Abstract
Aims
Calcium-activated chloride channels (CACCs) share common pharmacological properties with Kcnma1-encoded large conductance K+ channels (BKCa or KCa1.1) and it has been suggested that they may co-exist in a macromolecular complex. As KCa1.1 channels are known to localize to cholesterol and caveolin-rich lipid rafts (caveolae), the present study investigated whether Ca2+-sensitive Cl− currents in vascular myocytes were affected by the cholesterol depleting agent methyl-β-cyclodextrin (M-βCD).
Methods and results
Calcium-activated chloride and potassium currents were recorded from single murine portal vein myocytes in whole cell voltage clamp. Western blot was undertaken following sucrose gradient ultracentrifugation using protein lysates from whole portal veins. Ca2+-activated Cl− currents were augmented by 3 mg mL−1 M-βCD with a rapid time course (t0.5 = 1.8 min). M-βCD had no effect on the bi-modal response to niflumic acid or anthracene-9-carboxylate but completely removed the inhibitory effects of the KCa1.1 blockers, paxilline and tamoxifen, as well as the stimulatory effect of the KCa1.1 activator NS1619. Discontinuous sucrose density gradients followed by western blot analysis revealed that the position of lipid raft markers caveolin and flotillin-2 was altered by 15 min application of 3 mg mL−1 M-βCD. The position of KCa1.1 and the newly identified candidate for CACCs, TMEM16A, was also affected by M-βCD.
Conclusion
These data reveal that CACC properties are influenced by lipid raft integrity.
Keywords: Calcium-activated chloride channels, Vascular smooth muscle, TMEM16A, KCa1.1, Lipid raft
1. Introduction
Calcium-activated chloride channels (CACCs) underpin various physiological activities including smooth muscle contraction, secretion, neuronal firing, and cardiomyocyte depolarization. Study of CACCs has always been plagued by the molecular identity being unknown,1 the lack of truly selective tools2 and the complicated interaction of so called chloride channel blockers with native CACCs.2–5 Recent work has revealed a remarkable pharmacological overlap between calcium-activated chloride currents (IClCa) and the large conductance, calcium-activated potassium channel (BKCa or KCa1.1).2 Thus, a wide range of structurally disparate agents considered to be chloride channel blockers, such as niflumic acid (NFA), anthracene-9-carboxylate (A-9-C) and ethacrynic acid, enhance KCa1.1 currents.6–8 Furthermore, in vascular myocytes, the structurally different activators of KCa1.1 channels, NS1619 and isopimaric acid, augment IClCa,9 and the KCa1.1 blockers paxilline, penitrem A, and iberiotoxin inhibit IClCa.10 Moreover, tamoxifen, which blocks IClCa effectively,10 either blocks or activates KCa1.1 channels depending on the presence of a β-auxiliary subunit.11 In contrast, positive and negative modulators of small and intermediate conductance, Ca2+-activated K+ channels (KCa2.1 and KCa2.3) do not affect IClCa.10 These observations led to the postulate that CACCs and KCa1.1 contained a common structural motif or co-existed in a sufficiently narrow micro-domain to allow inter-channel activity.2
Much evidence is now available for the compartmentalization of protein complexes into highly organized cholesterol- and sphingolipid-enriched structures within the plasma membrane, termed lipid rafts.12 These moieties selectively include, but also exclude, certain proteins from macromolecular complexes in order to aid efficient signalling and trafficking. The presence of the protein caveolin (isoforms: 1–3) in these specialized lipid-rich domains results in the formation of caveolae, a subtype of lipid raft, that form membrane invaginations of 50–100 nm in diameter that can be visualized by electron microscopy. KCa1.1 channels have been shown to localize to lipid rafts within cell membranes13,14 and surface expression and conductance of KCa1.1 channels are modulated by interaction with caveolin-1 protein.15 Treatment of cells with cholesterol depleting agents, such as methyl-β-cyclodextrin (M-βCD), modulates KCa1.1 channel currents.14,16 The present study aimed to assess whether M-βCD affected the biophysical and pharmacological properties of CACCs. The data presented suggest that CACCs are present in lipid sub-domains. More strikingly, this study reveals that the distinctive pharmacology of CACCs relies upon intact lipid rafts. Overall, these data lend weight to the possibility that native CACCs exist as part of a multi-protein complex.
2. Methods
For detailed Methods see Supplementary material online, Methods.
2.1. Tissue collection
BALB/c mice (6–8 weeks) were sacrificed by cervical dislocation in accordance with schedule 1 of the UK Animals Act (1986) and conforms with the Guide and Care of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, 1996).
2.2. Cell dissociation
Individual smooth muscle myocytes were isolated from strips of mouse portal vein (PV) by incubation at 37°C for 20 min with 1.5 mg mL−1 collagenase XI followed by 10 min with 1 mg mL−1 protease XIV, followed by gentle trituration with a wide bore fire polished pipette.
