Abstract
Background
We have recently reported the presence of CD8+ and CD4/8 double negative (DN) Natural Killer T (NKT) lymphocytes in sooty mangabeys. To investigate differences in the two NKT cell subsets, we compared the phenotype and function of sooty mangabey CD8+ and DN NKT cells.
Methods
Flow sorted NKT lymphocytes from one SIV-negative sooty mangabey were subjected to limiting dilution cloning. Invariant NKT clones were characterized by flow cytometry and cytokine-ELISA.
Results
The majority of NKT clones displayed an effector memory phenotype and expressed CXCR3 and NKG2D. While CD8+ NKT subsets expressed significantly higher levels of granzyme B and perforin and produced more IFN-γ, the DN NKT subsets secreted significantly more IL-4, IL-13, and IL-10.
Conclusions
The Th1 and Th2 cytokine bias of CD8+ and DN NKT cells respectively indicates the presence of functionally heterogeneous populations of NKT cells in sooty mangabeys.
Keywords: NKT cell subsets, α-GalCer, 6B11 mAb, sooty mangabey
INTRODUCTION
Natural Killer T (NKT) cells are innate T lymphocytes that express NK cell markers and mediate potent immunoregulatory functions in a variety of disease settings including autoimmunity, cancer, infection, and tolerance [1, 2]. Classical NKT cells are characterized by an invariant TCR α-chain and restricted TCR Vβ usage. Invariant NKT cells consist of Vα24-Jα18 chain preferentially paired with Vβ11 in humans, and Vα14-Jα18 chain paired with Vβ8.2, Vβ7 or Vβ2 in mice [3-5]. NKT cells can rapidly respond to glycolipid antigens presented by the MHC Class-I-like CD1d molecule resulting in potent production of IL-10, TGF-β, and several Th1 and Th2 cytokines[6, 7]. Although they represent a very small fraction of T lymphocytes, NKT cells can modulate immune responses by rapid production of a wide array of cytokines, thereby influencing other innate and adaptive arms of the immune system such as DCs, NK cells, macrophages, CD4+ and CD8+ T lymphocytes, and B lymphocytes [1, 2, 8-10].
The importance of NKT cells in regulating a wide range of immune responses has been demonstrated over the past few years in different disease models. In certain disease settings, NKT cells mediate antitumor and anti-microbial immune responses through secretion of Th1 cytokines like IFN-γ [11-13]. On the other hand, NKT cells can also induce tolerance through secretion of Th2 cytokines like IL-4 and IL-10, and play an immunoregulatory role in suppressing autoimmune diabetes, preventing corneal graft rejection, and control of immunopathogenesis in microbial infections in mice [14-17]. Contrary to their role in antitumor responses, NKT cells have also been reported to inhibit tumor immunosurveillance through secretion of IL-13 [18].
The mechanisms by which NKT cells regulate the expression of Th1 or Th2 cytokines and either enhance or suppress immune responses are not completely understood. There is some evidence to suggest that the diverse immune activities of NKT cells could be attributed to functional differences of NKT cell subsets based on their surface expression of CD4 or CD8 molecules. Studies have indicated the association of human CD4− NKT cell subsets with cytolytic function and Th1 cytokine production, and CD4+ NKT cell subsets with immunoregulatory function and both Th1 and Th2 cytokine production [19-22]. While CD4+ and CD8+ NKT cells have been reported in Asian macaque species [23-25], we recently reported that sooty mangabey NKT cells consist of CD8+ and DN NKT lymphocytes but not CD4+ T lymphocytes (Rout et al., PLoS ONE, in press). In this study, we investigated whether the DN and CD8+ subsets of NKT cells in sooty mangabeys differ with regards to phenotype and function. Investigation of NKT clones showed that mangabey CD8+ NKT cells were more cytotoxic and had a dominant Th1 cytokine-secreting profile, while DN NKT cells showed skewing towards a Th2 cytokine-secreting profile. Our results demonstrate the functional heterogeneity of NKT cells in sooty mangabeys.
MATERIALS AND METHODS
Animals and sample collection
Blood samples from one SIV uninfected sooty mangabey housed at the Yerkes National Primate Research Center, Atlanta, were collected in heparin vacutainer tubes (Becton Dickinson Vacutainer systems, Franklin Lakes, NJ), and shipped overnight on ice to the New England Primate Research Center for processing. Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation (Lymphocyte Separation Medium; MP Biomedicals Inc., Solon, OH) at 1500 rpm for 45 minutes and used for phenotyping and cloning.
