells are stained in red and nuclei in blue with DAPI. Arrows denote TUNEL-positive cells. Magnification 400x. Bar = 100μm
F-actin immunocytochemistry
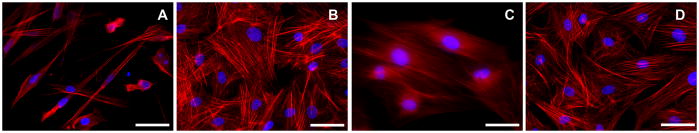
F-actin immunostaining of treatment groups was photographed (Figure 4). The ECF grown in absence of TGFβ1 (normal fibroblasts) showed mild F-actin staining in microfilament bundles (Figure 4A) whereas ECF grown in presence of TGFβ1 (myofibroblasts) showed prominent intracellular staining of F-actin in large microfilaments bundles of contractile apparatus (typical of myofibroblasts, Figure 4B). 4 A single 2 minute, 0.02% MMC application to ECF in the early treatment group demonstrated a significant decrease in F-actin staining, consistent with marked reduction in contractile apparatus formation (Figure 4C). However, the same MMC dose showed a moderate reduction in F-actin staining in late treatment group (Figure 4D).
Figure 4.
Immunocytochemical staining with phalloidin showing levels of F-actin in ECF control group (A; no MMC treatment), in the presence of TGFβ1 (B), early MMC treatment group (C) and late MMC treatment group (D). F-actin stained cells are red and nuclei are stained blue with DAPI. Magnification 400x. Bar = 100μm
αSMA immunocytochemistry, quantification and western blotting
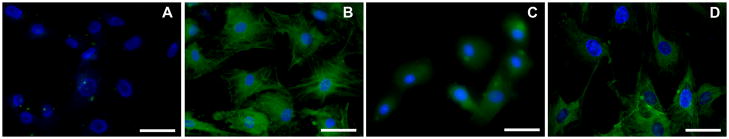
Immunocytochemistry of αSMA was performed on all experimental groups (Figure 5). No αSMA was detected in ECF grown in absence of TGFβ1 (Figure 5A). By contrast, high levels of αSMA were detected in ECF grown in presence of TGFβ1 (Figure 5B). One 2 minute application of 0.02% MMC to ECF on day-1 (early treatment group) showed substantial inhibition of TGFβ1-induced transdifferentiation of ECF to myofibroblasts (Figure 5C). However, similar MMC treatment performed on day-11 (late treatment group) did not appear effective and showed only moderate inhibition of ECF to myofibroblasts (Figure 5D).
Figure 5.
Immunocytochemical staining of α-SMA (green) showing anti-fibrotic effects of MMC on ECF. The TGFβ1 untreated cultures did not show any α-SMA (A) while the TGFβ1 treated cultures showed significant α-SMA staining (B). Early MMC treatment (C) showed significant inhibition in TGFβ1-induced myofibroblast formation in ECF whereas late MMC treatment showed moderate inhibition (D). The DAPI stained nuclei are shown in blue. Magnification 400x. Bar = 100μm
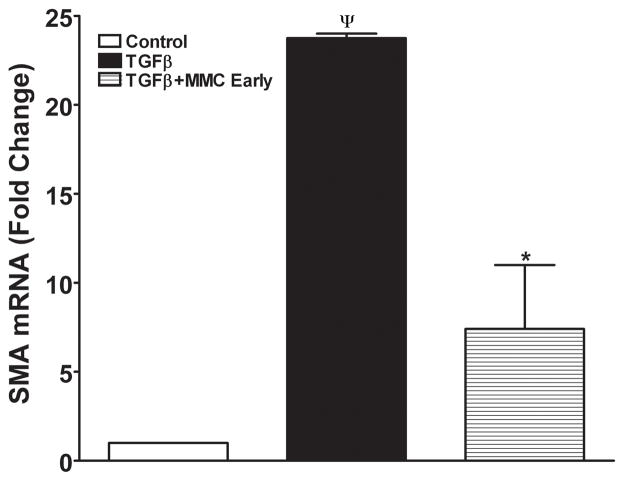
Quantification of αSMA immunocytochemical data is summarized in Figure 6. The ECF grown under serum-free conditions in the presence of TGFβ1 (1ng/ml) showed significantly high levels of αSMA staining (65–81%; p < 0.01) in the cells compared to control ECF grown in the absence of TGFβ1 (<2%). One 2 minute 0.02% MMC application to ECF significantly decreased TGFβ1-induced myofibroblast development in early (69% ± 5.8; p < 0.01) and late (28% ± 9.8) treatment groups (Figure 6).
