Abstract
Background Plasmacytoid dendritic cells (pDC) are depleted from blood of individuals with HIV infection associated with progression to disease. It has been postulated but not proven that pDC accumulate in lymph nodes and induce sustained immune activation characteristic of disease.
Methods The dynamics of the pDC response to acute pathogenic SIV infection of rhesus macaques were studied using methods to track recently divided cells.
Results pDC were lost from blood and lymph nodes in acute SIV infection despite rapid mobilization and recruitment. pDC had a low frequency of infection, were uniformly activated and had increased levels of apoptosis, while maintaining normal function.
Conclusions pDC mobilization into blood and lymph nodes in acute SIV infection does not keep pace with excessive pDC loss through activation and apoptosis. The depletion of pDC from lymphoid tissues in acutely infected rhesus macaques does not support a pathogenic role for pDC in disease.
Keywords: AIDS, cell dynamics, lymph node, non‐human primate
Introduction
The plasmacytoid dendritic cell (pDC) is a specialized subset of DC that is characterized by copious production of type I interferon (IFN) in response to virus exposure [16]. Human pDC are phenotypically defined by lack of expression of T and B cell and monocyte markers and by expression of MHC class II and the IL‐3 receptor CD123 [25, 52], and similar markers can be used to define pDC in non‐human primate species [8, 15, 37, 44, 58]. pDC recognize single‐stranded viral RNA via endosomal toll‐like receptor (TLR)7 [18, 35], and HIV and SIV activate pDC to produce IFN‐α through virus RNA engagement of this receptor, although a role for TLR9 remains a possibility [3, 39, 40]. pDC‐derived IFN directly controls virus infection in murine coronavirus and herpes simplex virus models [12, 36] and controls HIV infection in vitro [26, 41]. However, the role of pDC in either controlling HIV replication or exacerbating pathogenesis is controversial.
A series of studies has shown that pDC loss from the circulation in HIV‐infected individuals correlates with disease progression, suggesting that pDC depletion contributes to HIV immunopathology [2, 13, 20, 31, 43, 55]. It has been hypothesized that infection is associated with recruitment of pDC to inflamed lymph nodes that draw cells from blood resulting in a deficit of circulating pDC [2, 24, 42]. In mice, pDC are recruited to inflamed lymph nodes through CXCR3 interaction with CXCL9 and CXCL10 [11, 60], and expression of these chemokines is markedly increased in SIV infection of macaques [46, 47]. pDC activate T cells both through direct contact and elaboration of IFN‐α [5, 41], and the increased number of activated CD4+ T cells could negatively impact lentivirus pathogenesis by fueling virus replication and CD4+ T cell depletion. Substantial pDC recruitment has been reported to occur within 1 day of vaginal exposure of rhesus macaques to SIV associated with elaboration of pDC‐derived chemokines and attraction of large numbers of CD4+ T cell targets to mucosal tissues [32]. Recent studies have indicated that CD4+ T cells from HIV‐infected lymph nodes are hyperactive because of the effects of IFN and upregulated IFN‐stimulated genes [28, 50]. Moreover, comparative studies have shown that the acute IFN response is rapidly controlled in SIV‐infected African green monkeys and sooty mangabeys, which do not progress to disease, but is sustained in rhesus macaques, which do progress to disease [7, 29].
Together, these findings have raised the hypothesis that chronic innate immune activation potentially through sustained recruitment and stimulation of pDC in lymphoid tissues mediates HIV immunopathogenesis [6, 38, 39]. However, little is known about the kinetics of the pDC response to acute SIV and HIV infection, and in chronic progressive SIV and HIV infection there is profound depletion of pDC from lymph nodes [4, 9, 45]. The role of pDC recruitment to lymphoid tissues in AIDS pathogenesis thus remains to be determined and is likely to be complex [22].
In this study, we characterized the kinetics of the pDC response in bone marrow, blood and lymph nodes in rhesus macaques during the acute phase of progressive SIV infection. We enumerated pDC in different compartments [8] and used in vivo labeling with 5‐bromo‐2′‐deoxyuridine (BrdU) together with Ki67 staining to document mobilization and recruitment of recently divided cells that would otherwise be obscured by net cell loss [10]. Interestingly, we found that pDC were present in normal numbers in bone marrow but were lost from blood and lymph nodes within 14 days of intravenous infection with SIVmac251, despite evidence of a profound mobilization of pDC into blood and recruitment to lymph nodes. In lymph nodes, pDC were uniformly activated and more likely to be apoptotic than in uninfected animals, and a significant proportion of pDC were infected with virus [10]. These findings show that pDC are recruited to inflamed lymph nodes in SIV infection, as predicted. However, the hostile inflammatory environment within these tissues favors pDC activation and death that exceeds the capacity for pDC replacement, resulting in a net pDC loss.