2.3. Electrophysiology
Macroscopic IClCa were recorded from a total of 85 cells from 46 different mice using pipette solutions containing 106 mM CsCl, 20 mM TEA, 3 mM Na2ATP, 0.2 mM GTP-Na, 10 mM HEPES, 10 mM BAPTA, 1.1 mM MgCl2, and a sustained Cl− channel activation evoked by a fixed free [Ca2+] of 500 nM obtained through the addition of 7.8 mM CaCl2 as calculated by EqCal (Biosoft, Ferguson, MO, USA).4,9,10,17–19 The external solution contained 126 mM NaCl, 11 mM glucose, 10 mM HEPES, 10 mM TEA-Cl, 1.2 mM MgCl2, and 1.5 mM CaCl2 (pH was adjusted to 7.2 with NaOH). IClCa were recorded at different potentials using protocols described in Figure 1. Potassium currents (IK) were recorded in cells bathed in a solution containing 136 mM NaCl, 5 mM KCl, 11 mM glucose, 10 mM HEPES, 1.2 mM MgCl2, and 1.5 mM CaCl2. The pipette solution composed of 126 mM KCl, 10 mM HEPES, 3 mM Na2ATP, 0.2 mM GTP-Na, 10 mM BAPTA, 1.2 mM MgCl2, and a fixed free [Ca2+] of 250 nM obtained through the addition of 6.4 mM CaCl2. Cells were held at −50 mV and IK was recorded at different potentials by application of voltage ramps (200 mV s−1) from −100 to +100 mV every 15 s. All experiments were performed at room temperature.
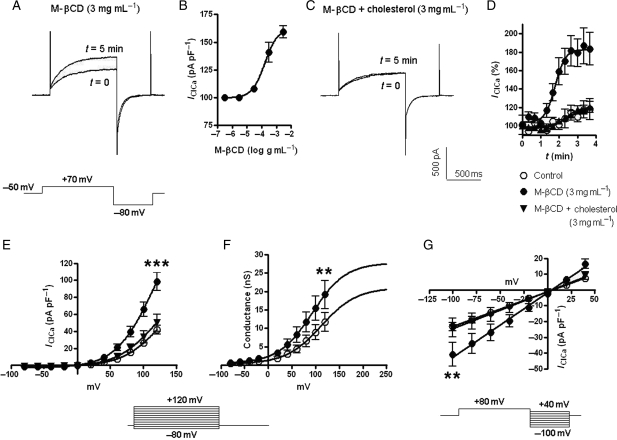
Figure 1.
Effects of M-βCD on IClCa. Examples of IClCa recorded in the absence and after 5 min application of 3 mg mL−1 M-βCD (A). IClCa was evoked by step depolarization to +70 mV followed by repolarization to −80 mV. (B) Concentration dependence of the increase in IClCa at +70 mV produced by M-βCD (mean of 3–9 cells). (C) Lack of effect of 3 mg mL−1 M-βCD when bound to cholesterol (M-βCD + cholesterol). (D) Time taken for a change in IClCa (expressed as a percentage of the maximum current at +70 mV prior to application of M-βCD). (E) I–V relationship of IClCa in the absence (open circle), or presence of M-βCD (filled circle), or M-βCD bound to cholesterol (filled inverted triangle). (F) Voltage-dependence of activation in the presence and absence of M-βCD. (G) Reversal potential of the evoked current was not affected by either M-βCD or cholesterol bound M-βCD. All protocols are shown as inserts. Each point comprises data from at least five cells with error bars depicting SEM **P < 0.01 and ***P < 0.001 for paired Student's t-test comparisons between data acquired prior to and post-application of M-βCD at the indicated voltage step.
2.4. Cholesterol modifying agents
Once IClCa had stabilized the role of lipid rafts was determined by application of M-βCD (0.03–3 mg mL−1), a cholesterol chelating agent which enhances aqueous solubility of cholesterol, as described previously.20–23 M-βCD pre-bound to cholesterol was used to rule out possible cholesterol independent effects.20
2.5. Lipid raft isolation
Portal veins (PV) from 30 mice were crushed in liquid nitrogen and homogenized in 1 ml lysis buffer (Sigma-Aldrich; Poole, UK) containing 1% Triton X-100. One half of the total lysate was incubated with 3 mg mL−1 M-βCD for 15 min, whereas the other half was incubated with the appropriate vehicle. Both lysates were fractionated using the Caveolae/Raft Isolation Kit (Sigma-Aldrich). A discontinuous sucrose gradient (0–40%) was constructed using different volumes of OptiPrep and centrifuged at 200 000 g for 4 h at 4°C. After centrifugation, 12 fractions of 1 mL were collected from the top (fraction 1) to the bottom (fraction 12) of the tube and stored at −20°C until required for western blot analysis.
2.6. Western blots
Protein samples were denatured at 95°C for 5 min in the presence of reducing agent (Invitrogen, UK), loaded onto a pre-cast sodium dodecyl sulphate-polyacrylamide gel (4–12% Bis–Tris, Invitrogen, UK), subjected to electrophoresis and then transferred onto PVDF membranes (Amersham Biosciences). The membranes were then probed for the lipid raft marker proteins caveolin (pan-caveolin24 1:10 000; BD Biosciences) and flotillin-225 (1:20 000; BD Biosciences), the non-lipid raft protein marker β-adaptin26 (1:1500; Santa Cruz), KCa1.1 (1:200; Alomone), and TMEM16A (ab53213; Abcam; a 1:5 dilution of a prediluted form). Protein bands were visualized using ECL (Thermo Scientific) and hyperfilm (Amersham Bioscience). All antibodies had been tested to determine effective concentrations and non-specific effects on samples of whole heart and whole PV in previous experiments (data not shown). Owing to low levels of protein, SignalBoost Immunoreaction Enhancer (Calbiochem; Nottingham, UK) was used with the anti-flotillin-2 antibody; it was not suitable/required for use with the other antibodies used in this investigation.