Immunophenotyping and flow cytometry of NKT cells
Multicolor flow cytometric analysis was performed on ex vivo and in vitro expanded cells according to standard procedures using anti-human mAbs that cross-react with sooty mangabeys (Rout et al., PLoS ONE, in press). NKT cell ligand PBS-57-loaded and unloaded human CD1d Tetramers (CD1d TM) conjugated with APC were obtained from the NIH Tetramer core facility. The following antibodies were obtained from BD Biosciences unless stated otherwise: anti-Vα24-PE (clone C15; Immunotech), 6B11–FITC (6B11), anti-CD3–APC-Cy7 (SP34-2), anti-CD4-Qdot605 (T4/19Thy5D7; custom/NHP Resource), anti-CD8–Alexa Fluor 700 (RPA-T8), anti-CD56–PE-Cy7 (NCAM16.2), anti-CD16–Alexa Fluor 700 (3G8; Invitrogen), anti-CD161–APC (DX12), anti-NKG2D–PE (ON72; Beckman Coulter), anti-CD95–PE-Cy5 (DX2), anti-CD28–PE TexasRed (CD28.2; Immunotech), anti-CCR7–biotin (150503; custom), anti-CXCR3–PE (1C6), anti-CD69–PE TexasRed (TP1.55.3; Beckman Coulter), anti-Perforin–FITC (B56), anti-GranzymeB–APC (GB12; Caltag), anti-IFN-γ–PE-Cy7 (B27), anti-IL-2–APC (MQ1-17H12), anti-TNF-α–Alexa Fluor 700 (MAb11).
For identification of NKT cells, PBMCs were surface stained for CD3 and anti-Vα24 combined with PBS-57 loaded CD1d TM or 6B11 antibody. APC-labeled unloaded CD1d TM controls were used in all experiments. Surface staining was carried out by standard procedures. Briefly, 2 to 4 million PBMC or >1000 cells of NKT clones re-suspended in 100 μl wash buffer (PBS with 2% FBS) were initially incubated with tetramers for 20 min at 4°C followed by addition of surface antibodies and further incubation for 30 min at 4°C. After washing, the cells were fixed in 2% paraformaldehyde. All intracellular cytokine staining (ICS) assays were carried out on cells that were stimulated overnight. Following 16 h incubation, cells were washed in PBS containing 2% FCS and 0.5 mM EDTA, stained for surface markers in wash buffer for 30 min at 4°C, washed, and then fixed and permeabilized using the Invitrogen Fix/Perm reagents (CALTAG™). Permeabilized cells were stained intracellularly with the requisite antibodies. Cells were then washed in wash buffer and fixed in 2% paraformaldehyde. Flow cytometric acquisition was performed on an LSR-II cytometer driven by the FACS DiVa software (version 5.2; BD). Analysis of the acquired data was performed using FlowJo software (version 8.8.3; TreeStar, Ashland, OR).
Medium and Reagents
The complete medium (R10 medium) used throughout was RPMI medium 1640 (Cellgro, Herndon, VA) supplemented with 10% FCS (Sigma-Aldrich, St. Louis, MO), 1% 1 M HEPES, 2 mM L-glutamine (Cellgro), 50 IU/ml penicillin (Cellgro), 50 μg/ml streptomycin (Cellgro). The NKT-ligand α-galactosylceramide (α-GalCer, Diagnocine LLC, Hackensack, NJ) was used at 100 ng/ml. Recombinant human IL-2 (Roche) was used at 10–50 IU/ml of medium for the expansion and maintenance of NKT cell clones.
In vitro expansion of NKT cells
6B11-positive lymphocytes were sorted on a FACSAria cell sorter (BD Biosciences, San Jose, CA, USA) and cloned by limiting dilution at 3 and 10 cells per well in 96-well, round-bottom, polystyrene plates (Corning, NY, USA). Cells were incubated in R10 medium containing 5 μg/ml ConA and 100 ng/ml of α-GalCer along with 100,000 cells/well of human feeder PBMC irradiated at 3000 rads. After two days, ConA was removed and 50 IU/ml recombinant human IL-2 was added. Wells with cell outgrowth were re-stimulated and expanded over a 2-4 week period. The presence of NKT clones was confirmed by staining with anti-Vα24 mAb and either PBS-57 loaded CD1dTM or 6B11 mAb. Positive NKT clones were maintained in complete media supplemented with 50 IU/ml IL-2. For functional analyses, the clones were gradually switched to resting stage (10 IU/ml IL-2) 48 h prior to the assay as previously described [26].
Functional Analysis of NKT cells
For NKT cell activation assays, 2 × 104 NKT cells were taken in a 96-well flat-bottom plate with either medium alone or with an equal number of stimulator cells that had been treated with α-GalCer at a final concentration of 100 ng/ml. 25 ng/ml PMA (Sigma-Aldrich, St. Louis, MO) with 1 μg/ml calcium ionophore (PMA/Ca) was used as a positive control stimulus. 20,000 CD1d-transfected C1R B cell lines (C1R.d) were γ-irradiated at 10,000 rads and used as APCs for the presentation of α-GalCer as previously described [27]. Irradiated mock-transfected C1R cells served as a negative control stimulus for NKT cells.
