Introduction
In the developing world, many patients either have a history of tuberculosis (TB) when they initiate antiretroviral therapy (ART), or they develop TB while receiving ART. ART diminishes the risk for TB as the CD4 count rises, yet the excess risk for TB is never eliminated even if CD4 levels return to normal levels [1-12].
TB has been shown to be associated with excess mortality in HIV-infected patients who are not treated with ART. However, when patients start ART, it has not been well delineated what influence a history of TB has on subsequent patient outcome, nor has it been clear what role incident TB has on their outcome, especially in patients who have consistent HIV suppression on ART. This report focuses on a large, well-defined HIV-infected patient population in South Africa which initiated ART under careful observation. The specific goal of this investigation was to examine risk factors for TB and mortality risk associated with TB [13]. This report emphasizes the importance of TB prevention programs if the outcome of ART is to be optimized.
Methods
PHIDSIA Study
Phidisa is a joint observational cohort and randomized HIV treatment study for the South African National Defence Force (SANDF) and their dependants which was designed and executed with close monitoring by South African and NIH regulatory authorities [13]. Phidisa II, the randomized component of PHIDISA, had a 2 × 2 factorial trial comparing initial therapy of Efavirenz with Lopinavir/Ritonavir and Zidovudine + Didanosine with Stavudine + Lamuvidine in treatment-naïve HIV-Infected persons with <200 CD4+ cells/mm3 or a prior AIDS diagnosis [13].
All eligible subjects were invited to enroll. The primary endpoint was the combined endpoint of HIV disease progression (new or recurrent AIDS defining illness, including TB) or death. The Phidisa II study enrolled 1,771 individuals from members of the military at six sites in South Africa between 2004-2007. Eligible persons were randomly allocated in a 1:1:1:1 allocation to one of four starting regimens: 1) EFV+ZDV+ddI; 2) EFV+d4T+3TC; 3) LPV/r+ZDV+ddI; or 4) LPV/r+d4T+3TC. Randomization was stratified by clinical site. All treatments were administered in an open-label manner. Patients with CD4 cell counts <200 cells/μl received pneumocystis pneumonia (PCP) prophylaxis with daily cotrimoxazole or dapsone.
No patients were known to have received isoniazid preventive therapy.
Patient Assessment
At enrollment, details regarding previous episodes of TB or treatment for current TB were self-reported by patients based on a questionnaire and interview. WHO clinical staging was documented. A comprehensive assessment was made to exclude active TB and other opportunistic diseases. Nebulized sputum induction was available across all sites. For patients entering the study with suspected or documented active TB, randomization was deferred until successful completion of an induction course of anti TB treatment as determined by the TB clinic. For patients who required anti TB treatment following randomization, Lopinavir/ritonavir was switched to Efavirenz and/or Rifabutin substituted for Rifampin. Patients with active TB were referred to the South African Military Health Service (SAMHS) for administration and monitoring of anti TB treatment according to the South African National Treatment Guidelines [14]. Subjects were followed monthly for the first three months and every 3 months thereafter until March 31, 2008 for a median follow-up of 24.7 months.
Tuberculosis Definitions
Baseline history of TB was defined as a history of pulmonary or extra-pulmonary TB, or a history of taking multidrug regimens for TB in the past or at the time of baseline evaluation. A TB event (pulmonary or extra-pulmonary) diagnosed during the study was reported as a study endpoint and was reviewed by an independent review committee. A confirmed pulmonary TB case had to include two of the following three criteria: A) fever, dyspnea, cough, weight loss or fatigue, B) positive AFB smear on 2 or more sputa, and C) culture or PCR positive for M. tuberculosis from sputum or bronchial lavage or lung tissue. A probable case of pulmonary TB had to meet the following four criteria: A) fever, dyspnea, cough, weight loss or fatigue, B) abnormal chest X-ray, C) AFBs seen in sputum or lavage or lung tissue but not grown in culture, and D) response to antituberculous treatment. A confirmed case of extra-pulmonary TB was defined by both of the following criteria: A) compatible symptoms, and B) culture or PCR from blood or affected tissue. A probable case of extra-pulmonary TB was defined as: A) compatible symptoms, plus either B) AFB seen in affected tissue or blood, C) concurrent diagnosis of pulmonary TB or D) responds to treatment. Tuberculosis endpoints reported during the study that were not confirmed or probable as defined above are referred to as possible TB events.
