Abstract
Background: New food biomarkers are needed to objectively evaluate the effect of diet on health and to check adherence to dietary recommendations and healthy eating patterns.
Objective: We developed a strategy for food biomarker discovery, which combined nutritional intervention with metabolic phenotyping and biomarker validation in a large-scale epidemiologic study.
Design: We administered a standardized diet to 8 individuals and established a putative urinary biomarker of fruit consumption by using 1H nuclear magnetic resonance (NMR) spectroscopic profiling. The origin of the biomarker was confirmed by using targeted NMR spectroscopy of various fruit. Excretion kinetics of the biomarker were measured. The biomarker was validated by using urinary NMR spectra from UK participants of the INTERMAP (International Collaborative Study of Macronutrients, Micronutrients, and Blood Pressure) (n = 499) in which citrus consumption was ascertained from four 24-h dietary recalls per person. Finally, dietary patterns of citrus consumers (n = 787) and nonconsumers (n = 1211) were compared.
Results: We identified proline betaine as a putative biomarker of citrus consumption. High concentrations were observed only in citrus fruit. Most proline betaine was excreted ≤14 h after a first-order excretion profile. Biomarker validation in the epidemiologic data showed a sensitivity of 86.3% for elevated proline betaine excretion in participants who reported citrus consumption and a specificity of 90.6% (P < 0.0001). In comparison with noncitrus consumers, citrus consumers had lower intakes of fats, lower urinary sodium-potassium ratios, and higher intakes of vegetable protein, fiber, and most micronutrients.
Conclusion: The biomarker identification and validation strategy has the potential to identify biomarkers for healthier eating patterns associated with a reduced risk of major chronic diseases. The trials were registered at clinicaltrials.gov as NCT01102049 and NCT01102062.
INTRODUCTION
Nutritional factors play a major underlying role in the causation of the global burden of chronic disease; specifically, a healthy diet rich in fruit and vegetables is associated with lower rates of cancers, diabetes, cardiovascular diseases, and related risk factors such as raised blood pressure and serum cholesterol (1–6). Focus has shifted from examining single nutrient relations with disease towards analyzing complex nutrient interactions and dietary patterns to define a more holistic relation between nutrition and associated diseases (7, 8). A food pattern high in fruit, vegetables, fish, whole grains, and legumes shows inverse correlations with features of metabolic syndrome (9), risk of colorectal cancer (10), and adverse blood pressure and serum lipid profiles (5, 11, 12).
Methods to assess dietary intakes of free-living populations rely mainly on questionnaire data that are subject to possible reporting and other biases (13). Objective measures that use biomarkers are needed to validate dietary assessment and check adherence to dietary recommendations and healthy eating patterns, but few such biomarkers are available (14), including markers for fruit and vegetable intake (15). The development of robust food biomarkers may help to improve disease risk stratification by better characterizing the metabolic phenotype at the individual level. Numerous studies that addressed single food components or nutrients have been conducted in small scale laboratory studies but few, if any, have been translated into free-living population studies. We show the use of high-throughput screening by 1H nuclear magnetic resonance (NMR) spectroscopy for citrus fruit biomarker discovery and its application to a large-scale population survey. High-resolution spectral analyses, typically NMR or mass spectrometry, have been used to generate metabolic signatures from biological samples and obtain complex profiles of a wide range of metabolite classes (16–18). Population-based studies have shown marked differences in metabolic profiles within and between populations that reflect, in part, dietary differences as key components of the complex interplay between environmental and genetic influences on disease risk (19); effects of dietary interventions on the metabolic phenotype have also been explored (20–23). In the current study, we outline a strategy for a biomarker discovery for healthy eating that is exemplified by citrus fruit consumption. Specifically, we combined nutritional intervention, metabolic profiling and biomarker cross-validation in large-scale epidemiologic data.
SUBJECTS AND METHODS
Fruit-intervention study
A laboratory study was designed to detect urinary biomarkers of fruit consumption by using a nontargeted metabolic profiling approach. The study was a fruit-meal intervention that involved 8 volunteers (7 women and 1 man; age range: 28–45 y) who met the following inclusion criteria: participants were healthy, aged 18–45 y, and nonsmokers and had a body mass index (BMI; in kg/m2) 18–25, an absence of regular drug intake and regular food supplement intake, and no antibiotic use within the previous 3 mo. Participants consumed a standardized breakfast, lunch, and dinner that comprised whole-grain bread and cheese (breakfast), a ham sandwich (lunch), and pasta and tomato sauce (dinner) from the run-in day (day 0) until lunch on day 3. In addition to the standard dinner, a supplementary mixed-fruit meal (apple, orange, grapes, and grapefruit) was introduced on the evening of day 2. Urine was collected 4 times/d (first morning urine, before lunch, before dinner, and at bed time), from the morning of day 1 until the evening of day 3. Urine specimens were collected into sterile tubes (Sterilin, Aberbargoed, United Kingdom) and stored at −40°C until analysis. The study was approved by Imperial College London Research Ethics Committee.
Food-analysis study
To ascertain the relative concentrations of proline betaine in fruit and fruit juices and to obtain an estimate of the specificity of proline betaine as a marker of citrus consumption, the relative concentrations of proline betaine were assessed in a number of fruit and commercially available fruit juices. Proline betaine quantification of fruit juices and fruit extractions was carried out with a fully relaxed water-presaturated 1H NMR technique. Three samples for each citrus fruit and citrus fruit juice and one sample for other fruit and fruit juices were analyzed. For the fruit extracts, whole fruit was homogenized, centrifuged for 5 min at 16,000 × g, and the supernatant fluid was used as a fruit extract. An aliquot of 200 μL fruit juice or fruit extract was dissolved in 800 μL phosphate D2O buffer (0.1 mol/L; pH 7.4) that contained 0.35 mmol sodium 3-(trimethylsilyl)-propionate-d4 (TSP). Samples were vortexed and centrifuged for 5 min at 16,000 × g. A 600-μL sample of the supernatant fluid was transferred into 5-mm outer-diameter NMR tubes for analysis by 1H NMR spectroscopy.
