Abstract
Aims
Nitric oxide (NO) plays a pivotal role in the regulation of cardiovascular physiology. Endothelial NO is mainly produced by the endothelial nitric oxide synthase (eNOS) enzyme. eNOS enzymatic activity is regulated at several levels, including Ca2+/calmodulin binding and the interaction of eNOS with associated proteins. There is emerging evidence indicating a role for the plasma membrane calcium ATPase (PMCA) as a negative regulator of Ca2+/calmodulin-dependent signal transduction pathways via its interaction with partner proteins. The aim of our study was to investigate the possibility that the activity of eNOS is regulated through its association with endothelial PMCA.
Methods and results
We show here a novel interaction between endogenous eNOS and PMCA in human primary endothelial cells. The interaction domains were located to the region 735–934 of eNOS and the catalytic domain of PMCA. Ectopic expression of PMCA in endothelial cells resulted in an increase in phosphorylation of the residue Thr-495 of endogenous eNOS. However, disruption of the PMCA–eNOS interaction by expression of the PMCA interaction domain significantly reversed the PMCA-mediated effect on eNOS phosphorylation. These results suggest that eNOS activity is negatively regulated via interaction with PMCA. Moreover, NO production by endothelial cells was significantly reduced by ectopic expression of PMCA.
Conclusion
Our results show strong evidence for a novel functional interaction between endogenous PMCA and eNOS in endothelial cells, suggesting a role for endothelial PMCA as a negative modulator of eNOS activity, and, therefore, NO-dependent signal transduction pathways.
Keywords: Plasma membrane calcium ATPase, eNOS, Functional interaction, Nitric oxide regulation
1. Introduction
Cardiovascular disease, one of the leading causes of mortality in western populations, encompasses a number of different conditions including coronary heart disease, stroke, and peripheral vascular disease. The underlying pathology for all these disorders is associated with endothelial dysfunction.1 Under normal conditions, the cells of the vascular endothelium transmit extracellular signals to the arterial wall to regulate cardiovascular physiology. The ability of the endothelium to synthesize and release the vasodilator nitric oxide plays a major role in this process.
In endothelial cells, NO is synthesized from the amino acid l-arginine and molecular oxygen by the constitutively expressed endothelial form of nitric oxide synthase (eNOS or NOS3).2 Normal function of eNOS and the tonic production of NO are associated with a fully functional endothelium. In contrast, reduced bioavailability of NO is thought to be one of the central factors common to vascular disease.1 eNOS enzymatic activity is regulated at different levels including Ca2+/calmodulin binding,3 post-translational lipid modifications,4 multi-site phosphorylation5 and its association with partner proteins.4
Studies in recent years have uncovered an increasingly important role for the plasma membrane calcium ATPase (PMCA) pump as a novel regulator of Ca2+/calmodulin-dependent signal transduction pathways via interaction with specific partner proteins.6 PMCAs are enzymatic low capacity, high-affinity systems involved in the extrusion of Ca2+ from the cell.7 Four different PMCA isoforms, PMCA1–4, have been identified in mammals and are encoded by four independent genes.8 It is thought that PMCA regulates the activity of associated proteins by tethering them to low Ca2+/calmodulin cellular microdomains created by the Ca2+ extrusion activity of the pump. In this sense, PMCA has been shown to interact and inhibit the activity of the Ca2+/calmodulin-dependent proteins nNOS,9–11 CASK,12 and calcineurin.13–15
The role of PMCA as a regulator of Ca2+/calmodulin-dependent enzymes and, in particular, its involvement in the regulation of NO production by nNOS in neuronal, cardiac, and smooth muscle cells9–11 prompted us to investigate the hypothesis that the activity of eNOS is regulated through its association with endothelial PMCA.
Here we show a novel interaction between endogenous PMCAs and eNOS in human umbilical vein endothelial cells (HUVEC) and human dermal microvascular endothelial cells (HDMEC). PMCA expression in HUVEC and HDMEC leads to a significant increase in the phosphorylation of residue Thr-495 of eNOS. Conversely, ectopic expression of PMCA results in a slight reduction on the phosphorylation of Ser-1177. Disruption of the PMCA–eNOS interaction (by expression of the PMCA interaction domain) reversed the PMCA-mediated increase in eNOS Thr-495 phosphorylation, demonstrating the functionality of this interaction. These PMCA-dependent modifications in the phosphorylation status of key regulatory residues of eNOS suggest that eNOS activity is negatively regulated via interaction with PMCA. In agreement with this hypothesis, expression of PMCA in HUVEC resulted in a significant reduction in NO production by endothelial cells.
Our results reinforce the emerging role of PMCA as a macromolecular complex organizer and a modulator of Ca2+/calmodulin-dependent signal transduction pathways. Moreover, this work reveals eNOS as a new interaction partner of PMCA and suggests a role for PMCA as a regulator of NO production by endothelial cells, what might open new avenues for future therapeutic approaches to treat patients suffering from cardiovascular diseases.
2. Methods
2.1. Cell culture
HEK 293 cells were maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% foetal calf serum, 1% penicillin/streptomycin and 1% l-glutamine. HUVEC (Clonetics or Promocell) were maintained in Endothelial Cell Growth Medium 2-Supplement mix (Promocell) supplemented with 1% penicillin/streptomycin. HDMEC (Promocell) were maintained in Endothelial Cell Growth Medium MV-Supplement mix (Promocell) supplemented with 1% penicillin/streptomycin. Endothelial cells were used at low passage numbers (3–5).