Figure 6.
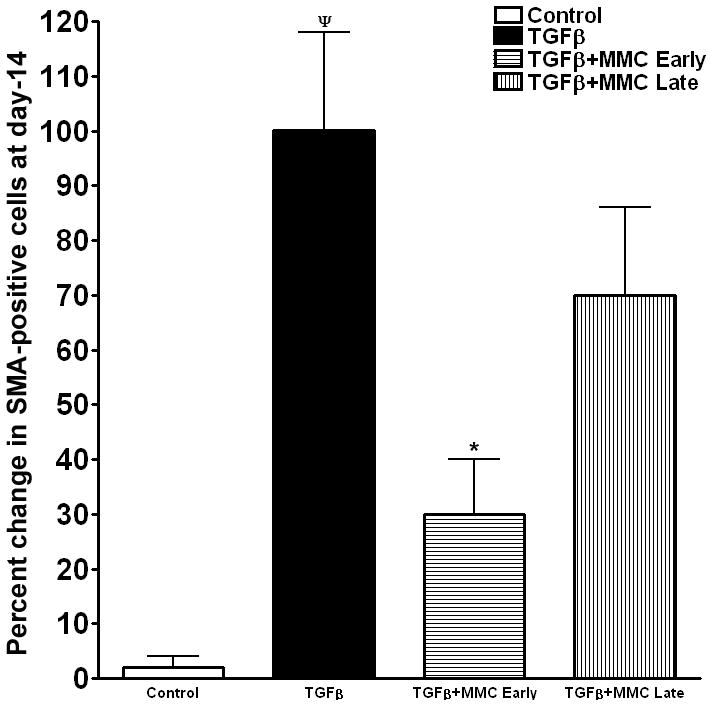
Quantification of α-SMA-stained cells in ECF cultures treated with or without MMC. Myofibroblast formation was significantly induced in the presence of TGFβ1. Early MMC treatment significantly decreased TGFβ1-induced myofibroblast formation in ECF compared to untreated controls, whereas late MMC treatment showed moderate reduction. ¬denotes p <0.01 (Con vs TGFβ1) and * represents p<0.01 (TGFβ1 vs TGFβ1+MMC).
Figure 7 demonstrates measurement of αSMA protein with western blot analysis. The analysis of data after normalization further confirmed the anti-fibrotic effect of MMC on ECF. The 0.02% MMC treatment to ECF grown in presence of TGFβ1 under serum-free conditions effectively suppressed αSMA expression in both the early (≤ 65%) and late (≤ 30%) treatment groups.
Figure 7.