Statistical Analysis
Comparisons between the NKT cell subsets within clones were performed by paired t-test and statistical significance was set at p <0.05.
RESULTS
Identification of invariant CD8+ and CD4−CD8− double negative (DN) NKT lymphocytes in sooty mangabeys
Invariant NKT lymphocytes were identified in sooty mangabeys by flow cytometric detection of Vα24 TCR-positive T lymphocytes that co-stained either with the 6B11 mAb directed against the invariant CDR3 region of the TCR α-chain [28], or with human CD1d tetramers (CD1d TM) loaded with ligand α-galactosylceramide (α-GalCer) analog PBS-57 [29, 30]. The CD8+ and DN NKT subsets were identified as recently described (Rout et al., PLoS ONE, in press).
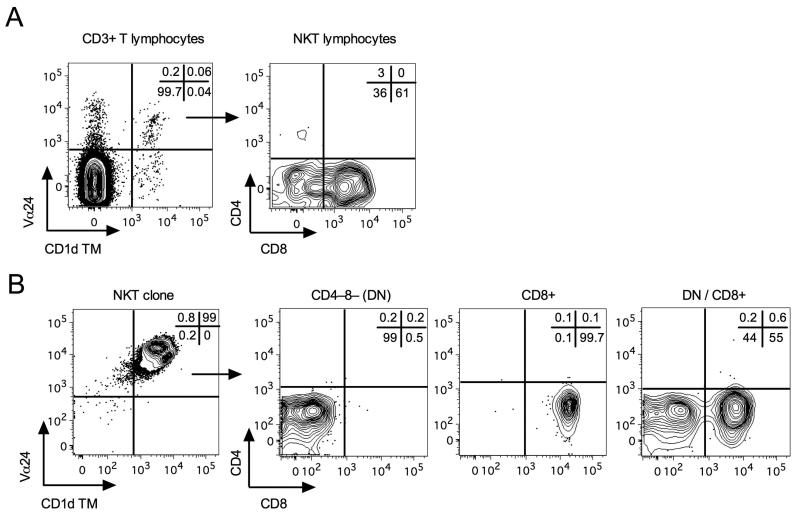
In one SIV-negative sooty mangabey, comprising a mixture of CD8+ and DN NKT lymphocytes in peripheral blood (Fig. 1A), 6B11-positive T lymphocytes were sorted by flow cytometry and subjected to limiting dilution cloning in the presence of irradiated human PBMC and α-GalCer pulsed C1R.d cells, and medium containing recombinant IL-2. Wells with cell outgrowth were examined for reactivity with Vα24 antibody and PBS-57 loaded CD1d TM. NKT clones were identified as Vα24+CD1d TM+ or Vα24+6B11+ cells (Fig. 1B). Of the 125 wells examined, 98 wells (78.4%) contained NKT cells at a frequency of ≥60% (median 93%; range of 60–100%). Analysis of CD4 and CD8 expression on NKT clones demonstrated relatively pure (>90%) CD8+ or DN T lymphocyte phenotypes in addition to a mixed CD8+ and DN T lymphocyte phenotype (Fig. 1B). Of the 98 NKT clones, 70.4% displayed a mixed CD8+ and DN phenotype, 22.4% were predominantly CD8+, and 7.1% displayed a dominant DN T lymphocyte phenotype (Fig. 2A).
Fig. 1. Identification of sooty mangabey NKT lymphocyte subsets.
A) Contour plots showing ex vivo peripheral blood NKT lymphocytes and their subsets in one SIV-negative sooty mangabey. On the CD3+ T lymphocyte population, the co-staining for Vα24 and CD1d tetramers loaded with PBS-57 (CD1d TM) and subsequent gating on the Vα24+CD1d TM+ cells for analysis of CD4 and CD8 surface expression is shown. B) Contour plot of one NKT clone stained with anti-Vα24 and CD1d TM and three representative contour plots showing clonal NKT subsets: CD4−8− (DN), CD8+4− (CD8+), and mixed (DN/CD8+) phenotypes. Frequencies of each quadrant are shown in the top right corner of each plot.
Fig. 2. T lymphocyte subset frequency and kinetics of sooty mangabey NKT clones.
A) Percentages of DN, CD8+ and CD8+/DN mixed NKT clones in a total of 98 clones examined. B) Percentages of CD8+ and DN cells in 8 NKT clones with CD8+/DN mixed phenotype at 1, 2, and 4 month of in vitro culture.