2.2. Plasmids
pcDNA3-hPMCA2b contains the human PMCA2b cDNA and was a gift from Professor Carafoli (University of Padova, Italy). pFlag-PMCA2b(462–684) and pFlag-PMCA2b(1143–1243) have been described previously.14 pcDNA3-hPMCA4b contains the human PMCA4b cDNA.13 pFlag-PMCA4b(1–92) and pFlag-PMCA4b(428–651) have been described previously.13 Plasmid pFlag-PMCA4b(652–840) has been previously reported.16 pcDNA3-eNOS contains the human eNOS cDNA. For details on the generation of plasmids pFlag-eNOS (401–934), pFlag-eNOS (193–735), pFlag-eNOS (735–934), and pFlag-eNOS (1–505), please see the expanded section Generation of plasmids in the Supplementary material on line.
2.3. Transient transfections
For immunoprecipitation experiments, HEK 293 cells were plated in 100 × 20 mm2 tissue culture dishes (4.5 × 106 cells/plate) the day before transfection. Cells were transfected with 10 μg of the indicated expression plasmids by using LipofectAMINE 2000 reagent (Invitrogen) as described previously.13
For transfection of primary endothelial cells, 1 × 106 HUVEC were transfected with 5 μg of the indicated expression plasmids in 100 μL of HUVEC nucleofector solution from a HUVEC nucleofector kit (Amaxa) following the recommendations of the manufacturer. Likewise, 1 × 106 HDMEC were transfected with 5 μg of the indicated expression plasmids in 100 μL of nucleofector solution from a Basic nucleofector Primary Endothelial cells kit (Amaxa) following the recommendations of the manufacturer. After transfection, endothelial cells were resuspended in fresh media, plated in six-well tissue culture plates, and incubated overnight. The following day, cells were washed with PBS and incubated for 24 h more in 5 mL of endothelial medium with supplements.
2.4. cGMP and NO determination
For cGMP determination, transfected HUVEC were saturated with l-arginine (30 min, 1 mM) and nascent NO was stabilized by addition of superoxide dismutase (100 U/mL of culture medium) (Sigma-Aldrich) 5 min before lyses. In parallel, 3-isobutyl-l-methylxanthine (IBMX, 1 mM; Sigma-Aldrich) was added to the culture 5 min before lyses (to inactivate phosphodiesterase activity), and NO synthesis was induced by addition of 0.5 μM A23487 calcium ionophore 3 min before lyses. cGMP content was determined by using a cGMP Enzymeimmunoassay Biotrak™ (EIA) system (Amersham) according to the manufacturers instructions.
For the determination of intracellular nitric oxide bioavailability, HUVEC were transfected as indicated earlier, except that cells were plated on laminin-coated, glass-bottomed, black-walled 24-well plates. After incubation, cells were loaded with 10 μM of the NO-sensitive fluorescence probe DAF-FM (Molecular Probes) at 37°C for 30 min. The medium, containing loading dye, was removed and replaced with 1 mL of EGM-2 medium and incubated for another 45 min at 37°C to ensure complete de-esterification. NO synthesis was induced by stimulation with acetylcholine (100 μM) for 5 min. Fluorescence was detected with a live cell imaging Leica AS MDW inverted fluorescence microscope in which the cells were kept at 37°C. Relative fluorescence intensity per cell was quantified using ImageJ software.
2.5. Immunoprecipitation
Primary endothelial cells (HUVEC, HDMEC) or transfected HEK293 cells were lysed using RIPA buffer (1x PBS, 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS, 20 μM PMSF, 500 ng/mL leupeptin, 1.0 μg/mL aprotein, 500 ng/mL pepstatin). To minimize unspecific binding, 1 mL of protein extracts were pre-cleared by incubation with 200 μL of protein A-agarose beads (Roche) at 4°C, for 1 h. Beads were removed by centrifugation, and pre-cleared protein extracts were incubated overnight with the corresponding immunoprecipitating antibodies and 40 μL of protein A-agarose beads (Roche), at 4°C, with shaking. Beads were recovered by centrifugation at 3000 r.p.m. and washed three times with 500 μL of RIPA buffer. Washed beads were resuspended in 60 μL of 2x SDS–PAGE-loading buffer (Invitrogen) and analysed by western blot.
2.6. Western blot
Samples were boiled and resolved as described previously.13 For a detailed description of antibody sources and conditions for western blot experiments, please see the expanded section Western blot antibodies in the Supplementary material online.
2.7. Statistical analysis
Numerical data are expressed as mean ± SEM and were analysed for statistical significance using Student's t-test. The chosen significance criterion was P < 0.05.
2.8. PMCA 4 knockdown in endothelial cells
HUVEC were plated on laminin-coated, glass-bottomed, black-walled 24-well plates. After overnight incubation at 37°C, cells were infected with Ad-PMCA4shRNA or Ad-CONTROLshRNA adenoviruses (MOI 25). Ad-PMCA4shRNA or Ad-CONTROLshRNA adenoviruses were generated by cloning sequence shPMCA4 (CAACGTTCTGGACCTCATATTCAAGAGATATGAGGTCCAGAACGTTG) (encoding a short hairpin RNA silencer for isoform PMCA4), or CONTROL (TTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAA) (irrelevant sequence used as a control), respectively, into plasmid pSilencer-U6 (Invitrogen). The resulting plasmids were used to generate recombinant adenoviruses Ad-PMCA4shRNA or Ad-CONTROLshRNA by using the ViraPower Adenoviral Gateway system (Invitrogen) following the recommendations of the manufacturer. Infected HUVEC were incubated at 37°C for 5 days before being analysed for NO production; during this time, the culture medium was replaced by fresh medium every 2 days. To measure intracellular NO levels, cells were loaded with 10 μM of the NO-sensitive fluorescence probe DAF-FM (Molecular Probes) at 37°C for 30 min. The medium, containing loading dye, was removed and replaced with 1 mL of EGM-2 medium and incubated for another 45 min at 37°C to ensure complete de-esterification. NO synthesis was induced by stimulation with acetylcholine (100 μM) for 5 min.