2.7. Statistical analysis
All data are means ± SEM taken from at least three animals. Statistical comparison was performed between the stable response observed prior to exposure to modulators (t = 0) and that obtained in the presence of modulators using either paired Student's t-test or ANOVA. All drugs were purchased from Sigma-Aldrich unless otherwise indicated.
3. Results
IClCa evoked by pipette solutions containing 500 nM free Ca2+ exhibited a rapid rundown immediately after membrane rupture and then remained constant for the duration of the experiment. At −50 mV, the mean inward current was −1.6 ± 0.2 pA pF−1 (n = 7) and depolarization to +70 mV yielded currents with distinctive outward kinetics (Figure 1A) that are characteristic of IClCa recorded by this technique.9,10,17–19 The current at +70 mV increased from a mean level of 2.4 ± 0.2 pA pF−1 immediately after depolarization to 10.6 ± 1.5 pA pF−1 after 750 ms with a τopen of 199 ± 13 ms (n = 10). Repolarization to −80 mV evoked an immediate inward current of −13.8 ± 1.9 pA pF−1 (I−80 mV) which decayed to −1.9 ± 0.3 pA pF−1 with a τclose of 54 ± 3 ms.
3.1. Effect of M-βCD on native Ca2+-activated Cl− currents in mPV myocytes
Application of M-βCD (3 mg mL−1) rapidly augmented IClCa (Figure 1A) with half maximal enhancement (t0.5) occurring after 1.8 min (Figure 1D). Figure 1B shows that augmentation of IClCa was also observed with 0.3 mg mL−1 M-βCD but not lower concentrations (0.03–0.1 mg mL−1, n ≥ 3). In contrast, application of 3 mg mL−1 M-βCD pre-bound with an equivalent concentration of cholesterol did not produce any changes in IClCa that were significant from vehicle effects (n = 6; Figure 1D and E). Application of 3 mg mL−1 M-βCD increased the current at −50 mV from −1.6 ± 0.2 pA pF−1 to −1.9 ± 0.3 pA pF−1 (n = 7; P < 0.05) and at all test potentials (Figure 1E, significance only at +120 mV shown for clarity). Maximal extrapolated whole-cell conductance increased from 21.1 to 27.8 nS, whereas V0.5 decreased from 113 to 93 mV (Figure 1F). The stimulatory effects of M-βCD were not reversed upon removal from the bath solution for up to 15 min (n = 3). The augmentation of IClCa was not due to the de novo recruitment of a new ionic conductance as neither the reversal potential (Figure 1G) nor the kinetics at +70 or −80 mV were affected by M-βCD. These data are the first to show that Ca2+-activated Cl− currents are affected by cholesterol depletion.
3.2. Effect of M-βCD on native Ca2+-activated K+ currents in mPV myocytes
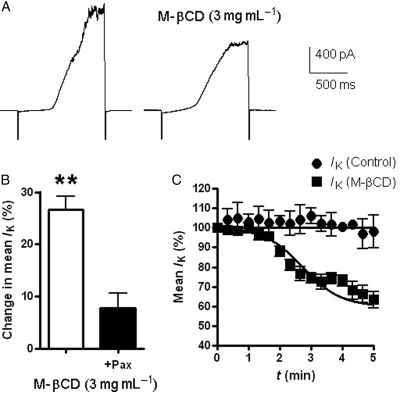
Experiments were performed to see if M-βCD also modulated large conductance KCa1.1 currents in mPV myocytes similar to previous findings in uterine smooth muscles cells and gliomas.14,16 As Figure 2A shows application of a depolarizing voltage ramp with pipette solutions containing [Ca2+] fixed at 250 nM evoked an outwardly rectifying IK superimposed by considerable current fluctuations at positive potentials that was inhibited by the KCa1.1 channel blocker 1 µM paxilline27,28 by 80 ± 2% (P < 0.05; n = 4). Application of M-βCD (3 mg mL−1) reduced IK (Figure 2A and B) with a time course similar to the effect on IClCa (t0.5 = 2.7 min, Figure 2C), which was not manifest in cells pre-treated with paxilline (Figure 2B). The effect of M-βCD on IK was also maintained after washout of the cyclodextrin (n = 3). Thus, KCa1.1 channels in vascular myocytes are inhibited by cholesterol depletion.
Figure 2.
M-βCD and IK. (A) Collection of traces elicited by a ramp protocol [−100 mV to +100 mV (at 200 mV s−1) taken from a cell prior to and after 5 min incubation in M-βCD (3 mg mL−1)]. (B) Change in mean IK amplitude evoked by M-βCD in the absence and presence of paxilline (1 µM). (C) Time course of M-βCD modulation of IK at +100 mV compared with control currents recorded over a similar time course. Each point comprises data from four cells with error bars depicting SEM. **P < 0.01 for paired Student's t-test comparisons between data acquired prior to and post-application of M-βCD.