Materials and methods
Animals and virus infection
Six adult Indian‐origin rhesus macaques were infected by intravenous inoculation with 1000 TCID50 of the pathogenic isolate SIVmac251 and compared to a cohort of uninfected controls, as described in detail previously [10].
Identification and enumeration of pDC
pDC were identified in bone marrow, blood and lymph nodes using standard flow cytometry‐based approaches [8, 9, 10, 44]. In general, cell suspensions were labeled with a panel of monoclonal antibodies to CD3, CD14 and CD20 (conjugated to the same fluorochrome and combined as a lineage cocktail), CD45, HLA‐DR and CD123 along with a live/dead viability dye to exclude dead cells [8]. In some experiments, additional antibodies to BrdU, Ki‐67, TNF‐α, IFN‐α or CD95 were used. Apoptosis was determined by labeling with 7‐AAD and Annexin‐V. To accurately count cells in blood, we developed a combined whole blood/peripheral blood leukocyte (PBL) assay [8]. This is a two‐step assay that involves (i) antibody staining and analysis of a precise volume of whole blood to identify and count CD45 + mononuclear cells in TruCOUNT tubes, which contain a known number of fluorescent beads that serve as an internal calibrator and (ii) multiparameter analysis of PBL to determine the percentage of mononuclear cells that are pDC. The pDC percentage in PBL is multiplied by the absolute number of mononuclear cells calculated from whole blood to determine the number of pDC in a unit volume of whole blood [8]. Analysis of pDC in PBL as opposed to whole blood is preferred as the lineage− MHC class II+ cells, and DC subsets are poorly discriminated in whole blood but are readily defined in PBL in our experience. This method has revealed that normal rhesus macaque blood contains an average of 2.5 pDC/ul, 20‐fold fewer than the number of myeloid DC [8].
Tracking recently divided pDC
To track recently divided pDC in vivo, BrdU (30 mg/kg) was given by intravenous injection at 24‐hour intervals for four doses starting at day 10 post infection, as described [10]. BrdU is a thymidine analog that is incorporated into actively dividing cells and can be identified within cells following permeabilization and antibody labeling. BrdU incorporation into pDC can be used to track the exodus of pDC from bone marrow and the entrance into blood and tissues, as murine studies have indicated that pDC do not undergo proliferation in peripheral tissues under physiologic conditions [34], and our preliminary data indicate that the same is true for pDC in blood and tissues of SIV‐infected macaques [10]. Hence, any BrdU+ pDC in blood or tissues will be marked as having been released from the bone marrow since the time of the BrdU pulse. BrdU staining was complemented by antibody staining against the nuclear antigen Ki‐67 that is expressed by cells in non‐G0 phase of the cell cycle. Ki‐67 positivity gives an estimate of the recent proliferative history of a given cell [49].
Determining pDC function and infection
To determine the functional capacity of pDC in blood and lymph node, unseparated cell suspensions were stimulated with the TLR7 agonist 3M‐007 and analyzed for production of TNF‐α and/or IFN‐α by intracellular cytokine staining and flow cytometry, as described [10]. To determine the proportion of lymph node pDC that was infected with SIV, pDC were sorted from lymph nodes, and SIV gag DNA in total cellular DNA was measured using albumin DNA quantity to determine the frequency of infected cells [10].
Results
Rapid loss of pDC despite massive mobilization in acute pathogenic SIV infection
In rhesus macaque monkeys that were infected with SIVmac251, there was a highly dynamic pDC response, with a significant increase in the number of pDC in blood between 3 and 6 days post infection followed at day 10 by a significant decrease in pDC number from baseline. Production of pDC in bone marrow was unchanged over this period, as judged by phenotypic labeling of bone marrow aspirates and Ki‐67 labeling, suggesting that the loss of pDC from blood at this acute stage was not a consequence of suppressed hematopoiesis [10]. To determine whether the drop in pDC was associated with an increase in pDC in lymph node that would reflect recruitment, we analyzed peripheral lymph node cell suspensions for pDC subsets. Surprisingly, the percentage of live pDC within the lineage− HLA‐DR+ gate decreased fourfold from prior to infection to 14 days post infection [10]. These data suggest that pDC were depleted from both the blood and the lymph nodes during acute SIV infection (Fig. 1A, B).