3. Results
3.1. Endogenous PMCA and eNOS interact in human endothelial cells
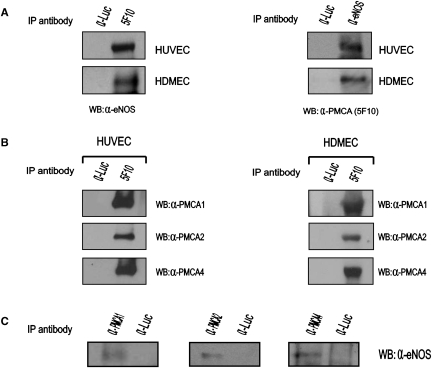
To investigate the interaction between endogenous PMCA and eNOS in endothelial cells, protein extracts from primary HUVEC or HDMEC were immunoprecipitated with the anti-PMCA 5F10 monoclonal antibody (Abcam). Immunoprecipitated proteins were probed by western blot with an anti-eNOS rabbit polyclonal antibody (Sigma). eNOS co-precipitated with PMCA in protein extracts isolated from both cellular types (Figure 1A, left panels). Converse immunoprecipitation of protein lysates with a rabbit polyclonal antibody raised against eNOS (Sigma), and subsequent western blot with the anti-PMCA 5F10 monoclonal antibody, showed co-precipitation of PMCA proteins (Figure 1A, right panels). These results demonstrate the interaction between endogenous eNOS and PMCA in human endothelial cells.
Figure 1.
Endogenous PMCA and eNOS interact in human endothelial cells. (A) eNOS and PMCA co-precipitate in endothelial cells. (Left panels) Protein lysates isolated from primary HUVEC or HDMEC were incubated with an anti-PMCA monoclonal antibody (5F10) and immunoprecipitated proteins analysed by western blot with an anti-eNOS rabbit polyclonal antibody. (Right panels) Endothelial protein lysates were immunoprecipitated with an anti-eNOS rabbit polyclonal antibody (α-eNOS) and immunoprecipitated proteins probed against the 5F10 anti-PMCA monoclonal antibody. (B) PMCA isoforms 1, 2, and 4 are expressed in endothelial cells. Protein lysates isolated from HUVEC or HDMEC were precipitated with an anti-PMCA monoclonal antibody (5F10) and precipitated proteins were probed with rabbit polyclonal antibodies recognizing specifically PMCA isoforms 1, 2, or 4. (C) Protein extracts isolated from HUVEC were immunoprecipitated with polyclonal antibodies recognizing specifically the isoforms 1 (α-PMCA1) or 2 (α-PMCA2) of PMCA. Immunoprecipitated proteins were probed with a mouse anti-eNOS antibody (Zymed). Likewise, protein extracts were precipitated with the JA9 anti-PMCA4 monoclonal antibody (α-PMCA4) and immunoprecipitated proteins subsequently probed with a rabbit anti-eNOS polyclonal antibody. In all cases, eNOS was present within the immunoprecipitated proteins, suggesting that PMCA 1, 2, and 4 isoforms interact with eNOS in endothelial cells. Immunoprecipitation with an irrelevant antibody against firefly luciferase (α-Luc) was included as a negative control in all immunoprecipitation experiments.
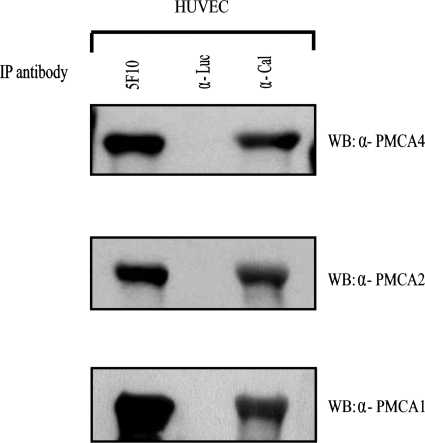
PMCA1 and 4 isoforms have been reported to be expressed in most tissues.7 PMCA2 has a more restricted expression profile and it has been detected in the cardiovascular system.17 Therefore, we decided to investigate whether these PMCA isoforms are expressed in HUVEC and HDMEC. For this purpose, we immunoprecipitated protein lysates isolated from these cellular types using the anti-PMCA 5F10 monoclonal antibody (that recognizes all PMCA isoforms). Western blot of the immunoprecipitated proteins with rabbit polyclonal antibodies reacting specifically with the isoforms 1, 2, or 4 of PMCA (Swant) revealed the presence of isoforms 1, 2, and 4 in human endothelial cells (Figure 1B).
In order to determine whether these PMCA isoforms interact with eNOS, we immunoprecipitated protein extracts isolated from HUVEC with polyclonal antibodies specific for PMCA1 or PMCA2 isoforms (Swant) and probed the immunoprecipitated proteins with an anti-eNOS monoclonal antibody (Zymed). Likewise, HUVEC protein lysates were immunoprecipitated with the JA9 anti-PMCA4 monoclonal antibody and immunoprecipitated proteins probed with an anti-eNOS rabbit polyclonal antibody (Sigma). Immunoprecipitation of specific PMCA isoforms resulted in co-precipitation of eNOS in all the cases (Figure 1C).
These results further demonstrate the interaction between endogenous PMCA and eNOS and indicate that isoforms PMCA1, -2 and -4 interact with eNOS in endothelial cells.
Control immunoprecipitations carried out in each experiment using an irrelevant antibody (anti-luciferase) precipitated no protein, confirming the selectivity of the interactions (Figure 1A–C).