Assessment of the excretion profile of proline betaine after orange juice consumption
A study was undertaken to characterize the excretion profile of proline betaine after orange juice consumption. Six volunteers (4 women and 2 men; age range: 24–36 y) who met the inclusion criteria of being healthy, aged 18–45 y, and nonsmokers and had a BMI of 18–25 kg/m2 and an absence of regular drug intake and regular food supplement intake were recruited. Participants consumed a restricted diet that excluded all citrus fruit, ethnic foods, alcohol, grain legumes, and cheese. Day 0 was a run-in day; spot urine specimens were collected on day 1 (ie, baseline samples, 6 times/d, collected at 0800, 1000, 1200, 1600, and 2000 and at bed time); an orange juice challenge (250 mL orange juice) was administered on day 2 at 1000, and spot urine was collected at the same times as on day 1 and continued until 1000 on day 3 (study finish). Urine specimens were collected in sterile tubes (Sterilin) and stored at −40°C until analysis. The study was approved by Imperial College London Research Ethics Committee.
1H NMR spectroscopic analyses
1H NMR spectra were acquired on a Bruker 600 MHz spectrometer (Bruker Analytische GmbH, Rheinstetten, Germany) that operated at 600.13 MHz. The spectrometer used a standard one-dimensional pulse-sequence [recycle delay (RD)-90°-t1-90°-mixing time (tm)-90°-acquire] free-induction decay (FID) with water-suppression irradiation during a RD of 2 s with tm set to 100 ms and a 90° pulse set to 10 μs. Spectra were acquired with 128 scans into 32-K data points with a spectral width of 12,000 Hz. Fruit spectra were acquired by using a standard one-dimensional pulse sequence [RD-90°-t1-90°-tm-90°-acquire FID] with water suppression that irradiated during a RD of 2 s with tm set to 100 ms and a 90° pulse set to 10 μs. The proline betaine and TSP resonances were fully relaxed after a delay time tau of 1.25 and 3 s, respectively, resulting in a spin-lattice relaxation time T1 (=tau/ln2) of 4.33 s. The interpulse delay time d1 (=5 × T1) was therefore set to 21.64 s (24). For spectral processing, FIDs were multiplied by an exponential function that corresponded to line broadening of 0.3 Hz before Fourier transformation. All spectra were manually phased, baseline corrected, and calibrated to TSP [δ (chemical shift) 0] and exported into Matlab software (2009a; The MathWorks Inc, Natick, MA) for data analysis. The spectra were normalized by using a probabilistic quotient normalization algorithm (25) to account for urinary dilution effects and aligned by using a recursive segment-wise peak-alignment algorithm (26).
Multivariate data analyses
All timed 24-h urine specimens of the nutrition study that were collected on day 1 in the morning until evening of day 2 (before the fruit meal) were defined as standard class; specimens collected after the fruit meal (bed time of day 2 until the evening of day 3) were defined as fruit class. Pairwise partial least-squares discriminant analysis (PLS-DA) (27) was carried out with Matlab software (2009a; The MathWorks Inc). The PLS-DA loading plots were created with an in-house Matlab (2009a; The MathWorks Inc) script according to the method of Cloarec et al (28).
Evaluation of urinary proline betaine excretion in an epidemiologic study and comparison with dietary recall data
The use of proline betaine excretion as a marker of citrus intake and as a surrogate marker of healthier eating patterns was evaluated by using a multipopulation epidemiologic study [International Collaborative Study of Macronutrients, Micronutrients, and Blood Pressure (INTERMAP)] with robust dietary recall questionnaire data. INTERMAP is a large-scale epidemiologic study that involves men and women aged 40–59 y from 17 population samples in China, Japan, the United Kingdom, and the United States. The study investigates the role of multiple dietary factors in causes of raised blood pressure, which includes the use of the metabolome-wide association approach to identify blood pressure–related biomarkers of dietary patterns (19). Individuals were selected randomly from population lists and stratified for age and sex. A 24-h urine specimen and 2 consecutive in-depth multipass 24-h dietary recalls were collected from each participant, and the procedure was repeated after 3 wk (29, 30). The two 24-h urine collections were obtained on days 2 and 4, which corresponded to the first and second and third and fourth dietary recalls, respectively. For the prediction of citrus fruit intake on the basis of proline betaine excretion, we used the Belfast (UK) sample (n = 220) as the training set and the West Bromwich (UK) sample (n = 279) as the validation set. We quantified proline betaine by peak integration (δ 3.106–3.116) by using the 24-h urinary 1H NMR spectral data from the first of the two 24-h collections from each participant. Standard sample-preparation procedures, 1H NMR–acquisition variables, and spectral processing methods were used as described by Holmes et al (19). INTERMAP received institutional ethics committee approval for each site, all participants gave written consent, and all procedures were in accordance with institutional guidelines. The 24-h dietary recall data for the 2 UK-population samples (Belfast and West Bromwich) were coded by using the UK FOODBASE database (version 1.3; Institute of Brain Chemistry, London, United Kingdom) (31). From this database, information on all citrus fruit and citrus fruit juices consumed was obtained. Citrus consumption was classified as any case in which citrus fruit had been reported as being consumed, regardless of the quantity, with the exception of lemon or lime juice used in cooking because the amount used in such cases generally was <10 g.
Receiver operating characteristicndashcurve comparison of 1H NMRndashcalculated urinary proline betaine excretion values with 24-h food-record data
A receiver operating characteristic (ROC) curve was constructed by using information on citrus fruit consumption in the 24-h food-record data for identification of citrus consumers and noncitrus consumers. Every participant who recorded citrus consumption was defined as a citrus consumer. The ROC curve was a plot of sensitivity compared with 1 − specificity for all possible values of cutoffs from proline betaine integrals of the NMR spectral data.