3.3. Effects of Cl− channel blockers on IClCa in the presence of M-βCD
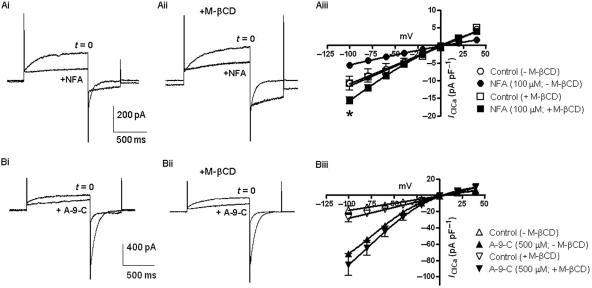
Experiments were undertaken with the Cl− channel blockers NFA and A-9-C to assess whether disruption of cholesterol levels affected the ability of these chloride channel blockers to modify IClCa. In vascular myocytes, these agents produce complex effects on sustained IClCa, which is manifest as a significant but not complete inhibition at positive potentials and a paradoxical stimulation of IClCa at negative potentials.3–5,9 Figure 3 shows that NFA (100 µM) produced effects, comparable to those observed previously in mPV myocytes by increasing holding current at −50 mV, inhibiting late outward current and increasing inward current upon repolarization to −80 mV (n = 4; Figure 3Ai). Similar effects were observed in the presence of M-βCD (Figure 3Aii). In the absence of M-βCD, NFA evoked a decrease in the outward time-dependent current of 2.6 ± 1.3 pA pF−1, whereas in the presence of M-βCD, a decrease of 3.9 ± 0.8 pA pF−1 was observed. In addition, the inward current at −80 mV in the presence of NFA was larger in M-βCD (Figure 3Aiii). Both observations reflect a heightened activation of the underlying CACCs. Application of A-9-C (500 µM) produced effects similar to those previously reported in the rabbit pulmonary artery4 that were manifest as a reduction in current at +70 mV and a marked augmentation of the current recorded upon repolarization to −80 mV (n = 3; Figure 3Bi), which were also apparent after application of 3 mg mL−1 M-βCD (n = 4; Figure 3Bii). However, the enhancement of the current at −80 mV produced by A-9-C increased from 6.1 ± 1.2 to 8.8 ± 3.8 pA pF−1 in M-βCD (n = 4, Figure 3Biii) again consistent with an increase in the availability of the underlying CACC. These data show that the distinctive bimodal effects of NFA and A-9-C on sustained IClCa were maintained after incubation with M-βCD.
Figure 3.
Effect of NFA and A-9-C in the presence of M-βCD. (A) Effect of 100 µM NFA on IClCa in the absence and presence of 3 mg mL−1 M-βCD. (Ai and Aii) Representative traces. (Aiii) Effect of NFA on IClCa amplitude recorded at different potentials, using the reversal protocol in Figure 1G, in the absence and presence of 3 mg mL−1 M-βCD (see insert). *P < 0.05 for paired Student's t-test comparisons between data acquired prior to and post-application of NFA at the indicated step. (Bi and Bii) Representative traces showing effects of 500 µM A-9-C in the absence and presence of 3 mg mL−1 M-βCD, respectively. (Biii) Mean effect of A-9-C on IClCa amplitude recorded at different potential, using the reversal protocol in Figure 1G, in the absence and presence of 3 mg mL−1 M-βCD (see insert). Each point comprises data from at least four cells with error bars depicting SEM.
3.4. Effects of KCa1.1 modulators on IClCa in the presence of M-βCD
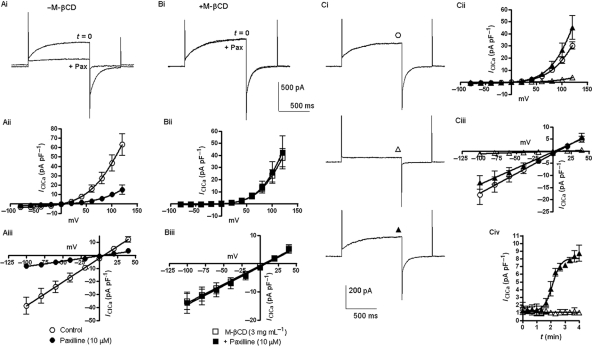
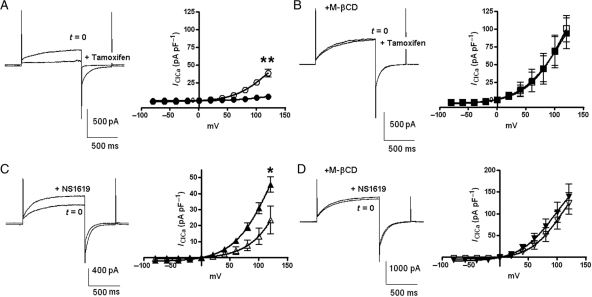
We have recently shown that the KCa1.1 inhibitor paxilline and the KCa1.1 activator NS1619 modulate IClCa.9,10 The observation, in the present study, that IClCa, as well as KCa1.1 current, are affected by M-βCD suggests that the pharmacological cross over could stem from a close physical interaction between the Cl− channel and KCa1.1 in the same microdomain. Consequently, disruption of the local environment by M-βCD might alter the ability of KCa1.1 channel modulators to affect IClCa. Similar to our previous report,10 1 µM paxilline reduced IClCa markedly at all potentials but did not affect the reversal potential of the evoked current (n = 4, Figure 4Ai–Aiii). In stark contrast, paxilline had no effect on IClCa in the presence of M-βCD (3 mg mL−1; n = 4; Figure 4Bi–Biii). Moreover, as Figure 4C shows addition of M-βCD (3 mg mL−1) reversed completely the inhibition of IClCa produced by paxilline with a time course similar to the stimulatory effect of M-βCD alone (τ = 2.1 min, Figure 4Civ, n = 4). M-βCD did not reverse the inhibitory effect of paxilline on IK (n = 3). Similarly tamoxifen (10 µM), another KCa1.1 modulator, which inhibits IClCa10 (n = 4), induced no change in IClCa after pre-incubation with M-βCD (3 mg mL−1; n = 4; Figure 5A and B). The presence of M-βCD also limited the stimulatory effect of the KCa1.1 channel activator NS1619 on IClCa (Figure 5C and D). Figure 5C shows that similar to Saleh et al.9 Thirty micromolar NS1619 increased IClCa at all potentials with the current at the end of a 750 ms step to +70 mV increasing from 7.7 ± 2.1 to 16.9 ± 2.9 pA pF−1 (P < 0.05, n = 4). In contrast, IClCa was unaffected by the application of 30 µM NS1619 when the myocytes had been previously treated with M-βCD (3 mg mL−1; n = 4; Figure 5D). These data provide evidence that the effects of KCa1.1 modulators on IClCa are reliant upon functional lipid microdomains.