3.2. The big, intracellular, catalytic domain of PMCA interacts with eNOS
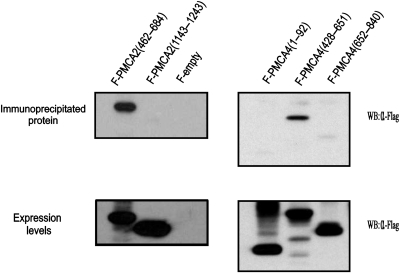
To map the domain of PMCA implicated in the interaction with eNOS, we transfected HEK293 cells with plasmids pFlag-PMCA4b(1–92), pFlag-hPMCA2b(1143–1243), pFlag-hPMCA2b(462–684), pFlag-PMCA4b(428–651), pFlag-PMCA4b(652–840), or pFlag-CMV7.1 empty (negative control). These plasmids encode PMCA N-terminal or C-terminal cytoplasmic tails, or different regions of its big catalytic intracellular domain. Commercially available recombinant human eNOS (Sigma) was added to protein lysates of the transfected cells, which were then immunoprecipitated with a rabbit polyclonal antibody against eNOS (Sigma), and analysed by western blot.
Flag-PMCA2(462–684), a Flag-tagged protein containing the proximal part of the big intracellular domain of PMCA2b, and Flag-PMCA4(428–651), containing the homologous region in PMCA4b, strongly co-precipitated with eNOS (Figure 2, upper panels). However, no precipitation was detected when Flag-PMCA4b(1–92), Flag-PMCA2b(1143–1243), or Flag-PMCA4b(652–840), Flag-tagged proteins containing the N-terminal intracellular domain of PMCA4b, the C-terminal intracellular domain of PMCA2b, or the distal part of the big intracellular domain of PMCA4b, respectively, were used in the assayed (Figure 2, upper panels).
Figure 2.
PMCA interacts with eNOS through the proximal part of the catalytic domain. Mapping of the region of PMCA involved in the interaction with eNOS. Expression vectors encoding Flag-tagged PMCA2b(462–684), PMCA2b(1143–1243), PMCA4b(1–92), PMCA4b(428–651), or PMCA4b(652–840) were transfected in HEK 293 cells. Transfection of p3xFlag-CMV7.1 empty vector (Sigma) (F-empty) was used as a negative control. Protein lysates were incubated with commercial recombinant eNOS (Sigma) (10 μM, final concentration), and complexes were precipitated with an anti-eNOS polyclonal antibody (Sigma). Flag-tagged immunoprecipitated proteins (upper panel) and the expression levels of the Flag-tagged proteins prior to immunoprecipitation (lower panel) were analysed by western blot with anti-Flag antibody.
All Flag-tagged PMCA-truncated proteins were expressed at roughly equivalent levels (Figure 2, lower panels), thus ruling out the scenario that poor expression led to the lack of interaction.
Cells transfected with empty vector p3xFlag-CMV7.1 were used as a negative control (Figure 2, upper panel).
These results show that eNOS interacts with the proximal part (amino acids 462–684 for PMCA2b, or 428–651 for PMCA4b) of the PMCA catalytic, big intracellular loop located between transmembrane regions 4 and 5.
3.3. The region 735–934 of eNOS is essential for interaction with PMCA
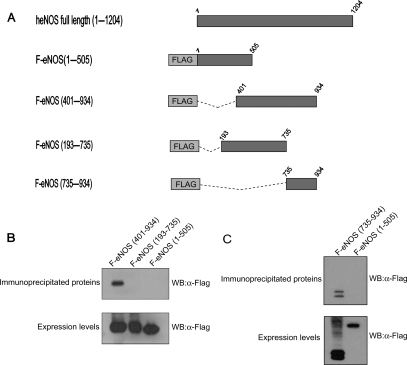
To map the domain of eNOS responsible for the interaction with PMCA, a series of Flag-tagged deletion mutants of eNOS were generated (Figure 3A) and then assayed by immunoprecipitation for their ability to interact with PMCA in mammalian cells. HEK 293 cells were co-transfected with pcDNA3-hPMCA2b and either plasmid pFlag-eNOS(401–934), pFlag-eNOS(193–735), or pFlag-eNOS(1–505), and protein extracts were immunoprecipitated with the 5F10 anti-PMCA monoclonal antibody. A truncated version of eNOS containing only amino acids 401–934 was still able to co-precipitate with PMCA, indicating that the domain of eNOS implicated in the interaction with PMCA must be included within this region (Figure 3B, upper panel). Overlapping mutants containing the regions 193–735 or 1–505 of eNOS failed to immunoprecipitate with PMCA (Figure 3B, upper panel), suggesting that the region of eNOS 735–934 is essential for the interaction with PMCA. In order to test this hypothesis, we generated a new expression plasmid, pFlag-eNOS(735–934), encoding a Flag-tagged version of eNOS containing amino acids from 735 to 934, and we analyse its capacity to interact with PMCA as described earlier. Flag-eNOS(735–934) co-precipitated with PMCA in immunoprecipitation assays (Figure 3C, upper panel).
Figure 3.
Interaction with PMCA maps to the region 735–934 of eNOS. (A) Schematic representation of Flag-tagged deletion mutants of eNOS. (B) Expression vectors encoding human PMCA2b and Flag-tagged deletion mutants of eNOS encoding amino acids 401–934, 193–735, or 1–505 were co-transfected in HEK 293 cells. Protein lysates were incubated with the 5F10 anti-PMCA monoclonal antibody. Immunoprecipitated proteins (upper panel) and the expression levels of Flag-tagged proteins prior to immunoprecipitation (lower panel) were probed by western blot with anti-Flag antibody. (C) Expression vectors encoding human PMCA2b and Flag-tagged deletion mutants encoding amino acids 735–934 or 1–505 were co-transfected in HEK 293 cells. Protein lysates were immunoprecipitated and probed by western blot as described in (B).