The optimal operating point [shortest distance from the optimal point (0,1) to the intersect of the ROC curve (32)] was used to measure the optimal cutoff for which specificity and sensitivity were calculated.
Classification of citrus consumption on the basis of urinary proline betaine concentrations and association of spectroscopically calculated citrus consumption with nutrient intakes, BMI, and blood pressure
Participants from the INTERMAP US and UK samples were classified as citrus consumers or noncitrus consumers by using the urinary proline betaine integral. Individuals with proline betaine >39.4 in both 24-h urine collections (n = 787) were classified as citrus consumers, and noncitrus consumers were defined as individuals with a urinary proline betaine integral ≤39.4 in both 24-h collections (n = 1211). Individuals with a urinary proline betaine integral >39.4 in one 24-h collection only (n = 645) were excluded. Mean values of selected nonnutrient and nutrient variables from all four 24-h dietary recalls were compared between the 2 groups with adjustment for age, sex, and country with SAS 9.1 software (SAS Institute, Cary, NC).
RESULTS
Untargeted 1H NMR metabolite profiling
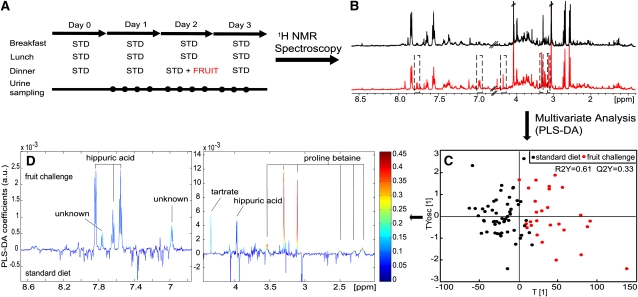
To identify novel biomarkers of fruit consumption, we conducted a human dietary intervention trial (n = 8) and evaluated the effect of a mixed-fruit meal, which consisted of apples, grapes, oranges, and grapefruit, on the urinary metabolome (Figure 1A). Participants' diets were standardized over 4 d, and the mixed-fruit meal was introduced on day 2. Urine was collected 4 times/d for 3 d and analyzed by using multivariate modeling of spectroscopic profile data. The urinary metabolite profiles, as measured by 600 MHz 1H NMR spectroscopy, revealed apparent metabolic changes in urine profiles between prefruit and postfruit meal consumption (Figure 1B). Further biochemical information was extracted via application of PLS-DA (Figure 1, C and D) in which we observed an increased excretion of proline betaine, tartaric acid, hippuric acid, and an unknown benzoic acid metabolite after fruit consumption compared with after consumption of a baseline diet (Figure 1D). Of those compounds, proline betaine showed the strongest correlation (r2 = 0.4; P < 0.0001) with fruit ingestion; therefore, we investigated its dietary origin and characterized its urinary excretion kinetics.
FIGURE 1.
Identification of putative biomarkers by using metabolite profiling and multivariate analysis. A: Study design for the dietary intervention study (n = 8). B: Representative 1H nuclear magnetic resonance (NMR) spectra of urine specimens in response to fruit consumption (red) compared with the standard (STD) meal (black). Apparent differences are highlighted (dashed rectangles). C: Partial least-squares discriminant analysis (PLS-DA) scores plot of urine specimens 0–24 h after fruit challenge, which shows a clear separation of the fruit and STD meals. All urine specimens from the morning of day 1 to the evening of day 2 were allocated to the STD diet, and all urine specimens collected after consumption of the fruit meal (bed time of day 2 until evening of day 3) were allocated to the fruit class. D: Loading plots of the fruit challenge compared with the STD meal indicated the following putative biomarkers for fruit consumption: hippuric acid (δ 2.97d, 7.55t, 7.64t, and 7.84d), proline betaine (δ 2.18m, δ 2.30m, δ 2.50m, δ 3.11, δ 3.31, and δ 3.54), tartaric acid (δ 4.34s), and unknown (δ 7.74d and δ 6.98d). The P value of proline betaine before fruit consumption compared with after fruit consumption was <0.0001. ppm, parts per million; a.u., arbitrary units; Y, response variable (classification identifier); R2Y, variation of Y modeled; Q2Y, cross-validated variation of Y predicted; T[1], first predictive PLS scores vector; Tyosc [1], first orthogonal PLS score vector.
Quantification of proline betaine in fruit and fruit juices
To assign the origin of urinary proline betaine excretion after the fruit challenge, we measured concentrations of proline betaine in selected fruit and commercially available fruit juices by using a standard 1H NMR experiment optimized for quantification of this compound (Table 1). All citrus fruit tested contained proline betaine. Concentrations of proline betaine varied depending on the type of citrus fruit (orange > lime > satsumas > grapefruit > lemon) and the method of juice processing (commercially processed > freshly squeezed) with values ≤1316 mg proline betaine/L for orange juice from concentrate. In contrast, the proline betaine signal was of a low intensity in spectra of the other commonly available fruit and fruit juices tested (Table 1).
TABLE 1.
Proline betaine concentrations in fruit and fruit juices measured by 1H nuclear magnetic resonance spectroscopy
| Juice/fruit description | Proline betaine |
| mg/L | |
| Citrus fruit juice (authentic; all n = 3) | |
| Orange juice | |
| From concentrate | 1316 ± 721 |
| Not from concentrate | 1189 ± 24 |
| Freshly squeezed | 1062 ± 81 |
| Grapefruit juice | 766 ± 93 |
| Citrus fruit juice (synthetic; all n = 1) | |
| Orange soft drink | 216 |
| Orange squash | 75 |
| Citrus fruit (all n = 3) | |
| Orange | 761 ± 89 |
| Lime | 730 ± 126 |
| Satsuma | 461 ± 55 |
| Lemon | 251 ± 153 |
| Other fruit juice (all n = 1) | |
| Pineapple juice | 57 |
| Red grape and raspberry juice | 46 |
| Pomegranate and blueberry juice | 18 |
| Peach, mango, passion fruit juice | 17 |
| Apple juice | 14 |
| Black currant juice | 12 |
| Other fruit (all n = 1) | |
| Kiwi | 66 |
| Grape | 51 |
| Melon | 34 |
| Banana | 28 |
| Strawberry | 22 |
| Pear | 14 |
| Apricot | 10 |
Mean ± SD (all such values); the SD is indicated when more than one sample was analyzed.