In vitro kinetics of NKT lymphocyte subsets
We next analyzed the effect of long-term in vitro culture conditions on the phenotype of NKT clones. NKT clones proliferated in response to stimulation with CD1d/α-GalCer and recombinant IL-2. Analysis of CD8 expression on the clones with a mixed CD8+ and DN T lymphocyte phenotype showed a gradual increase in the proportion of CD8+ NKT cells and a decrease in DN NKT subsets over a period of 4 months (Fig. 2B). Of the 11 dominantly DN NKT clones initially identified, only three clones survived over a period of 4 months, and none of them retained their dominant DN phenotype in culture. In contrast, 19 of the 32 dominant CD8+ NKT clones survived at the end of 4 months and 18 of them maintained their dominant CD8+ phenotype. Overall, the survival of pure DN NKT clones (27%) was significantly lower than that of pure CD8+ NKT clones (59%). These data suggest either a better survival or enhanced proliferative capacity of CD8+ NKT clones as compared to DN NKT clones in response to CD1d/α-GalCer and IL-2 stimulation.
Memory phenotype of CD8+ and DN NKT lymphocyte subsets
NKT lymphocyte subsets with CD4+ or CD8+ phenotypes have been shown to have distinct phenotypic and functional characteristics [19-21]. To investigate whether CD8+ and DN NKT subsets in sooty mangabeys were different, NKT clones were examined for the expression of the memory T lymphocyte markers, CD95 and CD28, as well as the chemokine receptors CCR7 and CXCR3. Both CD8+ and DN NKT subsets displayed a predominant CD95+CD28− effector memory phenotype (Fig. 3A,B). The CD8+ NKT subsets had a significantly higher CD95 mean channel fluorescence (MFI) compared to the DN subsets (Fig. 3C). Consistent with the finding in human NKT lymphocytes, both CD8+ and DN NKT lymphocytes in sooty mangabeys displayed high expression levels of CXCR3, indicating the ability to home to extra-lymphoid inflammatory sites. The majority of NKT clones did not express CCR7 (Fig. 3A,C). When present, CCR7 was almost exclusively expressed on the DN NKT cells (Fig. 3A,C). Furthermore, comparable frequencies of CD28 and CCR7 were observed in the DN subsets, suggesting the presence of a subset of CD28+CCR7+ central memory DN NKT cells with ability to traffic to lymphoid organs.
Fig. 3. Memory phenotype of CD8+ and DN NKT cell subsets.
A) Overlay histogram plots gated on representative NKT subsets showing surface expression of memory markers CD95 and CD28, and chemokine receptors CCR7 and CXCR3. B) Dot plots showing expression of CD95 and CD28 on CD8+ and DN NKT subsets. C) Bar graphs showing mean percentages of CD95, CD28, CCR7, and CXCR3 expression in the top panel and corresponding MFI of the DN (filled bars) and CD8+ (empty bars) NKT subsets in the bottom panel. Error bars denote SEM.
Expression of NK cell markers and cytolytic molecules in CD8+ and DN NKT lymphocyte subsets
Since NKT cells are associated with expression of various NK cell receptors, some of which have been reported to be associated with NKT effector functions [31, 32], we investigated the expression of CD161, CD16, CD56, and NKG2D on the NKT subsets. Analysis of the expression of NK cell markers on sooty mangabey NKT clones revealed that NKG2D was expressed at significantly higher levels on the CD8+ subsets in comparison to the DN subsets (p<0.0001), whereas CD161 expression was similar in both subsets (Fig. 4A,C). Two major populations of human and macaque NK cells including CD16bright CD56dim cells and CD16–/dim CD56bright cells, with distinct cytotoxic and cytokine activity are described [33, 34]. We examined the simultaneous expression of CD56 and CD16 on the NKT cells. Three major phenotypic subsets of NKT cells were observed including CD56+CD16−, CD56+CD16+ and 56−CD16− (Fig. 4B). It is worth noting that all the CD16+ NKT cells co-stained with CD56 and there were almost no CD16+CD56− cells in the NKT clones. While DN NKT cells contained higher frequencies of CD16+CD56+ (p=0.052), and CD56−CD16− (p<0.001) cells, CD8+ NKT cells contained higher frequencies of CD56+CD16− (p<0.001) cells (Fig. 4C). Human DN NKT clones do not express CD16 or CD56 [26].
Fig. 4. NK cell receptors expressed on CD8+ and DN NKT cell subsets.
A) Overlay histogram plots gated on representative NKT subsets showing surface expression of the NK cell markers CD16, CD56, CD161, and NKG2D. B) Dot plots showing expression of CD56 and CD16 on the CD8+ and DN NKT subsets. Frequencies of each quadrant are shown in the top right corner of each plot. C) Bar graphs showing mean percentages of CD56+CD16−, CD56+CD16+, CD56−CD16−, CD161+, and NKG2D+ cells in the DN (filled bars) and CD8+ (empty bars) NKT subsets. Error bars denote SEM.
