Abstract
Objective
The canonical Wnt signaling pathway, heavily studied in development and cancer, has recently been implicated in microvascular growth with the use of developmental and in vitro models. To date, however, no study exists showing the effects of perturbing the canonical Wnt pathway in a complete microvascular network undergoing physiological remodeling in vivo. Our objective was to investigate the effects of canonical Wnt inhibition on the microvascular remodeling of adult rats.
Methods
Canonical Wnt inhibitor DKK-1, Wnt inhibitor sFRP-1, BSA or saline was superfused onto the exteriorized mesenteric windows of 300g adult female Sprague-Dawley rats for 20 minutes. Three days following surgery, mesenteric windows were imaged intravitally and harvested for immunofluorescence staining with smooth muscle alpha-actin and BRDU.
Results
We observed prominent differences in the response of the mesenteric microvasculature amongst the various treatment groups. Significant increases in hemorrhage area, vascular density, and draining vessel diameter were observed in windows treated with Wnt inhibitors as compared to control-treated windows. Additionally, confocal imaging analysis showed significant increases in proliferating cells as well as evidence of proliferating smooth muscle cells along venules.
Conclusions
Together, our results suggest that canonical Wnt inhibition plays an important role in microvascular remodeling, specifically venular remodeling.
Keywords: Wnt, Dickkopf-1 (DKK-1), secreted frizzled-related protein 1 (sFRP-1), venular remodeling, hemorrhage
Introduction
Microvascular remodeling and vasculogenesis are regulated by myriad signaling pathways and growth factors, such as VEGF, Ang-1, Ang-2, and neuropilin [45]. A more recently investigated pathway garnering increased attention for its role in these processes is the Wnt pathway. Canonical Wnt signaling, activated by binding to Frizzled receptor/LRP co-receptor and inhibited by Dickkopf-1 (DKK-1), results in the accumulation of constitutively ubiquitinated beta-catenin. Subsequent translocation of beta-catenin to the nucleus results in binding to co-factor Tcf/LEF-1, which modulates transcription of many genes such as cyclin D1 and c-Myc [21,53,60]. Several non-canonical Wnt pathways exist that do not involve beta-catenin, although the canonical pathway is the focus of this manuscript. Canonical Wnt signaling has been implicated in many aspects of biological function, including cell proliferation, morphogenesis, cell polarity, stem cell fate, and cancer, as has been reviewed thoroughly [16,18,30,31,36,38,43,49,58]. The past several years have seen a large increase in the study of the role Wnt signaling plays in microvascular processes, yet there is still much to be learned, particularly in the setting of physiological and pathological adult microvascular growth and remodeling, including angiogenesis and arteriogenesis.
Genetic knockouts of Wnt and Wnt pathway-related genes have led to vasculogenic defects in a wide array of tissues: mouse coelomic vessels (Wnt-4) [23], mouse pulmonary vessels (Wnt-7b) [51], mouse placenta (Wnt-2) [37], and mouse yolk sac (Frizzled-5) [22]. In vitro studies have also revealed much about the role of Wnt in vascular cell function. In the vasculature, many proteins in the Wnt and Frizzled families have been shown to be secreted and expressed, respectively, by various primary endothelial cells [34,59]. Activation of the canonical and non-canonical Wnt pathways can lead to many alterations in endothelial cell function, including effects on proliferation, migration, cord formation and survival [10,34,35,59]. Related work in cancer models and other cell types have implicated Wnt signaling in the modulation of a large number of molecules associated with microvascular remodeling, including vascular endothelial growth factor (VEGF), fetal growth factor (FGF), matrix metalloproteinases (MMP), interleukin-8 (IL-8), endothelin-1 (ET-1), Eph/Ephrin, transforming growth factor beta (TGF-β), platelet-derived growth factor BB (PDGF-BB) and the Cyr61/CTGF/NOV (CCN) family [5,6,7,9,11,14,15,25,28,32,48,59,61].
Disruption of Frizzled-4 has been directly linked to familial exudative vitreoretinopathy (FEVR) – a hereditary disorder characterized by leaky defective retinal vascularization – in humans [25,48]. Overexpression of Wnt modulator sFRP-1, a secreted Wnt inhibitor capable of either binding to Frizzled receptors or directly binding to Wnt proteins [3], in mesenchymal stem cells resulted in increased vessel density in Matrigel plugs [14]. This molecule has been shown to increase vessel numbers in a CAM assay [13] and reduce infarct size in a myocardial infarction model [4].
While the Wnt signaling pathway and its related molecules have been studied extensively in recent years, until now, no study has investigated the role of Wnt signaling in a whole-mount preparation of adult microvascular remodeling. Using a whole-mount preparation, the effects of Wnt signaling can be monitored and measured throughout an entire intact microvascular network. In this study, a rat mesentery model was used to investigate the effects of Dickkopf-1 (DKK-1) and sFRP-1, two inhibitors of the canonical Wnt pathway, on microvascular remodeling.
DKK-1 is one of four members of the Dickkopf family of secreted proteins, with each having a separate profile of Wnt modulation [17,24,42]. The DKKs are heavily implicated in bone formation and cancer [29,39,55], as well as various aspects of development [42]. Their general structure and function have been recently reviewed [40]. In brief, DKK-1 inhibits Wnt signaling both by competitively binding Wnt co-recptor LRP. Conflicting theories exist about a second mechanism where DKK-1 forms a ternary complex with both Kremen and LRP which is then endocytosed [33,49,50]. DKK-1 has been shown to mobilize vascular progenitor cells and increase vessel number in implanted Matrigel plugs via activation of endosteal cells in the bone marrow niche [1]. Recently, DKK-4 has been shown to be a pro-angiogenic regulator of both normal endothelial cells and primary cancer cells [46]. Knock-down of DKK-3 in human cord blood-derived endothelial colony forming cells resulted in decreased migratory and cord forming capability [57].