Excretion kinetics of proline betaine after orange juice consumption
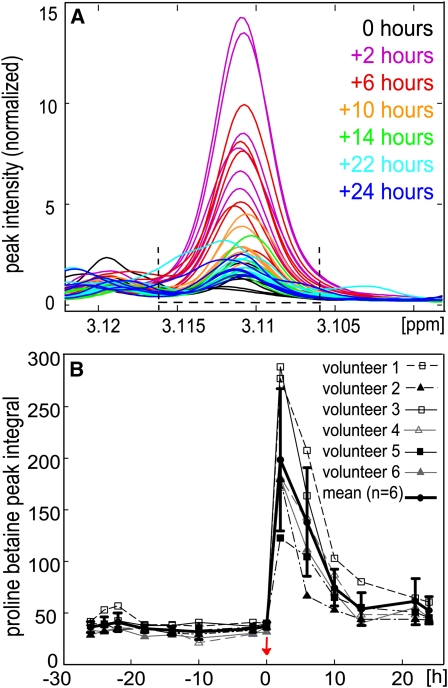
We investigated proline betaine as a potential biomarker for citrus fruit intake by measuring urinary proline betaine excretion in 6 individuals after consumption of 250 mL orange juice. Urine specimens were collected before (−26 to 0 h) and after (+2 to +24 h) the orange juice challenge (Figure 2, A and B). The singlet peak at δ 3.11, which represented the CH3 moiety of proline betaine, showed a minimal overlap with other peaks in the spectrum. Excretion of proline betaine was rapid and peaked in all individuals at the 2-h postintervention collection, as calculated from the spectral integral [spectra normalized to probabilistic quotient (25) over the region δ 3.106–3.116]. In all participants, concentrations declined to almost baseline after >24 h, with most proline betaine excretion occurring in the first 14 h (83% of 24-h excretion) (Figure 2B).
FIGURE 2.
Urinary excretion kinetics of proline betaine after orange juice consumption (n = 6). A: Proline betaine singlet at δ 3.11 was integrated over the spectral region δ 3.106–3.116 as shown where the peak overlap is minimal. B: Mean and SD proline betaine integral (solid bold line) and the proline betaine integral for each of the 6 volunteers plotted over time. The red arrow indicates the time of orange juice consumption. ppm, parts per million.
Validation in epidemiologic data of proline betaine as a biomarker for citrus fruit intake
To validate our experimental findings, we investigated proline betaine as a potential biomarker of citrus fruit consumption in a free-living human population by using 1H NMR spectral data obtained from the 2 INTERMAP UK urine collections per individual (29). The technical error of proline betaine quantification from 1H NMR spectra was 2.64% calculated from data on specimens split in the field and blinded to the laboratory (n = 40; 8% of total samples) by using the formula
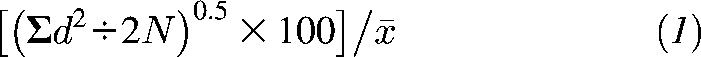 |
where d is the within-pair difference, N is the number of split-sample pairs, and is the mean of all split-sample values (33).
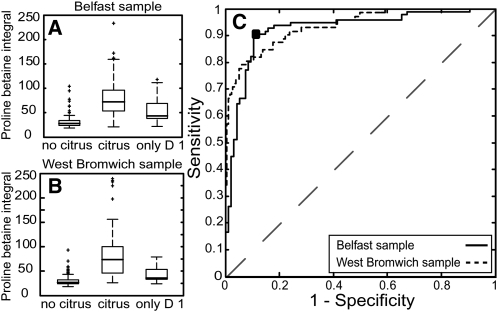
On the basis of the excretion kinetics (Figure 2B), which indicated that most urinary excretion of proline betaine occurred in ≤14 h, the citrus consumption reported for the morning of the previous day (>20 h before the start of urine collection) was not expected to affect urinary proline betaine concentrations, whereas citrus consumption on the previous evening (<12 h before the start of urine collection) might have resulted in the elevation of urinary proline betaine concentrations (see supplemental Figure 1 under “Supplemental data” in the online issue). Therefore, we categorized reported citrus fruit intake from the 24-h recall data into 3 subgroups as follows: no recorded citrus intake, recorded citrus intake (citrus intake on days 1 and 2 or day 2 only), and recorded citrus intake on day 1 only. Proline betaine concentrations differed significantly between individuals with no recorded citrus consumption and individuals with recorded citrus consumption of proline betaine in the training set and validation set (Belfast sample: P < 0.0001; West Bromwich sample: P < 0.0001; Figure 3, A and B). In noncitrus consumers who excreted proline betaine, we observed no consistent pattern of blue or brie cheese consumption or other foods reported to contain low amounts of proline betaine (34). ROC curves for proline betaine were derived for the training and validation sets with an area under the curve (AUC) of 92.3% and 93.5%, respectively (Figure 3C). A cutoff was calculated from the optimal operating point on the ROC curve of the training set with a threshold of 39.4 for the proline betaine integral. This optimal point had a specificity and sensitivity of 90.6% and 86.2% for the training set and 92.3% and 80.6% for the validation set, respectively. To provide further biomarker validation, we repeated these analyses with use of the ratio of proline betaine and creatinine. Sensitivity and specificity were similar for ROC curves constructed from the proline betaine:creatinine ratio (89.1% and 83.9%, respectively; AUC: 93.5%); similar results were also obtained for the second rather than the first 24-h urine collection (90.2% and 84.8%, respectively; AUC: 90.7%) (see supplemental Table 1 under “Supplemental data” in the online issue).