Figure 1.
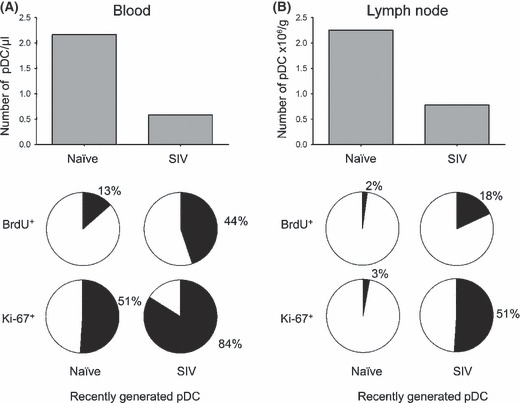
pDC loss from blood and lymph node despite marked mobilization in acute pathogenic SIV infection. (A, B) The median number of blood (A) and lymph node pDC (B) in naïve and acutely infected rhesus macaques (top). The proportion of blood (A) and lymph node pDC (B) in naïve and acutely infected rhesus macaques that are recently generated based on BrdU incorporation in vivo and Ki‐67 expression (bottom). Data are adapted from a recently published study [10].
To better understand the kinetics of the pDC response, we next identified recently divided pDC based on BrdU incorporation and Ki‐67 labeling. In SIV naïve monkeys, only 13% of pDC were BrdU+ in blood after four injections, whereas ∼20% of blood pDC were already BrdU+ in SIV‐infected macaques after a single injection and 44% were BrdU+ after four injections, reaching 60% in one animal. Similarly, while the median proportion of pDC that was Ki‐67+ prior to infection was 51%, this increased to 84% in acutely infected monkeys [10] (Fig. 1). These data reveal that as blood pDC numbers dropped with infection, the fraction of newly produced cells entering the circulation increased [10] (Fig. 2). When we analyzed lymph node pDC for BrdU incorporation after four pulses of BrdU, we found only 2% of pDC were BrdU+ in naive lymph nodes when compared to around 20% in the acutely infected lymph node. The proportion of Ki‐67+ pDC in lymph node increased from 3% prior to infection to a massive 51% following infection and was inversely correlated with the number of pDC, indicating that recruitment and loss were occurring concurrently [10] (Fig. 1B). Together, these data indicated that underlying the loss of pDC in blood and lymph node was a profound mobilization of pDC from bone marrow into blood and subsequent influx into lymph node (Fig. 2).
Figure 2.
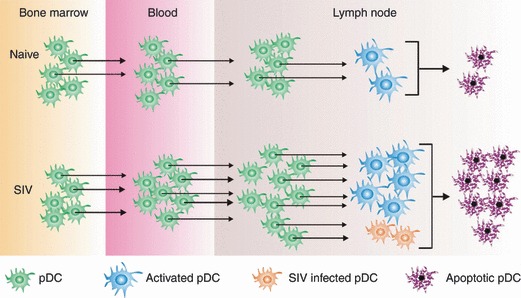
Lymph node pDC death through activation and infection exceeds replenishment from bone marrow and blood in acute pathogenic SIV infection. (Top) Homeostatic pDC production in bone marrow and migration into blood and lymph nodes in naïve rhesus macaques. A proportion of lymph node pDC undergoes activation and apoptosis at a rate that equals replenishment maintaining a steady state in all compartments. (Bottom) In acute SIV infection, pDC production in bone marrow is unchanged but mobilization into blood and lymph nodes is markedly increased. However, excessive pDC activation and a low frequency of infection lead to a high rate of apoptosis in lymph nodes that exceeds the rate of replenishment, leading to an overall pDC deficit in blood and lymph nodes.