Based on the aforementioned in vitro and in vivo studies performed with both DKK-1 and sFRP-1, we hypothesized that canonical Wnt inhibition would cause an increase in microvascular remodeling, both in capillaries and in larger vessels. We tested this hypothesis in a whole-mount model of adult inflammation-induced microvascular growth and remodeling in order to test the effects of Wnt inhibition on the structure of the entire microvascular network.
Materials and Methods
Angiogenesis Model: Rat Mesentery Exteriorization and Superfusion
All animal studies were approved by the Animal Research Committee at the University of Virginia and conformed to the American Heart Association Guidelines for the Use of Animals in Research. During normal development, microvessel networks develop in previously avascular mesenteric tissue via capillary sprouting from vessels in the surrounding fat [2,19,47]. In adult animals in the absence of a stimulus, microvessel networks are visible at the edges of the mesentery while the center is primarily avascular. Animals were anesthetized by an intramuscular (IM) injection of ketamine (80 mg/kg bw) and xylazine (8 mg/kg bw) and the mesentery was exposed by laparotomy under sterile conditions. Each 300g male Sprague-Dawley rat received treatment on one mesenteric window. A 100 µL (3.3ng/g bodyweight) bolus of recombinant DKK-1 (10µg/mL, R&D Systems, Minneapolis, MN) sFRP-1 (10µg/mL, St. Louis, MO), BSA (10µg/mL, Jackson ImmunoResearch, West Grove, PA), or warm saline was pipetted directly onto the window. Ten minutes later, another bolus was pipetted onto the same window. Ten minutes following the second bolus, the window was washed with warm Ringer’s solution for 5 minutes. Following intervention, the abdominal wall and skin were closed with non-absorbable 5-0 and absorbable 4-0 sutures, respectively, and the animal was allowed to recover. Mesenteric tissues were harvested from euthanized rats for immunofluorescence staining and analysis 3 days after surgery.
Acute Vasodilation Test
To test the acute vasodilatory capabilities of the treatments used in these studies, DKK-1 (10µg/mL), sFRP-1 (10µg/mL), or adenosine (100 µM, Sigma-Aldrich, St. Louis, MO) was dripped onto an exteriorized mesentery window in the following order: adenosine, saline wash, DKK-1/sFRP-1, saline wash, adenosine. Blood vessels in the window were examined at 10× magnification both before and after each treatment. In all cases, adenosine treatment resulted in immediate dilation of affected vessels, whereas DKK-1 and sFRP-1 treatments resulted in no dilation.
Harvest and Epifluorescence Microscopy
For tissues used in the examination of cell proliferation, a 100µM pulse of BRDU (Santa Cruz Biotechnology, Santa Cruz, CA) was applied for 2 hours before harvest. Harvested tissues were placed in methanol at −20C for 30 minutes before rinsing in PBS. BRDU-pulsed tissues were incubated twice in HCL – 1hr with 37C .1M HCl and 30 minutes with 37C 1M HCl – and rinsed in PBS with 0.1% saponin. Primary antibody (BRDU IIB5, 1:200, Millipore, Billerica, MA) was added overnight at 4C with 5% NGS in PBS with .1% saponin and 2% BSA. After washing for 30 minutes in PBS with 1% saponin, secondary and conjugated antibodies were added (goat anti-mouse 647, 1:200, Invitrogen, Carlsbad, CA; monoclonal anti-α-smooth muscle actin, Cy3 conjugate, 1:400, Sigma-Aldrich, Saint Louis, MO) for 1 hour at room temperature. After 30 minutes of washing in PBS with 1% saponin, slides were mounted in 1:1 glycerol:PBS for imaging.
Imaging and Analysis
Intravital imaging was performed using a Zeiss Axioskop inverted microscope. Complete intravital networks were captured using an air objective on a Zeiss Axioskop (×4) both directly prior to superfusion and prior to harvest after the 3 day recovery period. Networks were reconstructed using Autopano (Kolor, Challes-les-Eaux, France) image stitching software and Photoshop (Adobe Systems, San Jose, CA). Confocal microscopy was performed using a Nikon Eclipse C1si and a Nikon Eclipse TE300 with oil (×20) objectives. Z-stack images were acquired and compressed using Nikon NIS-Elements software. The compressed images were montaged using Autopano for analysis. Reconstructions used for analysis consisted of 4–6 fields of view per tissue. When measuring capillary lengths, a capillary was defined as any vessel or portion thereof with a diameter less than 10 µm. Conversely, arterioles and venules were defined as having diameters greater than or equal to 10 µm. Hemorrhage area was defined as the sum of the area covered by leaked blood in a given mesenteric window. Vascular density measurements were performed by first converting all vessels in a window to black pixels, and then taking the ratio of black pixels to total pixels in a given vascularized region. All measurements were performed using ImageJ software (NIH, Bethesda, MD).
Statistics
All statistical comparisons were made using the statistical analysis tools provided by Sigma Stat 3.5 (Systat Software, San Jose, CA, USA). Results are presented in the form of mean ± standard deviation (SD) Data were analyzed using paired T-test or one-way analysis of variance (ANOVA). In all cases, statistical significance was asserted at p < 0.05.