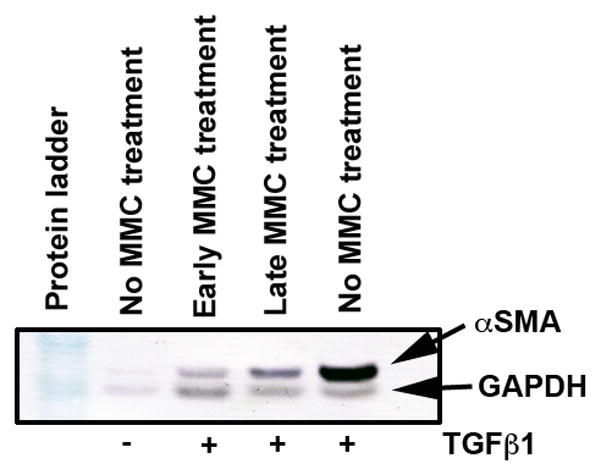
Western blot analysis showing quantitative measurement of α-SMA (myofibroblast marker) in ECF treated with or without TGFβ1 (1ng/ml) and 0.02% MMC. Equal quantity of protein (50 μg) was loaded in each lane. GAPDH was used as house keeping gene. A single early MMC treatment showed significant decrease in TGFβ1-induced myofibroblast formation in ECF compared to untreated controls. The late MMC treatment showed moderate reduction in α-SMA.
αSMA RNA quantification
Figure 8 shows quantification of αSMA RNA performed with real-time PCR. A statistically significant 23-fold increase in αSMA RNA was detected in ECF exposed to TGFβ1 and no change in ECF not exposed to TGFβ1 (Control). The early application of MMC demonstrated a significant 14-fold reduction of αSMA RNA (p < 0.01).
Figure 8.
Quantitative real-time PCR reactions showing measurements of α-SMA in MMC treated or untreated ECF. TGFβ1 exposure induced significant α-SMA formation in ECF and early MMC treatment significantly decreased TGFβ1-induced myofibroblast formation in ECF. Late MMC treatment showed moderate decrease in α-SMA RNA (data not shown). ¬indicates p <0.01 (Con vs TGFβ1) and * represents p<0.01 (TGFβ1 vs TGFβ1+MMC early).
DISCUSSION
Corneal scarring or fibrosis is a common sequela to corneal disease and frequently results in vision deficits.1,3 Historically in veterinary ophthalmology, therapeutic efforts have been focused on globe preservation and restoration of ocular comfort. Treatment options for horses specifically geared towards prevention and/or the reduction of corneal fibrosis are lacking. In-vitro studies represent a highly valuable tool to further our understanding of the physiologic mechanisms of corneal wound healing. In-vitro models represent an ideal controlled setting to test drug development with potentially clinically relevant applications. Corneal fibrosis involves keratocyte apoptosis, proliferation and transdifferentiation of fibroblasts into myofibroblasts.31,32 Smooth muscle actin expression and deposition of extracellular matrix protein in a random pattern are primarily responsible for corneal fibrosis and resultant visual opacity.7,32 Numerous studies in various animal models have demonstrated the role of TGFβ1 in the development of corneal fibrosis.10,11,32–34 Additionally, it has been established that anti-TGFβ1 antibodies have reduced the production of corneal haze in rabbits which had received photorefractive keratotomy (PRK).35 Several reports have been published demonstrating the effectiveness of MMC to reduce corneal fibrosis in rabbit, rodent and human corneas both in-vivo and in-vitro.10,29,30
Current literature reviews found only one report regarding the use of MMC on the equine cornea. Rayner and Van found MMC effective for equine corneal squamous cell carcinoma (0.4mg/ml MMC intraoperatively on the affected areas for 1 or 5 minutes).36 In that report, no mention was made pertaining to the anti-fibrotic effects of MMC. To the best of our knowledge this publication represents the first investigation of the anti-fibrotic effects of MMC on equine cornea using an in-vitro model. Our data demonstrate the ability of MMC to reduce myofibroblast production in ECF cultures and its effectiveness when applied at an early or late stage of disease (e.g., reduction in the αSMA and F-actin levels; Figures 4–8). Significant suppression of myofibroblast production was noted when MMC was applied at an early stage of disease compared to a late stage consistent with the fact that the anti-fibrotic effects of MMC are due to the inhibition of cellular proliferation and induction of apoptosis.5–11 Although relatively more apoptosis was detected in the late MMC group when compared to the early MMC group, the percent of apoptotic cells between the two groups was not statistically significant. We believe that lack of statistical difference is due to the higher ECF density in late treatment group (Figure 3C and 3D) compared to the early treatment group (Figure 3A and 3B) as evident from DAPI nuclear staining (Figure 3). The late ECF treatment group received MMC on day-11 whereas the early ECF treatment group received MMC on day-1. These findings are consistent with our in-vivo rabbit studies that showed reduction in corneal scarring with MMC was due to increased apoptosis and decreased cellular proliferation.10 Our data support previously published reports where significant inhibition of corneal scarring in humans and rabbits in-vitro and in-vivo has been demonstrated.8, 15–24 It is reasonable to expect that MMC may be useful clinically for the reduction of corneal fibrosis in equine patients. MMC treatment in the face of an established corneal scar may prove less beneficial compared to topical MMC application at the time of corneal ulceration diagnosis or keratectomy; however, clinical trials are needed to further assess the usefulness of its application in equine patients.