FIGURE 3.
Box plots of urinary proline betaine excretion of volunteers recording no citrus fruit consumption (no citrus), citrus fruit consumption (citrus), and citrus fruit consumption only on day 1 (only D 1). A: Proline betaine in the Belfast (UK) sample (no citrus: n = 96; citrus: n = 96; only D 1: n = 28). B: Proline betaine in the West Bromwich (UK) sample (no citrus: n = 181; citrus: n = 71; only D 1: n = 27). C: Receiver operating characteristic curves to assess the predictive ability of excretion of proline betaine for discrimination of citrus fruit intake and no citrus fruit intake as reported in the dietary recall data for the training set [International Collaborative Study of Macronutrients, Micronutrients, and Blood Pressure (INTERMAP) UK Belfast sample] and test set (INTERMAP UK West Bromwich sample). The optimal operating point (▪) for the training set was a peak integral value of 39.4 for proline betaine. This represented a specificity and sensitivity of 90.6% and 86.3%, respectively, for the training set and 92.3% and 80.6%, respectively, for the validation set.
Comparison of nutrient intakes, BMI, and blood pressure between citrus fruit consumers and nonconsumers
We classified participants from the INTERMAP US and UK samples as citrus consumers and noncitrus consumers on the basis of their urinary proline betaine excretions. Citrus consumers reported higher intakes of carbohydrates (49.2% compared with 45.5% of energy) and vegetable protein (5.8% compared with 5.4% of energy) and less total fat (31.0% compared with 34.2% of energy) and animal protein (9.7% compared with 10.2% of energy) (all P < 0.0001) compared with noncitrus consumers; intakes of total saturated fatty acids, monounsaturated fatty acids, trans fatty acids, polyunsaturated fatty acids, omega-6 fatty acids, and cholesterol were also lower in citrus consumers (Table 2). Most differences were favorable in terms of a healthier diet (ie, a low-fat and high-carbohydrate diet that is rich in fruit, vegetables, and fiber). Citrus consumers ingested more total sugars (25.9% compared with 22.2% of energy) derived mainly from higher fructose (5.2% compared with 3.6% of energy) and glucose (5.3% compared with 3.8% of energy) intakes; fiber and most vitamin intakes (vitamin A, β-carotene, thiamine, pantothenic acid, vitamin B-6, vitamin C, and folic acid) and mineral intakes (copper, iron, magnesium, and urinary potassium) were also higher in citrus consumers, whereas the urinary sodium-potassium ratio was lower (2.9 compared with 2.3) (Table 2). The mean vitamin C intake for consumers was 17.4 mg vitamin C/1000 kJ (150.7 mg vitamin C/d) compared with 7.7 mg vitamin C/1000 kJ (65.5 mg vitamin C/d) for nonconsumers; therefore, as a group, nonconsumers were not meeting the US National Academy of Sciences recommendations for vitamin C intake [75–90 mg vitamin C/d (35)]. In addition, citrus consumers had a higher socioeconomic status assessed by education years (14.5 compared with 13.3 y), lower BMI (27.6 compared with 28.7), and lower systolic blood pressure (118.5 compared with 120.2 mm Hg; P < 0.005) (Table 3).
TABLE 2.
Selected nutrients from four 24-h dietary recalls per person with comparison for proline betaine–predicted citrus and noncitrus consumers from International Collaborative Study of Macronutrients, Micronutrients, and Blood Pressure UK and US participants (n = 1998)1
| Variable | Predicted noncitrus consumers (n = 1211) | Predicted citrus consumers (n = 787) | P |
| Energy (kJ/24 h) | 9214.8 | 9286.4 | 0.53 |
| Total SFA (% of energy) | 11.9 | 10.7 | <0.0001 |
| Total MUFA (% of energy) | 12.1 | 10.9 | <0.0001 |
| Total PUFA (% of energy) | 6.9 | 6.2 | <0.0001 |
| Cholesterol (mg/1000 kJ) | 31.5 | 28.1 | <0.0001 |
| Starch (% of energy) | 24.0 | 24.0 | 0.95 |
| Total sugars (% of energy) | 22.2 | 25.9 | <0.0001 |
| Fructose (% of energy) | 3.6 | 5.2 | <0.0001 |
| Glucose (% of energy) | 3.8 | 5.3 | <0.0001 |
| Fiber (g/1000 kJ) | 2.4 | 2.7 | <0.0001 |
| Vegetable protein (% of energy) | 5.4 | 5.8 | <0.0001 |
| Animal protein (% of energy) | 10.2 | 9.7 | 0.0003 |
| Urinary sodium (mmol/24 h) | 155.7 | 150.7 | 0.05 |
| Urinary potassium (mmol/24 h) | 59.2 | 69.2 | <0.0001 |
| Urinary sodium-potassium ratio (mmol:mmol) | 2.9 | 2.3 | <0.0001 |
| Calcium (mg/1000 kJ) | 95.5 | 99.9 | 0.003 |
| Magnesium (mg/1000 kJ) | 34.9 | 38.0 | <0.0001 |
| Phosphorus (mg/1000 kJ) | 148.8 | 151.9 | 0.02 |
| β-Carotene (μg/1000 kJ) | 315.8 | 427.8 | <0.0001 |
| Vitamin C (mg/1000 kJ) | 7.7 | 17.4 | <0.0001 |
| Folic acid (μg/1000 kJ) | 31.2 | 36.9 | <0.0001 |
All values are means and were adjusted for age, sex, and country by least-squares means from all 4 visits. SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid. Predicted citrus consumers had urinary proline betaine integrals >39.4 in both 24-h collections; predicted noncitrus consumers had urinary proline betaine integrals ≤39.4 in both 24-h collections. Individuals with a urinary proline betaine integral >39.4 in one 24-h collection only (n = 645) were excluded.