Results
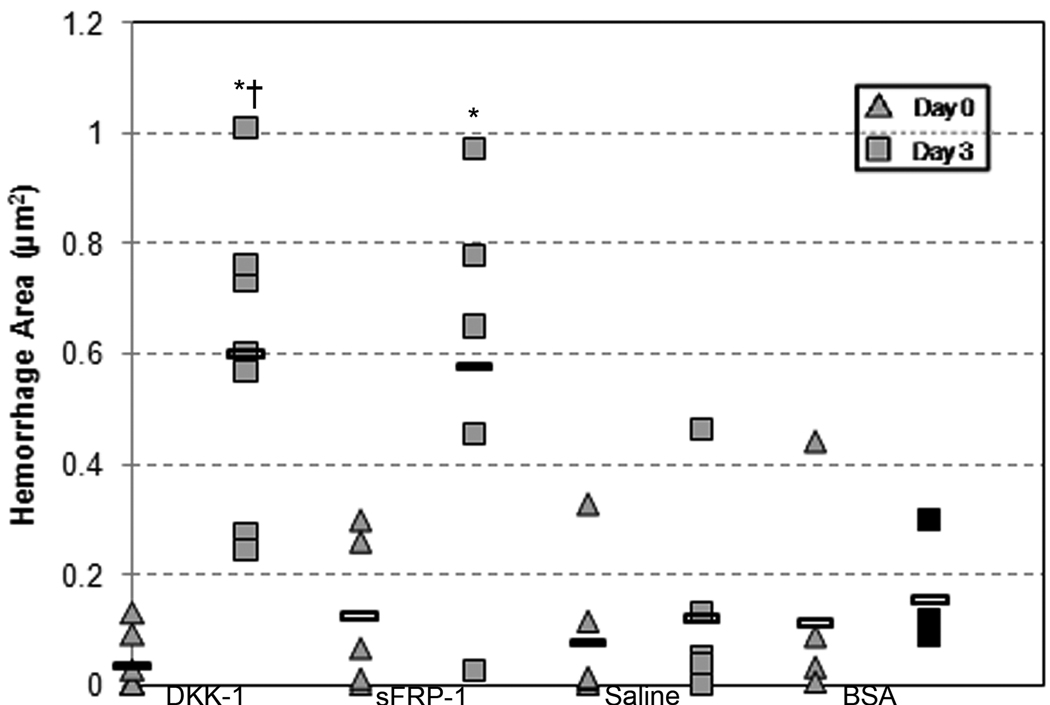
DKK-1 and sFRP-1 treatments significantly increase mean hemorrhage area
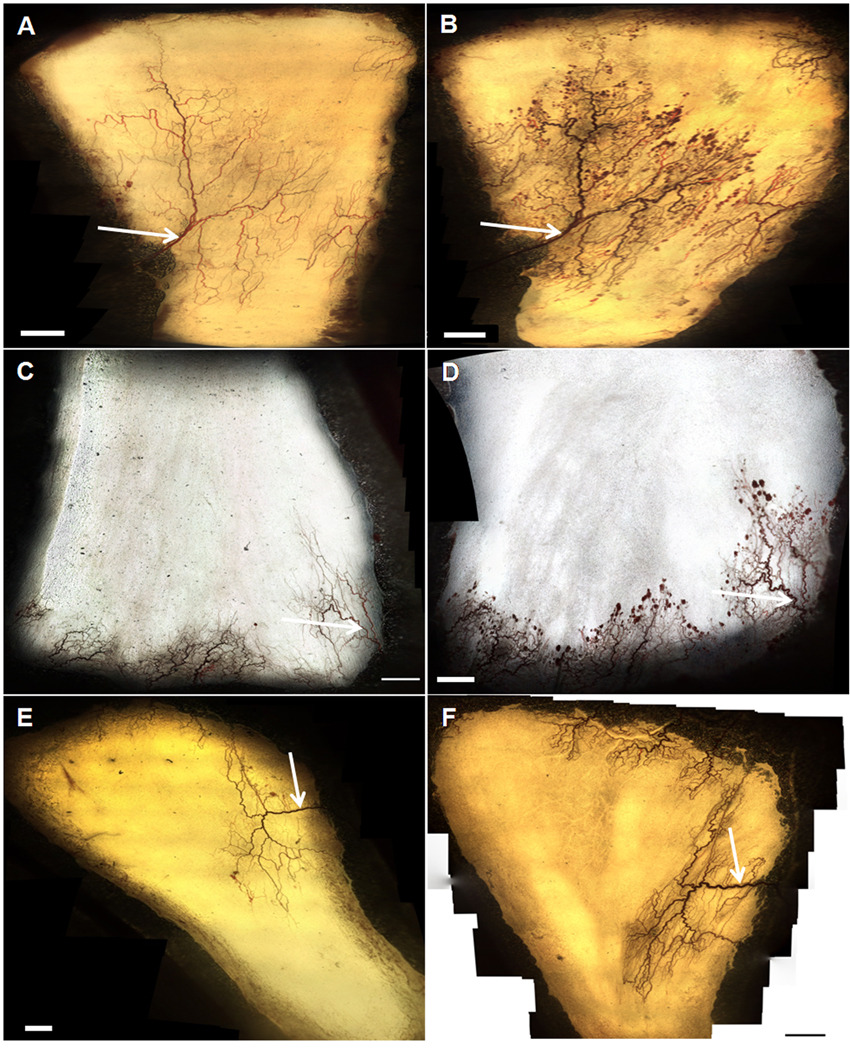
There was a striking visual difference between the microvascular beds of mesenteric windows treated with DKK-1 and sFRP-1 as compared to the BSA and saline control groups (Figure 1, A–F). These differences were characterized by the presence of numerous diffuse hemorrhages throughout the microvascular networks. In order to quantify this observation, we measured the total area of hemorrhages in each tissue. Figure 1A–F shows representative images of DKK-1, sFRP-1 and saline control-treated windows at Day 0 and Day 3. We calculated the area enclosed by each hemorrhage and plotted the total hemorrhages for each window on the dot plot in Figure 1G. While no statistical difference existed between the mean hemorrhage area of the four treatment types at Day 0, both sFRP-1 (.58 mm2) and DKK-1-treated windows (.60 mm2) exhibited a mean total hemorrhage area significantly greater than that of their Day 0 counterparts (.13 mm2 and .04 mm2, respectively), as well as the Day 3 saline control-treated windows (.12 mm2). In addition, DKK-1-treated windows had a significantly larger mean hemorrhage area than the negative control protein-treated windows (.15 mm2) at Day 3. Lastly, there was no significant difference between the Day 3 hemorrhage area of windows treated with saline and PBS. These results indicate that the addition of Wnt inhibitors, but not saline or a negative control protein, creates abundant hemorrhaging.