Statistical analysis
The association of history of TB at baseline with other baseline patient characteristics was assessed using Fisher's exact test for categorical variables and two-sample t-test or a nonparametric Wilcoxon for continuous variables. Baseline factors considered were: Phidisa clinical site, gender, age, body mass index (BMI), CD4 count, HIV viral load, hemoglobin, white blood cell count (WBC), SGOT and SGPT. The event rate (number of cases per 100 person-years) for TB during follow-up was assessed by endpoint review status (confirmed/probable versus possible) and timing of the event (0-3, 3-6, 6-12, 12-24, 24+ months).
The unadjusted association between TB history and risk of TB during follow-up was assessed by the log-rank test. The association between risk of TB during follow-up with baseline risk factors was also assessed using a multivariate Cox regression model including: baseline history of TB, age, gender, BMI, baseline CD4, HIV viral load (on the log-scale), hemoglobin, WBC and SGOT and stratifying on study site. Separate analyses were done for confirmed/probable TB and all TB events. A similar analysis was performed to assess whether history of TB at baseline was associated with an increased mortality risk during follow-up.
Several exploratory Cox regression models were considered to understand better which individuals were at risk for getting TB during follow-up, these models included baseline WHO stage III/IV (yes/no) as a risk factor, reflecting a history of serious AIDS defining illnesses (including tuberculosis), along with the other aforementioned baseline risk factors and CD4 and viral load as time-varying covariates. Separate models examining associated risk factors for early TB events (< 6 months) and late TB events (> 6 months) were also considered.
The impact of newly diagnosed TB on the mortality hazard was estimated by including history of TB diagnosed during follow-up as a time-varying covariate in a multivariate Cox regression model stratified on study site and adjusting for baseline risk factors, and CD4 and HIV viral load as time varying covariates, as described above. This analysis was performed separately for all reported TB events and those designated as confirmed/probable TB by the endpoint review committee. The potentially time-varying nature of the mortality hazard associated with newly diagnosed TB was explored by 1) estimating the hazard associated with TB separately for events in the first 6 months of follow-up compared to after 6 months, and 2) testing for whether there is a different short-term risk associated with TB (within first 6 months of getting diagnosed) versus a long-term risk of TB (>6 months after diagnosis).
Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC) and R version 2.8.1 (R Development Core Team, Vienna, Austria). All statistical tests were two-sided, and performed at the 0.05 significance level.
Results
Tuberculosis Risk
The PHIDISA II cohort included 1,771 HIV+ individuals with median follow-up of 24.7 months ((inter quartile range (IQR) 12.6, 39.9). This cohort was 36 years of age (mean) and 32% female. At baseline, the median CD4 count was 106 (IQR; 44, 154) cells/mm3 and viral load was 144,000 (IQR; 53,500, 307,000) copies/ml. There was little loss to follow-up in the Phidisa II study, with only about 5% of subjects having more than 8 months since the last contact (roughly two visits) at the end of the study
Table 1 presents clinical factors by baseline history of TB. In the unadjusted analyses, baseline history of TB was associated with PHIDISA clinical site, being male, lower CD4 count, higher viral load, lower hemoglobin, and higher SGOT. In the multivariate logistic model, adjusting simultaneously for all individually significant factors, baseline history of TB was still significantly associated with clinical site (p<0.001), being male (p<0.001), lower BMI (0.006), lower baseline CD4 counts (p<0.001), higher baseline HIV viral load (p<0.001), and lower hemoglobin (p<0.001). This suggests each of these risk factors have independent effects that were significantly associated with a history of TB.
Table 1.