While the effectiveness of MMC against corneal haze is well-established in both rabbits and humans, conflicting reports regarding its safety in human patients can be found.25–30 Several different protocols, involving different concentrations and exposure times, have been suggested or used by clinicians to minimize unwanted side effects of MMC which include increased stromal loss and corneal malacia as the result of increased cellular toxicity.10,37,38 This pilot study addressed the safety of MMC for the equine cornea in an in-vitro model by evaluating the response of MMC on ECF morphology and the proliferation and cell death using various biochemical assays. In this study, the 0.02% dose of MMC was deemed safe for the equine cornea because it did not induce significant apoptosis or alter ECF proliferation or phenotype (Figures 1–3). MMC may prove beneficial in the reduction of corneal fibrosis secondary to corneal injury in the equine patient and improve long-term visual outcome. Further studies are warranted to determine optimal MMC dosing for treatment of corneal fibrosis in the clinical patient.
Acknowledgments
Funding for this project was provided through the RO1EY017294 (RRM) grant from the National Eye Institute, National Institutes of Health, Bethesda, MD; Clinician Scientist Grant (EG, DB, RRM) from the College of Veterinary Medicine, University of Missouri-Columbia; and an Unrestricted grant from the Research to Prevent Blindness, New York, NY.
References
- 1.Haber M, Cao Z, Panjwani N, et al. Effects of growth factors (EGF, PDGF-BB and TGF-beta 1) on cultured equine epithelial cells and keratocytes: implications for wound healing. Veterinary Ophthalmology. 2003;6:211–217. doi: 10.1046/j.1463-5224.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 2.Michau TM, Schwabenton B, Davidson MG, et al. Superficial, nonhealing corneal ulcers in horses: 23 cases (1989–2003) Veterinary Ophthalmology. 2003;6:291–297. doi: 10.1111/j.1463-5224.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 3.Nasisse MP, Nelms S. Equine ulcerative keratitis. Vet Clinics North America Equine Practice. 1992;8:537–555. doi: 10.1016/s0749-0739(17)30440-6. [DOI] [PubMed] [Google Scholar]
- 4.Buss DG, Giuliano EA, Sharma A, et al. Isolation and cultivation of equine corneal keratocytes, fibroblasts and myofibroblasts. Veterinary Ophthalmology. 2010;13:37–42. doi: 10.1111/j.1463-5224.2009.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buss DG, Sharma A, Giuliano EA, et al. Gene delivery in the equine cornea: a novel therapeutic strategy. American Journal of Veterinary Research. 2009 doi: 10.1111/j.1463-5224.2010.00813.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vij N, Sharma A, Thakkar M, et al. PDGF-driven proliferation, migration, and IL8 chemokine secretion in human corneal fibroblasts involve JAK2-STAT3 signaling pathway. Molecular Vision. 2008;14:1020–1027. [PMC free article] [PubMed] [Google Scholar]
- 7.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Progress in Retinal Eye Research. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SE, Mohan RR, Ambrosio R, Jr, et al. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Progress in Retinal Eye Research. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 9.Netto MV, Mohan RR, Ambrosio R, Jr, et al. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- 10.Netto MV, Mohan RR, Sinha S, et al. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. Journal of Refractive Surgery. 2006;22:562–574. doi: 10.3928/1081-597x-20060601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jester JV, Huang J, Barry-Lane PA, et al. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Investive Ophthalmology and Visual Science. 1999;40:1959–1967. [PubMed] [Google Scholar]