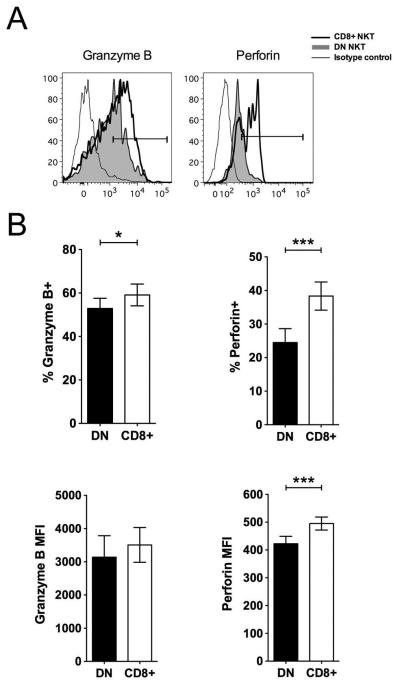
The cytolytic potential of the NKT subsets was compared by intracellular staining for the cytolytic molecules, granzyme B and perforin. On average, there was a high level of granzyme B (49.3% ± 38.7) and perforin (43.5% ± 20) in the NKT clones. However, the CD8+ NKT cells contained a significantly higher frequency of granzyme B and perforin compared to DN NKT cells (Fig. 5A,B).
Fig. 5. Cytolytic molecules expressed in CD8+ and DN NKT cell subsets.
A) Overlay histogram plots showing intracellular cytokine staining of representative NKT subsets with antibodies against granzyme B and perforin. B) Bar graphs showing mean percentages of granzyme B and perforin expression in the top panel and corresponding MFI of the DN (filled bars) and CD8+ (empty bars) NKT subsets in the bottom panel. Error bars denote SEM.
Cytokine production by sooty mangabey CD8+ and DN NKT lymphocyte subsets
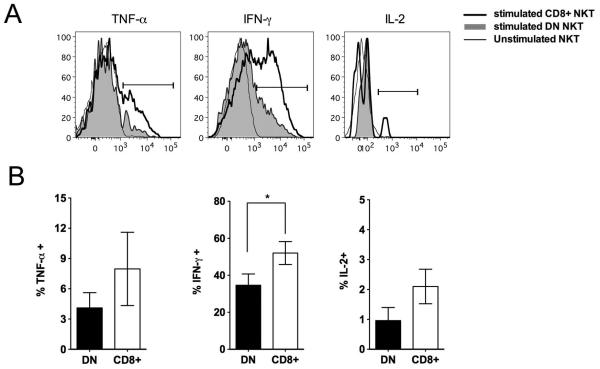
To further compare the functional characteristics of sooty mangabey NKT lymphocyte subsets, the cytokine profile of NKT clones following stimulation with CD1d-transfected cell lines pulsed with α-GalCer (C1R.d/αGC) were analyzed as previously described [26]. In vitro stimulation of NKT clones with C1R.d/αGC resulted in production of the Th1 cytokines TNF-α, IFN-γ, and IL-2 by both subsets as observed by intra-cellular cytokine flow cytometry (Fig. 6A). The frequency of Th1 cytokine-producing cells in response to NKT ligand stimulation tended to be higher in the CD8+ NKT cells compared to the DN NKT cells, but the differences were statistically significant only for IFN-γ production (Fig. 6B).
Fig. 6. Intracellular cytokine production by sooty mangabey CD8+ and DN NKT cells.
A) Intracellular cytokine staining of representative NKT subsets with antibodies against TNF-α, IFN-γ, and IL-2 following 16 hours in vitro stimulation with ligand α-GalCer presented on APC. Unstimulated cells served as a negative control. B) Bar graphs showing mean percentages of cytokine-positive NKT cells in the DN (filled bars) and CD8+ (empty bars) NKT subsets. Data on 9 NKT clones shown. Error bars denote SEM.
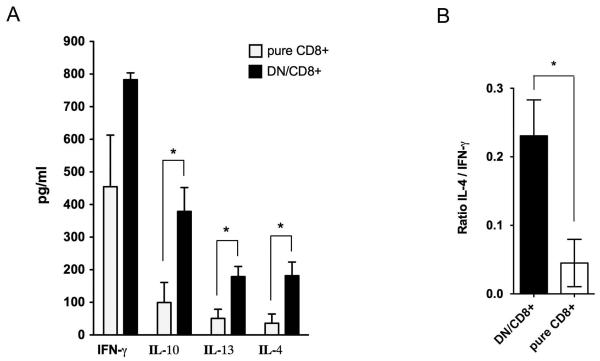
To further examine the cytokine secretion pattern of sooty mangabey NKT lymphocyte subsets, the culture supernatants of pure CD8+ and DN/CD8+ mixed NKT clones responding to C1R.d/αGC stimulation were analyzed for Th1 and Th2 cytokines by cytokine-ELISA. Pure DN NKT clones could not be assayed due to their poor survival and unavailability of required number of cells. After a 24-48 hour stimulation period, supernatants of stimulated clones showed the presence of IFN-γ, IL-10, IL-13, and IL-4 (Fig. 7A). Significantly higher levels of the Th2 cytokines IL-10, IL-13, and IL-4 were secreted by DN/CD8+ mixed NKT clones in comparison to pure CD8+ NKT clones, suggesting that the DN NKT cells were responsible for the increased Th2 cytokine production. Consistent with this finding, the DN/CD8+ mixed NKT clones showed a significantly higher ratio of IL-4 to IFN-γ compared to the pure CD8+ NKT clones (Fig. 7B), suggesting that DN NKT clones are skewed towards Th2 cytokine production.