Figure 4.
M-βCD modulates the effect of paxilline on IClCa. (A) Effect of 10 µM paxilline (Pax) on IClCa. (Ai) Representative currents recorded in the absence and presence of paxilline (5 min). (Aii and Aiii) I–V relationships and reversal potential of IClCa in the absence (open circle) and presence of paxilline (filled circle). (B) Effect of 10 µM paxilline on IClCa in the absence (open square) and presence of 3 mg mL−1 M-βCD (filled square). (C) Reversal of paxilline modulation by M-βCD. (Ci) Representative traces, (Cii) I–V relationships, (Ciii) Reversal potentials of IClCa, and (Civ) Time course for the effect of M-βCD in the continued presence of 10 µM paxilline. In each graph, control currents are (open circle) +paxilline (open triangle) and paxilline plus M-βCD (filled triangle). Each point comprises data from at least four cells from different animals with error bars depicting SEM.
Figure 5.
Effect of tamoxifen and NS1619 in the presence of M-βCD. (A) Representative currents and the I–V relationship recorded in the absence (open circle) and presence of tamoxifen (5 min; filled circle) in the absence of M-βCD. (B) Recordings performed in the absence (open square) and presence of tamoxifen (filled square) in cells pre-incubated in 3 mg mL−1 M-βCD. (C) Representative traces and the I–V relationship recorded prior to (open triangle) and post-exposure to NS1619 (filled triangle) min the absence of M-βCD. (D) In the presence of 3 mg mL−1 M-βCD, traces and the I–V relationship recorded before (inverted triangle) and after exposure to NS1619 (filled inverted triangle). Each point comprises data from at least three cells with error bars depicting SEM. *P < 0.05 and **P < 0.01 for paired Student's t-test comparisons between data acquired prior to and post-application at the indicated step.
3.5. Lipid fractionation studies
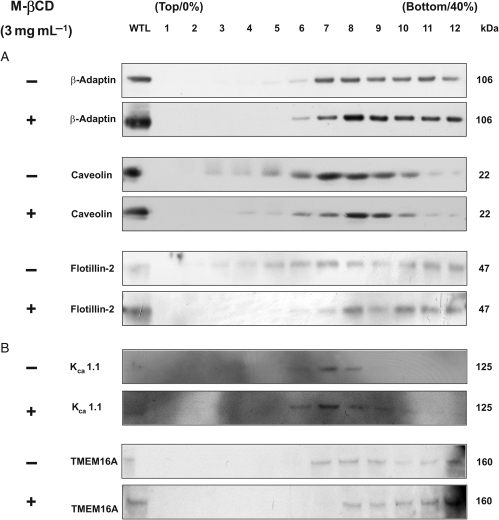
In the present study, electrophysiological effects were observed with a relatively mild treatment with M-βCD compared with previous studies where higher concentrations (5–10 mg mL−1) and longer application times (1–3 h) have been used.22,23,25,26 Thus, a series of experiments were undertaken to assess whether the M-βCD treatment used in the electrophysiology studies could affect the distribution of lipid raft markers. Figure 6A shows representative western blot analysis following discontinuous sucrose density ultracentrifugation of mPV tissue. Immunodetection with antibodies directed against β-adaptin and caveolin produced a localization profile similar to previous work in rat aorta.23,26 The localization pattern for flotillin-2 was also consistent with earlier work when the same concentration of Triton-X (1%) was used, indicating that flotillin-2-enriched lipid rafts are susceptible to glycerophospholipid depletion.25 Treatment of the protein lysate for 15 min incubation with M-βCD (3 mg mL−1) produced an obvious reduction in density of the bands for caveolin and flotillin-2 at lower fractions and the appearance of bands in later fractions (Figure 6A). The non-raft marker β-adaptin was affected considerably less by incubation with M-βCD (Figure 6A). These data show that the electrophysiological effects of M-βCD are associated with changes in the buoyancy of lipid raft markers.