- 12.Jester JV, Huang J, Fisher S, et al. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life human corneal fibroblasts. Invest Ophthalmology and Visual Science. 2003;44:1850–1858. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- 13.Maltseva O, Folger P, Zekaria D, et al. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Investigative Ophthalmology and Visual Science. 2001;42:2490–2495. [PubMed] [Google Scholar]
- 14.Boote C, Dennis S, Newton RH, et al. Collagen fibrils appear more closely packed in the prepupillary cornea: optical and biomechanical implications. Investigative Ophthalmology and Visual Science. 2003;44:2941–2948. doi: 10.1167/iovs.03-0131. [DOI] [PubMed] [Google Scholar]
- 15.Conrad GW, Funderburgh JL. Eye development and the appearance and maintenance of corneal transparency. Transactions of the Kansas Academy of Science. 1992;95:34–38. [PubMed] [Google Scholar]
- 16.Maurice DM. The transparency of the corneal stroma. Vision Research. 1970;10:107–108. doi: 10.1016/0042-6989(70)90068-4. [DOI] [PubMed] [Google Scholar]
- 17.Mohan RR, Hutcheon AE, Choi R, et al. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Experimental Eye Research. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 18.Mohan RR, Stapleton WM, Sinha S, et al. A novel method for generating corneal haze in anterior stroma of the mouse eye with the excimer laser. Experimental Eye Research. 2008;86:235–240. doi: 10.1016/j.exer.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leccisotti A. Mitomycin C in photorefractive keratectomy: effect on epithelialization and predictability. Cornea. 2008;27:288–291. doi: 10.1097/ICO.0b013e31815c5a51. [DOI] [PubMed] [Google Scholar]
- 20.Mohan RR, Liang Q, Kim WJ, et al. Apoptosis in the cornea: further characterization of Fas/Fas ligand system. Experimental Eye Research. 1997;65:575–589. doi: 10.1006/exer.1997.0371. [DOI] [PubMed] [Google Scholar]
- 21.Galm U, Hager MH, Van Lanen SG, et al. Antitumor antibiotics: bleomycin, enediynes, and mitomycin. Chemical Reviews. 2005;105:739–758. doi: 10.1021/cr030117g. [DOI] [PubMed] [Google Scholar]
- 22.Nuyts RM, Pels E, Greve EL. The effects of 5-fluorouracil and mitomycin C on the corneal endothelium. Current Eye Research. 1992;11:565–570. doi: 10.3109/02713689209001812. [DOI] [PubMed] [Google Scholar]
- 23.Jampel HD. Effect of brief exposure to mitomycin C on viability and proliferation of cultured human Tenon’s capsule fibroblasts. Ophthalmology. 1992;99:1471–1476. doi: 10.1016/s0161-6420(92)31781-6. [DOI] [PubMed] [Google Scholar]
- 24.Kang SG, Chung H, Yoo YD, et al. Mechanism of growth inhibitory effect of Mitomycin-C on cultured human retinal pigment epithelial cells: apoptosis and cell cycle arrest. Current Eye Research. 2001;22:174–181. doi: 10.1076/ceyr.22.3.174.5513. [DOI] [PubMed] [Google Scholar]
- 25.Wu KY, Hong SJ, Huang HT, et al. Toxic effects of mitomycin-C on cultured corneal keratocytes and endothelial cells. Journal of Ocular Pharmacology and Therapeutics. 1999;15:401–411. doi: 10.1089/jop.1999.15.401. [DOI] [PubMed] [Google Scholar]