Fig. 7. Th1 and Th2 cytokine secretion by sooty mangabey CD8+ and DN NKT cells.
A) Cytokine ELISA for IFN-γ, IL-10, IL-13, and IL-4 with culture supernatants collected 24 hours post-stimulation of NKT clones (mean and SEM of 5-6 clones). Filled bars denote DN/CD8+ mixed clones and empty bars denote pure CD8+ NKT clones B) Mean Ratios of IL-4 to IFN-γ in the DN/CD8+ mixed NKT and pure CD8+ clones. Error bars denote SEM.
DISCUSSION
This study demonstrates that the two major NKT cell subsets in sooty mangabeys consisting of CD8+ and DN T cells display phenotypic and functional heterogeneity. Since NKT cells are an extremely rare population of peripheral blood mononuclear cells in sooty mangabeys, we used in vitro expanded NKT clones in this study for investigating the differential phenotypic and functional properties of the two NKT subsets. Although these results are representative of in vitro expanded clonal NKT subsets, it is noteworthy that significant differences between the two subsets were observed even after prolonged in vitro culture. Our data reveal that CD8+ and DN NKT cell subsets in sooty mangabeys differ from each other with respect to expression of NK cell receptors, cytolytic molecules, and production of Th1/Th2 cytokines.
We observed that CD8+ NKT subsets had better survival following specific stimulation with α-GalCer presented by APC in the presence of IL-2. Previous studies have shown that cytokines such as IL-2, IL-7, or IL-15 are required for the expansion of human NKT cells [35, 36]. A more detailed study of the effects of IL-2, IL-7, IL-12, and IL-15, on the in vitro expansion of different NKT subsets isolated from human PBMC demonstrated that IL-7 and IL-15 preferentially expanded DN NKT cells whereas IL-2 induced preferential expansion of CD4+ NKT cells [37]. While there are reports of CD4+ NKT cells expressing high-affinity IL-2Rα, and preferential expansion of these subsets in the presence of IL-2 [19, 21], there are no such data on the CD8+ subsets, possibly due to the paucity of CD8+ NKT subsets in humans. Our results suggest that sooty mangabey CD8+ NKT subsets also require IL-2 for expansion. However, it remains to be determined whether CD8+ NKT cells express higher levels of IL-2 receptors compared to DN NKT cells accounting for the improved survival of CD8+ NKT clones in this study. It also remains to be determined whether similar to their human counterparts, mangabey DN NKT cells require IL-7 and/or IL-15 for expansion.
The memory phenotype of the NKT cell subsets was consistent with that reported in humans and rhesus macaques [38, 39] with high expression of CD95 and lower expression of CD28 suggesting an effector memory phenotype. The sooty mangabey CD8+ NKT subset phenotype was more Th1 type than DN NKT. Also the higher intensities of CD95 and CXCR3 on the CD8+ NKT subset suggest that sooty mangabey CD8+ NKT cells show preferential homing to inflammatory sites. Of note, a fraction of the DN NKT subsets appeared to retain a central memory phenotype even under in vitro culture conditions indicating potential homing to lymphoid organs. It would be interesting to examine if ex vivo un-stimulated DN and CD8+ NKT cells display a similar distinct pattern of memory and homing markers.
One of the characteristic features of NKT cells is the expression of NK lineage receptors, which can regulate cellular activation by their involvement in TCR signaling. Using an array of NK receptor antibodies, we found that CD8+ and DN NKT subsets have similarities and differences in the surface expression of NK receptors. The DN and CD8+ NKT subsets were similar with regards to the expression levels of CD161, a molecule that has a role in co-stimulating NKT TCR activation [40], but differed in the expression levels of CD16, CD56, and NKG2D. The CD56 and CD16 distribution on sooty mangabey NKT cells was distinct from the characteristic distribution observed on circulating macaque NK cells [34]. Thus, unlike NK cells, mangabey NKT cells did not contain a CD16+CD56− subset. Instead, the CD16+ NKT cells were almost exclusively CD16+CD56+. Human CD56+CD16+ NK cells have potent cytolytic activity and moderate cytokine production, while CD56+CD16− NK cells are cytokine producers with little cytolytic activity [33]. Likewise, in macaques only CD16+ NK cells are cytolytic [34]. Even though mangabey DN NKT cells showed a higher frequency of CD56+CD16+ cells compared to CD8+ NKT cells, the frequency and intensity of granzyme B and perforin was higher on the CD8+ NKT cells, suggesting that the expression and function of NK receptors on NKT cells is distinct from that on NK cells. Consistent with higher intracellular levels of granzyme B and perforin, the higher expression of NKG2D on the CD8+ NKT subsets also suggests elevated cytotoxic potential because NKG2D is an activating receptor associated with cytolysis of infected/neoplastic cells that express stress-induced antigens [41, 42].