Baseline clinical characteristics by baseline history of tuberculosis (TB). Unless otherwise noted, mean (SD) are presented (N=1769*).
| Characteristics | History of TB N=479 |
No History of TB N=1290 |
P-value† |
|---|---|---|---|
| Age | 36.2 (5.2) | 35.8 (5.6) | 0.142 |
| Male (n,%) | 365 (76.2%) | 838 (65.0%) | <0.001 |
| Body Mass Index - women | 23.3 (5.3) | 26.0 (5.7) | <0.001 |
| Body Mass Index - men | 21.8 (3.7) | 23.1 (4.1) | <0.001 |
| CD4 count | 77.0 (61.1) | 111.9 (64.9) | <0.001 |
| Viral load | 298,394 (275,026) | 196,700 (215,383) | <0.001 |
| Hemoglobin | 11.9 (1.9) | 12.9 (2.0) | <0.001 |
| WBC | 4.43 (2.1) | 4.61 (1.9) | 0.102 |
| SGOT (IU/L) | 50.8 (37.2) | 43.9 (44.8) | 0.001 |
| SGPT (IU/L) | 42.1 (43.6) | 37.5 (46.1) | 0.056 |
Only 1,769/1,771 subjects had TB history sufficiently collected on the baseline medically history form.
P-values are unadjusted from the Fisher exact test for binary variables and the two-sample t-statistic with unequal variances for continuous covariates, except viral RNA for which a non-parametric Wilcoxon test was used.
Table 2 presents the number of subjects and event rate (number per 100 person-years) for incident TB during follow-up. There were 254 individuals with at least one incident TB event during follow-up.
Table 2.
TB incidence and mortality rates during follow-up as number per 100 person-years along with 95% confidence interval and number of events (n), broken down by timing of event.
| 0-3 months | 3-6 month | 6-12 months | 12-24 months | > 24 months | |
|---|---|---|---|---|---|
| Confirmed/Probable TB | 8.42 (6.07,11.67) |
3.24 (1.88,5.58) |
1.98 (1.17,3.34) |
2.21 (1.48, 3.30) |
1.95 (1.26,3.02) |
| n=36 | n=13 | n=14 | n=24 | n=20 | |
| TB – All events | 19.88 (16.05,24.6) |
8.75 (6.25,12.25) |
6.06 (4.46,8.23) |
5.50 (5.23,7.14) |
4.11 (3.00,5.62) |
| n=84 | n=34 | n=41 | n=56 | n=39 | |
| Death | 16.89 (13.42,21.24) |
8.29 (5.92,11.60) |
5.78 (4.27,7.82) |
3.54 (2.60,4.83) |
1.75 (1.12, 2.75) |
| n=73 | n=34 | n=42 | n=40 | n=19 | |
Baseline history of TB was associated with increased risk for TB (all events), with unadjusted hazard ratio 1.36 (95% CI: 1.04, 1.78) and log-rank p-value (0.02) (Figure 1). When baseline factors of CD4, log-viral load, age, BMI, gender, WBC, SGOT, and hemoglobin are included in the Cox model for risk, stratified on Phidisa site, baseline TB history was no longer significantly associated with risk of incident TB (all events or confirmed/probable); however, the association with hemoglobin, being male, and BMI remained significant. This suggests that baseline hemoglobin, BMI, and gender have important independent effects on the risk of TB during follow-up; whereas baseline history of TB is correlated with poor baseline health status and is not associated with an independent effect on risk of incident TB during follow-up over and above the risk associated with poor health. When baseline advanced WHO stage (stage III/IV – yes/no) was included in the multivariate model in place of baseline history of TB, results were similar and advanced WHO stage was also an independent predictor. In this model, keeping other factors the same, the hazard ratio (95% CI) for newly diagnosed TB associated with being male was 1.42 (1.02, 1.99), advanced WHO stage 1.35 (1.01, 1.80), a unit increase in BMI 1.06 (1.02, 1.09), and a unit increase in hemoglobin 1.10 (1.02, 1.18). For confirmed/probable TB events, there were no significant associations with baseline risk factors, with only low BMI and advanced WHO stage as marginally significant in the multivariate model. When viral load and CD4 were included in the multivariate model for all TB events as time-varying covariates, instead of baseline levels, they were both more strongly associated significant (p<0.0001 for both); both were also significant in the model for confirmed/probable TB (p=0.02 and p<0.0001, respectively).