TABLE 3.
Blood pressure, BMI, and educational status in proline betaine–predicted citrus consumers and noncitrus consumers from International Collaborative Study of Macronutrients, Micronutrients, and Blood Pressure UK and US participants (n = 1998)1
| Variable | Predicted noncitrus consumers (n = 1211) | Predicted citrus consumers (n = 787) | P |
| BMI (kg/m2) | 28.7 ± 6.7 | 27.6 ± 6.4 | <0.0001 |
| Systolic blood pressure (mm Hg) | 120.2 ± 15.8 | 118.5 ± 15.1 | 0.005 |
| Diastolic blood pressure (mm Hg) | 75.3 ± 10.8 | 75.5 ± 10.3 | 0.64 |
| Education (y) | 13.3 ± 3.4 | 14.5 ± 3.2 | <0.0001 |
All values are means ± SDs and were adjusted for age, sex, and country by least-squares means from all 4 visits. Predicted citrus consumers had urinary proline betaine integrals >39.4 in both 24-h collections. Predicted noncitrus consumers had urinary proline betaine integrals ≤39.4 in both 24-h collections. Individuals with a urinary proline betaine integral >39.4 in one 24-h collection only (n = 645) were excluded.
DISCUSSION
We present a strategy for food biomarker discovery on the basis of untargeted metabolic profiling of urine specimens from a nutritional intervention study and the subsequent validation of the candidate biomarker by using epidemiologic data. We identify urinary excretion of proline betaine as a specific and sensitive biomarker of citrus fruit intake. Although there are several nutritional biomarkers, such as total urinary nitrogen or urea for protein intake and 24-h urinary sodium and potassium for sodium and potassium intake (30, 36), there are few validated biomarkers for specific foods, the most cited example being increased excretion of resveratrol after wine consumption (37).
Sample sizes used in nutritional intervention studies tend to be small, and dietary interventions are usually given in a controlled setting. Therefore, proposed food or nutrient biomarkers derived from such studies cannot always be extrapolated to population studies in free-living individuals. In the current study, we used the rich data resource of a well-validated large-scale epidemiologic study to establish whether the biomarker for citrus fruit is generalizable to populations and to ascertain whether it can act as a surrogate indicator of healthier eating patterns.
We first identified proline betaine as a candidate citrus fruit biomarker by analyzing urine specimens from a food intervention trial by 1H NMR untargeted metabolite profiling (ie, without preselection and prior knowledge of the metabolites to be measured). Our follow-up kinetics study showed that proline betaine was excreted rapidly in urine, and urinary excretion was nearly complete after 24 h, which indicated that proline betaine is metabolically inert or minimally metabolized in humans, which is in agreement with other reports in the literature (38). Because proline betaine is not metabolized, and the CH3 signal used in the measurement is in a relatively uncrowded spectral region and, therefore, not compromised by other metabolite signals, it is a robust indicator of citrus fruit intake and the quantity of citrus fruit consumed. Thus, the use of proline betaine as a biomarker gives a quantitative and qualitative measure of citrus fruit intake, (see supplemental Figure 2 under “Supplemental data” in the online issue) which is an important advantage over many questionnaire approaches that are qualitative or semiquantitative in nature (39, 40).
Proline betaine is known to act as an osmoprotectant in citrus fruit, alfalfa sprouts (41), molluscs (42), and bacteria (43). Previous reports identified proline betaine in orange juice (44), and in humans, proline betaine concentrations were reported to be increased in plasma and urine after orange juice consumption (45). From analysis of fruit and commercial juices, we ascertained that proline betaine concentrations varied according to the type of citrus fruit and the type of fruit processing (commercially processed compared with freshly squeezed juice), which suggests the importance of, eg, pulp extraction and pasteurization (46). This is consistent with reports that phenolic compounds and vitamin C concentrations increase with more vigorous juice extraction and squeezing techniques (47). We observed a higher (>6 times) proline betaine content in authentic (100%) orange juice than in orange soft drinks and orange squash that allowed us to differentiate between citrus fruit juices and synthetic citrus drinks, which may not always be correctly differentiated by participants in dietary recall studies.
Urinary excretion of proline betaine was also shown to have high specificity and sensitivity as a marker of citrus fruit intake in high-quality epidemiologic data by using the multipass 24-h dietary recall method as an independent measure of citrus fruit intake (30). Increased excretion of proline betaine after consumption of brie or blue cheese (34) and alfalfa sprouts has also been reported (48) but at substantially lower concentrations (approximately one-fifth the concentration of citrus fruit). Interrogation of the INTERMAP data did not find an association between blue or brie cheese consumption and proline betaine concentrations, and none of the participants in the INTERMAP UK samples reported consuming alfalfa sprouts. Thus in the INTERMAP sample, the measured proline betaine excretion was related overwhelmingly to citrus fruit intake because other dietary sources did not contribute to the proline betaine concentrations, and proline betaine is not synthesized or metabolized in the lumen.
We observed proline betaine to be a robust biomarker of citrus fruit consumption in Western populations. However, to our knowledge, this biomarker has not been validated in Eastern populations. Furthermore, because proline betaine is predominantly excreted ≤24 h after consumption, it can only be used as evidence of an acute intake of citrus fruit.