Levels of the Flag proteins prior to immunoprecipitation were analysed by western blot to ensure differences were not attributable to differential expression of the fusion proteins (Figure 3B and C, lower panels).
These results demonstrate that the region 735–934 of eNOS is necessary and sufficient to maintain the interaction with PMCA.
3.4. PMCA modulates eNOS phosphorylation in regulatory residues Thr-495 and Ser-1177
Compelling evidence has recently revealed a novel role for PMCA proteins as regulators of Ca2+/calmodulin-dependent enzymes.6 It is thought that PMCA inhibits the activity of these enzymes by tethering them to low calcium cellular microenvironments created by the calcium extrusion function of PMCA. The identification of eNOS as a PMCA interaction partner prompted us to investigate the functional consequences of this interaction upon eNOS activity and, therefore, NO production in endothelial cells.
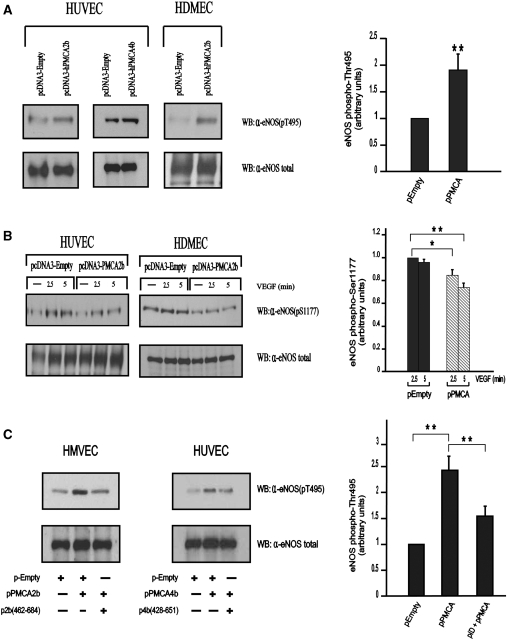
Multi-site phosphorylation is one of the mechanisms involved in the regulation of eNOS activity.5 Phosphorylation at Ser-1177 of eNOS has been shown to increase its activity, whereas phosphorylation at Thr-495 is inhibitory.5 To investigate the effect of ectopic expression of PMCA on the activity of eNOS, we transfected HUVEC and HDMEC with an expression vector encoding human PMCA2b or PMCA4b and analysed by western blot the phosphorylation status of endogenous eNOS at Thr-495 and Ser-1177 by using the corresponding phospho-specific antibodies. Ectopic expression of PMCA2b in endothelial cells significantly increased phosphorylation at Thr-495 of eNOS when compared with that of control cells transfected with empty plasmid (Figure 4A, upper panels). Likewise, ectopic expression of isoform PMCA4b yielded an identical result (Figure 4A, upper panels). Phosphorylation of Ser-1197 was, however, slightly reduced by ectopic expression of PMCA2b in endothelial cells (Figure 4B, upper panels).
Figure 4.
PMCA modulates eNOS phosphorylation in regulatory residues Thr-495 and Ser-1177. (A) Ectopic expression of PMCA2b or 4b in endothelial cells increases the phosphorylation status of endogenous eNOS in Thr-495. HUVEC or HDMEC were transfected with 5 μg of plasmids pcDNA3-hPMCA2b, pcDNA-hPMCA4b, or p-cDNA3-Empty (negative control) and proteins analysed by western blot with a mouse antibody recognizing specifically eNOS phosphorylated in the residue Thr-495 (α-eNOS(pT495)). Histogram shows data as mean ± SEM of seven independent experiments performed with four different batches of cells. **P < 0.01. (B) Expression of PMCA2b in endothelial cells leads to a reduction in eNOS Ser-1177 phosphorylation. HUVEC or HDMEC were transfected with 5 μg of plasmids pcDNA3-hPMCA2b or pcDNA3-Empty (negative control). Transfected cells were incubated with VEGF (25 ng/mL) for the times indicated to induce phosphorylation of eNOS in Ser-1177 as described.25 Protein lysates were analysed by western blot with a mouse antibody recognizing specifically eNOS phosphorylated in Ser-1177 (α-eNOS(pS1177). Histogram shows data as mean ± SEM of six independent experiments performed with four different batches of cells. *P < 0.05, **P < 0.01. (C) Disruption of the PMCA–eNOS interaction reverses the PMCA-dependent effect on eNOS Thr-495 phosphorylation. HMVEC (left panels) or HUVEC (right panels) were transfected with either 10 μg of empty vector; 5 μg of empty vector plus 5 μg of a vector encoding PMCA2b or PMCA4b as indicated; or 5 μg of an expression vector for the indicated PMCA protein plus 5 μg of a pFlag vector encoding the corresponding interaction domain. Western blot was performed as described in (A). Histogram shows data as mean ± SEM of five independent experiments performed with three different batches of cells. **P < 0.01. In all experiments, total levels of eNOS protein were determined by analysing lysates from transfected cells using a polyclonal rabbit anti-eNOS antibody (α-eNOS total).
These results strongly suggest that PMCA negatively regulates eNOS activity by promoting a significant increase in phosphorylation of Thr-495 (inhibitory phosphorylation) and a slight decrease in phosphorylation of Ser-1177 (activating phosphorylation).
To investigative whether the interaction between PMCA and eNOS plays a significant role in the observed increase in eNOS phosphorylation at Thr-495, we blocked the interaction between PMCA and eNOS by co-expressing PMCA2b or 4b, together with Flag-PMCA2(462–684) or Flag-PMCA4(428–651), respectively. In both cases, blockage of the PMCA–eNOS interaction (by overexpression of the corresponding interaction domain) reversed the PMCA-mediated increase in Thr-495 phosphorylation (Figure 4C, upper panels). These results demonstrate that the PMCA–eNOS interaction is essential for the PMCA-mediated increase in eNOS inhibitory phosphorylation.