Figure 1.
A–F: Representative intravital microscopy images (4×) of a rat mesentery window. A (DKK-1), C (sFRP-1), E (Saline): Vasculature of an entire network at the time of surgery. B (DKK-1), D (sFRP-1), F (Saline) Vasculature of the same network three days following surgery. Arrows indicate venules measured for diameter changes. Scale bar = 1mm. G: Hemorrhage area in mesenteric windows for DKK-1 (n=7), sFRP-1 (n=5), saline (n=6) and BSA (n=4) treatments. Each data point represents a window, and each column represents a particular treatment and day. Rectangular bars indicate the mean for each column. * - Significant compared to respective Day 0 treatment mean with p < .05. † - Significant compared to Saline at Day 3 with p < .05.
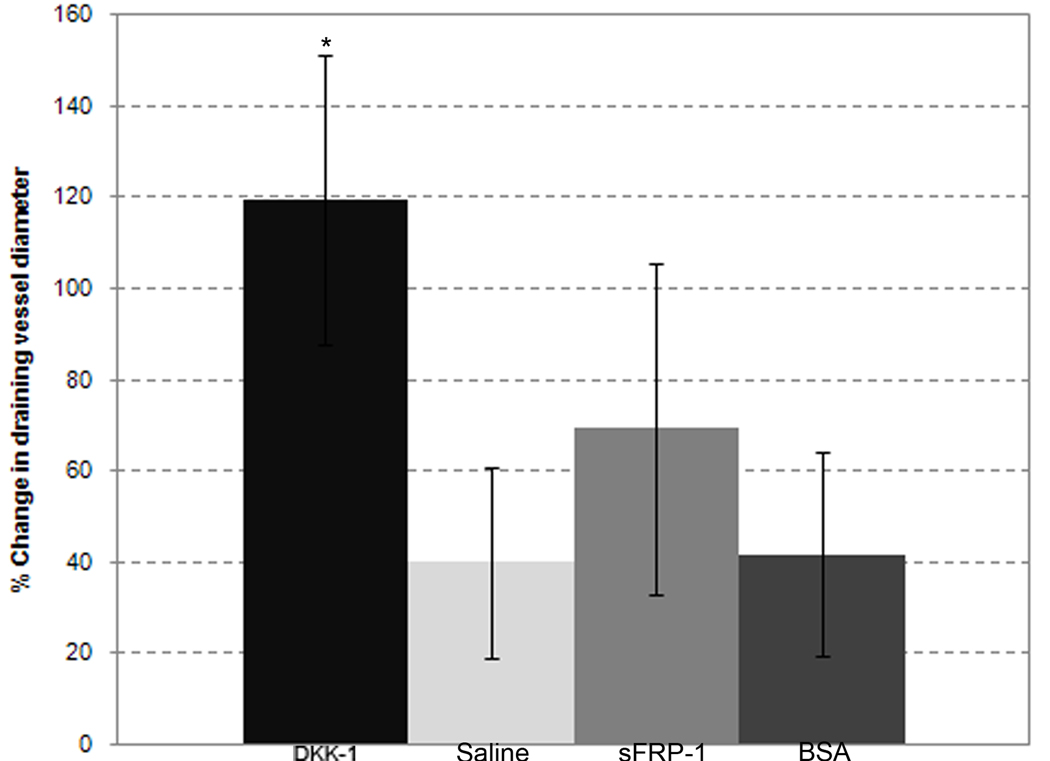
DKK-1 treatment significantly increases draining vessel diameter
We qualitatively observed changes in draining venule diameters as opposed to feeding arterioles in the remodeling microvascular networks. To quantify DKK-1’s effects on remodeling in these larger vessels, intravital images from treated windows were evaluated by measuring the diameter of the largest draining venule in each window. Figure 2 shows the change in draining vessel diameter as a percent change from Day 0 to Day 3. DKK-1 (119 +/− 31% increase) treated windows experienced a significantly larger change in draining vessel diameter than the draining vessel diameters of either saline control (40 +/− 20% increase) or negative control protein-treated (41 +/− 22% increase) windows. The draining vessels of sFRP-1 (69 +/− 34% increase) treated windows, however, did not show significant increases in diameter as compared to either of the control groups. These data indicate that aspects of Wnt inhibitor DKK-1's activities in this model that differ from those of Wnt inhibitor sFRP-1 result in changes in how major draining vessels remodel in the rat mesentery.
Figure 2.
Changes in draining venule diameters. Each column represents the mean change +/− SD over three days of the largest draining vessel in the mesentery windows of the DKK-1 (n=7), sFRP-1 (n=5), saline (n=6) and BSA (n=4) treatments, respectively. * - Significant compared to both saline and BSA with p < .05.