Figure 6.
Effect of M-βCD on lipid raft-enriched fractions prepared from murine portal vein. (A) Western blot analysis of membrane proteins separated by a discontinuous sucrose density gradient (0, 20, 25, 30, 35, 40% sucrose) in the absence (−) and presence of 3 mg mL−1 M-βCD for 15 min (+). (B) A representative western blot for fractionated proteins probed with antibodies against KCa1.1 and TMEM16A ± M-βCD for 15 min.
The molecular identity of CACCs is unknown but recently the gene TMEM16A has been proposed as a strong candidate for this channel.29–31 Consequently, we ascertained whether TMEM16A could be detected in mPV lysates and whether it existed in the same lipid fractions as KCa1.1. Figure 6B shows that TMEM16A and KCa1.1 immunoreactivity was detected in PV lysates. KCa1.1 staining appeared most abundant in fractions that overlapped with caveolin and not with β-adaptin, suggesting that this ion channel may be located in the caveolae fraction of lipid rafts similar to other smooth muscles. TMEM16A staining was apparent in some of the same fractions as KCa1.1 but was generally present in less buoyant fractions (Figure 6B). Treatment with M-βCD caused a small, but obvious shift in the appearance of TMEM16A and KCa1.1 to less buoyant fractions.
4. Discussion
The work of the present study shows that the amplitude and pharmacology of CACCs in vascular smooth muscle cells is drastically altered by short application of M-βCD, an agent shown to deplete cholesterol levels. Western blot analysis after sucrose gradient ultracentrifugation showed that lipid raft markers caveolin and flotillin-2 migrated to less buoyant fractions upon treatment with M-βCD. This agent had no effect on the non-raft marker β-adaptin but had a subtle effect on the staining pattern for KCa1.1 and the recently identified molecular candidate for CACCs, TMEM16A. This study, by analogy with past work, suggests that CACCs exist in localized lipid microdomains or rafts where an interaction with KCa1.1 may dictate biophysical and pharmacological properties.
KCa1.1 channels have been identified in caveolin-enriched lipid microdomains in endothelial cells,32,33 glioma cells14 as well as ureter and uterine smooth muscle.16,26,34 Physical interaction with caveolin-1 protein in these microdomains acts as a tether and a regulator.15,33 Disruption of cholesterol-rich lipid domains by cyclodextrins reduces KCa1.1 activity in glioma and uterine smooth muscle cells,14,16 which may be due to dismemberment from IP3-mediated Ca2+ release sites14 as caveolin and the sarcoplasmic reticulum make physical nanocontacts.35 The present study shows that a brief application of M-βCD, at relatively low concentrations (3 mg mL−1) compared with previous studies,22–26 also reduced macroscopic paxilline-sensitive K+ currents in PV myocytes. Importantly, the present study also shows that the same low concentration of M-βCD increased the amplitude of IClCa with the same, rapid time course as the effect on K+ currents and caused translocation of the lipid raft markers caveolin and flotillin-2 to less buoyant fractions. No stimulation of IClCa was observed when M-βCD had been pre-incubated with cholesterol showing that the effect on IClCa was due to cholesterol depletion rather than an effect of the cyclodextrin itself. The similar time course of the effect of M-βCD on IK and IClCa suggests a common biochemical event is involved, although this may represent a subtle alteration in the macromolecular complex rather than complete disruption of the lipid raft.
An even more striking observation was that cholesterol depletion by M-βCD abolished the effect of agents known to modulate KCa1.1 channels on IClCa. Hence, the selective KCa1.1 blocker paxilline, which abolishes IClCa at all voltages in mPV myocytes10 and in the present study, was totally ineffective in the presence of M-βCD. Similarly, tamoxifen, which activates or inhibits KCa1.1 depending on the presence of an auxiliary subunit,11 abolished IClCa in the absence but not in the presence of M-βCD10 and in the present study. Moreover, the KCa1.1 opener NS1619, shown to enhance IClCa in vascular myocytes,9 had no effect on IClCa in the presence of M-βCD. This inability to augment IClCa was not due to M-βCD increasing the current to saturating levels, because Saleh et al.9 showed that NS1619 augmented IClCa generated by a higher activating [Ca2+] (1 µM vs. 500 nM). In contrast, the bimodal inhibition and stimulation of IClCa reported for the so-called Cl− channel blockers NFA and A-9-C3–5 were generally unaffected by M-βCD, although the stimulatory effect of these agents was greater in M-βCD. This reflects a greater availability of the underlying CACCs and may be related to the impact of phosphorylation on the pharmacology of Ca2+-activated Cl− channels shown recently.36 Overall, the present data provide support for KCa1.1 and Ca2+-activated Cl− channel proteins existing in a restricted space to allow pharmacological overlap. Disruption of cholesterol by M-βCD uncouples these two proteins and causes the loss of the pharmacological overlap.