In all cases, the levels of total eNOS in the samples were analysed using a rabbit polyclonal anti-eNOS antibody (Sigma) to rule out the possibility that the differences detected in the phosphorylation levels of eNOS were attributable to differential levels of the protein in the samples after transfection (Figure 4A–C, lower panels).
3.5. PMCA significantly inhibits NO production by endothelial cells
To determine whether the observed inhibitory effect exerted by PMCA on eNOS activity led to a reduction on NO production in endothelial cells, we transfected HUVEC with plasmid pcDNA3-hPMCA2b or pcDNA3-hPMCA4b.
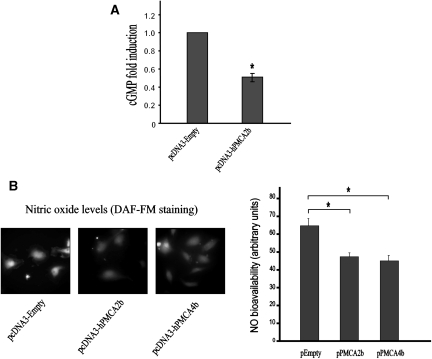
NO production was determined by measuring cGMP as a surrogate marker. Ectopic expression of human PMCA2b in HUVEC resulted in a significant reduction (43% inhibition, P ≤ 0.05) in NO production when compared with the levels of NO produced by HUVEC transfected with the pcDNA3-Empty vector (negative control) (Figure 5A). To further confirm the decrease in NO production in endothelial cells transfected with PMCA, intracellular NO levels were measured by loading cells with 10 μM of the NO-sensitive fluorescence probe DAF-FM (Molecular Probes). NO synthesis was stimulated by treating transfected cells with acetylcholine (100 μM) for 5 min. Acetylcholine-induced NO production in endothelial cells expressing PMCA2b or PMCA4b was significantly lower (28.2 and 30.6%, respectively, P ≤ 0.01) than that of cells transfected with the pcDNA3-Empty vector (Figure 5B).
Figure 5.
PMCA negatively regulates NO production in endothelial cells. Human PMCA2b or PMCA4b were ectopically expressed in HUVEC by transfection of five μg of the corresponding expression vector (A). NO synthesis was induced by addition of the A23187 ionophore (0.5 μM) 3 min before lyses. NO-dependent cGMP production was calculated in relation to HUVEC transfected with 5 μg of pcDNA3-Empty vector. Mean ± SEM values of six independent experiments are shown. *P < 0.05. (B) Intracellular nitric oxide level in transfected HUVEC was assessed by staining cells with the NO-sensitive dye DAF-FM. NO synthesis was stimulated by addition of acetylcholine (100 μM) for 5 min. Representative microscopy fields of DAF-FM staining in cells are shown in left panels. Mean ± SEM values of fluorescence units measured from 25 different microscopy fields taken from six independent preparations are shown. **P < 0.01.
Together, our results demonstrate a relevant role for PMCA as a negative regulator of NO production in primary endothelial cells via an inhibitory association with eNOS.
3.6. PMCA and calcineurin interact in human endothelial cells
We have previously reported the interaction of PMCA with the Ca2+/calmodulin-dependent phosphatase calcineurin in breast cancer cells.14 The interaction in endothelial cells of PMCA with eNOS (another Ca2+/calmodulin-dependent enzyme) that we show in this work prompted us to investigate whether PMCA also interacts with calcineurin in human endothelial cells. For this purpose, we immunoprecipitated protein lysates from HUVEC with the 5F10 anti-PMCA monoclonal antibody. Immunoprecipitated proteins were analysed by western blot using antibodies recognizing specifically PMCA isoforms 1, 2, or 4. PMCA1, 2, and 4 were found within the immunoprecipitated proteins (Figure 6), demonstrating that endogenous PMCA interacts with calcineurin in human endothelial cells.
Figure 6.
Endogenous PMCA and calcineurin interact in endothelial cells. Protein lysates from HUVEC were incubated with an anti-PMCA monoclonal antibody (5F10), anti-Luciferase antibody (α-Luc), or an anti-calcineurin antibody (α-Cal) as indicated. Immunoprecipitated proteins were analysed by western blot with antibodies recognizing specifically isoform 1 (α-PMCA1), 2 (α-PMCA2), or 4 (α-PMCA4) of PMCA. The presence of the PMCA isoforms within the immunoprecipitated proteins indicates that endogenous PMCA and calcineurin interact in endothelial cells.
4. Discussion
Through the production of NO, eNOS represents a crucial regulator of cardiovascular physiology. In this work, we describe a novel role for the plasma membrane Ca2+/calmodulin ATPase pump, PMCA, as a negative regulator of NO production in endothelial cells. Endogenous eNOS and PMCA interact in primary HUVEC and HDMEC, leading to a decrease in eNOS activity and subsequent NO production by activated endothelial cells. In agreement with our observations on the functional relevance of the PMCA–eNOS interaction as a novel mechanism for eNOS regulation, inhibitory interactions between eNOS and the intracellular domains of other plasma membrane proteins such as caveolin-1,18 the bradykinin B2 receptor,19 the endothelin-1 ETB receptor,20 and the angiotensin II AT1 receptor20 have been reported previously.
We demonstrate in this paper that PMCA1, 2, and 4 interact with eNOS in endothelial cells (Figure 1C). The three isoforms have calcium extrusion catalytic activity and, therefore, it is likely that there might be redundancy in their function as negative regulators of eNOS. In fact, supporting this hypothesis, knockdown of PMCA4 in endothelial cells by infection with an adenovirus expressing a short hairpin RNA silencer specific for PMCA4 did not alter NO production in either basal or acetylcholine-stimulated conditions (see Supplementary material on line, Figure S1).