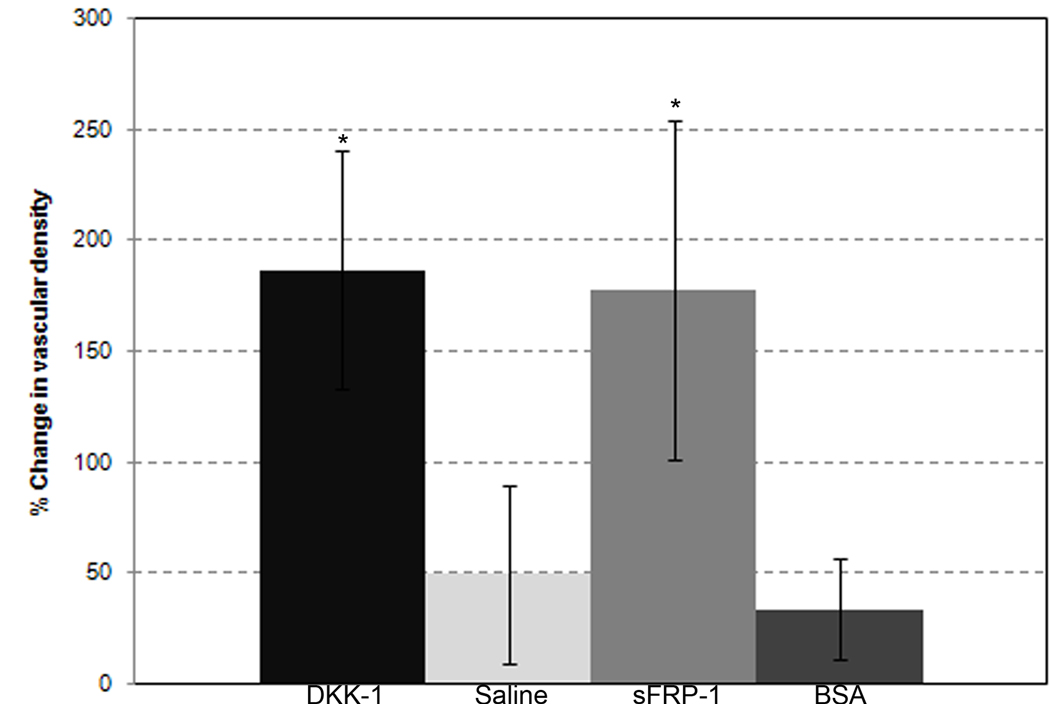
DKK-1 and sFRP-1 treatments significantly increase vascular density
To examine DKK-1’s effects on the vascular density of mesenteric windows, intravital images from DKK-1, sFRP-1, negative control protein and saline control-treated windows were evaluated. Figure 3 shows the percent change in vessel density from Day 0 to Day 3 for each treatment group, indicating the portion of a given vascularized area that contains vessels. Both DKK-1 (186 +/− 53% increase) and sFRP-1 (177 +/− 76% increase) treated windows experienced a significantly larger change in vascular density than either saline (50 +/− 40% increase) or BSA-treated (34 +/− 22%) windows. In addition, the negative control protein-treated group was statistically similar to the saline-treated group. Changes in this metric most likely reflect diameter changes (i.e. increased thickness) described in the previous section, as opposed to new vessel formation via angiogenic sprouting. Further measurements examining changes in vessel branch point density, capillary length density, and large (> 10µm diameter) vessel length density were all not significant between DKK-1, sFRP-1 and control groups (Supplementary Figure 1).
Figure 3.
Changes in vascular density. Each column represents the mean change +/− SD over three days of vascular density in the mesentery windows of the DKK-1 (n=7), sFRP-1 (n=5), saline (n=6) and BSA (n=4) treatments, respectively. The vascular density measurement was described in the Materials and Methods. * - Significant compared to both saline and BSA with p < .05.
DKK-1 treatment increases cellular proliferation
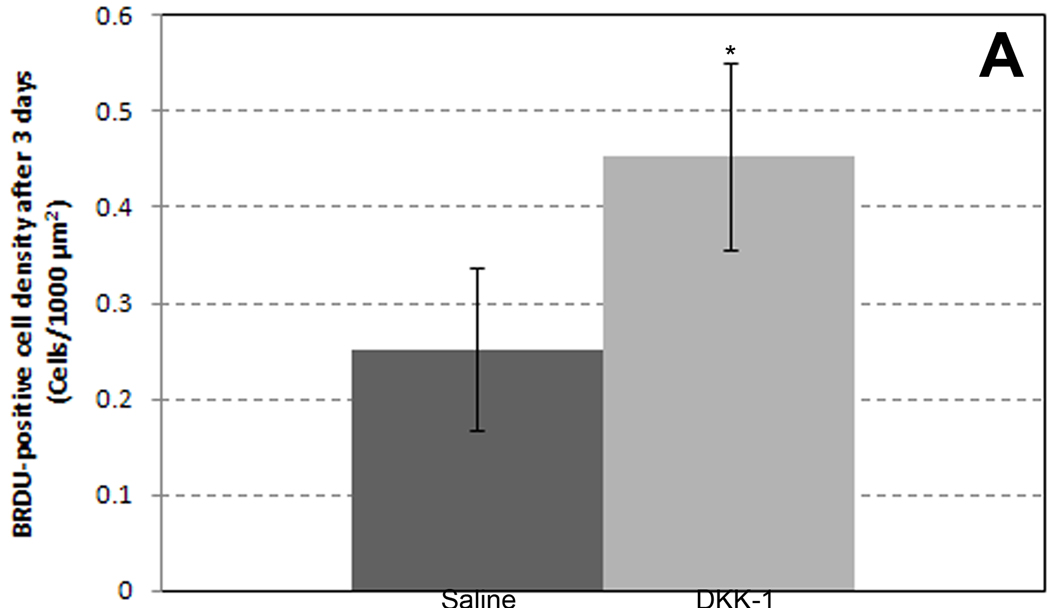
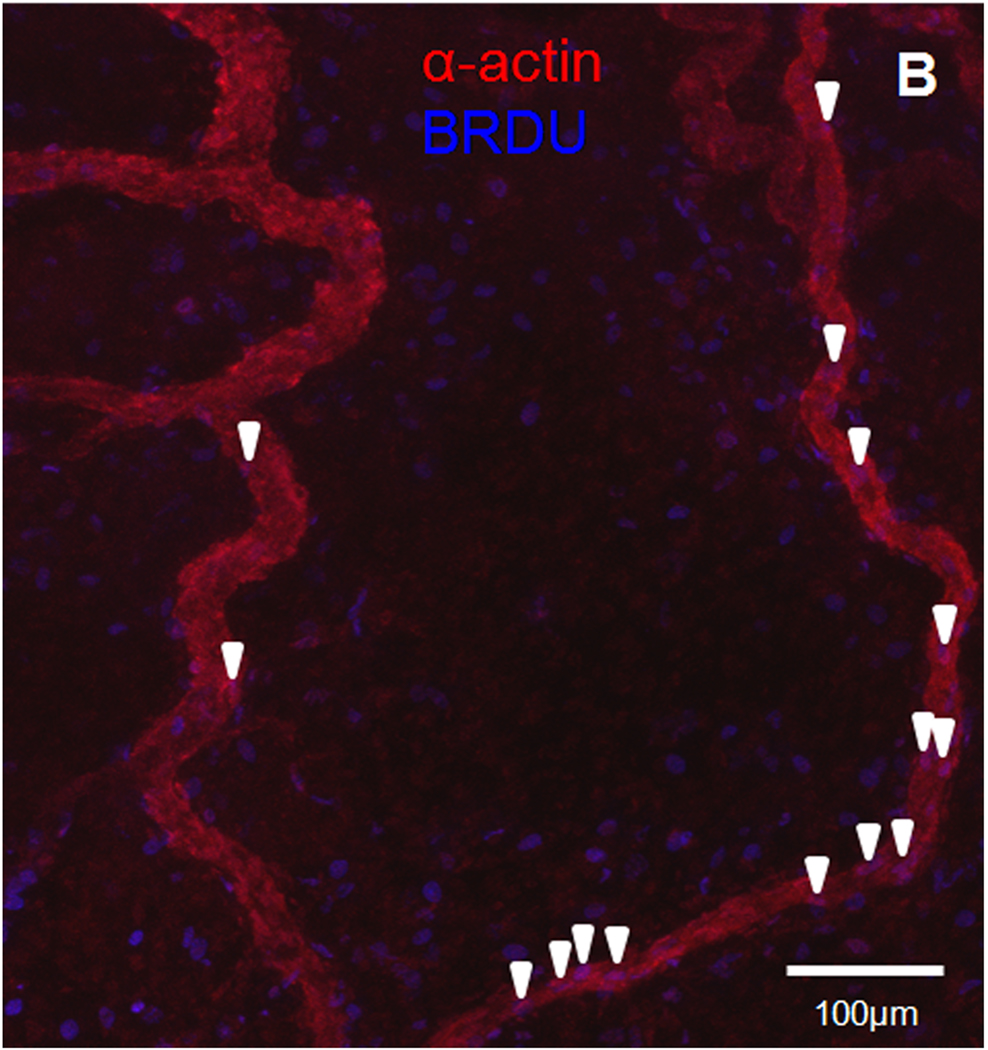
Finally, to determine if DKK-1 influenced proliferation in this model, we immunostained harvested mesentery windows for the proliferative marker BRDU. We observed a significant increase in proliferating cell density in DKK-1-treated windows (.45 ± .1 cells/1000 µm2) compared to saline–treated controls (.25 ± .08 cells/1000 µm2) (Figure 4A). In DKK-1-treated windows immunostained for both smooth muscle alpha-actin and