It is tempting to speculate that the native Ca2+-activated Cl− channel exists as a multimeric assemblage consisting of a pore forming subunit, possibly TMEM16A after recent publications29–31 and the expression data of the present study, KCa1.1 and various signalling moieties such as CaMKII, calcineurin, and PP1 known to regulate vascular IClCa.1,18,19,37 However, the sucrose gradient work of the present study suggests that TMEM16A is distributed more evenly in the plasma membrane rather than just localized in rafts, even though some overlap with caveolin was observed and the pattern of TMEM16A staining was altered by mild treatment with M-βCD. These data suggest that if TMEM16A constitutes the native CACCs in smooth muscle then the nature of any molecular interaction with KCa1.1 is more subtle or complex than simple consolidation in a discrete microdomain. Ongoing experiments are aimed at ascertaining whether TMEM16A expression products contribute to native CACCs in smooth muscle.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
W.R.S. and A.J.D. were funded by British Heart Foundation grants to I.A.G. (PG/05/038 and PG/07/127/24235). This study was supported by a grant to N.L. from the National Institutes of Health (Grant 5 RO1 HL 075477) and from the National Center for Research Resources (NCRR 5 P20 RR15581).
Supplementary Material
Acknowledgements
Thanks to Mr Thomas Jepps for technical support and the advice of Profs Johnstone and Whitely at St George's, University of London. The advice of Drs C. Dart (University of Liverpool) and C. Vial (University of Leicester) is acknowledged.
Conflict of interest: none declared.
References
- 1.Leblanc N, Ledoux J, Saleh SN, Sanguinetti A, Angermann J, O'Driscoll K, et al. Regulation of calcium-activated chloride channels—a complex picture emerges. Can J Physiol Pharmacol. 2005;83:541–556. doi: 10.1139/y05-040. doi:10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood IA, Leblanc N. Overlapping pharmacology of Ca2+-activated Cl− and K+ channels. TiPS. 2007;28:1–5. doi: 10.1016/j.tips.2006.11.004. doi:10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- 3.Piper AS, Greenwood IA, Large WA. Dual effect of blocking agents on Ca2+-activated Cl− currents in rabbit pulmonary artery smooth muscle cells. J Physiol. 2002;539:119–131. doi: 10.1113/jphysiol.2001.013270. doi:10.1113/jphysiol.2001.013270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piper AS, Greenwood IA. Anomalous effect of anthracene-9-carboxylic acid on calcium-activated chloride currents in rabbit pulmonary artery smooth muscle cells. Br J Pharmacol. 2003;138:31–38. doi: 10.1038/sj.bjp.0705000. doi:10.1038/sj.bjp.0705000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledoux J, Greenwood IA, Leblanc N. Dynamics of Ca2+-dependent Cl− channel modulation by niflumic acid in rabbit coronary arterial myocytes. Mol Pharmacol. 2005;67:163–173. doi: 10.1124/mol.104.004168. doi:10.1124/mol.104.004168. [DOI] [PubMed] [Google Scholar]
- 6.Ottolia M, Toro L. Potentiation of large conductance KCa channels by niflumic, flufenamic, and mefenamic acids. Biophys J. 1994;67:2272–2279. doi: 10.1016/S0006-3495(94)80712-X. doi:10.1016/S0006-3495(94)80712-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenwood IA, Large WA. Comparison of the effects of the fenamates on Ca-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. Br J Pharmacol. 1995;116:2939–2948. doi: 10.1111/j.1476-5381.1995.tb15948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toma C, Greenwood IA, Helliwell RM, Large WA. Activation of potassium currents by inhibitors of calcium-activated chloride conductance in rabbit portal vein smooth muscle cells. Br J Pharmacol. 1996;118:513–520. doi: 10.1111/j.1476-5381.1996.tb15432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saleh SN, Angermann JE, Sones WR, Leblanc N, Greenwood IA. Stimulation of Ca2+-gated Cl− currents by the calcium-dependent K+ channel modulators NS1619 and isopimaric acid. J Pharmacol Exp Ther. 2007;321:1075–1083. doi: 10.1124/jpet.106.118786. doi:10.1124/jpet.106.118786. [DOI] [PubMed] [Google Scholar]
- 10.Sones WR, Leblanc N, Greenwood IA. Inhibition of vascular calcium-gated chloride currents by blockers of KCa1.1 but not by modulators of KCa2.1 or KCa2.3 channels. Br J Pharmacol. 2009;158:521–531. doi: 10.1111/j.1476-5381.2009.00332.x. doi:10.1111/j.1476-5381.2009.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dick GM, Rossow CF, Smirnov S, Horowitz B, Sanders KM. Tamoxifen activates smooth muscle BK channels through the regulatory beta 1 subunit. J Biol Chem. 2001;276:34594–34599. doi: 10.1074/jbc.M104689200. doi:10.1074/jbc.M104689200. [DOI] [PubMed] [Google Scholar]
- 12.Lai EC. Lipid rafts make for slippery platforms. J Cell Biol. 2003;162:365–370. doi: 10.1083/jcb.200307087. doi:10.1083/jcb.200307087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam RS, Shaw AR, Duszyk M. Membrane cholesterol content modulates activation of BK channels in colonic epithelia. Biochim Biophys Acta. 2004;1667:241–248. doi: 10.1016/j.bbamem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Weaver AK, Olsen ML, McFerrin MB, Sontheimer H. BK channels are linked to inositol 1,4,5-triphosphate receptors via lipid rafts: a novel mechanism for coupling [Ca2+]i to ion channel activation. J Biol Chem. 2007;282:31558–31568. doi: 10.1074/jbc.M702866200. doi:10.1074/jbc.M702866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alioua A, Lu R, Kumar Y, Eghbali M, Kundu P, Toro L, et al. Slo1 caveolin-binding motif, a mechanism of caveolin-1-Slo1 interaction regulating Slo1 surface expression. J Biol Chem. 2008;283:4808–4817. doi: 10.1074/jbc.M709802200. doi:10.1074/jbc.M709802200. [DOI] [PubMed] [Google Scholar]