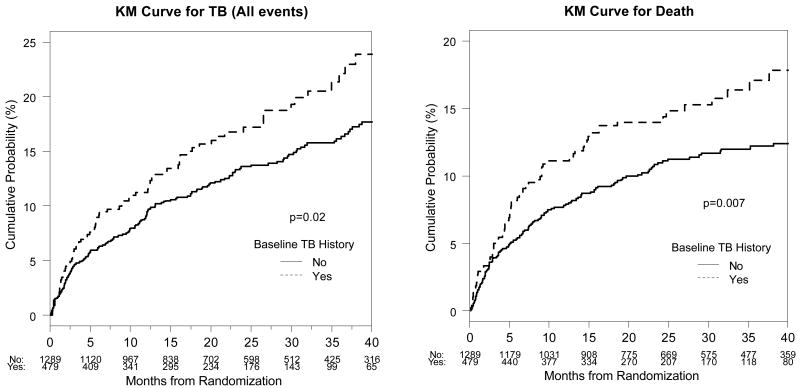
Figure 1.
Kaplan-Meier (KM) curves for the cumulative probability (%) of tuberculosis (TB) during follow-up (all events) and mortality by baseline history of TB. The log-rank p-value for the difference in survival is also given.
For the early occurring TB events (<6 months), baseline BMI, viral load, WBC, and hemoglobin were significant factors. Late occurring TB risk (>6 months) was associated with the 6-month level of viral load, CD4 count, hemoglobin, being male and advanced baseline WHO stage marginally significant (p=0.06 for both). (Data not shown.)
Virologic failure is defined as having a viral load >400 copies/mL on any occasion three or more months post therapy initiation. At 3 months, 37% of subjects were still not under virologic control. Considering the history of virologic failure as a time-varying covariate in the Cox regression model for TB risk, adjusting for baseline covariates and CD4 as time-varying, a history of virologic failure is associated with a 1.54 times higher risk (95% CI: 1.05, 2.27) of being diagnosed with TB during follow-up compared to someone with a history of sustained viral control. Note, the analogous associated risk of virologic failure for other OI was not significant 1.18 (0.63, 2.19).
112/1,771 (6%) individuals were observed to have non-TB opportunistic infection(s) during follow-up: 38/112 (34%) occurred in individuals who also had a TB event during follow-up. Of the individuals with both types of events, 7/38 had the event within 2 weeks of each other, 17 had TB first and 14 had the other OI first. The probability for individuals who had both to have TB first was not significantly different from 0.5 (McNemar's test, p=0.72). Having another OI besides TB was associated with a 2.85 fold risk (95% CI: 1.75, 4.64) for a later TB event compared to not having another OI. Conversely, having a TB event during the follow-up was associated with a 2.71 (95% CI: 1.56, 4.70) times higher relative risk of a subsequent other opportunistic infection compared to having no prior TB during follow-up.
Mortality Risk
Figure 1 shows the Kaplan-Meier survival curves for overall mortality by baseline TB history. Baseline TB was individually associated with risk of death (log-rank p=0.007). This association was no longer significant when adjusting for other baseline risk factors, suggesting that history of TB was not having an effect on mortality risk independent of poor baseline health status. Baseline factors that were significantly associated with increased mortality risk in the multivariate model included low BMI, low hemoglobin, high WBC, and low CD4. Similar results were obtained when examining the association between baseline TB and TB-free survival (data not shown). Similar results were also obtained when advanced WHO stage was included in the multivariate model in place of baseline TB history, with WHO stage also significantly associated with mortality risk. In this model, the mortality hazard ratio (95% CI) associated with baseline advanced WHO stage was 1.56 (1.12, 2.17), with a 50 unit decrease in baseline CD4 1.42 (1.22, 1.65), a unit decrease in BMI 1.07 (1.03, 1.12), unit decrease in hemoglobin 1.13 (1.05, 1.22), and a unit increase in WBC 1.07 (1.01, 1.14).