Although it is important to ascertain the effects of single nutrients or foods with respect to risk of diseases such as cardiovascular disease or cancers, more recently, there has been a shift in emphasis to address the relatively greater effect of dietary patterns with respect to disease risk. Thus, a food pattern dominated by fruit, vegetables, fish, whole grains, and legumes correlates negatively with attributes of the metabolic syndrome (9), cardiovascular disease risk factors (49), risk of colorectal cancer (10), and blood pressure and serum lipids (12). Our analyses of the INTERMAP UK and US samples showed that there were significant differences in dietary patterns for citrus fruit consumers than for nonconsumers, as confirmed by NMR-detected urinary proline betaine excretion. Citrus fruit consumers had a diet lower in total fats and urinary sodium-potassium ratios, higher intakes of vegetable protein, most micronutrients, and fiber, and lower BMI values and systolic blood pressure measurements, which conferred a lower risk of cardiovascular disease, than did noncitrus consumers (50).
In conclusion, we showed the use of a strategy that combines metabolome-wide association via untargeted metabolic profiling in nutritional intervention studies with validation in free-living populations to identify and validate biomarkers of food intake, which was exemplified in the current study by proline betaine for citrus fruit intake. This nutrimetabonomics approach to biomarker identification and verification should facilitate the evaluation of individual diets toward healthier eating to promote healthy lifestyles and longevity.
Supplementary Material
Acknowledgments
We thank TMD Ebbels and H Keun for providing in-house Matlab software and E Maibaum for performing 1H NMR acquisition. We also thank the principal investigators responsible for the INTERMAP UK West Bromwich (G Beevers, G Lip) and Belfast (A Evans, J Yarnell) population sample collections.
The authors' responsibilities were as follows—SSH: developed the analytical strategy, wrote the manuscript, and conducted the experiment, data analysis, and interpretation of the data; IJB: analyzed the data and wrote the manuscript; QC: developed the analytical strategy and analyzed the data; MB: helped conceptualize the INTERMAP 1H NMR spectral data; M-ED: provided statistical support; SK: helped conceptualize the design of the nutritional studies; JS: designed the INTERMAP study and provided input for writing the manuscript; EH: helped design and implement the NMR data analysis of the INTERMAP samples and provided input for the writing of the manuscript; PE: designed the INTERMAP study, obtained funds for the NMR data analysis of the INTERMAP samples and provided input for the writing of the manuscript; and JKN: obtained funds for the NMR data analysis of the INTERMAP samples and provided input for the writing of the manuscript. None of the authors declared a conflict of interest.
REFERENCES
- 1.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009;169:659–69 [DOI] [PubMed] [Google Scholar]
- 2.Schulz M, Lahmann PH, Boeing H, et al. Fruit and vegetable consumption and risk of epithelial ovarian cancer: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 2005;14:2531–5 [DOI] [PubMed] [Google Scholar]
- 3.van Duijnhoven FJB, Bueno-De-Mesquita HB, Ferrari P, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2009;89:1441–52 [DOI] [PubMed] [Google Scholar]
- 4.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr 2003;78:559S–69S [DOI] [PubMed] [Google Scholar]
- 5.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24 [DOI] [PubMed] [Google Scholar]
- 6.Marmot M, Elliott P. Coronary heart disease epidemiology: From aetiology to public health. 2nd ed Oxford, United Kingdom: Oxford University Press, 2005 [Google Scholar]
- 7.Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids – results of the OmniHeart randomized trial. JAMA 2005;294:2455–64 [DOI] [PubMed] [Google Scholar]
- 8.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–9 [DOI] [PubMed] [Google Scholar]
- 9.Panagiotakos D, Pitsavos C, Chrysohoou C, Stefanadis C. The association between food patterns and the metabolic syndrome using principal components analysis: the ATTICA study. J Am Diet Assoc 2007;107:979–87; quiz 997 [DOI] [PubMed] [Google Scholar]
- 10.Flood A, Rastogi T, Wirfalt E, et al. Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am J Clin Nutr 2008;88:176–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 2001;344:3–10 [DOI] [PubMed] [Google Scholar]
- 12.Sadakane A, Tsutsumi A, Gotoh T, et al. Dietary patterns and levels of blood pressure and serum lipids in a Japanese population. J Epidemiol 2008;18:58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingham SA. Limitations of the various methods for collecting dietary intake data. Ann Nutr Metab 1991;35:117–27 [DOI] [PubMed] [Google Scholar]
- 14.Bates CJ. Biochemical markers of nutrient intake. : Margetts BM, Nelson M, Design concepts in nutritional epidemiology. Oxford, United Kingdom: Oxford University Press, 1991:192–265 [Google Scholar]
- 15.Al-Delaimy WK, Ferrari P, Slimani N, et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr 2005;59:1387–96 [DOI] [PubMed] [Google Scholar]
- 16.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999;29:1181–9 [DOI] [PubMed] [Google Scholar]
- 17.Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed 2005;18:143–62 [DOI] [PubMed] [Google Scholar]
- 18.Lindon JC, Nicholson JK. Spectroscopic and statistical techniques for information recovery in metabonomics and metabolomics. Ann Rev Anal Chem 2008;1:45–69 [DOI] [PubMed] [Google Scholar]
- 19.Holmes E, Loo RL, Stamler J, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008;453:396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stella C, Beckwith-Hall B, Cloarec O, et al. Susceptibility of human metabolic phenotypes to dietary modulation. J Proteome Res 2006;5:2780–8 [DOI] [PubMed] [Google Scholar]