- 16.Shmygol A, Noble K, Wray S. Depletion of membrane cholesterol eliminates the Ca2+-activated component of outward potassium current and decreases membrane capacitance in rat uterine myocytes. J Physiol. 2007;581:445–456. doi: 10.1113/jphysiol.2007.129452. doi:10.1113/jphysiol.2007.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Britton F, Ohya S, Horowitz B, Greenwood IA. Comparison of the properties of CLCA1 generated currents and ICl(Ca) in murine portal vein smooth muscle cells. J Physiol. 2002;539:107–117. doi: 10.1113/jphysiol.2001.013170. doi:10.1113/jphysiol.2001.013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca2+-activated Cl− currents in rabbit arterial and portal vein smooth muscle cells by Ca2+ /calmodulin-dependent kinase. J Physiol. 2001;534:395–408. doi: 10.1111/j.1469-7793.2001.00395.x. doi:10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angermann JE, Sanguinetti AR, Kenyon JL, Leblanc N, Greenwood IA. Mechanism of the inhibition of Ca2+-activated-chloride currents by phosphorylation in pulmonary arterial smooth muscle cells. J Gen Physiol. 2006;128:73–87. doi: 10.1085/jgp.200609507. doi:10.1085/jgp.200609507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 21.Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, et al. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. doi:10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 22.Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains. Br J Pharmacl. 2007;151:332–340. doi: 10.1038/sj.bjp.0707222. doi:10.1038/sj.bjp.0707222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson LJ, Davies LM, Barrett-Jolley R, Standen NB, Dart C. Angiotensin II-activated protein kinase C targets caveolae to inhibit aortic ATP-sensitive potassium channels. Cardiovasc Res. 2007;76:61–70. doi: 10.1016/j.cardiores.2007.05.020. doi:10.1016/j.cardiores.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol-Cell Physiol. 2005;289:C49–C57. doi: 10.1152/ajpcell.00399.2004. doi:10.1152/ajpcell.00399.2004. [DOI] [PubMed] [Google Scholar]
- 25.Vial C, Evans RJ. Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J Biol Chem. 2005;280:30705–30711. doi: 10.1074/jbc.M504256200. doi:10.1074/jbc.M504256200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localizes protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ Res. 2004;95:1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. doi:10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacol. 1996;35:963–968. doi: 10.1016/0028-3908(96)00137-2. doi:10.1016/0028-3908(96)00137-2. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Cheung DW. Effects of paxilline on K+ channels in rat mesenteric arterial cells. Eur J Pharmacol. 1999;372:103–107. doi: 10.1016/s0014-2999(99)00188-0. doi:10.1016/S0014-2999(99)00188-0. [DOI] [PubMed] [Google Scholar]
- 29.Caputo A, Caci E, Ferrera L, Pedemonte NM, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. doi:10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. doi:10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. doi:10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 32.Bravo-Zehnder M, Orio P, Norambuena A, Wallner M, Meera P, Toro L, et al. Apical sorting of a voltage- and Ca2+-sensitive K+ channel a-subunit in Madin–Darby canine kidney cells is independent of N-glycosylation. Proc Natl Acad Sci. 2000;97:13114–13119. doi: 10.1073/pnas.240455697. doi:10.1073/pnas.240455697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, et al. Caveolae targeting and regulation of large conductance Ca2+-activated K+ channels in vascular endothelial cells. J Biol Chem. 2005;280:11656–11664. doi: 10.1074/jbc.M410987200. doi:10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- 34.Babiychuk EB, Smith RD, Burdyga TV, Babiychuk VS, Wray S, Draeger A. Membrane cholesterol selectively regulates smooth muscle phasic contraction. J Membr Biol. 2004;198:95–101. doi: 10.1007/s00232-004-0663-1. doi:10.1007/s00232-004-0663-1. [DOI] [PubMed] [Google Scholar]
- 35.Gherghiceanu M, Popescu LM. Electron microscope tomography: further demonstration of nanocontacts between caveolae and smooth muscle sarcoplasmic reticulum. J Cell Mol Med. 2007;11:1416–1418. doi: 10.1111/j.1582-4934.2007.00166.x. doi:10.1111/j.1582-4934.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wichawr M, Ayon R, Greenwood IA, Leblanc N. The pharmacology of Ca2+-activated Cl− channels in rabbit pulmonary arterial smooth muscle cells is altered by phosphorylation. Br J Pharmacol. 2009;158:1356–1365. doi: 10.1111/j.1476-5381.2009.00405.x. doi:10.1111/j.1476-5381.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayon R, Sones W, Forrest AS, Wiwchar M, Valencik M, Sanguinetti AR, et al. Complex phosphatase regulation of Ca2+ -activated Cl− currents in pulmonary artery smooth muscle cells. J Biol Chem. 2009;284:32507–32521. doi: 10.1074/jbc.M109.050401. doi:10.1074/jbc.M109.050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.