Activation, apoptosis, infection and function of pDC
The rapid depletion of pDC in acutely infected lymph nodes was associated with a significant increase in apoptotic pDC, as measured by uptake of the live/dead indicator as well as Annexin‐V and 7‐AAD staining, and by uniform upregulation of CD95 [10], which in mice is linked to apoptotic death of pDC [57]. Interestingly, we could not detect increases in TNF‐related apoptosis‐inducing ligand (TRAIL) expression despite reports indicating that HIV induces expression of TRAIL in pDC in vitro in an IFN‐dependent manner [27]. We also measured the frequency of SIV‐infected pDC in lymph node using a quantitative real‐time PCR assay to detect integrated gag DNA in highly purified sorted cells. These data showed for the first time that more than 4% of lymph node pDC harbored SIV DNA during acute infection, likely resulting in pDC death [10]. Together, our findings are consistent with activation‐induced apoptosis and to a lesser extent direct virus infection accounting for pDC death that exceeds the rate of recruitment (Fig. 2).
A pressing question is whether pDC have normal function in pathogenic SIV infection and whether they contribute to the marked IFN response in the lymph node [7, 29, 30]. To examine this, we stimulated unseparated lymph node cells taken at day 14 post infection or prior to infection with a TLR7 agonist ex vivo and then measured IFN‐α and TNF‐α production in the pDC subset by multicolor flow cytometry. Approximately half of all pDC produced both cytokines in response to this stimulation regardless of infection status, although lymph node pDC from SIV‐infected monkeys had a significantly reduced capacity to produce TNF‐α alone [10]. These findings indicate that despite a net loss of pDC, the remaining cells in blood and lymph node retained largely normal functional responses to exogenous TLR7‐mediated stimulation during acute infection.
Discussion
We combined precise methods for analyzing and counting pDC with approaches to identify proliferating cells in vivo to show unequivocally that pDC in the rhesus macaque are highly responsive to acute SIV infection, with rapid mobilization into blood and a 10‐ to 20‐fold increase in recruitment into lymph nodes coinciding with peak viremia at 1–2 weeks post infection [10]. This is consistent with an increase in lymph node expression of inflammatory chemokines in lymph nodes and with maturation of circulating pDC [14, 46, 60]. Nevertheless, the absolute number of pDC in both compartments dropped markedly during this time, associated with widespread pDC activation and apoptosis and infrequent but significant pDC infection [10]. This finding is consistent with several reports showing that HIV induces pDC death both through direct effects and through pDC fusion with virus‐infected cells [23, 41, 48] and with the finding that pDC activation in vivo precedes apoptosis [57]. The overall picture is that pDC are highly responsive to pathogenic SIV infection, but the overwhelming death of cells in lymphoid tissues exceeds this response leading to a net pDC loss (Fig. 2).
Taken with previous studies [9, 45], these findings suggest that pDC are lost from both blood and lymphoid tissues from the earliest stages of pathogenic SIV infection through to AIDS. Data in the human also indicate that pDC are lost from lymphoid tissues in chronic HIV infection [4] and show that the extent of blood pDC loss is predictive of progression to disease [2, 13, 19, 20, 21, 43, 55]. Together, these findings are consistent with pDC playing an antiviral role in lymphoid tissues that is compromised in HIV and SIV infection because of progressive pDC depletion, resulting in lack of virus control and exacerbation of disease. The overwhelming loss of pDC from lymph nodes does not support the opposing scenario that pDC are pathogenic in SIV infection.
It is widely accepted that sustained immune activation is pathognomonic for HIV‐associated immune dysfunction [6, 17, 53, 56], and recent comparative studies in progressive and non‐progressive non‐human primate SIV hosts support this paradigm [7, 29, 30]. However, the role for pDC and in particular pDC‐derived IFN in this pathogenesis is unclear. Our studies revealed that pDC responsiveness to exogenous TLR7 stimulation was largely unimpaired in SIV‐infected rhesus macaques [10], but we still do not know the contribution of the limited number of remaining pDC to the endogenous levels of IFN and other inflammatory cytokines in acutely and chronically infected lymph nodes. Certainly, other cells including myeloid DC, macrophages, NK cells and activated T cells could contribute to this sustained inflammatory response [54]. Moreover, even if pDC are seen to be the primary producers of type I IFN in lymph nodes during pathogenic SIV and HIV infection, it is still not clear whether IFN is itself detrimental or beneficial in disease or simply a marker of immune activation. Notably, mice receiving chronic administration of TLR7 agonist have pronounced lymph node changes resembling HIV immunopathology, but these effects are independent of IFN [1], suggesting that in this species other cytokines or factors may be central to immune activation.