Functionally, both NKT cell subsets were capable of Th1 and Th2 cytokine production upon stimulation with CD1d/α-GalCer. However, there was significantly greater IFN-γ production by CD8+ NKT cells. While the DN NKT cells retained the ability to produce high IFN-γ, higher levels of the Th2 cytokines IL-4, IL-13, and IL-10, were produced by the DN/CD8+ mixed clones as compared to the pure CD8+ NKT clones. These data suggest a Th2 cytokine bias in the DN NKT cells as compared to the CD8+ NKT cells. Even though studies in humans have shown that Th2 cytokine production is restricted to the CD4+ NKT subset [19-22], our results demonstrate that the absence of CD4+ NKT subsets in the sooty mangabeys is not a barrier to Th2 cytokine secretion. Thus, the cytokine profile of CD8+ NKT subsets in sooty mangabeys appears to be similar to that of human DN NKT subsets in being predominantly Th1 type, while the cytokine profile of mangabey DN NKT cells resembles that of human CD4+ NKT cells.
In summary, the analysis of NKT clones in sooty mangabeys showed higher cytotoxicity and a bias towards Th1-type cytokine secretion in CD8+ NKT cells, while DN NKT cells showed more Th2 cytokine production. DN NKT cells were also unique in consisting of a subpopulation of central memory cells suggesting that they can traffic to lymphoid organs. Overall, this study suggests that sooty mangabey NKT cells consist of a heterogeneous population of cells with distinct functional and homing properties. These studies need to be extended to analysis of ex vivo NKT cells in SIV-negative and SIV-infected sooty mangabeys.
Acknowledgments
We thank Tracy Meeker of the Yerkes National Primate Research Center for coordinating the sooty mangabey blood sample collections, and the flow cytometry core facility staff at the New England Primate Research Center, for cell sorting and flow cytometric analysis. We thank the NIH Tetramer Core Facility for providing PBS-57-loaded and empty human CD1d tetramers.
Funding: Public Health Service grants RR00168, RR00165, DK066917, AI049809, and AI084810
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koseki H, Asano H, Inaba T, Miyashita N, Moriwaki K, Lindahl KF, Mizutani Y, Imai K, Taniguchi M. Dominant expression of a distinctive V14+ T-cell antigen receptor alpha chain in mice. Proc Natl Acad Sci U S A. 1991;88:7518–7522. doi: 10.1073/pnas.88.17.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, Locksley RM, Kronenberg M. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003;100:8395–8400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 9.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 11.Ashkar AA, Rosenthal KL. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J Virol. 2003;77:10168–10171. doi: 10.1128/JVI.77.18.10168-10171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8(+) T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002;76:4294–4303. doi: 10.1128/JVI.76.9.4294-4303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YG, Choisy-Rossi CM, Holl TM, Chapman HD, Besra GS, Porcelli SA, Shaffer DJ, Roopenian D, Wilson SB, Serreze DV. Activated NKT cells inhibit autoimmune diabetes through tolerogenic recruitment of dendritic cells to pancreatic lymph nodes. J Immunol. 2005;174:1196–1204. doi: 10.4049/jimmunol.174.3.1196. [DOI] [PubMed] [Google Scholar]
- 15.Dieli F, Taniguchi M, Kronenberg M, Sidobre S, Ivanyi J, Fattorini L, Iona E, Orefici G, De Leo G, Russo D, Caccamo N, Sireci G, Di Sano C, Salerno A. An anti-inflammatory role for V alpha 14 NK T cells in Mycobacterium bovis bacillus Calmette-Guerin-infected mice. J Immunol. 2003;171:1961–1968. doi: 10.4049/jimmunol.171.4.1961. [DOI] [PubMed] [Google Scholar]
- 16.Sonoda KH, Faunce DE, Taniguchi M, Exley M, Balk S, Stein-Streilein J. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J Immunol. 2001;166:42–50. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- 17.Watte CM, Nakamura T, Lau CH, Ortaldo JR, Stein-Streilein J. Ly49 C/I-dependent NKT cell-derived IL-10 is required for corneal graft survival and peripheral tolerance. J Leukoc Biol. 2008;83:928–935. doi: 10.1189/jlb.0807579. [DOI] [PubMed] [Google Scholar]