There were 54 deaths (21.3%) among the 254 individuals with TB events during follow-up. When TB is included in the Cox regression model as a time-varying covariate, adjusted for baseline risk factors and CD4 and log viral load also as time-varying covariates, confirmed/probable TB during follow-up is associated with a mortality hazard ratio of 2.40 (95% CI: 1.34, 4.31) compared to having no prior TB event (of any type), and possible TB is associated with a hazard ratio of 2.54 (95% CI; 1.62, 3.99). There was little difference in the risk associated with confirmed/probable TB versus unconfirmed TB, with an overall mortality hazard ratio for any TB of 2.49 (95% CI: 1.68 3.69). That is, at a given time, those individuals alive with prior TB during follow-up had about 2.5 times the risk of impending mortality compared to those with similar risk factors who did not have a TB event.
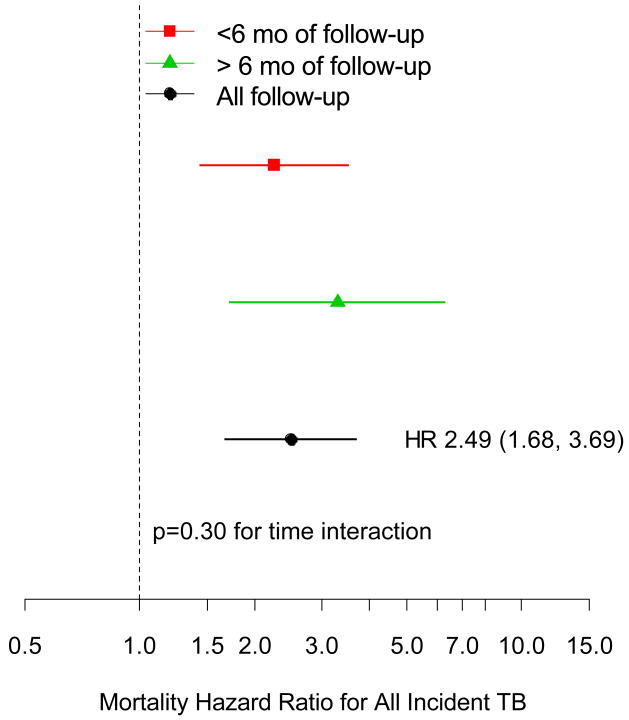
Table 2 shows the incidence of TB events and deaths by time intervals 0-3 months, 3-6 months and >6 months. Observed rates for death and TB were higher earlier during follow-up. When the preceding analysis was repeated including a time interaction for the effect of TB on mortality risk and whether a TB event occurred in the first 6 months or after 6 months of follow-up, there was no significant difference in risk associated with the timing of the TB event (Figure 2). Similar results were obtained when considering confirmed/probable TB events only (data not shown). Another way that the TB effect on risk could vary over time is that there could be a different mortality risk shortly after the TB diagnosis compared to a long-term risk. The Cox model that estimated these separate effects found a non-significantly higher risk of mortality within the first 6 months compared to later (p=0.16). Compared to someone with no prior TB event, someone within the first 6 months after their incident TB had a 2.91 fold greater risk of death (95% CI; 1.88, 4.48), and someone who had a TB diagnosis more than 6 months prior had a 1.74 fold increase in risk (95% CI: 0.90, 3.35). These results are suggestive of an increased mortality risk associated with a TB diagnosis that wanes over time, perhaps due to successful treatment.
Figure 2.
Mortality hazard ratio and 95% confidence interval associated with a prior TB diagnosis during follow-up (all events) compared to no TB event, adjusted for CD4 and log-HIV RNA level, and other baseline risk factors, broken down by timing of event.
Discussion
In many parts of the world, tuberculosis and HIV infection are inextricably linked in the general population. This study focused on a South African population that is representative of many patient groups where tuberculosis and HIV infection both occur with high incidence. The study was large, and included 3,777 person years of follow-up (mean 2.1 years per person) among individuals who volunteered for ART, and had CD4 counts <200 cells or a history of an AIDS defining illness at study entry. These patients were typical of many patients in high endemic areas: they were young, mostly male, entered the study with relatively low CD4 counts (median 106; IQR 44, 154 cells/mm3) and high viral loads (median 144,000; IQR 53,500, 307,000 copies/ml). None were documented to have received ART or isoniazid prophylaxis prior to study enrollment.