Given the apparently conflicting reports in the literature, studies aimed at directly manipulating pDC activation and the IFN pathway in both non‐progressive and progressive SIV hosts are now needed to clarify the role of pDC in virus control and immune activation in both acute and chronic phases of infection. A detailed analysis of the pDC response to antiretroviral therapy may also be revealing, given that effective therapy rapidly shuts down immune activation in lymph nodes [33]. Ultimately, the role of pDC in control or exacerbation of SIV infection may only be determined following antibody‐mediated depletion of this subset, as has been used to great effect in murine infectious disease models [51, 59].
This work was supported by research grant AI071777 to SMBB and training grant AI065380 to KNB from the US National Institutes of Health.
References
- 1. Baenziger S, Heikenwalder M, Johansen P, Schlaepfer E, Hofer U, Miller RC, Diemand S, Honda K, Kundig TM, Aguzzi A, Speck RF: Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV‐mediated pathology. Blood 2009; 113:377–88. [DOI] [PubMed] [Google Scholar]
- 2. Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC: Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1‐infected individuals. J Infect Dis 2003; 187:26–37. [DOI] [PubMed] [Google Scholar]
- 3. Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N: Endocytosis of HIV‐1 activates plasmacytoid dendritic cells via Toll‐like receptor‐viral RNA interactions. J Clin Invest 2005; 115:3265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, Debernardo R, Garate K, Rodriguez B, Margolis LB, Lederman MM: Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV‐1. Blood 2007; 109:4272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boasso A, Hardy AW, Anderson SA, Dolan MJ, Shearer GM: HIV‐induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS ONE 2008; 3:e2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boasso A, Shearer GM: Chronic innate immune activation as a cause of HIV‐1 immunopathogenesis. Clin Immunol 2008; 126:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ: Global genomic analysis reveals rapid control of a robust innate response in SIV‐infected sooty mangabeys. J Clin Invest 2009; 119:3556–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown KN, Barratt‐Boyes SM: Surface phenotype and rapid quantification of blood dendritic cell subsets in the rhesus macaque. J Med Primatol 2009; 38:272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown KN, Trichel A, Barratt‐Boyes SM: Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol 2007; 178:6958–67. [DOI] [PubMed] [Google Scholar]
- 10. Brown KN, Wijewardana V, Liu X, Barratt‐Boyes SM: Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog 2009; 5:e1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M: Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med 1999; 5:919–23. [DOI] [PubMed] [Google Scholar]
- 12. Cervantes‐Barragan L, Zust R, Weber F, Spiegel M, Lang KS, Akira S, Thiel V, Ludewig B: Control of coronavirus infection through plasmacytoid dendritic‐cell‐derived type I interferon. Blood 2007; 109:1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ: Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV‐infected individuals. J Immunol 2002; 168:4796–801. [DOI] [PubMed] [Google Scholar]
- 14. Choi YK, Fallert BA, Murphey‐Corb MA, Reinhart TA: Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood 2003; 101:1684–91. [DOI] [PubMed] [Google Scholar]
- 15. Coates PT, Barratt‐Boyes SM, Zhang L, Donnenberg VS, O’Connell PJ, Logar AJ, Duncan FJ, Murphey‐Corb M, Donnenberg AD, Morelli AE, Maliszewski CR, Thomson AW: Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood 2003; 102:2513–21. [DOI] [PubMed] [Google Scholar]
- 16. Colonna M, Trinchieri G, Liu YJ: Plasmacytoid dendritic cells in immunity. Nat Immunol 2004; 5:1219–26. [DOI] [PubMed] [Google Scholar]
- 17. Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM: Immune activation set point during early HIV infection predicts subsequent CD4+ T‐cell changes independent of viral load. Blood 2004; 104:942–7. [DOI] [PubMed] [Google Scholar]
- 18. Diebold SS, Kaisho T, Hemmi H, Akira S, Reis ESousaC: Innate antiviral responses by means of TLR7‐mediated recognition of single‐stranded RNA. Science 2004; 303:1529–31. [DOI] [PubMed] [Google Scholar]
- 19. Donaghy H, Gazzard B, Gotch F, Patterson S: Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV‐1. Blood 2003; 101:4505–11. [DOI] [PubMed] [Google Scholar]