BRDU, proliferating smooth muscle cells were observed along venules (Figure 4B). Proliferating endothelial cells, as indicated by lectin-positive cells on a vessel co-staining for BRDU, however, were rare (not shown). These results suggest DKK-1 causes an increase in proliferation in venular smooth muscles cells, as well as possibly other non-vascular, interstitial cells.
Figure 4.
A: Quantification of BRDU+ cells three days after saline (n=3) or DKK-1 treatment (n=4). * - Significant compared with saline with p < .05. B: Representative confocal image (20×) of mesenteric vascular networks. Image shows smooth muscle alpha-actin (red) and BRDU (blue) three days following DKK-1 treatment. Purple cells (arrowheads) co-localizing actin and BRDU along vessels indicate proliferating smooth muscle cells. Scale bar = 100 µm.
Discussion
The purpose of our study was to test the hypothesis that interfering with Wnt signaling would alter the microvascular remodeling response in the setting of normal mesenteric exteriorization. The striking hemorrhages in the DKK-1 and sFRP-1-treated windows confirm that significant changes in the local microvasculature are taking place. This hemorrhagic response is typically attributed to VEGF overexpression [54]. Therefore we first speculated that DKK-1 may cause increased expression of VEGF. However, the lack of significant changes in capillary length density, which would also be an outcome of increased VEGF signaling, leads us to believe that VEGF is not being overexpressed due to the addition of Wnt inhibitors. The most plausible scenario is that both sFRP-1 and DKK-1 – and therefore canonical Wnt signaling – are upstream of some signaling pathway responsible for maintaining homotypic and heterotypic cell-cell interactions and/or regulating endothelial cell permeability. This hemorrhaging vessel phenotype is also a characteristic of tumor vascular beds, and it is obvious to speculate that DKK-1 regulation of Wnt signaling may play an important role in generating tumor vessel hemorrhages. However, the current literature is inconclusive with regards to DKK-1’s effects on various cancers [26,52,62].