The PMCA interaction domain is located in the proximal region of the big intracellular catalytic domain defined by trans-membrane segments 4 and 5. PMCA2b and 4b interact with eNOS through the same homologous region: 462–684 in PMCA2, or 428–651 in PMCA4 (Figure 2). Given the high percentage of amino acid homology shown by the PMCA isoforms in this region, it is very likely that PMCA1 also interacts with eNOS through the domain equivalent to those mapped for PMCA2 and 4.
Our group has previously reported an inhibitory interaction between endogenous PMCA and the Ca2+/calmodulin-dependent phosphatase calcineurin A in breast cancer cells.14 In this work, we demonstrate that PMCA also interacts with calcineurin A in human endothelial cells. The interaction between PMCA and eNOS takes place through the same domains of PMCA2 and 4 that reported for the PMCA–calcineurin interaction.14 The interaction of another Ca2+/calmodulin-dependent protein via the same domain of PMCA suggests an important role for this region in the association with partner proteins that are regulated by Ca2+.
We have also located the interaction domain with PMCA to the region 735–934 of eNOS (Figure 3). An examination of the sequences corresponding to the interaction domains in eNOS (this work) and calcineurin A13 did not show any significant homology between the two regions, suggesting that, probably, eNOS and calcineurin interact with two different small fragments within the interaction domain of PMCA. This leads to the intriguing possibility that PMCA, eNOS, and calcineurin might be present in endothelial cells as a ternary complex.
In this work, we demonstrate that ectopic expression of PMCA results in a significant increment in the phosphorylation of residue Thr-495 of eNOS (Figure 4A). The molecular mechanism behind the PMCA-mediated increase in eNOS phosphorylation is not understood at the moment. Interestingly, Harris et al.21 reported that calcineurin mediates dephosphorylation of Thr-495 of eNOS. We have previously reported that PMCA inhibits calcineurin activity,13,14 thus, if PMCA, calcineurin, and eNOS are part of a macromolecular ternary complex, PMCA-dependent inhibition of calcineurin would result in a decrease in calcineurin-mediated dephosphorylation of Thr-495 contributing to eNOS inhibition. The possibility that PMCA, calcineurin, and eNOS are forming a ternary macromolecular complex, introducing a new level of regulation in NO production by endothelial cells, requires further investigation. Supporting this hypothesis, PMCA has been reported to participate in the organization of a macromolecular protein complex formed by endogenous PMCA, α-1 syntrophin, and nNOS in cardiac cells.22 Likewise, eNOS has been found to be part of a ternary complex together with caveolin-1 and NOSTRIN.23
Interestingly, when we disrupted the PMCA–eNOS interaction by co-expressing PMCA together with the PMCA interaction domain, the effect on eNOS Thr-495 phosphorylation was reversed (Figure 4C). This result rules out the possibility that the PMCA inhibitory effect on eNOS activity was due to general Ca2+ clearance resulting from PMCA ectopic expression, and demonstrates the functional significance of the PMCA–eNOS interaction. These data are in agreement with the idea of PMCA inhibiting the activity of Ca2+/calmodulin-dependent proteins by tethering them to low Ca2+/calmodulin cellular microdomains created by the Ca2+ extrusion activity of the pump.
The role of Ca2+ in the PMCA–eNOS interaction is not known at present, but it is tempting to speculate with the possibility that the levels of intracellular Ca2+ could be implicated in the regulation of the PMCA–eNOS interaction. In this context, it has been reported that increments in the intracellular levels of Ca2+/calmodulin disrupt the interaction between eNOS and caveolin-1, leading to the activation of eNOS.24 In a similar way, increments in intracellular Ca2+ levels might disrupt the PMCA–eNOS interaction leading to eNOS activation. This increase in intracellular Ca2+ would also trigger PMCA activation. As a result of PMCA activation, Ca2+ would be removed around the PMCA microenvironment, and the local reduction in intracellular Ca2+ levels would restore the interaction between eNOS and PMCA, and the subsequent inhibition of eNOS activity. The involvement of intracellular Ca2+ levels in the regulation of the PMCA–eNOS interaction, and in the potential formation of a ternary complex PMCA/eNOS/calcineurin, deserves further investigation.
In conclusion, this work shows an inhibitory interaction between endogenous PMCA and eNOS in human endothelial cells and suggests PMCA as an important regulator of NO signalling in endothelial cells. Considering the relevant role of NO in cardiovascular physiology and pathophysiology, the implications of this interaction are far-reaching. Loss of NO synthesis and bioavailability is a hallmark of cardiovascular disease.1 Disruption of the PMCA–eNOS interaction might, therefore, have an important significance as a potential therapeutic target to modulate NO bioactivity in patients with cardiovascular disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Research Institute in Healthcare Science (RIHS), School of Applied Sciences, University of Wolverhampton. M.H. is the recipient of a PhD studentship funded by RIHS, University of Wolverhampton. M.E. is funded by Wellcome Trust Grant (085132/Z/08/Z).
Conflict of interest. none declared.