- 20. Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S: Loss of blood CD11c(+) myeloid and CD11c(‐) plasmacytoid dendritic cells in patients with HIV‐1 infection correlates with HIV‐1 RNA virus load. Blood 2001; 98:2574–6. [DOI] [PubMed] [Google Scholar]
- 21. Feldman S, Stein D, Amrute S, Denny T, Garcia Z, Kloser P, Sun Y, Megjugorac N, Fitzgerald‐Bocarsly P: Decreased interferon‐alpha production in HIV‐infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol 2001; 101:201–10. [DOI] [PubMed] [Google Scholar]
- 22. Fitzgerald‐Bocarsly P, Jacobs ES: Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol 2010; 87:609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fong L, Mengozzi M, Abbey NW, Herndier BG, Engleman EG: Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J Virol 2002; 76:11033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A, Liu YJ, Lifson JD, Littman DR, Bhardwaj N: Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol 2004; 78:5223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ: The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)‐3 and CD40‐ligand. J Exp Med 1997; 185:1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gurney KB, Colantonio AD, Blom B, Spits H, Uittenbogaart CH: Endogenous IFN‐alpha production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV‐1 infection. J Immunol 2004; 173:7269–76. [DOI] [PubMed] [Google Scholar]
- 27. Hardy AW, Graham DR, Shearer GM, Herbeuval JP: HIV turns plasmacytoid dendritic cells (pDC) into TRAIL‐expressing killer pDC and down‐regulates HIV coreceptors by Toll‐like receptor 7‐induced IFN‐alpha. Proc Natl Acad Sci U S A 2007; 104:17453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herbeuval JP, Hardy AW, Boasso A, Anderson SA, Dolan MJ, Dy M, Shearer GM: Regulation of TNF‐related apoptosis‐inducing ligand on primary CD4+ T cells by HIV‐1: role of type I IFN‐producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 2005; 102:13974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre‐Sinoussi F, Benecke A, Muller‐Trutwin MC: Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest 2009; 119:3544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG: Transcriptional profiling in pathogenic and non‐pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog 2009; 5:e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levy JA, Scott I, Mackewicz C: Protection from HIV/AIDS: the importance of innate immunity. Clin Immunol 2003; 108:167–74. [DOI] [PubMed] [Google Scholar]
- 32. Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz‐Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT: Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009; 458:1034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Q, Schacker T, Carlis J, Beilman G, Nguyen P, Haase AT: Functional genomic analysis of the response of HIV‐1‐infected lymphatic tissue to antiretroviral therapy. J Infect Dis 2004; 189:572–82. [DOI] [PubMed] [Google Scholar]
- 34. Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M: Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol 2007; 8:578–83. [DOI] [PubMed] [Google Scholar]
- 35. Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA: Recognition of single‐stranded RNA viruses by Toll‐like receptor 7. Proc Natl Acad Sci USA 2004; 101:5598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lund JM, Linehan MM, Iijima N, Iwasaki A: Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol 2006; 177:7510–4. [DOI] [PubMed] [Google Scholar]
- 37. Malleret B, Karlsson I, Maneglier B, Brochard P, Delache B, Andrieu T, Muller‐Trutwin M, Beaumont T, McCune JM, Banchereau J, Le GrandR, Vaslin B: Effect of SIVmac infection on plasmacytoid and CD1c+ myeloid dendritic cells in cynomolgus macaques. Immunology 2008; 124:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Manches O, Bhardwaj N: Resolution of immune activation defines nonpathogenic SIV infection. J Clin Invest 2009; 119:3512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, Barrat FJ, Coffman RL, Staprans SI, Feinberg MB: Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med 2008; 14:1077–87. [DOI] [PubMed] [Google Scholar]