Another interesting observation from our data is that venules appear to be the vessel subset whose diameters are most impacted by Wnt-regulation in microvascular remodeling. Venules exhibitied diameter enlargement, which was further reflected in the vascular density metric, and supported by the observation of abundant proliferating smooth muscle cells along venules. Moreover, we confirmed that measured diameter enlargement was not merely the result of vasodilation by application of an acute vasodilator, strengthening the conclusion that structural changes to the venular walls were taking place. In addition, the increase in vascular density was shown to not be a function of capillary growth and remodeling, as there was a lack proliferating endothelial cells along capillaries (data not shown). Venular remodeling has been investigated with regard to remodeling during venous arterialization after graft implantation and to treat vascular access dysfunction in hemodialysis patients [8,27], giving the idea ample precedent. The idea of a Wnt-inhibitor-induced shift towards venular remodeling leads to myriad follow-up studies, including investigation of the possible connection between venular remodeling and hemorrhaging as well as the other molecular mediators of smooth muscle cell proliferation, such as PDGF-BB, in the context of Wnt signaling.
Another point of note regarding the proliferation results is that while there were numerous proliferating venular smooth muscle cells, there were also many proliferating cells in the interstitial space in DKK-1-treated mesentery windows. The identity and function of these cells is unknown, although several possibilities exist. One possibility for the proliferating interstitial cell pool is inflammatory cells. DKK-1 has been shown to be present in several models of inflammation [12,56], and the mesenteric exteriorization is generally considered to be an inflammatory stimulus [41]. A second potential pool for these proliferating interstitial cells is bone marrow-derived cells. Recent work in a mouse model of hypoxia-induced angiogenesis has shown that bone marrow cells are frequently present in perivascular positions, as well as in the interstitial space [44]. Those two locations are where the majority of BRDU-positive cells were observed in the DKK-1-treated mesentery windows, indicating that bone marrow cells could be proliferating in our model. A final, related potential pool of cells is the mesenchymally-derived stem cells present in the mesentery [20]. These primordial mesoderm cells could have been recruited by DKK-1 treatment towards any number of lineages relevant to microvascular remodeling. It is worth pointing out that all three of these cell types would be thought to provide paracrine support to the remodeling process. This would mean that DKK-1 is capable of modulating the production of support cells related to microvascular remodeling, as well as directly amplifying the production of vascular cells.
Finally, we were intrigued by the differences between DKK-1 and sFRP-1 treatments in the rat mesentery microvasculature. Notably, the DKK-1 treatment caused significant changes in draining vessel diameter while the sFRP-1 treatment did not. One explanation for the differences is simply that DKK-1 is more potent than sFRP-1, as the same concentration of drug was examined for the two molecules.
Alternatively, if DKK-1 has a more powerful effect on draining vessel diameter changes than sFRP-1, the manners in which the two molecules respectively act on Wnt signaling are likely causes. DKK-1 is mainly a canonical Wnt inhibitor, as it binds to the Fzd receptor, preventing Wnt molecules from binding. SFRP-1, on the other hand, is a complete Wnt inhibitor, as it acts directly on Wnt molecules. Given these differences, non-canonical Wnt signaling could play an important role in the microvascular response of the rat mesenteric networks.
In conclusion, our results show that the addition of Wnt inhibitors onto a rat mesentery microvasculature clearly impacts the remodeling process in terms of main draining vessel diameters, vascular density, and hemorrhaging. The microvascular changes induced by Wnt inhibitors appear to be borne out at least partially by an increase in proliferating venular smooth muscle cells. Overall, this descriptive study lays the ground work for future studies to further investigate the impact of this exciting, far-reaching signaling pathway on the microvasculature. Future studies could uncover exactly which molecules and signaling pathways are being influenced by the addition of Wnt inhibitors, as well as delving further into the role of venules and vessel-specific markers in Wnt-regulated microvascular remodeling. The addition of a mouse model would greatly aid in visual reporting of Wnt signaling parameters, and alternative models of microvascular remodeling could shed new light on length density and other quantitative metrics.
Supplementary Material
Acknowledgments
The authors would like to thank Ji Song, Ph.D. and Chris Anderson, Ph.D. for their assistance with surgical procedures, and Lydia Glaw for her contributions towards manuscript preparation.
Support: NIH-5R01HL082838-03 (S.M.P), NIH-HL065958 (T.C.S.)