Supplementary Material
References
- 1.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. doi:10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. Bioenerg. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. doi:10.1016/S0005-2728(99)00016-X. [DOI] [PubMed] [Google Scholar]
- 3.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. doi:10.1016/S0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 4.Kone BC, Kuncewicz T, Zhang W, Yu ZY. Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am J Physiol Renal Physiol. 2003;258:F178–F190. doi: 10.1152/ajprenal.00048.2003. [DOI] [PubMed] [Google Scholar]
- 5.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. doi:10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oceandy D, Stanley PJ, Cartwright EJ, Neyses L. The regulatory function of plasma-membrane Ca(2+)-ATPase (PMCA) in the heart. Biochem Soc Trans. 2007;35:927–930. doi: 10.1042/BST0350927. doi:10.1042/BST0350927. [DOI] [PubMed] [Google Scholar]
- 7.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Di Leva F, Domi T, Fedrizzi L, Lim D, Carafoli E. The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch Biochem Biophys. 2008;476:65–74. doi: 10.1016/j.abb.2008.02.026. doi:10.1016/j.abb.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Schuh K, Uldrijan S, Telkamp M, Röthlein N, Neyses L. The plasmamembrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase I. J Cell Biol. 2001;155:201–205. doi: 10.1083/jcb.200104131. doi:10.1083/jcb.200104131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuh K, Quaschning T, Knauer S, Hu K, Kocak S, Roethlein N, et al. Regulation of vascular tone in animals overexpressing the sarcolemmal calcium pump. J Biol Chem. 2003;278:41246–41252. doi: 10.1074/jbc.M307606200. doi:10.1074/jbc.M307606200. [DOI] [PubMed] [Google Scholar]
- 11.Oceandy D, Cartwright EJ, Emerson M, Prehar S, Baudoin FM, Zi M, et al. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation. 2007;115:483–492. doi: 10.1161/CIRCULATIONAHA.106.643791. doi:10.1161/CIRCULATIONAHA.106.643791. [DOI] [PubMed] [Google Scholar]
- 12.Schuh K, Uldrijan S, Gambaryan S, Roethlein N, Neyses L. Interaction of the plasma membrane Ca2+ pump 4b/CI with the Ca2+/calmodulin-dependent membrane-associated kinase CASK. J Biol Chem. 2003;78:9778–9783. doi: 10.1074/jbc.M212507200. doi:10.1074/jbc.M212507200. [DOI] [PubMed] [Google Scholar]
- 13.Buch MH, Pickard A, Rodriguez A, Gillies S, Maass AH, Emerson M, et al. The sarcolemmal calcium pump inhibits the calcineurin/nuclear factor of activated T-cell pathway via interaction with the calcineurin A catalytic subunit. J Biol Chem. 2005;80:29479–29487. doi: 10.1074/jbc.M501326200. doi:10.1074/jbc.M501326200. [DOI] [PubMed] [Google Scholar]
- 14.Holton M, Yang D, Wang W, Mohamed TM, Neyses L, Armesilla AL. The interaction between endogenous calcineurin and the plasma membrane calcium-dependent ATPase is isoform specific in breast cancer cells. FEBS Lett. 2007;581:4115–4119. doi: 10.1016/j.febslet.2007.07.054. doi:10.1016/j.febslet.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Chang B, Blair NS, Sargent M, York AJ, Robbins J, et al. Plasma membrane Ca2+-ATPase isoform 4 antagonizes cardiac hypertrophy in association with calcineurin inhibition in rodents. J Clin Invest. 2009;119:976–985. doi: 10.1172/JCI36693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armesilla AL, Williams JC, Buch MH, Pickard A, Emerson M, Cartwright EJ, et al. Novel functional interaction between the plasma membrane Ca2+ pump 4b and the proapoptotic tumor suppressor Ras-associated factor 1 (RASSF1) J Biol Chem. 2004;279:31318–31328. doi: 10.1074/jbc.M307557200. doi:10.1074/jbc.M307557200. [DOI] [PubMed] [Google Scholar]
- 17.Santiago-García J, Mas-Oliva J, Saavedra D, Zarain-Herzberg A. Analysis of mRNA expression and cloning of a novel plasma membrane Ca(2+)-ATPase splice variant in human heart. Mol Cell Biochem. 1996;155:173–182. doi: 10.1007/BF00229314. [DOI] [PubMed] [Google Scholar]
- 18.García-Cardeña G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. doi:10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 19.Ju H, Venema VJ, Marrero MB, Venema RC. Inhibitory interactions of the bradykinin B2 receptor with endothelial nitric-oxide synthase. J Biol Chem. 1998;273:24025–24029. doi: 10.1074/jbc.273.37.24025. doi:10.1074/jbc.273.37.24025. [DOI] [PubMed] [Google Scholar]
- 20.Marrero MB, Venema VJ, Ju H, He H, Liang H, Caldwell RB, et al. Endothelial nitric oxide synthase interactions with G-protein-coupled receptors. Biochem J. 1999;343:335–340. doi:10.1042/0264-6021:3430335. [PMC free article] [PubMed] [Google Scholar]
- 21.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, et al. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. doi:10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 22.Williams JC, Armesilla AL, Mohamed TM, Hagarty CL, McIntyre FH, Schomburg S, et al. The sarcolemmal calcium pump, alpha-1 syntrophin, and neuronal nitric-oxide synthase are parts of a macromolecular protein complex. J Biol Chem. 2006;281:23341–23348. doi: 10.1074/jbc.M513341200. doi:10.1074/jbc.M513341200. [DOI] [PubMed] [Google Scholar]
- 23.Schilling K, Opitz N, Wiesenthal A, Oess S, Tikkanen R, Müller-Esterl W, et al. Translocation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol Biol Cell. 2006;17:3870–3880. doi: 10.1091/mbc.E05-08-0709. doi:10.1091/mbc.E05-08-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feron O, Michel JB, Sase K, Michel T. Dynamic regulation of endothelial nitric oxide synthase: complementary roles of dual acylation and caveolin interactions. Biochemistry. 1998;37:193–200. doi: 10.1021/bi972307p. doi:10.1021/bi972307p. [DOI] [PubMed] [Google Scholar]
- 25.Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, et al. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. doi:10.1016/S0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.