- 40. Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, Teigen N, Streeck H, Stellbrink HJ, Hellman J, Van LunzenJ, Altfeld M: MyD88‐dependent immune activation mediated by human immunodeficiency virus type 1‐encoded Toll‐like receptor ligands. J Virol 2007; 81:8180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meyers JH, Justement JS, Hallahan CW, Blair ET, Sun YA, O’Shea MA, Roby G, Kottilil S, Moir S, Kovacs CM, Chun TW, Fauci AS: Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS ONE 2007; 2:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muller‐Trutwin M, Hosmalin A: Role for plasmacytoid dendritic cells in anti‐HIV innate immunity. Immunol Cell Biol 2005; 83:578–83. [DOI] [PubMed] [Google Scholar]
- 43. Pacanowski J, Kahi S, Baillet M, Lebon P, Deveau C, Goujard C, Meyer L, Oksenhendler E, Sinet M, Hosmalin A: Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV‐1 infection. Blood 2001; 98:3016–21. [DOI] [PubMed] [Google Scholar]
- 44. Reeves RK, Fultz PN: Characterization of plasmacytoid dendritic cells in bone marrow of pig‐tailed macaques. Clin Vaccine Immunol 2008; 15:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reeves RK, Fultz PN: Disparate effects of acute and chronic infection with SIVmac239 or SHIV‐89.6P on macaque plasmacytoid dendritic cells. Virology 2007; 365:356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reinhart TA, Fallert BA, Pfeifer ME, Sanghavi S, Capuano S 3rd, Rajakumar P, Murphey‐Corb M, Day R, Fuller CL, Schaefer TM: Increased expression of the inflammatory chemokine CXC chemokine ligand 9/monokine induced by interferon‐gamma in lymphoid tissues of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. Blood 2002; 99:3119–28. [DOI] [PubMed] [Google Scholar]
- 47. Sarkar S, Kalia V, Murphey‐Corb M, Montelaro RC, Reinhart TA: Expression of IFN‐gamma induced CXCR3 agonist chemokines and compartmentalization of CXCR3+ cells in the periphery and lymph nodes of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. J Med Primatol 2003; 32:247–64. [DOI] [PubMed] [Google Scholar]
- 48. Schmidt B, Scott I, Whitmore RG, Foster H, Fujimura S, Schmitz J, Levy JA: Low‐level HIV infection of plasmacytoid dendritic cells: onset of cytopathic effects and cell death after PDC maturation. Virology 2004; 329:280–8. [DOI] [PubMed] [Google Scholar]
- 49. Scholzen T, Gerdes J: The Ki‐67 protein: from the known and the unknown. J Cell Physiol 2000; 182:311–22. [DOI] [PubMed] [Google Scholar]
- 50. Sedaghat AR, German J, Teslovich TM, Cofrancesco J Jr, Jie CC, Talbot CC Jr, Siliciano RF: Chronic CD4+ T‐cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon‐mediated disruption of T‐cell dynamics. J Virol 2008; 82:1870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shen H, Iwasaki A: A crucial role for plasmacytoid dendritic cells in antiviral protection by CpG ODN‐based vaginal microbicide. J Clin Invest 2006; 116:2237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shortman K, Liu YJ: Mouse and human dendritic cell subtypes. Nat Rev Immunol 2002; 2:151–61. [DOI] [PubMed] [Google Scholar]
- 53. Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, Staprans SI, Feinberg MB: Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high‐level viremia. Immunity 2003; 18:441–52. [DOI] [PubMed] [Google Scholar]
- 54. Sodora DL, Silvestri G: Immune activation and AIDS pathogenesis. Aids 2008; 22:439–46. [DOI] [PubMed] [Google Scholar]
- 55. Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu YJ: Depletion of circulating natural type 1 interferon‐producing cells in HIV‐infected AIDS patients. Blood 2001; 98:906–12. [DOI] [PubMed] [Google Scholar]
- 56. Sousa AE, Carneiro J, Meier‐Schellersheim M, Grossman Z, Victorino RM: CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV‐1 and HIV‐2 but only indirectly to the viral load. J Immunol 2002; 169:3400–6. [DOI] [PubMed] [Google Scholar]
- 57. Stranges PB, Watson J, Cooper CJ, Choisy‐Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, Chervonsky AV: Elimination of antigen‐presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 2007; 26:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Teleshova N, Kenney J, Jones J, Marshall J, Van NestG, Dufour J, Bohm R, Lifson JD, Gettie A, Pope M: CpG‐C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN‐gamma‐secreting simian immunodeficiency virus‐specific T cells. J Immunol 2004; 173:1647–57. [DOI] [PubMed] [Google Scholar]
- 59. Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, Narumi S, Lu B, Gerard C, Ishikawa S, Matsushima K: Plasmacytoid DCs help lymph node DCs to induce anti‐HSV CTLs. J Exp Med 2005; 202:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, Narumi S, Morikawa S, Ezaki T, Lu B, Gerard C, Ishikawa S, Matsushima K: Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol 2004; 16:915–28. [DOI] [PubMed] [Google Scholar]