- 21.Solanky KS, Bailey NJC, Beckwith-Hall BM, et al. Application of biofluid H-1 nuclear magnetic resonance-based metabonomic techniques for the analysis of the biochemical effects of dietary isoflavones on human plasma profile. Anal Biochem 2003;323:197–204 [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Tang H, Nicholson JK, Hylands PJ, Sampson J, Holmes E. A metabonomic strategy for the detection of the metabolic effects of chamomile (Matricaria recutita L.) ingestion. J Agric Food Chem 2005;53:191–6 [DOI] [PubMed] [Google Scholar]
- 23.Rezzi S, Ramadan Z, Martin FPJ, et al. Human metabolic phenotypes link directly to specific dietary preferences in healthy individuals. J Proteome Res 2007;6:4469–77 [DOI] [PubMed] [Google Scholar]
- 24.Saito T, Nakaie S, Kinoshita M, et al. Practical guide for accurate quantitative solution state NMR analysis. Metrologia 2004;41:213–8 [Google Scholar]
- 25.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures: application in H-1 NMR metabonomics. Anal Chem 2006;78:4281–90 [DOI] [PubMed] [Google Scholar]
- 26.Veselkov KA, Lindon JC, Ebbels TMD, et al. Recursive segment-wise peak alignment of biological 1H NMR spectra for improved metabolic biomarker recovery. Anal Chem 2009;81:56–66 [DOI] [PubMed] [Google Scholar]
- 27.Trygg J, Wold S. PLS regression on wavelet compressed NIR spectra. Chemom Intell Lab Syst 1998;42:209–20 [Google Scholar]
- 28.Cloarec O, Dumas ME, Trygg J, et al. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in H-1 NMR spectroscopic metabonomic studies. Anal Chem 2005;77:517–26 [DOI] [PubMed] [Google Scholar]
- 29.Stamler J, Elliott P, Dennis B, et al. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary). J Hum Hypertens 2003;17:591–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dennis B, Stamler J, Buzzard M, et al. INTERMAP: the dietary data—process and quality control. J Hum Hypertens 2003;17:609–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conway R, Robertson C, Dennis B, Stamler J, Elliott P, Grp IR. Standardised coding of diet records: experiences from INTERMAP UK. Br J Nutr 2004;91:765–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metz CE. Basic principles of ROC analysis. Semin Nucl Med 1978;8:283–98 [DOI] [PubMed] [Google Scholar]
- 33.Dyer AR, Greenland P, Elliott P, et al. Estimating laboratory precision of urinary albumin excretion and other urinary measures in the international study on macronutrients and blood pressure. Am J Epidemiol 2004;160:287–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slow S, Donaggio M, Cressey PJ, Lever M, George PM, Chambers ST. The betaine content of New Zealand foods and estimated intake in the New Zealand diet. J Food Compost Anal 2005;18:473–85 [Google Scholar]
- 35.Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of DRIs, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: The National Academies Press, 2000 [Google Scholar]
- 36.Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr 2003;133:921S–4S [DOI] [PubMed] [Google Scholar]
- 37.Zamora-Ros R, Urpi-Sarda M, Lamuela-Raventos RM, et al. Resveratrol metabolites in urine as a biomarker of wine intake in free-living subjects: The PREDIMED Study. Free Radic Biol Med 2009;46:1562–6 [DOI] [PubMed] [Google Scholar]
- 38.Lever M, Sizeland PCB, Bason LM, Hayman CM, Chambers ST. Glycine betaine and proline betaine in human blood and urine. Biochim Biophys Acta 1994;1200:259–64 [DOI] [PubMed] [Google Scholar]
- 39.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med 2001;134:1106–14 [DOI] [PubMed] [Google Scholar]
- 40.Kumanyika S, Tell GS, Shemanski L, Polak J, Savage PJ. Eating patterns of community-dwelling older adults: the Cardiovascular Health Study. Ann Epidemiol 1994;4:404–15 [DOI] [PubMed] [Google Scholar]
- 41.Trinchant JC, Boscari A, Spermato G, Van de Sype G, Le Rudulier D. Proline betaine accumulation and metabolism in alfalfa plants under sodium chloride stress. Exploring its compartmentalization in nodules. Plant Physiol 2004;135:1583–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierce SK, Edwards SC, Mazzocchi PH, Klingler LJ, Warren MK. Proline betaine - a unique osmolyte in an extremely euryhaline osmoconformer. Biol Bull 1984;167:495–500 [DOI] [PubMed] [Google Scholar]
- 43.Amin US, Lash TD, Wilkinson BJ. Proline betaine is a highly effective osmoprotectant for staphylococcus aureus. Arch Microbiol 1995;163:138–42 [DOI] [PubMed] [Google Scholar]
- 44.Rapp A, Markowetz A, Niebergall H. [Application of 13C-NMR spectroscopy for detection and quantitative determination of amino acids in wine and fruit juices.] Z Lebensm Unters Forsch 1991;192:1–6 (in German) [Google Scholar]
- 45.Atkinson W, Downer P, Lever M, Chambers ST, George PM. Effects of orange juice and proline betaine on glycine betaine and homocysteine in healthy male subjects. Eur J Nutr 2007;46:446–52 [DOI] [PubMed] [Google Scholar]
- 46.Le Gall G, Puaud M, Colquhoun IJ. Discrimination between orange juice and pulp wash by H-1 nuclear magnetic resonance spectroscopy: identification of marker compounds. J Agric Food Chem 2001;49:580–8 [DOI] [PubMed] [Google Scholar]
- 47.Gil-Izquierdo A, Gil MI, Ferreres F. Effect of processing techniques at industrial scale on orange juice antioxidant and beneficial health compounds. J Agric Food Chem 2002;50:5107–14 [DOI] [PubMed] [Google Scholar]
- 48.de Zwart FJ, Slow S, Payne RJ, et al. Glycine betaine and glycine betaine analogues in common foods. Food Chem 2003;83:197–204 [Google Scholar]
- 49.Berg CM, Lappas G, Strandhagen E, et al. Food patterns and cardiovascular disease risk factors: the Swedish INTERGENE research program. Am J Clin Nutr 2008;88:289–97 [DOI] [PubMed] [Google Scholar]
- 50.Stamler JNJ, Garside DB, Daviglus ML. Current status: six established major risk factors—and low risk. : Marmot M, Elliott P, Coronary heart disease epidemiology: from aetiology to public health. Oxford, United Kingdom: Oxford University Press, 2005:32–69 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.