- 18.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CH, Butcher EC, Johnston B. Distinct subsets of human Valpha24-invariant NKT cells: cytokine responses and chemokine receptor expression. Trends Immunol. 2002;23:516–519. doi: 10.1016/s1471-4906(02)02323-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi T, Nieda M, Koezuka Y, Nicol A, Porcelli SA, Ishikawa Y, Tadokoro K, Hirai H, Juji T. Analysis of human V alpha 24+ CD4+ NKT cells activated by alpha-glycosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2000;164:4458–4464. doi: 10.4049/jimmunol.164.9.4458. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez CS, Chan AC, Kyparissoudis K, De Rose R, Godfrey DI, Kent SJ. Peripheral NKT cells in simian immunodeficiency virus-infected macaques. J Virol. 2009;83:1617–1624. doi: 10.1128/JVI.02138-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashiwase K, Kikuchi A, Ando Y, Nicol A, Porcelli SA, Tokunaga K, Omine M, Satake M, Juji T, Nieda M, Koezuka Y. The CD1d natural killer T-cell antigen presentation pathway is highly conserved between humans and rhesus macaques. Immunogenetics. 2003;54:776–781. doi: 10.1007/s00251-002-0527-8. [DOI] [PubMed] [Google Scholar]
- 25.Motsinger A, Azimzadeh A, Stanic AK, Johnson RP, Van Kaer L, Joyce S, Unutmaz D. Identification and simian immunodeficiency virus infection of CD1d-restricted macaque natural killer T cells. J Virol. 2003;77:8153–8158. doi: 10.1128/JVI.77.14.8153-8158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Exley MA, Balk SP, Wilson SB. Isolation and functional use of human NK T cells. Curr Protoc Immunol. 2003:11. doi: 10.1002/0471142735.im1411s52. Chapter 14:Unit 14. [DOI] [PubMed] [Google Scholar]
- 27.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Exley MA, Hou R, Shaulov A, Tonti E, Dellabona P, Casorati G, Akbari O, Akman HO, Greenfield EA, Gumperz JE, Boyson JE, Balk SP, Wilson SB. Selective activation, expansion, and monitoring of human iNKT cells with a monoclonal antibody specific for the TCR alpha-chain CDR3 loop. Eur J Immunol. 2008;38:1756–1766. doi: 10.1002/eji.200737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, 3rd, Ravkov EV, Ibegbu CC, Altman JD, Teyton L, Bendelac A, Savage PB. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Balato A, Unutmaz D, Gaspari AA. Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J Invest Dermatol. 2009;129:1628–1642. doi: 10.1038/jid.2009.30. [DOI] [PubMed] [Google Scholar]
- 32.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 33.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 34.Webster RL, Johnson RP. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology. 2005;115:206–214. doi: 10.1111/j.1365-2567.2005.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishi N, van der Vliet HJ, Koezuka Y, von Blomberg BM, Scheper RJ, Pinedo HM, Giaccone G. Synergistic effect of KRN7000 with interleukin-15, -7, and -2 on the expansion of human V alpha 24+V beta 11+ T cells in vitro. Hum Immunol. 2000;61:357–365. doi: 10.1016/s0198-8859(99)00181-0. [DOI] [PubMed] [Google Scholar]
- 36.van der Vliet HJ, Nishi N, Koezuka Y, von Blomberg BM, van den Eertwegh AJ, Porcelli SA, Pinedo HM, Scheper RJ, Giaccone G. Potent expansion of human natural killer T cells using alpha-galactosylceramide (KRN7000)-loaded monocyte-derived dendritic cells, cultured in the presence of IL-7 and IL-15. J Immunol Methods. 2001;247:61–72. doi: 10.1016/s0022-1759(00)00272-6. [DOI] [PubMed] [Google Scholar]
- 37.Lin H, Nieda M, Nicol AJ. Differential proliferative response of NKT cell subpopulations to in vitro stimulation in presence of different cytokines. Eur J Immunol. 2004;34:2664–2671. doi: 10.1002/eji.200324834. [DOI] [PubMed] [Google Scholar]
- 38.D’Andrea A, Goux D, De Lalla C, Koezuka Y, Montagna D, Moretta A, Dellabona P, Casorati G, Abrignani S. Neonatal invariant Valpha24+ NKT lymphocytes are activated memory cells. Eur J Immunol. 2000;30:1544–1550. doi: 10.1002/1521-4141(200006)30:6<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 39.Gansuvd B, Hubbard WJ, Hutchings A, Thomas FT, Goodwin J, Wilson SB, Exley MA, Thomas JM. Phenotypic and functional characterization of long-term cultured rhesus macaque spleen-derived NKT cells. J Immunol. 2003;171:2904–2911. doi: 10.4049/jimmunol.171.6.2904. [DOI] [PubMed] [Google Scholar]
- 40.Exley M, Porcelli S, Furman M, Garcia J, Balk S. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V alpha 24 J alpha Q T cell receptor alpha chains. J Exp Med. 1998;188:867–876. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 42.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci U S A. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]