A history of TB at study baseline clearly identified a group with a poorer health status than the group without a history of TB in terms of BMI, CD4 count, HIV viral load, and hemoglobin. By multivariate logistic model, however, TB was not an independent risk factor for subsequent TB or death. Similar trends had been reported in a smaller case control study from Uganda [15]. Thus, a history of TB is a marker for poor health status and identifies a patient population that will derive particular benefit from ART.
In this study, 254 patients (14%) had one or more TB events during follow-up while assigned to ART. The annualized rate of TB was 10.7 per 100 person-years (95% CI; 9.2, 12.5) in the first year and 4.8 (4.0, 5.9) after 1 year for all reported TB, which follows a similar trend reported from Cape Town and from Asia between 1996-2005 [7, 10, 16]. Thus, as in prior studies, ART reduced but did not eliminate the incidence of subsequent TB episodes. Patients with virologic failure had a substantially higher (55%) risk for developing TB than patients with virologic response past 12 weeks, which reinforces the plausible link between optimal virologic control and optimal TB prevention.
Patients who developed TB during follow up had lower CD4 counts, higher viral loads, a higher likelihood of ART failure, and a higher occurrence of non-TB opportunistic infections than patients who did not develop TB. Adjusting for baseline factors, subjects who developed another AIDS defining event had a relative risk of developing TB of 2.85 (95% CI: 1.75, 4.64) compared to similar subjects without such an event in the multivariate model. Conversely, those with a prior TB event had a 2.71 (95% CI: 1.56, 4.70) times higher risk for an AIDS defining event than those without TB. This supports the concept that the development of TB correlates with a global immune defect that increases the likelihood of both TB and other AIDS defining events [5, 15, 16].
While baseline TB did not predict death as an independent risk factor, incident TB was associated with a hazard ratio for death of 2.49 (95% CI: 1.68, 3.69). Zhou et al. found a similar hazard ratio in Asia for patients on ART although patients entered that trial with a higher baseline CD4 count (majority over 300 cells compared to a mean in this study of 106 cells) [16]. This hazard ratio was similar to that of another South African cohort that was not receiving ART, (2.3) [17]. There were 54 deaths among the 254 individuals who developed TB during follow-up, but unfortunately the cause of death could not be definitively determined for most patients, nor were there objective data in most cases to indicate whether their TB had responded to therapy.
This study did have limitations that are shared by most studies in sub-Saharan Africa: while a large number of TB cases were confirmed microbiologically, many TB diagnoses were based on empiric criteria; there was less intense documentation of likely AIDS defining endpoints than in studies from North America or Europe; and there was a relatively short period of follow-up.
Thus, for patients in this geographic area who start ART with low CD4 counts, TB at baseline is a marker for poor health status, and this poor health status correlates with subsequent TB and death. Once ART is started, incident TB is independently associated with poor outcome. ART can substantially reduce the impact of incident TB, especially when patients have optimal virologic responses, but ART does not eliminate incident TB [18]. This study reinforces the concept that TB has a strong impact on the outcome of patients with HIV infection, and re-emphasizes the importance of active TB screening prior to ART as well as during ART, the importance of nutrition, and the relevance of measures that both enhance availability of ART and enhance multifaceted approaches to reduce the transmission of TB in health care facilities as well as the community.
Acknowledgments
Stephanus KOMATI had a primary role in study design, patient care, data analysis and manuscript preparation.
Pamela A. SHAW was the statistician for the study involved in study design, analysis and manuscript preparation.
Nomso STUBBS, Monkwe Jethro MATHIBEDI, Lizette MALAN had major roles in patient care and data collection
Phumelele SANGWENI and Julia A. METCALF supervised laboratory study implementation and analysis.
Henry MASUR participated in study design, data analysis, and manuscript development.
Shaheen HASSIM supervised and provided patient care, supervised collection of data, and participated in data analysis and manuscript preparation.