References
- 1.Aicher A, Kollet O, Heeschen C, Liebner S, Urbich C, Ihling C, Orlandi A, Lapidot T, Zeiher AM, Dimmeler S. The Wnt antagonist Dickkopf-1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ Res. 2008;103:796–803. doi: 10.1161/CIRCRESAHA.107.172718. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CR, Ponce AM, Price RJ. Immunohistochemical identification of an extracellular matrix scaffold that microguides capillary sprouting in vivo. J Histochem Cytochem. 2004;52:1063–1072. doi: 10.1369/jhc.4A6250.2004. [DOI] [PubMed] [Google Scholar]
- 3.Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999;274:16180–16187. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- 4.Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplaa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 5.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 6.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles AK, Gresham GA. Histopathological changes in venous grafts and in varicose and non-varicose veins. J Clin Pathol. 1993;46:603–606. doi: 10.1136/jcp.46.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, McLean S, Carter DE, Leask A. The gene expression profile induced by Wnt 3a in NIH 3T3 fibroblasts. J Cell Commun Signal. 2007;1:175–183. doi: 10.1007/s12079-007-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng CW, Smith SK, Charnock-Jones DS. Wnt-1 signaling inhibits human umbilical vein endothelial cell proliferation and alters cell morphology. Exp Cell Res. 2003;291:415–425. doi: 10.1016/j.yexcr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 12.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 13.Dufourcq P, Couffinhal T, Ezan J, Barandon L, Moreau C, Daret D, Duplaa C. FrzA, a secreted frizzled related protein, induced angiogenic response. Circulation. 2002;106:3097–3103. doi: 10.1161/01.cir.0000039342.85015.5c. [DOI] [PubMed] [Google Scholar]
- 14.Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamaziere JM, Couffinhal T, Duplaa C. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells. 2008;26:2991–3001. doi: 10.1634/stemcells.2008-0372. [DOI] [PubMed] [Google Scholar]
- 15.Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, Randazzo F, Gundel R, Warren RS, Escobedo J, Aukerman SL, Taylor RN, Fantl WJ. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–3153. [PubMed] [Google Scholar]
- 16.Fuerer C, Nusse R, Ten Berge D. Wnt signalling in development and disease. Max Delbruck Center for Molecular Medicine meeting on Wnt signaling in Development and Disease. EMBO Rep. 2008;9:134–138. doi: 10.1038/sj.embor.7401159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 18.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 19.Hansen-Smith FM, Joswiak GR, Baustert JL. Regional differences in spontaneously occurring angiogenesis in the adult rat mesentery. Microvasc Res. 1994;47:369–376. doi: 10.1006/mvre.1994.1029. [DOI] [PubMed] [Google Scholar]
- 20.Hatanaka K, Imakita M, Go S, Yamamoto A. Effects of degranulation of mast cells on proliferation of mesenchymal cells in the mesentery of mice. Cell Tissue Res. 1986;246:53–56. doi: 10.1007/BF00218998. [DOI] [PubMed] [Google Scholar]
- 21.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- 23.Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- 24.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 25.Kaykas A, Yang-Snyder J, Heroux M, Shah KV, Bouvier M, Moon RT. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat Cell Biol. 2004;6:52–58. doi: 10.1038/ncb1081. [DOI] [PubMed] [Google Scholar]
- 26.Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene. 2006;25:5027–5036. doi: 10.1038/sj.onc.1209508. [DOI] [PubMed] [Google Scholar]
- 27.Kwei S, Stavrakis G, Takahas M, Taylor G, Folkman MJ, Gimbrone MA, Jr, Garcia-Cardena G. Early adaptive responses of the vascular wall during venous arterialization in mice. Am J Pathol. 2004;164:81–89. doi: 10.1016/S0002-9440(10)63099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy L, Neuveut C, Renard CA, Charneau P, Branchereau S, Gauthier F, Van Nhieu JT, Cherqui D, Petit-Bertron AF, Mathieu D, Buendia MA. Transcriptional activation of interleukin-8 by beta-catenin-Tcf4. J Biol Chem. 2002;277:42386–42393. doi: 10.1074/jbc.M207418200. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 31.Macdonald BT, Semenov MV, He X. SnapShot: Wnt/beta-catenin signaling. Cell. 2007;131:1204. doi: 10.1016/j.cell.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 32.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 34.Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 35.Masckauchan TN, Kitajewski J. Wnt/Frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology (Bethesda) 2006;21:181–188. doi: 10.1152/physiol.00058.2005. [DOI] [PubMed] [Google Scholar]
- 36.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 37.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 38.Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 39.Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 40.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 41.Norrby K. In vivo models of angiogenesis. J Cell Mol Med. 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nusse R. Developmental biology. Making head or tail of Dickkopf. Nature. 2001;411:255–256. doi: 10.1038/35077199. [DOI] [PubMed] [Google Scholar]
- 43.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 44.O'Neill TJ, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97:1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 45.Peirce SM, Skalak TC. Microvascular remodeling: a complex continuum spanning angiogenesis to arteriogenesis. Microcirculation. 2003;10:99–111. doi: 10.1038/sj.mn.7800172. [DOI] [PubMed] [Google Scholar]
- 46.Pendas-Franco N, Garcia JM, Pena C, Valle N, Palmer HG, Heinaniemi M, Carlberg C, Jimenez B, Bonilla F, Munoz A, Gonzalez-Sancho JM. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–4477. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 47.Ponce AM, Price RJ. Angiogenic stimulus determines the positioning of pericytes within capillary sprouts in vivo. Microvasc Res. 2003;65:45–48. doi: 10.1016/s0026286202000146. [DOI] [PubMed] [Google Scholar]
- 48.Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, Zhang LH, Singaraja RR, Guernsey DL, Zheng B, Siebert LF, Hoskin-Mott A, Trese MT, Pimstone SN, Shastry BS, Moon RT, Hayden MR, Goldberg YP, Samuels ME. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32:326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- 49.Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Semenov MV, Zhang X, He X. DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J Biol Chem. 2008;283:21427–21432. doi: 10.1074/jbc.M800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi N, Fukushima T, Yorita K, Tanaka H, Chijiiwa K, Kataoka H. Dickkopf-1 is overexpressed in human pancreatic ductal adenocarcinoma cells and is involved in invasive growth. Int J Cancer. 2009 doi: 10.1002/ijc.24865. [DOI] [PubMed] [Google Scholar]
- 53.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 54.Thurston G. Complementary actions of VEGF and angiopoietins on blood vessel permeability and growth in mice. J Anat. 2002;200:529. doi: 10.1046/j.1469-7580.2002.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 56.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, Gullestad L, Oie E, Hansson GK, Aukrust P. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 57.Untergasser G, Steurer M, Zimmermann M, Hermann M, Kern J, Amberger A, Gastl G, Gunsilius E. The Dickkopf-homolog 3 is expressed in tumor endothelial cells and supports capillary formation. Int J Cancer. 2008;122:1539–1547. doi: 10.1002/ijc.23255. [DOI] [PubMed] [Google Scholar]
- 58.van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- 59.Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Commun. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- 60.You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–6054. [PubMed] [Google Scholar]
- 62.Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, Li J, Yan X, Liu Y, Shao C, Zhao RC. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23:925–933. doi: 10.1038/leu.2008.384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.