- 26.Thornton I, Xu M, Krueger RR. Comparison of standard (0.02%) and low dose (0.002%) mitomycin C in the prevention of corneal haze following surface ablation for myopia. Journal of Refractive Surgery. 2008;24:S68–76. doi: 10.3928/1081597X-20080101-13. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan S, Drake A, Herzig S. Photorefractive keratectomy with 0.02% mitomycin C for treatment of residual refractive errors after LASIK. Journal of Refractive Surgery. 2008;24:S64–67. doi: 10.3928/1081597X-20080101-12. [DOI] [PubMed] [Google Scholar]
- 28.Shalaby A, Kaye GB, Gimbel HV. Mitomycin C in photorefractive keratectomy. Journal of Refractive Surgery. 2009;25:S93–97. doi: 10.3928/1081597X-20090115-03. [DOI] [PubMed] [Google Scholar]
- 29.Rajan MS, O’Brart DP, Patmore A, et al. Cellular effects of mitomycin-C on human corneas after photorefractive keratectomy. Journal of Cataract Refractive Surgery. 2006;32:1741–1747. doi: 10.1016/j.jcrs.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Nassaralla BA, McLeod SD, Nassaralla JJ., Jr Prophylactic mitomycin C to inhibit corneal haze after photorefractive keratectomy for residual myopia following radial keratotomy. Journal of Refractive Surgery. 2007;23:226–232. doi: 10.3928/1081-597X-20070301-04. [DOI] [PubMed] [Google Scholar]
- 31.Wilson SE, Mohan RR, Hong JW, et al. The wound healing response after laser in situ keratomileusis and photorefractive keratectomy: elusive control of biological variability and effect on custom laser vision correction. Archives of Ophthalmology. 2001;119:889–896. doi: 10.1001/archopht.119.6.889. [DOI] [PubMed] [Google Scholar]
- 32.Sharma A, Mehan MM, Sinha S, et al. Trichostatin a inhibits corneal haze in vitro and in vivo. Investigative Ophthalmology and Visual Science. 2009;50:2695–2701. doi: 10.1167/iovs.08-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C, Michelini-Norris B, Stevens S, et al. Measurement of mRNAs for TGFss and extracellular matrix proteins in corneas of rats after PRK. Investigative Ophthalmology and Visual Science. 2000;41:4108–4116. [PubMed] [Google Scholar]
- 34.Tuli SS, Liu R, Chen C, et al. Immunohistochemical localization of EGF, TGF-alpha, TGF-beta, and their receptors in rat corneas during healing of excimer laser ablation. Current Eye Research. 2006;31:709–719. doi: 10.1080/02713680600837390. [DOI] [PubMed] [Google Scholar]
- 35.Moller-Pedersen T, Cavanagh HD, Petroll WM, et al. Neutralizing antibody to TGFbeta modulates stromal fibrosis but not regression of photoablative effect following PRK. Current Eye Research. 1998;17:736–747. [PubMed] [Google Scholar]
- 36.Rayner SG, Van Zyl N. The use of mitomycin C as an adjunctive treatment for equine ocularsquamous cell carcinoma. Australian Veterinary Journal. 2006;84:43–46. doi: 10.1111/j.1751-0813.2006.tb13124.x. [DOI] [PubMed] [Google Scholar]
- 37.Majmudar PA, Forstot SL, Dennis RF, et al. Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery. Ophthalmology. 2000;107:89–94. doi: 10.1016/s0161-6420(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 38.Carones F, Vigo L, Scandola E, et al. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. Journal of Cataract Refractive Surgery. 2002;28:2088–2095. doi: 10.1016/s0886-3350(02)01701-7. [DOI] [PubMed] [Google Scholar]