This study was funded by the National Institutes of Health. The authors acknowledge the help with thanks of the South African Medical Services, the Phidisa staff, and the study volunteers.
Source of support: This study was funded by the National Institutes of Health.
References
- 1.Kirk O, Gatell JM, Mocroft A, Pedersen C, Proenca R, Brettle RP, et al. Infections with Mycobacterium tuberculosis and Mycobacterium avium among HIV-infected patients after the introduction of highly active antiretroviral therapy. EuroSIDA Study Group JD. Am J Respir Crit Care Med. 2000;162:865–872. doi: 10.1164/ajrccm.162.3.9908018. [DOI] [PubMed] [Google Scholar]
- 2.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282:2220–2226. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 3.Jones JL, Hanson DL, Dworkin MS, DeCock KM. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4:1026–1031. [PubMed] [Google Scholar]
- 4.Girardi E, Antonucci G, Vanacore P, Libanore M, Errante I, Matteelli A, et al. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14:1985–1991. doi: 10.1097/00002030-200009080-00015. [DOI] [PubMed] [Google Scholar]
- 5.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 6.Ralph AP, Anstey NM, Kelly PM. Tuberculosis into the 2010s: is the glass half full? Clin Infect Dis. 2009;49:574–583. doi: 10.1086/600889. [DOI] [PubMed] [Google Scholar]
- 7.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–333. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global tuberculosis control. Epidemiology, strategy, financing. World Health Organization Conference; Geneva. 2009.World Health Organization; [Google Scholar]
- 9.Seyler C, Toure S, Messou E, Bonard D, Gabillard D, Anglaret X. Risk factors for active tuberculosis after antiretroviral treatment initiation in Abidjan. Am J Respir Crit Care Med. 2005;172:123–127. doi: 10.1164/rccm.200410-1342OC. Epub 2005 Apr 2001. [DOI] [PubMed] [Google Scholar]
- 10.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 11.Santoro-Lopes G, de Pinho AM, Harrison LH, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34:543–546. doi: 10.1086/338641. Epub 2002 Jan 2007. [DOI] [PubMed] [Google Scholar]
- 12.Girardi E, Antonucci G, Vanacore P, Palmieri F, Matteelli A, Iemoli E, et al. Tuberculosis in HIV-infected persons in the context of wide availability of highly active antiretroviral therapy. Eur Respir J. 2004;24:11–17. doi: 10.1183/09031936.04.00109303. [DOI] [PubMed] [Google Scholar]
- 13.The Phidisa II Study Group: South African National Defence Force-RSA, National Institute of Allergy and Infectious Diseases-USA, University of New South Wales-AUS, University of Minnesota-USA for Project Phidisa. A Randomized, Open Label Factorial Trial Comparing Efavirenz to Lopinavir/r and Zidovudine + Didanosine to Stavudine + Lamivudine in Treatment-Naïve HIV-Infected Persons with <200 CD4+ cells/mm3 in South Africa. 17th Conference on Retroviruses and Opportunistic Infections (CROI); Montreal, Canada. 2009. [Google Scholar]
- 14.National Department of Health South Africa. [2/19/10];National Antiretroviral Treatment Guidelines. 2004 http://www.doh.gov.za/docs/factsheets/guidelines/artguidelines04/index.html.
- 15.Whalen CC, Nsubuga P, Okwera A, Johnson JL, Hom DL, Michael NL, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Elliott J, Li PC, Lim PL, Kiertiburanakul S, Kumarasamy N, et al. Risk and prognostic significance of tuberculosis in patients from The TREAT Asia HIV Observational Database. BMC Infect Dis. 2009;9:46. doi: 10.1186/1471-2334-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badri M, Ehrlich R, Wood R, Pulerwitz T, Maartens G. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int J Tuberc Lung Dis. 2001;5:225–232. [PubMed] [Google Scholar]
- 18.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. MMWR Recomm Rep. Vol. 58. 2009. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC,the National Institutes of Health and the HIV Medicine Association of the Infectious Diseases Society of America; pp. 1–207. quiz CE201-204. [PubMed] [Google Scholar]